Introduction
A frequent problem in cancer chemotherapy is the
development of multidrug resistance (MDR) which renders tumors
unresponsive to a diverse array of compounds. There are a number of
mechanisms by which a cell can acquire MDR, one of the most common
being overexpression of members of the ATP-binding cassette (ABC)
transporter family. This is a group of plasma membrane proteins
that actively extrude a broad range of substrates from cells. They
are predominantly found in areas such as the epithelial cells of
the intestine, hepatocytes, capillary epithelial cells of the
blood-brain barrier, and in various stem cells where the
physiological role of these ATPase efflux ‘pumps’ is to protect
cells from damage by rapidly extruding xenobiotics (1). Unfortunately, their unwanted
expression in tumor cells can lead to chemotherapy resistance in
these cells (2).
Most research concerning ABC
transporter-overexpressing MDR cancer cells have focused on
molecules or signaling pathways that regulate expression of these
transporters (3,4), and a few potential therapeutic target
molecules have been identified. In recent years, it has been
reported that suppression of ABC transporters inhibits cancer cell
proliferation, particularly in transporter-overexpressing MDR
cancer cells (5,6). However, there are no reports
concerning the relationship between inhibition of ABC transporters
and cell death.
In the present study, we demonstrated that treatment
with NP-1250, an ABCG2 inhibitor, alone induced apoptotic cell
death via a caspase-independent pathway in MDR MCF7 breast cancer
cells (MCF7/MX). MCF7/MX cells (1)
have the MDR phenotype characterized by high levels of the ABCG2
transporter. We speculated that NP-1250 would have an effect on
molecules involved in growth and survival, whose expression levels
have been elevated in the process of acquiring resistance to
anticancer drugs. Using a proteomics approach, we identified three
candidate molecules: peroxiredoxin-2, protein disulfide-isomerase
A4 and prohibitin-2. These molecules may be potential candidates
for the treatment of MDR cancers.
Materials and methods
Materials
Pan-caspase inhibitor (Z-VAD-FMK) and
caspase-3/7-specific inhibitor (Ac-DEVD-CHO) were purchased from
Promega (Madison, WI, USA). Doxorubicin was obtained from Kyowa
Hakko Kirin (Tokyo, Japan) and mitoxantrone hydrochloride was
obtained from Wyeth Lederle Japan, Ltd. (Tokyo, Japan). NP-1250 was
synthesized at Nippon Chemiphar Co., Ltd. (Saitama, Japan). It is
still under development, therefore, disclosure of the structure of
this compound is prohibited.
Cell cultures and treatment
The MCF7 human breast cancer cell line, the HL-60
human promyelocytic leukemia cell line, the multidrug-resistant
HL-60/MX1 cell line, the MES-SA human uterus sarcoma cell line, and
the multidrug-resistant MES-SA/Mx2 and MES-SA/Dx5 cancer cell lines
were obtained from American Type Culture Collection (ATCC,
Rockville, MD, USA) and cultured in the recommended medium
containing 10% heat-inactivated fetal bovine serum (FBS) at 37°C in
a 95% air/5% CO2 atmosphere. Mitoxantrone-resistant MCF7
(MCF7/MX) cells (7) were a gift
from Dr Masayuki Nakagawa (Department of Urology, Kagoshima
University, Kagoshima, Japan) and doxorubicin-resistant MCF7
(MCF7/DOX) cells were a gift from Dr Takao Yamori (Division of
Molecular Pharmacology, Cancer Chemotherapy Center, Japanese
Foundation for Cancer Research, Tokyo, Japan). Prior to any
experiments, MCF7/MX cells and MCF7/DOX cells were maintained in
drug-free medium for one passage. Other resistant cells were
cultured in the absence of anticancer drugs. ABCG2-overexpressing
Flp-In-293 (Flp-In-293/ABCG2) cells and mock-transfected Flp-In-293
(Flp-In-293/Mock) cells were established and cultured as previously
described (8).
Evaluation of the inhibitory effect on
the ABCG2 transporter
To evaluate ABCG2-NP-1250 interaction, plasma
membrane vesicles prepared from ABCG2-expressing Sf9 cells were
incubated with [3H]methotrexate (MTX). Transport into
the vesicles was measured by counting the radioactivity remaining
on the filter of MultiScreen plates, as previously described
(9).
Cell viability assay
To evaluate the effectiveness of NP-1250 in
overcoming ABCG2-mediated drug resistance, Flp-In-293/ABCG2 and
MCF7/MX cells were incubated with anticancer drug SN-38 and
NP-1250, and the cell viabilities were measured.
Tumor cells were seeded in 96-well plates. After 24
h, the medium was replaced with medium containing different
concentrations of NP-1250 in triplicate. After a 72-h incubation at
37°C, cell viability was determined at the indicated times, as
previously described (10).
TUNEL staining
MCF7/WT and MCF7/MX cells were seeded in 35-mm
glass-bottom dishes. After 24 h, the medium was replaced with
medium containing 10, 20 or 40 μM of NP-1250. After a 48-h
incubation at 37°C, TUNEL staining was performed as previously
described (10). The frequency (%)
of TUNEL-positive cells per DAPI-positive cells was determined in
five different fields in duplicate. Two replicate experiments were
performed.
Annexin V/PI staining
The Annexin V-FITC fluorescence microscopy kit
(Pharmingen, San Diego, CA, USA) was used to detect apoptosis.
MCF7/MX cells were seeded in 35-mm glass-bottom dishes. After 6,
12, 24 or 48 h of incubation with 20 μM of NP-1250 at 37°C, Annexin
V/propidium iodide (PI) staining was performed according to the
manufacturer's protocol. Stained cells were visualized using a
confocal microscope (Leica).
RNA isolation and cDNA synthesis
Total RNA was prepared using the
FastPure® RNA kit (9190; Takara, Shiga, Japan) according
to the manufacturer's protocol, and RNA concentrations were
measured at an absorbance A260. Reverse transcription
(RT) was carried out with 1 μg RNA using a ReverTraAce®
qPCR RT kit (FSQ-101; Toyobo, Osaka, Japan).
Real-time PCR
Real-time PCR was carried out using Fast SYBR-Green
Master Mix (4385612, Applied Biosystems, Life Technologies Corp.,
Carlsbad, CA, USA) using a 7500 Fast Real-Time PCR system (Applied
Biosystems). All primers used for real-time PCR are described in
Table I. PCR was carried out with
an initial 20-sec denaturation at 95°C followed by 40 cycles of PCR
(3 sec at 95°C and 30 sec at 60°C). Reactions were performed in
triplicate for three biological replicates, and the amount of each
cDNA was normalized to GAPDH gene expression. Melt-curve analysis
was performed to ensure that the mRNA-specific fragments were
amplified and data were analyzed using the standard curve
method.
 | Table ISequence of primers for real-time
PCR. |
Table I
Sequence of primers for real-time
PCR.
| Gene | Direction | Primer sequence
(5′-3′) | Product size
(bp) |
|---|
| GAPDH | Forward |
GCCATCAATGACCCCTTC | 114 |
| Reverse |
GATGACAAGCTTCCCGTTC | |
| ABCB1 | Forward |
AACTTCCGAACCGTTGTTTC | 110 |
| Reverse |
CCAAAGATGTGTGCTTTCCTC | |
| ABCC1 | Forward |
CTACCTCCTGTGGCTGAATC | 150 |
| Reverse |
ATCAGCTTGATCCGATTGTC | |
| ABCG2 | Forward |
ACAGGTGGAGGCAAATCTTC | 94 |
| Reverse |
GCGGTGCTCCATTTATCAG | |
| PRDX1 | Forward |
CTGTCATCTAGCATGGGTCAAT | 106 |
| Reverse |
CCCATAATCCTGAGCAATG | |
| PRDX2 | Forward |
GTCCGTGCGTCTAGCCTTTG | 128 |
| Reverse |
CAGCTTCACCTCTTTGAAGG | |
| LGUL | Forward |
CCAAGGATTTTCTATTGCAG | 97 |
| Reverse |
TGGATTAGCGTCATTCCAAGA | |
| GLTP | Forward |
CCCTTCTTCGATTGCCTTG | 107 |
| Reverse |
AACTTGGCTGGGTTGGTGTC | |
| TAGL2 | Forward |
CCAAACTGTGGACCTCTGG | 143 |
| Reverse |
GATTCTCCTTGGATTTCTTAGGG | |
| PSB5 | Forward |
CCACCCTGGCCTTCAAGTTC | 132 |
| Reverse |
CCATGGTGCCTAGCAGGTATG | |
| PEBP1 | Forward |
GCCCACCCAGGTTAAGAATAG | 145 |
| Reverse |
GACCACCAGGAAATGATGC | |
| GNA1 | Forward |
CCTGTGCTAAGAGAGGAAGAGT | 143 |
| Reverse |
TGGTAGACATTCAAGGGTAATC | |
| DCTP1 | Forward |
CTTTCAGTGGAAAACCGATG | 144 |
| Reverse |
ACTGCTAGCGGCAGATCCAC | |
| VDAC2 | Forward |
GATTTGGTTTTGGGTTGGTG | 115 |
| Reverse |
TCCAAGGTCCCAGTAACTTT | |
| P5CR1 | Forward |
TGGACCTGGCCACAGTTT | 103 |
| Reverse |
TTCACAGCCAGGAAGAGCACAT | |
| SFXN1 | Forward |
TTGGCTTCTGTTTGGTGTTT | 113 |
| Reverse |
GCTCTCTTGGATCTTAGCTTGC | |
| PDIA4 | Forward |
CCGCTAACAACCTGAGAGAAG | 105 |
| Reverse |
CAGGCTGCATTACAACCAAC | |
| HNRPM | Forward |
AAGGGTGAAGGAGAACGACCTG | 111 |
| Reverse |
GGCTCTGTATCTTTTAGTTGGA | |
| ECHA | Forward |
AACTCTCCCAATTCAAAGGT | 117 |
| Reverse |
TGATGAGATAAGGACGGCACT | |
| GPDM | Forward |
TCACCAGAGGACTAAAAACAGC | 91 |
| Reverse |
ACACCACCATGGATCAATTT | |
| IPYR | Forward |
AATGGAGATTGCTACAAAGGAC | 142 |
| Reverse |
TGGGTCTTCCCAAGTCTG | |
| OTUB1 | Forward |
CAGCGGTTCAAGGCTGTGT | 133 |
| Reverse |
CGACAGAGGTCTGCTTCTCC | |
| PHB2 | Forward |
CTCCAAAGACCTACAGATGGTG | 130 |
| Reverse |
GTTGACAATGGACGGCAACA | |
| GGCT | Forward |
CCGCCTGCAGGATTTTAAG | 98 |
| Reverse |
CGCCAGGACTCTGAAAAATG | |
| LDHA | Forward |
CTGTCATGGGTGGGTCCTT | 106 |
| Reverse |
CCCTAAATCTGGGTGCAGAGTC | |
Immunofluorescence staining of ABCG2
Cells were fixed in 4% paraformaldehyde,
permeabilized with 0.2% Triton X-100, and blocked with 1% bovine
serum albumin and 10% normal goat serum in phosphate-buffered
saline (PBS). Incubation with primary monoclonal antibody for ABCG2
(ALX-801-029; Wako, Osaka, Japan) at a dilution of 1:200 was
carried out overnight at 4°C, followed by incubation with the Alexa
Fluor-conjugated secondary antibody (A11001; Molecular Probes,
Inc., Carlsbad, CA, USA) for 1 h at room temperature.
Immunofluorescence was detected and analyzed using a Leica TCS SP5
Laser Scanning Confocal Microscope system.
Western blot analysis
Cells were washed two times with PBS, harvested by
scraping from the culture dishes in lysis buffer and collected.
Protein concentrations were determined using the DC protein assay
kit according to the manufacturer's instructions (500-0116;
Bio-Rad, Hercules, CA, USA). Equal amounts of proteins were
denatured by boiling and subjected to SDS-polyacrylamide gel
electrophoresis. The separated proteins were transferred to
polyvinylidene difluoride membranes. The membranes were incubated
overnight at 4°C with primary polyclonal antibody at a dilution of
1:1000 for caspase-3, cleaved-PARP, cleaved-caspase-7 or
cleaved-caspase-9 (9665, 9541, 9491, 9501; Cell Signaling
Technology, Beverly, MA, USA). Membranes were then incubated with
goat anti-rabbit IgG-HRP secondary antibody for 1 h at room
temperature and visualized using an enhanced chemiluminescence
detection system.
Proteomics
SDS-PAGE and detection by MS
compatible silver staining
MCF7/WT and MCF7/MX cells were fractionated using
the ProteoExtract® Subcellular Proteome Extraction kit
(Merck KGaA, Darmstadt, Germany). Protein samples (5 μg) were
separated by SDS-PAGE. Upon completion, the gel was transferred to
a clean and dry glass chamber for silver staining using the PlusOne
Silver Staining kit, Protein (GE Healthcare, Hino, Japan).
Identification of protein
In-gel digestion using trypsin was performed as
previously described (11). The
tryptic peptides were used for identification of proteins by mass
spectrometry. The digested peptides were analyzed using a nanoflow
LC-MS/MS system with a direct nanoflow LC system (DiNa; KYA
Technologies, Tokyo, Japan) and a LTQ Orbitrap XL-ETD mass
spectrometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Data were searched against NCBI human sequence database using
Mascot (Matrix Science, Ltd., London, UK).
Statistical analysis
The results are expressed as means ± SD. Each value
represents the mean ± SD of two or three independent experiments.
One-way analysis of variance (ANOVA) with Dunnett multiple
comparison test and t-test were performed, and P<0.01 was
considered to indicate a statistically significant difference.
Results and Discussion
NP-1250 is an ABCG2 inhibitor and
treatment alone reduces the cell viability of ABCG2-overexpressing
MCF7/MX cells
We measured ATP-dependent [3H]MTX
transport in plasma membrane vesicles, prepared from
ABCG2-expressing Sf9 cells, in the presence of a potassium salt of
NP-1250. Potassium salt of NP-1250 inhibited ABCG2-mediated MTX
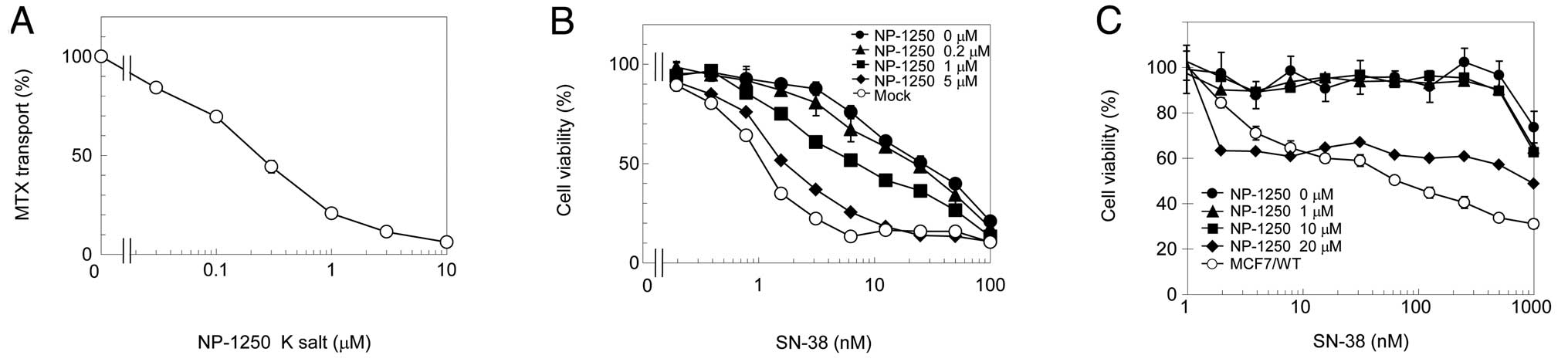
transport in a dose-dependent manner (Fig. 1A). This degree of inhibition was
comparable to gefitinib (9),
suggesting that NP-1250 is a powerful ABCG2 inhibitor. To examine
whether NP-1250 overcomes ABCG2-mediated resistance, the cytotoxic
effect of ABCG2-substrate SN-38 in the presence of NP-1250 on
Flp-In-293/ABCG2 cells was measured. NP-1250 dose-dependently
reversed SN-38 resistance in Flp-In-293/ABCG2 cells (Fig. 1B). We then evaluated NP-1250 on
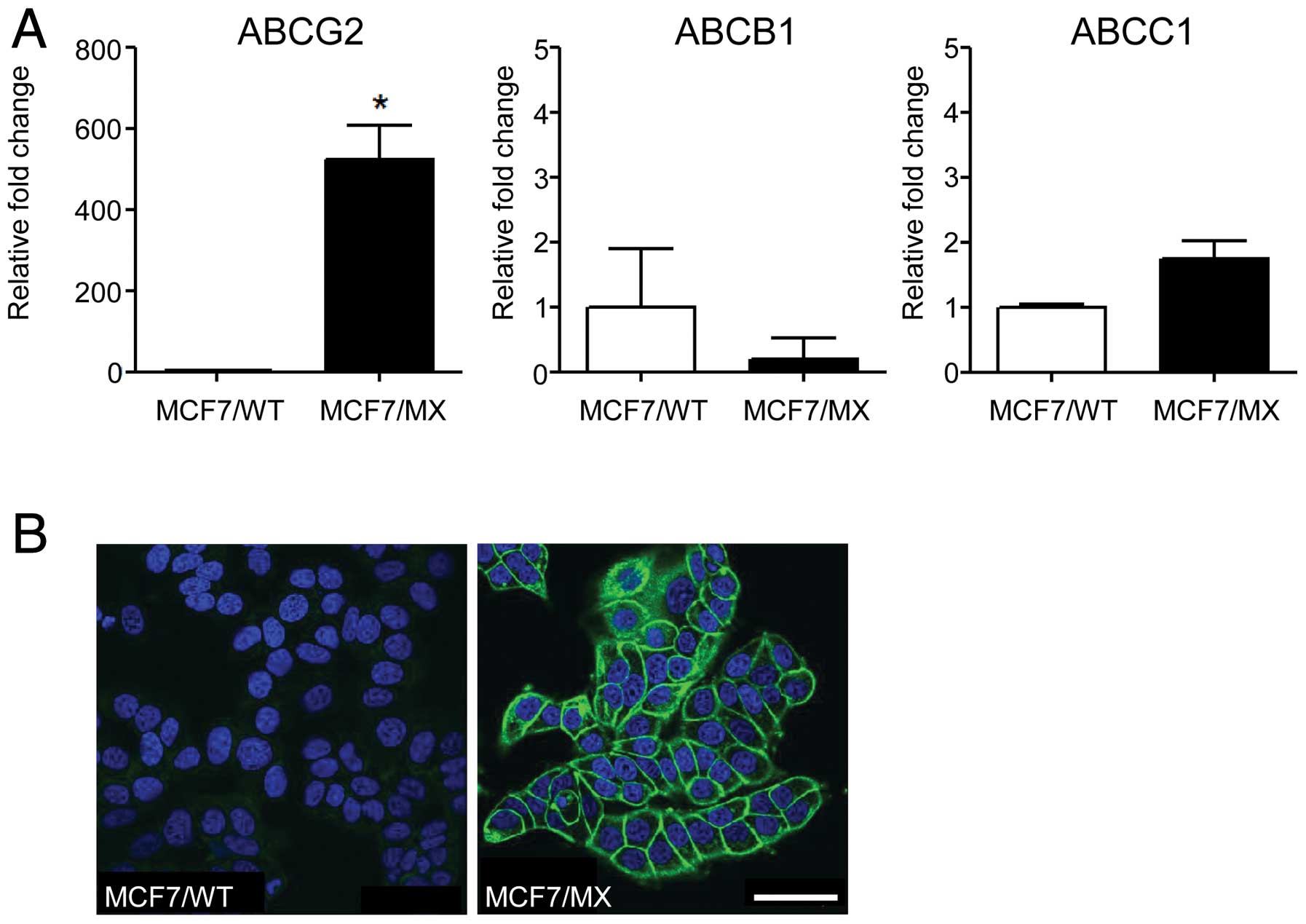
MCF7/MX cells, a mitoxantrone-resistant cell line in which ABCG2
was amplified and overexpressed (Fig.
2) by chronic exposure to mitoxantrone. Differing from our
expectation, NP-1250 exhibited no reversing effect on MCF7/MX cells
at concentrations <20 μM (Fig.
1C). Notably, 20 μM of NP-1250 inhibited cell viability with a
very low dose of SN-38. These results suggest that ABCG2-inhibitor
NP-1250 itself may have a cytotoxic effect.
NP-1250 induces apoptotic cell death in
MCF7/MX cells
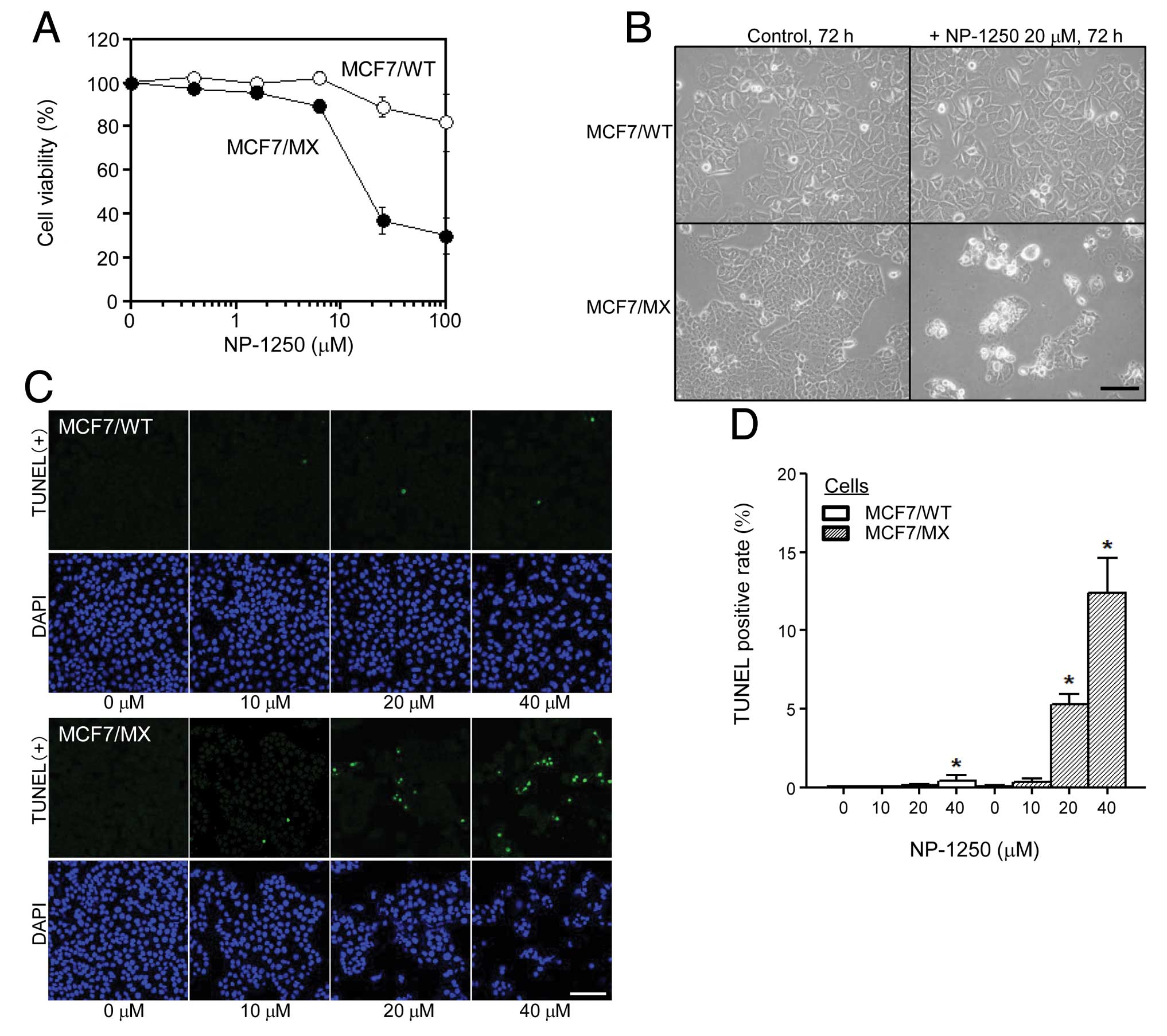
We compared the chemosensitivity to NP-1250 of
drug-sensitive MCF7/WT cells and multidrug-resistant MCF7/MX cells
by examining the cell viability and cell morphological changes. We
discovered that NP-1250 alone reduced the cell viability of MCF7/MX
cells, notably, more than that of MCF7/WT cells (Fig. 3A). For the cell morphological study,
the number of MCF7/MX cells was significantly less than the number
of cells in the control and the adherent cells displayed large
vacuoles in the cytoplasm, exhibited atrophic changes or were
almost dying (Fig. 3B). In
contrast, little change was observed in MCF7/WT cells after a 72-h
treatment (Fig. 3B). To confirm
these initial observations, we analyzed apoptosis by TUNEL assay
and phosphatidylserine (PS) externalization. After a 48-h treatment
with NP-1250, in MCF7/MX cells, the number of TUNEL-positive cells
was elevated (Fig. 3C) and the
TUNEL-positive rate was significantly increased in a dose-dependent
manner (Fig. 3D). Apoptotic cell
death observed in the MCF7/MX cells upon treatment with NP-1250 was
further confirmed by determining Annexin V binding to PS
translocated from the inner to the outer membrane surface of the
plasma membrane, a hallmark of early stage apoptosis (Fig. 3E).
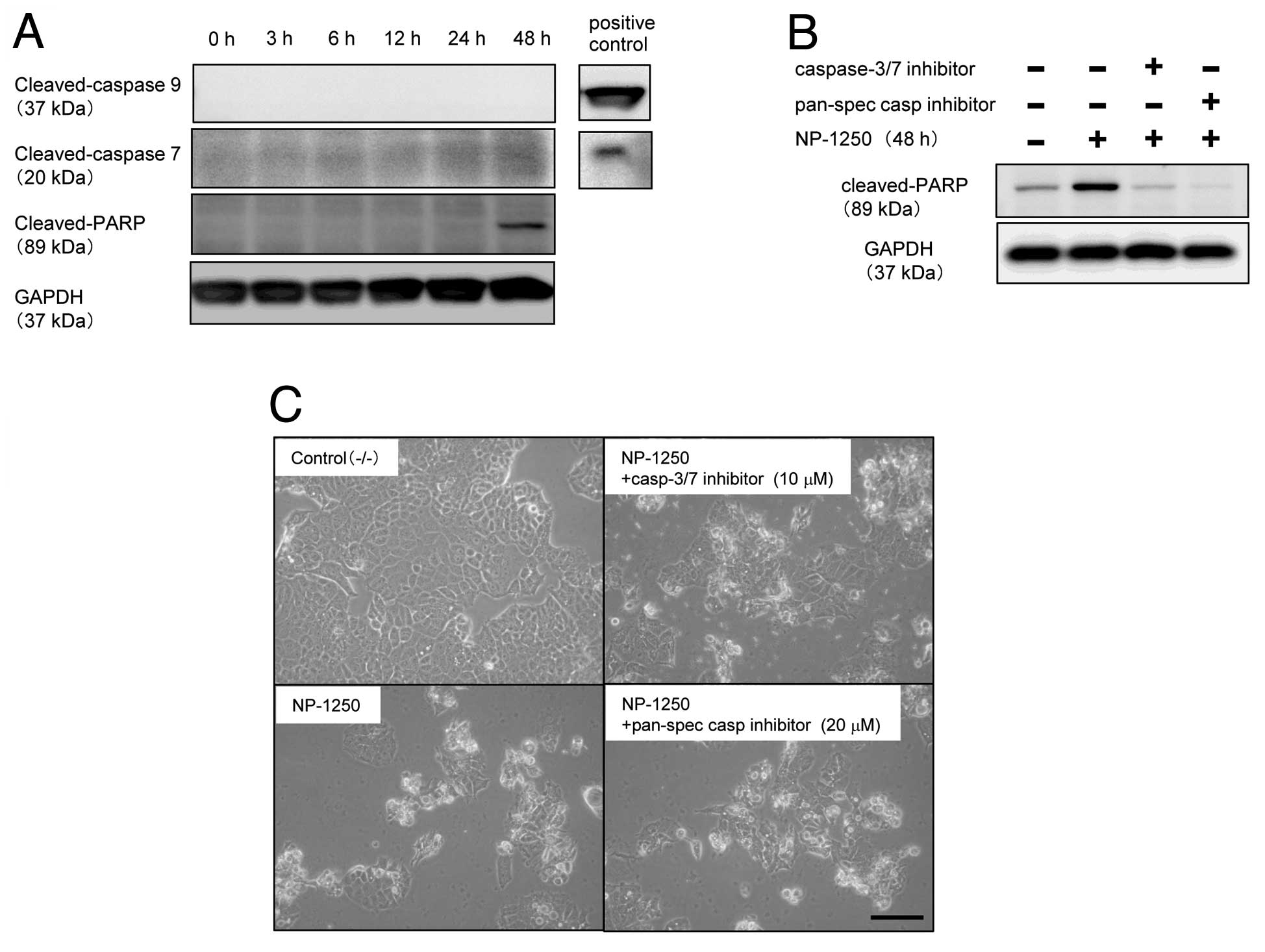
NP-1250-induced cell death is
‘caspase-independent apoptosis’
After 48 h of treatment with NP-1250, cleavage of
PARP was detected using an antibody recognizing the 89-kDa fragment
(Fig. 4A). Although PARP is a
well-known substrate of caspases, particularly caspase-3 and
caspase-7, MCF7 cells do not express caspase-3 due to the deletion
of 125 nucleotides in the CASP-3 mRNA (12). We confirmed the lack of caspase-3
protein in these MCF7/WT and MCF7/MX cells (data not shown). We,
therefore, examined whether caspase-7 and caspase-9 were cleaved
prior to cleavage of PARP, but we did not detect either one
(Fig. 4A). To further ascertain
whether NP-1250-induced apoptosis of MCF7/MX cells was
caspase-dependent or -independent, MCF7/MX cells were pretreated
with a caspase inhibitor prior to NP-1250 treatment, and cleavage
of PARP was subsequently examined. Pretreatment with the
pan-specific inhibitor completely abolished the cleavage of PARP,
and the pharmacological inhibitor specific for caspase-3/7 also
showed a significant inhibition of the cleavage of PARP (Fig. 4B). Under the same experimental
conditions, pretreatment of MCF7/MX cells with these inhibitors did
not block the production of apoptotic cells (Fig. 4C). These results suggest that
caspases, which cleave PARP, are cleaved and activated upon
exposure of MCF7/MX cells to NP-1250 but that this is not the main
apoptotic cascade in the death of MCF7/MX cells.
To evaluate the effects of NP-1250 on other MDR cell
lines that exhibit increased expression of ABC transporters, we
treated several MDR cell lines and their drug-sensitive parental
cell lines with NP-1250. We did not observe growth inhibition by
NP-1250 in any of the cell lines (data not shown). We also treated
several other cancer cell lines, specifically human hepatocellular
carcinoma cell line HuH7, human cervical adenocarcinoma cell line
HeLa and human mesothelioma cell lines H226 and MESO4, with
NP-1250, but we did not confirm any effect (data not shown). Taken
together, these results suggest that the
antiproliferative/apoptotic effect of NP-1250 may be limited to
MCF7/MX cells.
Search for target proteins by
proteomics
There are no reports relating inhibition of ABC
transporters to cell death, therefore, we speculated that NP-1250
may have other targets in MCF7/MX cells.
We excised several bands that were observed in the
protein electrophoretograms of MCF7/MX cells. As a control, we also
excised the band that appeared to be ABCG2, ~74 kDa, as observed in
the membrane/organelle fraction of MCF7/MX cells. ABCG2 was
identified in this band with a Mascot score of 192, thus we
excluded proteins whose Mascot scores were <100. We also
excluded proteins observed at positions >15 kDa different from
their original molecular weight. Candidate proteins that passed our
identification criteria are listed in Table II. We selected molecules
preferentially involved in cell growth and survival from these
proteins and performed real-time PCR to confirm whether the
expression of genes encoding these proteins was increased in the
MCF7/MX cells when compared to the MCF7/WT cells. Among the 46
proteins, we analyzed the gene expression of 22 proteins, including
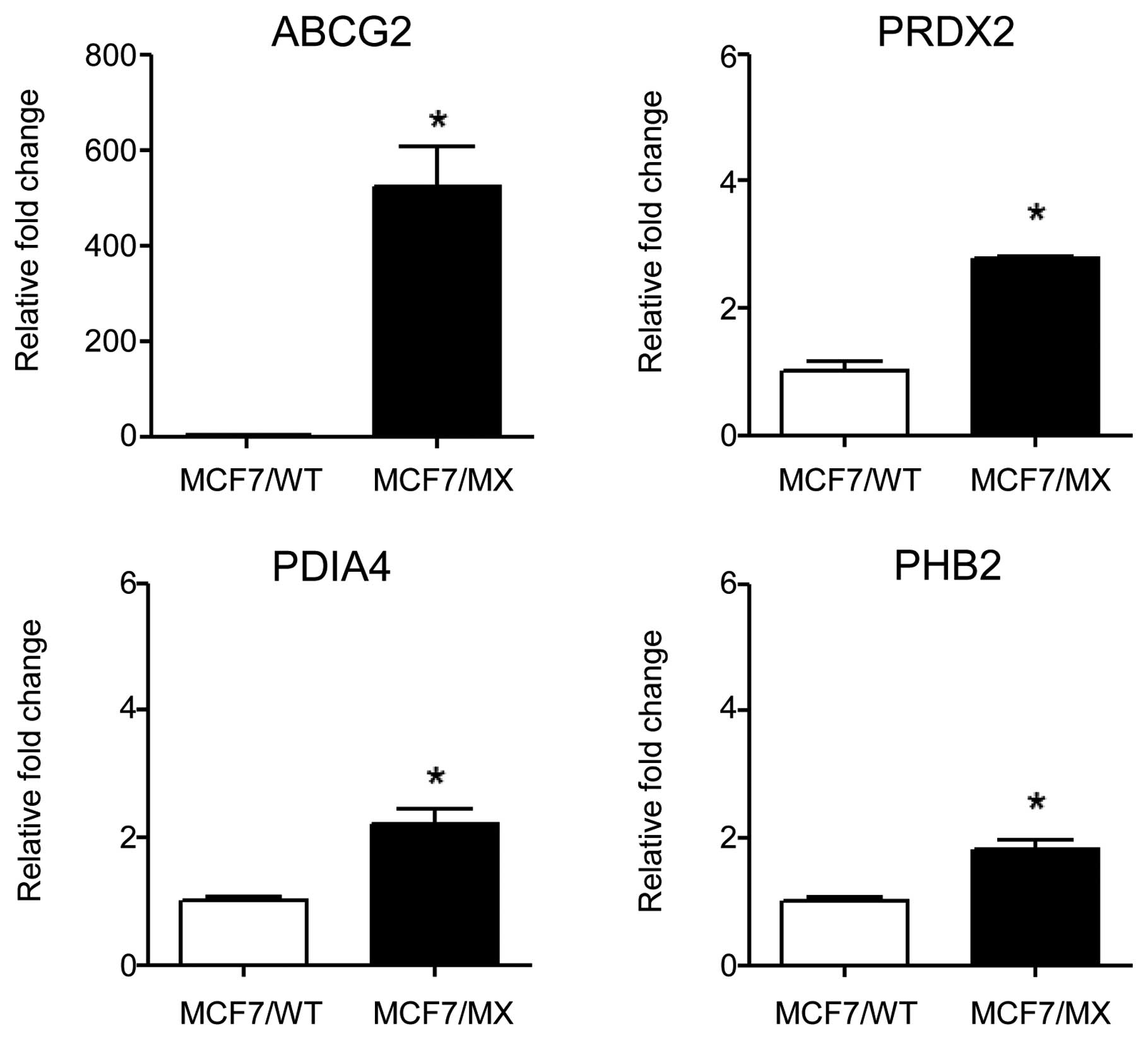
ABCG2. Three of these proteins, peroxiredoxin-2 (PRDX2), protein
disulfide-isomerase A4 (PDIA4) and prohibitin-2 (PHB2), showed
significantly elevated mRNA expression in MCF7/MX cells compared to
the expression levels in MCF7/WT cells (Fig. 5). PDIA4 is a protein disulfide
isomerase (PDI), an enzyme that catalyzes disulfide formation and
isomerization and a chaperone that inhibits aggregation (13) and a member of a large family of
dithiol/disulfide oxidoreductases, the thioredoxin superfamily.
While it has been reported that PDI shows an inverse correlation
with resistance (14), Liu et
al reported that increased expression of PDI was found in
multidrug-resistant MCF7/AdVp3000 cells when compared to MCF7 cells
(15). PHB2, known as a repressor
of estrogen receptor (ER) activity, has been shown to interact with
and inhibit the transcriptional activity of the ER (16). While PHB2 has been implicated in a
previously uncharacterized pathway of multidrug resistance in
Caenorhabditis elegans(17),
Keenan et al found that PHB2 was inversely correlated with
resistance in a squamous lung cancer cell line (14). PRDX2 has been previously known as a
natural killer-enhancing factor B (18) and is induced by various oxidative
stimuli. It plays an important protective role against oxidative
radical damage by reactive oxygen and nitrogen species (19). Its expression is correlated with
resistance to apoptosis induced by radiation therapy or anticancer
drugs (20), highlighting the
potential clinical importance of PRDX2 in chemotherapy resistance
in cancer. However, the association of PRDX2 and MCF7/MX cells has
not been previously reported. Thus further studies, such as
knockdown experiments, are warranted.
 | Table IIThe candidate target proteins of
NP-1250 identified by mass spectrometry. |
Table II
The candidate target proteins of
NP-1250 identified by mass spectrometry.
| No. | Prot_acca | Identified
proteina | Prot_pIb | Prot_massb | Prot_scorec |
|---|
| 1 | PRDX2_HUMAN |
Peroxiredoxin-2 | 5.66 | 22,091 | 533 |
| 2 | RL9_HUMAN | 60S ribosomal
protein L9 | 9.96 | 21,992 | 274 |
| 3 | RL18_HUMAN | 60S ribosomal
protein L18 | 11.73 | 21,763 | 232 |
| 4 | RAB7A_HUMAN | Ras-related protein
Rab-7a | 6.40 | 23,830 | 191 |
| 5 | RS5_HUMAN | 40S ribosomal
protein S5 | 9.73 | 23,075 | 256 |
| 6 | LGUL_HUMAN | Lactoylglutathione
lyase | 5.12 | 21,048 | 145 |
| 7 | RHOA_HUMAN | Transforming
protein RhoA | 5.83 | 22,180 | 184 |
| 8 | COMD3_HUMAN | COMM
domain-containing protein 3 | 5.63 | 22,421 | 118 |
| 9 | RAB10_HUMAN | Ras-related protein
Rab-10 | 8.59 | 22,811 | 150 |
| 10 | PRDX1_HUMAN |
Peroxiredoxin-1 | 8.27 | 22,380 | 146 |
| 11 | GLTP_HUMAN | Glycolipid transfer
protein | 6.90 | 24,048 | 112 |
| 12 | TAGL2_HUMAN | Transgelin-2 | 8.41 | 22,590 | 648 |
| 13 | PSB5_HUMAN | Proteasome subunit
β type-5 | 6.43 | 28,675 | 420 |
| 14 | PEBP1_HUMAN |
Phosphatidylethanolamine-binding protein
1 | 7.01 | 21,186 | 366 |
| 15 | RS7_HUMAN | 40S ribosomal
protein S7 | 10.09 | 22,113 | 248 |
| 16 | RS9_HUMAN | 40S ribosomal
protein S9 | 10.66 | 22,649 | 262 |
| 17 | GGCT_HUMAN |
γ-glutamylcyclotransferase | 5.07 | 21,278 | 185 |
| 18 | GNA1_HUMAN | Glucosamine
6-phosphate N-acetyltransferase | 8.17 | 21,162 | 147 |
| 19 | DCTP1_HUMAN | dCTP
pyrophosphatase 1 | 4.93 | 18,811 | 105 |
| 20 | RCL_HUMAN | Deoxyribonucleoside
5′-monophosphate N-glycosidase | 4.97 | 19,239 | 110 |
| 21 | DCD_HUMAN | Dermcidin | 6.08 | 11,419 | 100 |
| 22 | LDHA_HUMAN | L-lactate
dehydrogenase A chain | 8.44 | 37,021 | 566 |
| 23 | PCNA_HUMAN | Proliferating cell
nuclear antigen | 4.57 | 29,177 | 346 |
| 24 | RL5_HUMAN | 60S ribosomal
protein L5 | 9.73 | 34,625 | 333 |
| 25 | IPYR_HUMAN | Inorganic
pyrophosphatase | 5.54 | 33,207 | 284 |
| 26 | RL6_HUMAN | 60S ribosomal
protein L6 | 10.59 | 32,779 | 289 |
| 27 | RA1L3_HUMAN | Putative
heterogeneous nuclear ribonucleoprotein A1-like 3 | 9.23 | 34,415 | 180 |
| 28 | OTUB1_HUMAN | Ubiquitin
thioesterase OTUB1 | 4.85 | 31,549 | 176 |
| 29 | CHIP_HUMAN | E3
ubiquitin-protein ligase CHIP | 5.61 | 35,403 | 110 |
| 30 | AN32E_HUMAN | Acidic leucine-rich
nuclear phosphoprotein 32 family member E | 3.77 | 30,958 | 106 |
| 31 | PP4C_HUMAN |
Serine/threonine-protein phosphatase 4
catalytic subunit | 4.91 | 35,768 | 115 |
| 32 | NACA_HUMAN | Nascent
polypeptide-associated complex subunit α | 4.52 | 23,370 | 117 |
| 33 | PHB2_HUMAN | Prohibitin-2 | 9.83 | 33,276 | 489 |
| 34 | VDAC2_HUMAN | Voltage-dependent
anion-selective channel protein 2 | 7.49 | 32,186 | 319 |
| 35 | ROA1_HUMAN | Heterogeneous
nuclear ribonucleoprotein A1 | 9.17 | 38,865 | 238 |
| 36 | P5CR1_HUMAN |
Pyrroline-5-carboxylate reductase 1,
mitochondrial | 7.18 | 33,624 | 356 |
| 37 | EFTS_HUMAN | Elongation factor
Ts, mitochondrial | 8.62 | 35,794 | 157 |
| 38 | ELAV1_HUMAN | ELAV-like protein
1 | 9.23 | 36,282 | 143 |
| 39 | SFXN1_HUMAN | Sideroflexin-1 | 9.22 | 35,952 | 108 |
| 40 | PDIA4_HUMAN | Protein
disulfide-isomerase A4 | 4.96 | 73,313 | 1,623 |
| 41 | HNRPM_HUMAN | Heterogeneous
nuclear ribonucleoprotein M | 8.84 | 77,819 | 566 |
| 42 | ECHA_HUMAN | Trifunctional
enzyme subunit α, mitochondrial | 9.16 | 83,870 | 331 |
| 43 | ABCG2_HUMAN | ATP-binding
cassette sub-family G member 2 | 8.91 | 73,120 | 192 |
| 44 | GPDM_HUMAN |
Glycerol-3-phosphate dehydrogenase,
mitochondrial | 7.57 | 81,441 | 262 |
| 45 | TRAP1_HUMAN | Heat shock protein
75 kDa, mitochondrial | 8.30 | 80,415 | 117 |
| 46 | GRP78_HUMAN | Glucose-regulated
protein 78 kDa | 5.07 | 72,431 | 248 |
In the present study, NP-1250 showed no cytostatic
effect on other MDR cancer cell lines. However, it may be effective
against specific tumors that are clinically similar to MCF7/MX
cells and there is still a possibility that it may lead to the
identification of new therapeutic molecules for MDR cancers.
Acknowledgements
We thank Dr Takaichi Hamano (Nippon Chemiphar Co.,
Ltd.) for the helpful discussions. This study was supported by a
Grant-in-Aid for Cancer Research and Grants-in Aid for Scientific
Research from the Ministry of Education, Culture, Sports, Science
and Technology of Japan, and the Ministry of Health, Labor and
Welfare of Japan.
Abbreviations:
|
ABC
|
ATP-binding cassette
|
|
Flp-In-293/ABCG2 cells
|
ABCG2-overexpressing Flp-In-293
cells
|
|
Flp-In-293/Mock cells
|
mock-transfected Flp-In-293 cells
|
|
MCF7/MX cells
|
mitoxantrone-resistant MCF7 cells
|
|
MCF7/WT cells
|
wild-type MCF7 cells
|
|
MDR
|
multidrug resistance
|
|
MTX
|
methotrexate
|
|
PARP
|
poly(ADP-ribose) polymerase
|
|
PDIA4
|
protein disulfide-isomerase A4
|
|
PHB2
|
prohibitin-2
|
|
PRDX2
|
peroxiredoxin-2
|
References
|
1
|
Scheffer GL, Maliepaard M, Pijnenborg AC,
et al: Breast cancer resistance protein is localized at the plasma
membrane in mitoxantrone- and topotecan-resistant cell lines.
Cancer Res. 60:2589–2593. 2000.PubMed/NCBI
|
|
2
|
Kuo MT: Roles of multidrug resistance
genes in breast cancer chemoresistance. Adv Exp Med Biol.
608:23–30. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo X, Ma N, Wang J, Song J, Bu X, Cheng
Y, et al: Increased p38-MAPK is responsible for chemotherapy
resistance in human gastric cancer cells. BMC Cancer. 8:3752008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Honma K, Iwao-Koizumi K, Takeshita F,
Yamamoto Y, Yoshida T, Nishio K, et al: RPN2 gene confers docetaxel
resistance in breast cancer. Nat Med. 14:939–948. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katoh SY, Ueno M and Takakura N:
Involvement of MDR1 function in proliferation of tumour cells. J
Biochem. 143:517–524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Z, Liu F, Ren Q, Zhao Q, Ren H, Lu S,
et al: Suppression of ABCG2 inhibits cancer cell proliferation. Int
J Cancer. 126:841–851. 2010.PubMed/NCBI
|
|
7
|
Nakagawa M, Schneider E, Dixon KH, Horton
J, Kelley K, Morrow C and Cowan KH: Reduced intracellular drug
accumulation in the absence of P-Glycoprotein (mdr1) overexpression
in mitoxantrone-resistant human MCF-7 breast cancer cells. Cancer
Res. 52:6175–6181. 1992.PubMed/NCBI
|
|
8
|
Wakabayashi K, Nakagawa H, Adachi T, Kii
I, Kobatake E, Kudo A and Ishikawa T: Identification of cysteine
residues critically involved in homodimer formation and protein
expression of human ATP-binding cassette transporter ABCG2: a new
approach using the flp recombinase system. J Exp Ther Oncol.
5:205–222. 2006.
|
|
9
|
Saito H, Hirano H, Nakagawa H, Fukami T,
Oosumi K, Murakami K, et al: A new strategy of high-speed screening
and quantitative structure-activity relationship analysis to
evaluate human ATP-binding cassette transporter ABCG2-drug
interactions. J Pharmacol Exp Ther. 317:1114–1124. 2006. View Article : Google Scholar
|
|
10
|
Wang T, Kajino K, Abe M, Tan K, Maruo M,
Sun G, et al: Suppression of cell death by the secretory form of
N-terminal ERC/mesothelin. Int J Mol Med. 26:185–191.
2010.PubMed/NCBI
|
|
11
|
Fujimura T, Shinohara Y, Tissot B, Pang
PC, Kurogochi M, Saito S, et al: Glycosylation status of
haptoglobin in sera of patients with prostate cancer vs. benign
prostate disease of normal subjects. Int J Cancer. 122:39–49. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Janicke RU, Sprengart ML, Wati MR and
Porter AG: Caspase-3 is required for DNA fragmentation and
morphological changes associated with apoptosis. J Biol Chem.
273:9357–9360. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wilkinson B and Gilbert HF: Protein
disulfide isomerase. Biochim Biophys Acta. 1699:35–44. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Keenan J, Murphy L, Henry M, Meleady P and
Clynes M: Proteomic analysis of multidrug-resistance mechanisms in
adriamycin-resistant variants of DLKP, a squamous lung cancer cell
line. Proteomics. 9:1556–1566. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Liu H, Han B and Zhang JT:
Identification of 14-3-3sigma as a contributor to drug resistance
in human breast cancer cells using functional proteomic analysis.
Cancer Res. 66:3248–3255. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Montano MM, Ekena K, Delage-Mourroux R,
Chang W, Martini P and Katzenellenbogen BS: An estrogen
receptor-selective coregulator that potentiates the effectiveness
of antiestrogens and represses the activity of estrogens. Proc Natl
Acad Sci USA. 96:6947–6952. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zubovych I, Doundoulakis T, Harran PG and
Roth MG: A missense mutation in Caenorhabditis elegans
prohibitin 2 confers an atypical multidrug resistance. Proc Natl
Acad Sci USA. 103:15523–15528. 2006.PubMed/NCBI
|
|
18
|
Shau H and Kim A: Identification of
natural killer enhancing factor as a major antioxidant in human red
blood cells. Biochem Biophys Res Commun. 199:83–88. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kowaltowski AJ, Netto LE and Vercesi AE:
The thiol-specific antioxidant enzyme prevents mitochondrial
permeability transition evidence for the participation of reactive
oxygen species in this mechanism. J Biol Chem. 273:12766–12769.
1998. View Article : Google Scholar
|
|
20
|
Chung YM, Yoo YD, Park JK, Kim YT and Kim
HJ: Increased expression of peroxiredoxin II confers resistance to
cisplatin. Anticancer Res. 21:1129–1133. 2001.PubMed/NCBI
|