Introduction
Ovarian cancer remains a leading cause of mortality
from gynecological malignancy, with >204,000 new cases and
125,000 deaths each year, accounting for 4% of all cancer cases and
4.2% of all cancer deaths in women around the world (1). The incidence of ovarian cancer
increases with age and >70% of the patients are diagnosed with
late stage disease after distant metastasis has occurred. The
5-year survival rate for the patients diagnosed with late stage
disease is <20%, even with extensive surgery and chemotherapy
(2,3). Chemotherapy with the administration of
cisplatin (cis-diamminedichloroplatinum (II)) or cisplatin in
combination with taxanes is the current standard of care (4,5).
Although the majority of the ovarian tumors were initially
sensitive to chemotherapy (6,7),
long-term administration of cisplatin has been shown to result in
the development of chemotherapeutic drug resistance in the cancer
cell population (8,9). Cisplatin resistance is a major
obstacle to the successful therapy of recurrent ovarian tumors and
responsible for poor long-term overall survival (6,7). The
suggested mechanisms for cisplatin resistance include the increase
in intracellular thiols in the redox pathway (10), defects in the apoptotic pathway and
the altered activation of signaling pathways, such as PI3K/Akt
(11), MAPK (12) or NF-κB (13). Investigators have targeted these
pathways in an attempt to circumvent cisplatin resistance (13,14).
Salinomycin is a 751 Da monocarboxylic polyether
antibiotic belonging to the group of ionophores that is produced by
Streptomyces albus (strain no. 80614) (15). It is commonly used as a coccidiostat
in poultry and other livestock and is fed to ruminants to improve
nutrient absorption and feed efficiency (16). Recently, salinomycin has been
reported to selectively deplete human breast cancer stem cells from
tumorspheres and to inhibit mammary tumor growth and metastasis
in vivo(17). In another
report, salinomycin has been shown to induce apoptosis in human
cancer cells, including those showing wild-type p53 or p53 mutation
and multi-drug resistance due to the overexpression of Bcl-2,
P-glycoprotein or 26S proteasomes with deregulated proteolytic
activity (18). These results
strongly suggested that salinomycin should be considered an
anticancer compound. The mechanism of the anticancer action of
salinomycin has yet to be delineated. Salinomycin has been shown to
activate a particular apoptotic pathway not accompanied by cell
cycle arrest and independent of tumor suppressor protein p53,
caspase activation, the CD95/CD95L system or the proteasome
(18). More recently, salinomycin
has been reported to overcome ABC transporter-mediated multidrug
and apoptosis resistance (19) and
act as a potent inhibitor of multidrug resistance gp170 (20). Furthermore, salinomycin has been
shown to inhibit the activity of the Wnt signaling pathway,
recently appointed as an essential regulator of cancer stem cell
(CSC) properties in chronic lymphocytic leukemia cells (21).
The present study aimed to determine the biological
anticancer activity of salinomycin towards the cisplatin-resistant
human ovarian cancer cell line and its tumor xenograft model, as
well as to derive mechanistic insights into the action of
salinomycin. The results showed that salinomycin inhibited
cell-growth and induced apoptosis in a cisplatin-resistant human
ovarian cancer cell line in vitro and suppressed tumor
growth in vivo. The salinomycin-induced apoptosis in the
cisplatin-resistant ovarian cancer cell line may correlate with an
increase in the activation of p38 MAPK.
Materials and methods
Cell lines and culture
The ovarian cancer cell lines used in this study
were OV2008, C13, A2780, A2780-cp (A/CP), SKOV3 (p53-negative) and
OVCAR3 (p53-mutant). Two pairs of cisplatin-sensitive and
cisplatin-resistant ovarian cancer cell lines (OV2008 and C13,
A2780 and A/CP, respectively) were kindly provided by Dr Gaetano
Marverti (University of Modena and Reggio Emilia, Italy). The cell
lines were routinely grown in a humidified atmosphere at 5%
CO2 and 37°C, and then incubated with RPMI-1640 standard
medium supplemented with 10% fetal bovine serum (FBS), antibiotics
(100 IU/ml penicillin and 100 μg/ml streptomycin) and L-glutamine
(2 mM). Exponentially growing cells were used in the study. These
reagents were provided by Invitrogen (Carlsbad, CA, USA).
Growth inhibition assay
The growth inhibitory effects of salinomycin or
cisplatin on the six ovarian cancer cell lines were determined by
measuring cell viability using the resazurin reduction assay.
Briefly, cells were seeded in 100-μl media on 96-well microtitre
plates at a density of 5,000 cells/well. Subsequent to overnight
incubation, the cells were exposed to a range of different
concentrations of salinomycin (S4526; Sigma-Aldrich, St. Louis, MO,
USA) or cisplatin (Ebewe 0.5 mg/ml, Ebewe Pharma Schweiz AG, Cham,
Switzerland) and grown at 37°C under a 5% CO2 atmosphere
for 24–72 h. Resazurin [(5 μl) of 0.02% (w/v)] (Sigma-Aldrich,
R7017) in phosphate-buffered saline (PBS) was then added to each
well and incubation was continued for an additional 2 h. At the
end, fluorescence was read using a Spectramax Gemini XS microplate
reader (λexc=544 nm, λem=590 nm).
Cell apoptosis detection
Cell apoptosis was detected using the Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis
assay kit (BD Pharmingen, San Diego, CA, USA) in combination with
flow cytometry (CyAn ADP; Dako, Carpinteria, CA, USA). Subsequent
to pre-treatment with salinomycin for 12, 24 and 36 h, as well as
solvent control [0.1 % dimethyl sulfoxide (DMSO)], respectively,
the cells were collected by quick trypsinization to minimize
potentially high Annexin V background levels in adherent cells. The
cells were then washed twice with cold PBS and re-suspended in
binding buffer at a concentration of 1×106 cells/ml. The
cells (100 μl) were stained with 5 μl Annexin V/FITC and 5 μl PI
and were incubated in the dark at room temperature for 15 min.
Subsequent to the addition of 400 μl binding buffer, the cells were
analyzed by flow cytometry. Cells negative for both Annexin V and
PI were viable, Annexin V+/PI- cells were in
early apoptosis, while Annexin V+/PI+ cells
were necrotic or in late apoptosis. The percentages of apoptotic
cells were analyzed by the FlowJo software (Tree Star, Ashland, OR,
USA).
Cell cycle distribution analysis
To evaluate cell cycle profile, cells
(~1×106), pre-treated with salinomycin for 12 and 24 h
(0.1% DMSO as the solvent control), were harvested, washed twice
with PBS, then fixed and stored in ice-cold 70% (v/v) ethanol at
−20°C. Prior to analysis, the samples were washed again with PBS,
and then incubated in PI/RNAse staining buffer (BD Pharmingen) at
room temperature in the dark for at least 15 min. Subsequent to
filtration with a view to remove cellular debris, the single-cell
suspensions were analyzed using a flow cytometer. The cell cycle
parameters were analyzed using the FlowJo software.
Phosphoprotein assay
A panel of phosphoproteins was measured in
duplicate, using a bead-based multiplex assay (Bio-Plex
Phosphoprotein Detection, Bio-Rad Laboratories, Hercules, CA, USA),
according to the manufacturer's instructions (22,23).
Briefly, the cells were treated with salinomycin or with the
solvent control (0.1% DMSO) for the indicated time interval and
then the cell lysates were collected with the Bio-Plex Cell Lysis
kit. The protein concentration was measured with a detergent
compatible (DC) protein assay (Bio-Rad Laboratories) and adjusted
to 600 μg/ml. Coupled beads (50 μl), recognizing phosphorylated
Akt, IκB-α, ERK1/2, JNK and p38 MAPK, respectively, were added to
the 96-well filter plate, and were then washed twice. The same
volume of the cell lysates was added and incubated with the beads
for 15–18 h (overnight). Then, 25 μl of biotin-labelled detection
antibodies were added after washing and then incubated for 30 min.
Streptavidin-PE (50 μl) was added subsequent to washing then
incubated in the dark for 10 min. After rinsing, 125 μl of
re-suspension buffer was added, and the phosphoproteins were
analyzed by a Bio-Plex 200 system and the Bio-Plex Manager software
(Bio-Rad Laboratories). In this assay, the lysates of the
phosphotase-treated HeLa cells, TNF-α-treated HeLa cells,
UV-treated HEK293 cells and EGF-treated HEK293 cells, provided by
the Bio-Plex phosphoprotein assay, were used as the background and
the positive control of phospho-IκB-α (Ser32/Ser36), phospho-p38
MAPK (Thr180/Tyr182), phospho-JNK (Thr183/Tyr185), phospho-Akt
(Ser473), as well as phospho-ERK1/2 (Thr202/Tyr204, Thr185/Tyr187).
This experiment was repeated in duplicate.
Ovarian cancer tumor xenografts in
mice
Female mice of NOD/SCID were bred in-house in the
Animal Center (Tierversuchsstation) of the Department of
Biomedicine of the University Hospital of Basel, and were used at 6
weeks of age. The procedures were approved by the Cantonal
Veterinary Office (Kantonales Veterinäramt) and performed in
accordance with the regulations concerning animal experiments. For
the in vivo salinomycin treatment study, cultured ovarian
cancer cells (2×106 cells/mouse in 0.1 ml saline) were
subcutaneously injected into the back of NOD/SCID mice. The
following day, mice were randomized into two groups (n=5/group).
The treatment was initiated 24 h subsequent to injection. The two
experimental groups were administered salinomycin (5 mg/kg)
(17) and 5% ethanol (vehicle),
respectively, through intraperitoneal injection every other day for
three weeks. The size of the tumor was measured every two days
using a digital vernier caliper. The tumor volume was estimated
using the formula: volume= (a × b2) × π/6, where a and b
are major and minor axes of the tumor.
Statistical analysis
The data were expressed as the mean values ±
standard deviation. The growth-inhibitory curve was analyzed using
the GraphPad Prism 5.01 software. The Student's t-test was carried
out to compare the groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Growth-inhibitory effect of salinomycin
in ovarian cancer cell lines
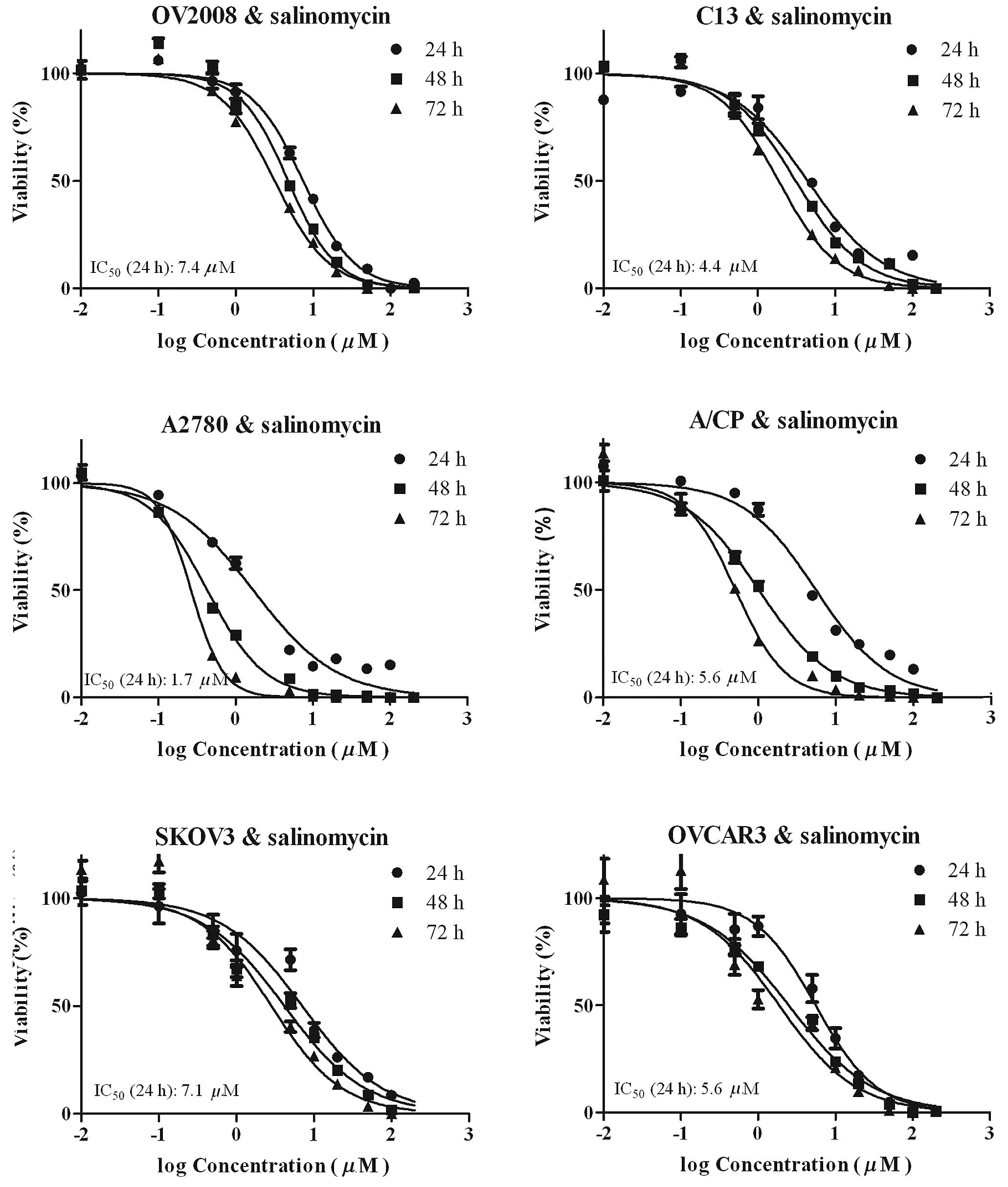
The growth-inhibitory effect of salinomycin on
OV2008, C13, A2780, A/CP, SKOV3 and OVCAR3 cell lines is shown in
Fig. 1. The effect of incubation
time and concentration on the viability of the ovarian cancer cell
lines by salinomycin was studied. The cells were exposed for 24, 48
or 72 h to salinomycin at a (0.01–200 μM) concentration range, and
cell viability was measured by the resazurin reduction assay. In
the six cell lines studied, the inhibition ratio of cell viability
showed a concentration- and time-dependence pattern. The
IC50 value of salinomycin or cisplatin on the six
ovarian cancer cell lines is shown in Table I. This finding demonstrated that
salinomycin was slightly more potent in A2780 compared to the rest
of the cell lines and was almost equipotent in the remaining five
cell lines, including cisplatin-resistant ovarian cancer cells,
such as C13, A/CP and SKOV3. The IC50 (24 h) range of
salinomycin on the six ovarian cancer cell lines was 1.7–7.4 μM. In
addition, salinomycin was more potent in C13 cells, approximately
9-fold resistant to cisplatin, compared to its parent OV2008 cells
(cisplatin-sensitive cells). Thus, the C13 cisplatin-resistant
human epithelial ovarian cancer cell line attracted more attention
and was used in most parts of the study.
 | Table IIC50 of cisplatin or
salinomycin on ovarian cancer cell lines. |
Table I
IC50 of cisplatin or
salinomycin on ovarian cancer cell lines.
| Cisplatin | Salinomycin |
|---|
|
|
|
|---|
| Cell lines | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h |
|---|
| OV2008 |
| IC50
(μM) | 8.08 | 2.49 | 0.60 | 7.44 | 4.78 | 3.20 |
| 95% CI | 6.15–10.63 | 2.09–2.97 | 0.52–0.68 | 6.80–8.14 | 4.12–5.55 | 2.90–3.53 |
| C13 |
| IC50
(μM) | 77.10 | 24.29 | 9.69 | 4.42 | 3.10 | 1.86 |
| 95% CI | 65.95–90.13 | 21.85–27.01 | 8.19–11.46 | 3.62–5.39 | 2.67–3.59 | 1.67–2.06 |
| A2780 |
| IC50
(μM) | 6.48 | 1.60 | 1.03 | 1.70 | 0.43 | 0.27 |
| 95% CI | 5.34–7.86 | 1.30–1.96 | 0.53–2.00 | 1.40–2.07 | 0.39–0.48 | 0.21–0.33 |
| A/CP |
| IC50
(μM) | 26.09 | 3.35 | 1.84 | 5.56 | 1.02 | 0.51 |
| 95% CI | 23.53–28.91 | 2.75–4.09 | 1.32–2.55 | 4.75–6.51 | 0.91–1.13 | 0.44–0.58 |
| SKOV3 |
| IC50
(μM) | 54.55 | 11.39 | 2.09 | 7.08 | 4.17 | 2.83 |
| 95% CI | 38.97–76.35 | 7.17–18.10 | 1.69–2.59 | 5.33–9.40 | 3.47–5.01 | 2.14–3.76 |
| OVCAR3 |
| IC50
(μM) | 13.23 | 2.12 | 0.63 | 5.56 | 2.50 | 1.87 |
| 95% CI | 10.90–16.06 | 1.83–2.46 | 0.55–0.72 | 4.30–7.18 | 2.09–2.98 | 1.32–2.67 |
Effect of salinomycin on tumor cell
apoptosis and the cell cycle
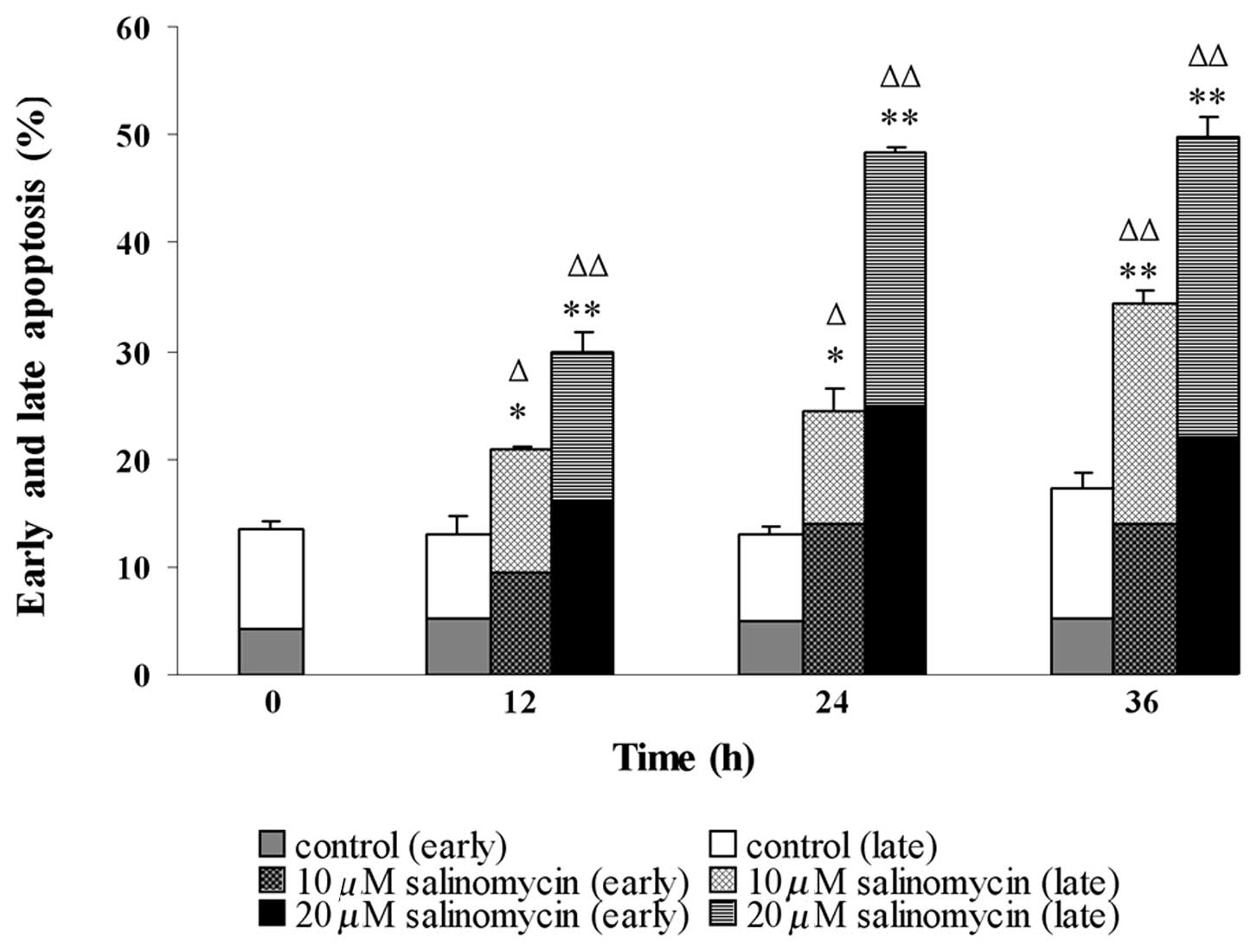
Salinomycin-treated C13 cells were analyzed by flow
cytometry to distinguish between early or late cell apoptosis,
subsequent to simultaneous staining with Annexin V and PI. Compared
to the control, salinomycin treatment significantly increased the
percentages of apoptotic cells in C13, demonstrating a
concentration- and time-dependent pattern (Fig. 2). In the control culture, 4.25±0.46%
cells were in the early, whereas 9.31±0.12% cells were in the late
apoptotic stage. Subsequent to treatment of cells with 20 μM
salinomycin for 12 h, the percentages of apoptotic cells in an
early phase increased to 16.2±0.68%, while that of the late phase
increased to 13.7±1.17%. By contrast, when cells were treated with
salinomycin for 24 and 36 h, 25.0±0.70 and 22.1±1.91% of them were
in early, and 23.3±1.08 and 27.6±1.13% in late apoptosis. These
results clearly indicated that salinomycin evoked apoptosis in C13
cells.
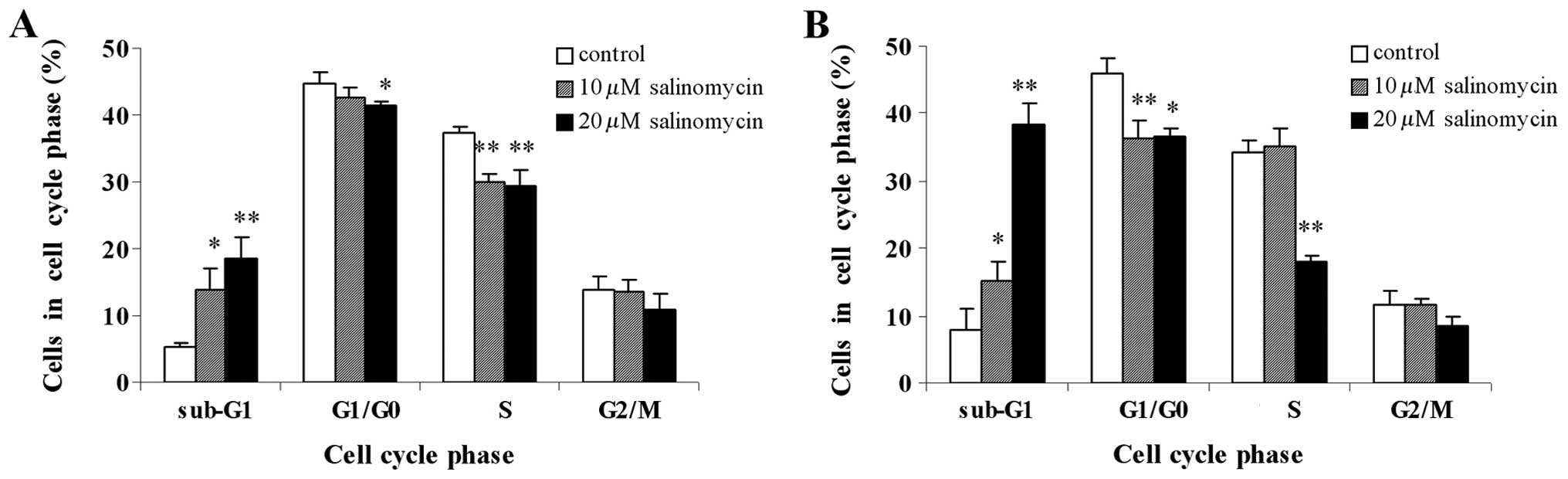
The effect of different concentrations of
salinomycin (10 and 20 μM) on the cell cycle phases was
investigated in C13 cells cultured over different times (12 and 24
h) by DNA content analysis, in a flow cytometer. The results showed
significantly higher percentages of the cell population in the
sub-G1 phase in salinomycin-treated C13 cells in a
concentration-dependent manner, whereas the percentages of cells in
other phases (G1/G0, S and G2/M phases) were almost reduced, when
compared to the control (Fig. 3).
These effects were similar at 12 and 24 h (Fig. 3). The marked accumulation of cells
in sub-G1 phase was another marker for apoptosis, further
confirming the results of the Annexin V/PI assay. Additionally,
there was no cell cycle arrest in the G1/G0, S and G2/M phases in
salinomycin-treated and control cells, suggesting that salinomycin
inhibited the proliferation of C13 cells not accompanied by cell
cycle arrest.
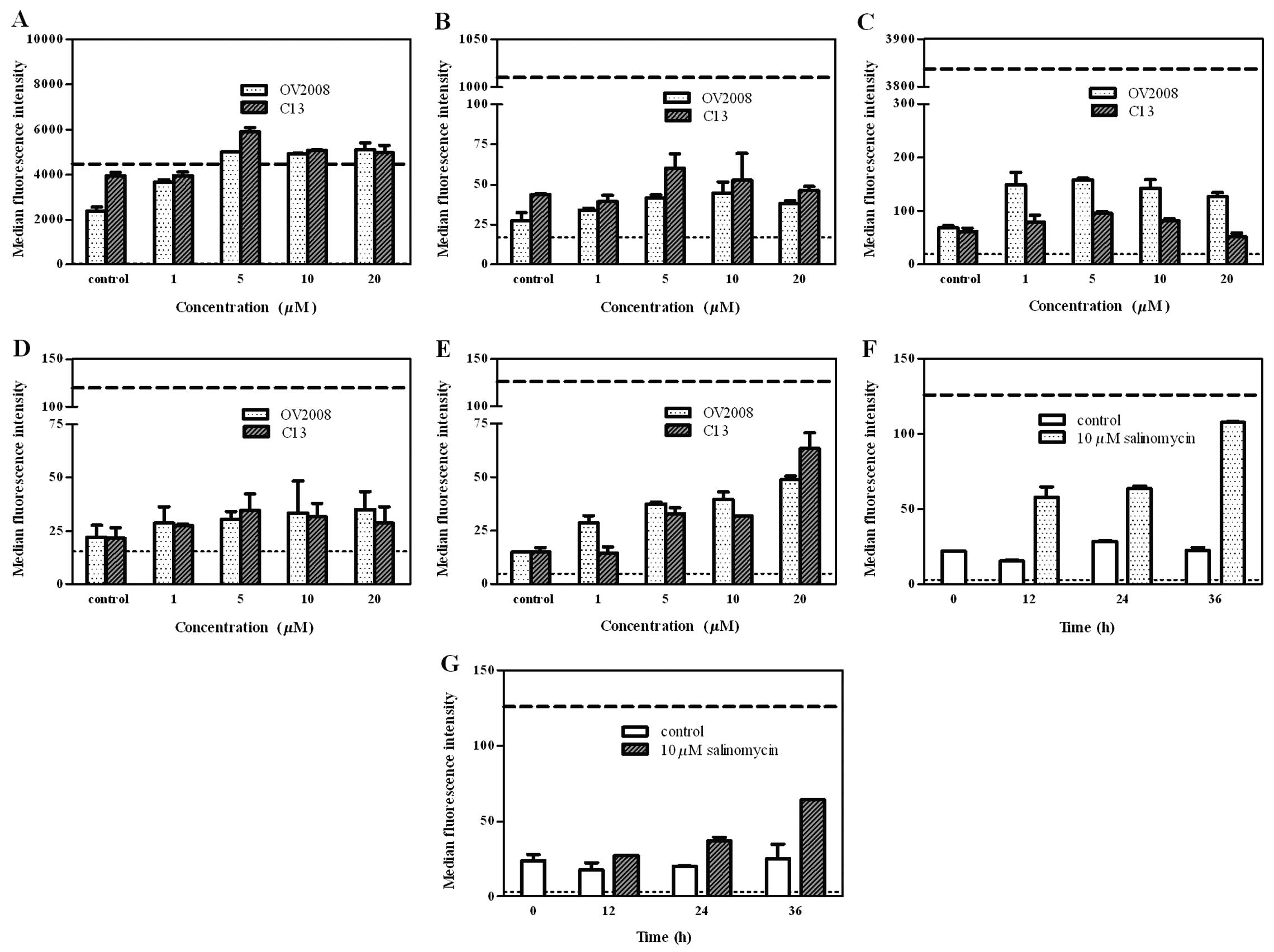
Effect of salinomycin on phosphoproteins
levels in OV2008 and C13 cells
To investigate the effect of salinomycin on the
phosphoproteins levels in the ovarian cancer cell lines, OV2008 and
C13, the regulation of phosphorylation by salinomycin in five
proteins was examined using the Bio-Plex phosphoprotein assay. The
results showed higher Akt and IκB-α phosphorylation basal levels in
untreated C13 compared to untreated OV2008 cells (~1.7- and
~1.6-fold, respectively) (Fig. 4A and
B). An increase in the phosphorylation of Akt in response to
salinomycin was observed in OV2008 (~2.2-fold) and C13 cells
(~1.3-fold) (Fig. 4A). ERK was
phosphorylated in OV2008 (~2-fold) and C13 cells (~1.4-fold)
subsequent to the addition of salinomycin, although the level was
independent of salinomycin dose and treatment time (Fig. 4C). There was no clear alteration of
the phosphorylation of IκB-α (Fig.
4B) and JNK (Fig. 4D) after
salinomycin treatment in either type of cell lines. However, a
marked concentration-dependent increase was observed in the p38
MAPK phosphorylation in the two cell lines, subsequent to
salinomycin exposure for 24 h (Fig.
4E). p38 MAPK phosphorylation was also enhanced by salinomycin
(10 μM) after 12, 24 and 36 h of incubation with OV2008 (Fig. 4F) or C13 cells (Fig. 4G), showing a time-dependent pattern.
These findings suggested that the salinomycin-induced
growth-inhibitory effect and apoptosis in both cell lines is likely
to be mediated through the alteration of p38 MAPK
phosphorylation.
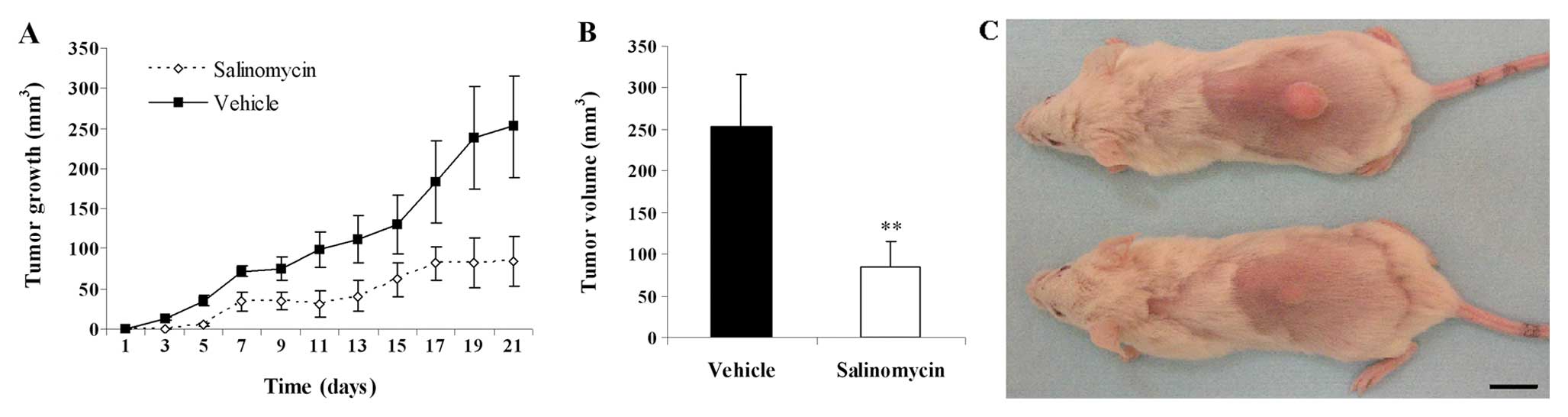
Evaluation of antitumor activity of
salinomycin in vivo
Based on the in vitro results, showing
significant cytotoxicity of salinomycin to human ovarian cancer
cell lines, the in vivo antitumor efficacy of salinomycin
was further evaluated in a cisplatin-resistant human ovarian tumor
(C13) xenograft grown in the back of mice. The mice were treated
with salinomycin and the change in the tumor volume after the first
injection was monitored for 21 days (Fig. 5A). Compared with the vehicle-treated
controls, a significant reduction in the tumor volume was observed
in the salinomycin-treated mice (Fig.
5C). At the emd of the test, the tumor volume of
salinomycin-treated groups and the controls, in the C13 tumor
model, was 84.2±30.8 and 252.5±63.4 mm3, respectively
(P<0.01; Fig. 5B).
Discussion
Recent findings suggest that salinomycin kills human
breast cancer stem-like cells (17), induces apoptosis and overcomes
multiple mechanisms of resistance to apoptosis in human cancer
cells, mainly human haematological tumor cells (18). The aim of the present study was to
examine the effects of salinomycin on human ovarian cancer cell
lines, including cisplatin-resistant cell lines. As shown in
Fig. 1, salinomycin demonstrated a
strong growth-inhibitory effect on ovarian cancer cell lines in a
concentration- and time-dependent manner. Six ovarian cancer cell
lines were selected for this study including cisplatin-resistant
cell lines, such as C13, A/CP and SKOV3, which are advanced and
refractory ovarian cancer cells. Salinomycin proved to be almost
equipotent in these cisplatin-resistant cells, exhibiting more
growth-inhibitory activity towards cisplatin-resistant C13,
compared to cisplatin-sensitive OV2008 cells (Table I). These results are consistent with
a previous study on breast cancer cell line, suggesting that
drug-resistant cells remain sensitive to salinomycin treatment
(17).
Apoptosis (programmed cell death), is an important
homeostatic mechanism balancing cell division and cell death, while
maintaining the appropriate cell number in the body (24). Therefore, searching for agents that
trigger the apoptosis of tumor cells has become an attractive
strategy in anticancer medication research (25). In the present investigation,
subsequent to salinomycin treatment, flow cytometry results, from
both the Annexin V/PI assay (Fig.
2) and sub-G1 populations in cell cycle analysis (Fig. 3), showed a concentration- and
time-dependent increase in the percentage of apoptotic C13 cell
subpopulations. Consistent with a previous report on various human
cancer cells, such as leukemia cells (18,19),
these results demonstrated that salinomycin triggered apoptosis in
C13 cells.
Cell cycle control is highly involved in the
regulation of tumor cell proliferation. Several anticancer and
DNA-damaging agents arrest the cell cycle in the G0/G1, S or G2/M
phase and then induce apoptotic cell death (26,27).
The results of the present study demonstrated that no cell cycle
arrest was observed in the G1/G0, S and G2/M phases in
salinomycin-treated C13 or control cells (Fig. 3), confirming previous findings
demonstrating that salinomycin activates a particular apoptotic
pathway not accompanied by cell cycle arrest (18).
To better understand the signaling pathways involved
in salinomycin-induced growth-inhibitory effects and apoptosis in
cisplatin-resistant ovarian cancer cells, the activity of Akt,
IκB-α, ERK1/2, JNK and p38 MAPK in cisplatin-resistant C13 cells
was investigated, compared to cisplatin-sensitive OV2008 cells. To
address this issue, multiple phosphoproteins were determined by the
Bio-Plex assays with Luminex technology. A recent report showed
that the results achieved using Bio-Plex phosphoprotein array
analyses significantly correlated (P<0.001) with those obtained
with numerized western blot analyses (23). Furthermore, Bland-Altman analyses
clearly demonstrated that Bio-Plex phosphoprotein array may be used
instead of western blot analysis providing a unique method of
analyzing the multiple phosphoprotein expression in small
specimens.
The PI3-kinase/Akt pathway contributed to tumor
formation by elevating the activity of the anti-apoptotic action of
Akt. Akt inhibited apoptosis through the phosphorylation of Bad,
GSK3 and caspase-9, as well as the activation of transcriptional
factors, such as Forkhead (FOXO1) and NF-κB (28). Cisplatin resistance has been
reported to be associated with the altered activation of PI3K/Akt
signaling pathways in an ovarian cancer cell line (11). The suppression of Akt activation was
likely to lead to the activation of pro-apoptotic signaling
pathways (29,30). The present study showed that the
basal levels of phospho-Akt in untreated cisplatin-resistant C13
cells were higher compared to cisplatin-sensitive OV2008 cells,
while salinomycin enhanced the phospho-Akt levels in both C13 and
OV2008 cells (Fig. 4A). However,
the growth inhibitory effect of salinomycin on C13 cells showed no
significant difference, compared to OV2008 cells. Moreover, IκB-α
is a downstream Akt substrate. Through the phosphorylation of IκB
kinase, Akt activates NF-κB, a transcription factor that has been
involved in cell survival (31,32).
Data indicated that the NF-κB has been linked with cell
proliferation, invasion, angiogenesis, metastasis, suppression of
apoptosis and chemoresistance in multiple tumors (33). In ovarian cancer cells, the
increased phosphorylation of IκB-α and the constitutive activation
of NF-κB have been shown to mediate cisplatin resistance, while the
inhibition of NF-κB activation sensitizes the ovarian cancer cells
to cisplatin (13). In the current
study, the basal levels of phospho-IκB-α in untreated
cisplatin-resistant C13 cells were higher compared to untreated
cisplatin-sensitive OV2008 cells. However, salinomycin did not
induce IκB-α phosphorylation (Fig.
4B). These results indicated that in cisplatin-resistant C13
cells, salinomycin induces apoptosis through
non-Akt/IκB-α-dependent pathways. The exact mechanism of the
salinomycin-enhanced phospho-Akt remains unknown and requires
additional investigation.
MAPKs are essential components of the signaling
transduction mechanism, while being highly involved in cell growth,
differentiation and cell apoptosis (34). Recent studies have suggested
apoptotic stimuli to be transmitted to caspases through the
activation of MAPKs, such as p38 MAPK and JNK (35). Therefore, whether MAPK activation is
involved in salinomycin-induced apoptosis in ovarian cancer cell
lines (OV2008 and C13) was examined. Data resulting from this study
showed that as opposed to JNK or ERK, p38 MAPK is associated with
the pro-apoptotic activity of salinomycin (Fig. 4C-G). The p38 MAPK pathway is
involved in cancer cell apoptosis and is induced by several
chemotherapeutic drugs (36,37).
The loss of the capacity to activate p38 MAPK in response to
cisplatin treatment was also reported to be a potential mechanism
of chemoresistance (38). Marked
time- and concentration-dependent increases were detected in the
phosphorylation of p38 MAPK subsequent to salinomycin treatment in
the two cell lines (Fig. 4E-G).
This result suggests that p38 MAPK activation contributes to the
pro-apoptotic effect of salinomycin in ovarian cancer cell lines
and that the activation of the p38 MAPK pathway is likely to be
involved in the salinomycin-induced apoptosis in ovarian cancer
cell lines. However, detailed downstream and upstream signaling
molecules of salinomycin-modulated p38 MAPK are unknown and require
further investigation.
In the present study, the xenografts of human
cisplatin-resistant ovarian cancer (C13) model showed very good
efficacy when treated with salinomycin (Fig. 5). Although, the mechanism of cell
death in the xenograft tumor model has not yet been ascertained,
salinomycin-induced cell apoptosis is likely to account for part of
the observed reduction in tumor growth rate and requires further
investigation. Additionally, more studies with salinomycin alone in
different characterized ovarian cancer cell lines or in combination
with other conventional medicatons in vitro and in
vivo are still necessary to enhance our understanding of this
promising antitumorigenic compound.
In summary, the present study demonstrated that
salinomycin inhibits the growth and induces apoptosis in the
cisplatin-resistant human ovarian cancer cell line C13 in
vitro and exhibits significant in vivo efficacy in the
tumor (C13) xenograft model. The pro-apoptotic effects of
salinomycin are not mediated through Akt-dependent pathways, rather
possibly associated with p38 MAPK activation. To address the
pathway involved additional investigations are required.
Acknowledgements
The authors would like to thank Dr Gaetano Marverti
(University of Modena and Reggio Emilia, Italy) for his kindly
supplying cisplatin-resistant ovarian cancer cell lines, Professor
Raija Lindberg, Professor Christoph Rochlitz, Dr Serdar Korur, Dr
LiFen Xu, Dr Bin Fan, Dr HaiFeng Ye, Mr. Jan Voelzmann, Mr. Lei
Fang, Ms. Zeinab Barekati, Mrs. Corina Kohler, Mr. Ramin Radpour,
Mrs. HongBo Chen and Mrs. Vivian Kiefer for their kind help. This
study was partly financed by the Swiss National Science Foundation
(320030_124958/1) Basel, Switzerland and Dr Hans Altschueler
Stiftung.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Morrison J: Advances in the understanding
and treatment of ovarian cancer. J Br Menopause Soc. 11:66–71.
2005. View Article : Google Scholar
|
|
3
|
Munkarah A, Chatterjee M and Tainsky MA:
Update on ovarian cancer screening. Curr Opin Obstet Gynecol.
19:22–26. 2007. View Article : Google Scholar
|
|
4
|
McGuire WP III and Markman M: Primary
ovarian cancer chemotherapy: current standards of care. Br J
Cancer. 89(Suppl): S3–S8. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bristow RE and Chi DS: Platinum-based
neoadjuvant chemotherapy and interval surgical cytoreduction for
advanced ovarian cancer: a meta-analysis. Gynecol Oncol.
103:1070–1076. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cannistra SA: Cancer of the ovary. N Engl
J Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kelland LR: Emerging drugs for ovarian
cancer. Expert Opin Emerg Drugs. 10:413–424. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harries M and Gore M: Part II:
chemotherapy for epithelial ovarian cancer-treatment of recurrent
disease. Lancet Oncol. 3:537–545. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harries M and Gore M: Part I: chemotherapy
for epithelial ovarian cancer-treatment at first diagnosis. Lancet
Oncol. 3:529–536. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jansen BA, Brouwer J and Reedijk J:
Glutathione induces cellular resistance against cationic dinuclear
platinum anticancer drugs. J Inorg Biochem. 89:197–202. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee S, Choi EJ, Jin C and Kim DH:
Activation of PI3K/Akt pathway by PTEN reduction and PIK3CA mRNA
amplification contributes to cisplatin resistance in an ovarian
cancer cell line. Gynecol Oncol. 97:26–34. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi KC, Auersperg N and Leung PC:
Mitogen-activated protein kinases in normal and (pre)neoplastic
ovarian surface epithelium. Reprod Biol Endocrinol. 1:712003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mabuchi S, Ohmichi M, Nishio Y, et al:
Inhibition of NFkappaB increases the efficacy of cisplatin in in
vitro and in vivo ovarian cancer models. J Biol Chem.
279:23477–23485. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rudin CM, Yang Z, Schumaker LM, et al:
Inhibition of glutathione synthesis reverses Bcl-2-mediated
cisplatin resistance. Cancer Res. 63:312–318. 2003.PubMed/NCBI
|
|
15
|
Miyazaki Y, Shibuya M, Sugawara H,
Kawaguchi O and Hirsoe C: Salinomycin, a new polyether antibiotic.
J Antibiot. 27:814–821. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Callaway TR, Edrington TS, Rychlik JL, et
al: Ionophores: their use as ruminant growth promotants and impact
on food safety. Curr Issues Intest Microbiol. 4:43–51.
2003.PubMed/NCBI
|
|
17
|
Gupta PB, Onder TT, Jiang G, et al:
Identification of selective inhibitors of cancer stem cells by
high-throughput screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fuchs D, Heinold A, Opelz G, Daniel V and
Naujokat C: Salinomycin induces apoptosis and overcomes apoptosis
resistance in human cancer cells. Biochem Biophys Res Commun.
390:743–749. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fuchs D, Daniel V, Sadeghi M, Opelz G and
Naujokat C: Salinomycin overcomes ABC transporter-mediated
multidrug and apoptosis resistance in human leukemia stem cell-like
KG-1a cells. Biochem Biophys Res Commun. 394:1098–1104. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Riccioni R, Dupuis ML, Bernabei M, et al:
The cancer stem cell selective inhibitor salinomycin is a
P-glycoprotein inhibitor. Blood Cells Mol Dis. 45:86–92. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu D, Choi MY, Yu J, Castro JE, Kipps TJ
and Carson DA: Salinomycin inhibits Wnt signaling and selectively
induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl
Acad Sci USA. 108:13253–13257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chergui F, Chretien AS, Bouali S, et al:
Validation of a phosphoprotein array assay for characterization of
human tyrosine kinase receptor downstream signaling in breast
cancer. Clin Chem. 55:1327–1336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martin SJ and Green DR: Apoptosis and
cancer: the failure of controls on cell death and cell survival.
Crit Rev Oncol Hematol. 18:137–153. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reed JC: Apoptosis-targeted therapies for
cancer. Cancer Cell. 3:17–22. 2003. View Article : Google Scholar
|
|
26
|
Schwartz GK and Shah MA: Targeting the
cell cycle: a new approach to cancer therapy. J Clin Oncol.
23:9408–9421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: a review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Katso R, Okkenhaug K, Ahmadi K, White S,
Timms J and Waterfield MD: Cellular function of phosphoinositide
3-kinases: implications for development, homeostasis, and cancer.
Annu Rev Cell Dev Biol. 17:615–675. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fraser M, Leung BM, Yan X, Dan HC, Cheng
JQ and Tsang BK: p53 is a determinant of X-linked inhibitor of
apoptosis protein/Akt-mediated chemoresistance in human ovarian
cancer cells. Cancer Res. 63:7081–7088. 2003.PubMed/NCBI
|
|
30
|
Weir NM, Selvendiran K, Kutala VK, et al:
Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant
human ovarian cancer cells by modulating Akt and p38 MAPK. Cancer
Biol Ther. 6:178–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Medema RH, Kops GJ, Bos JL and Burgering
BM: AFX-like Forkhead transcription factors mediate cell-cycle
regulation by Ras and PKB through p27kip1. Nature. 404:782–787.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aggarwal BB: Nuclear factor-kappaB: the
enemy within. Cancer Cell. 6:203–208. 2004.PubMed/NCBI
|
|
34
|
Ono K and Han J: The p38 signal
transduction pathway: activation and function. Cell Signal.
12:1–13. 2000. View Article : Google Scholar
|
|
35
|
Park SJ and Kim IS: The role of p38 MAPK
activation in auranofin-induced apoptosis of human promyelocytic
leukaemia HL-60 cells. Br J Pharmacol. 146:506–513. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bulavin DV and Fornace AJ Jr: p38 MAP
kinase's emerging role as a tumor suppressor. Adv Cancer Res.
92:95–118. 2004.
|
|
38
|
Brozovic A, Fritz G, Christmann M, et al:
Long-term activation of SAPK/JNK, p38 kinase and fas-L expression
by cisplatin is attenuated in human carcinoma cells that acquired
drug resistance. Int J Cancer. 112:974–985. 2004. View Article : Google Scholar : PubMed/NCBI
|