Introduction
The human epidermal growth factor receptor 2 (HER-2)
gene, also called ErbB-2, is one of the most confirmed and commonly
amplified genes in breast cancer. Overexpression of ErbB-2 usually
results in malignant transformation of cells and accounts for
approximately 25% of all breast cancer cases (1). ErbB-2 is consistently associated with
more aggressive tumor phenotypes, a greater likelihood of lymph
node involvement, and increased resistance to chemotherapy and
endocrine therapy (2–4). The overall survival rate and time of
relapse for ErbB-2-positive breast cancer patients are
significantly shorter than for patients without ErbB-2
overexpression. Therefore, ErbB-2 is a logical target for breast
cancer therapy, and inhibition of ErbB-2 expression leads to the
apoptosis of tumor cells (2,5–7).
A monoclonal humanized antibody against ErbB-2 (trastuzumab) has
been successfully applied in the treatment of ErbB-2-positive
breast cancer. ErbB-2 is a cell surface receptor tyrosine kinase
(RTK) and becomes internalized upon ligand binding which may
trigger a multitude of signaling pathways, such as MAPKs and PI3K.
Consequently, ErbB-2 has been shown to trigger signal transduction
leading to cell growth and differentiation. Tumors exhibiting
ErbB-2 amplification/overexpression have been shown to demonstrate
increased aggressiveness and metastatic potential and are
associated with decreased overall patient survival (8,9).
Therefore, agents targeted to ErbB-2 can be utilized for breast
cancer treatment.
Members of the transforming acidic coiled-coil
(TACC) family of proteins have all been implicated in human cancer
(10,11). TACC1 is located on a chromosomal
region that is amplified in 10–15% of human breast cancers
(12), and is a gene cloned from
the breast cancer amplicon 8p11 (11). The TACC1 mRNA is ubiquitously
expressed and encodes a protein with an apparent molecular mass of
125 kDa; it is cytoplasmic and mainly perinuclear (11). A full-length cDNA of 7758 bp
encoding human TACC1 was found in the databases (GenBank,
AF049910). Recently, research on TACC1 found that TACC1 is involved
in the regulation of interaction between centrosomes and
microtubules (13). Overexpression
of TACC1 in mouse fibroblasts results in cellular transformation
and anchorage independent growth (11). Moreover, research revealed that
TACC1 mRNA and/or protein expression is downregulated in various
types of tumors, such as breast and lung cancers. Thus, it was
speculated that downregulation of TACC1 may alter the control of
mRNA homeostasis in polarized cells and may participate in
oncogenic processes (14). Ovarian
tumors (78.5%) lack appreciable expression of TACC1, confirming
inferences made from published SAGE analysis that TACC1 can be
upregulated or lost in cancer (15,16).
Another investigator described that the interaction of the TACC1
protein with several protein partners makes it a good candidate to
participate in microtubule-associated processes in normal and
tumoral cells (17).
An opposing view raises the possibility that
amplification of TACC1 promotes malignant growth, thereby making
TACC1 an attractive candidate gene for promoting tumorigenicity in
human breast cancers. Still et al(11) found that deregulation of TACC1 gene
expression, directly, through amplification of the entire gene, or
through disruption of transcriptional regulatory elements,
contributes to the aggressive phenotype noted in breast tumors, and
the amplification of TACC1 may contribute to cancer. TACC1 was
found to be significantly overexpressed in samples from ER-positive
breast cancer patients who relapsed after tamoxifen treatment when
compared with samples from patients who did not. TACC1 was found to
be upregulated and to act as an oncogene in breast cancer, and
TACC1 was correlated with significantly shorter relapse-free
survival (18). Another study
identified TACC1, NOV and PTTG1 as new candidate genes associated
with endocrine therapy resistance in breast cancer (19). TACC1 has also been associated with
other genes, such as FGFR1, BRCA1, Aurora B, BRCA2, AZU-1 and WT1
(17,20,21).
Accordingly, we hypothesized that TACC1 is highly expressed in some
types of tumors, but downregulated in others. Wilson et al
used cDNA microarray technology that allows for the simultaneous
evaluation of expression, at the mRNA level, of thousands of genes.
A limited number of additional genes were found to be upregulated
or downregulated by ErbB-2. These included TACC1, which was found
to be downregulated both in ErbB2-positive breast cancer cell lines
and in ErbB2-overexpressing breast cancers (22). Therefore, in the present study, we
investigated the relationship between TACC1 and ErbB-2.
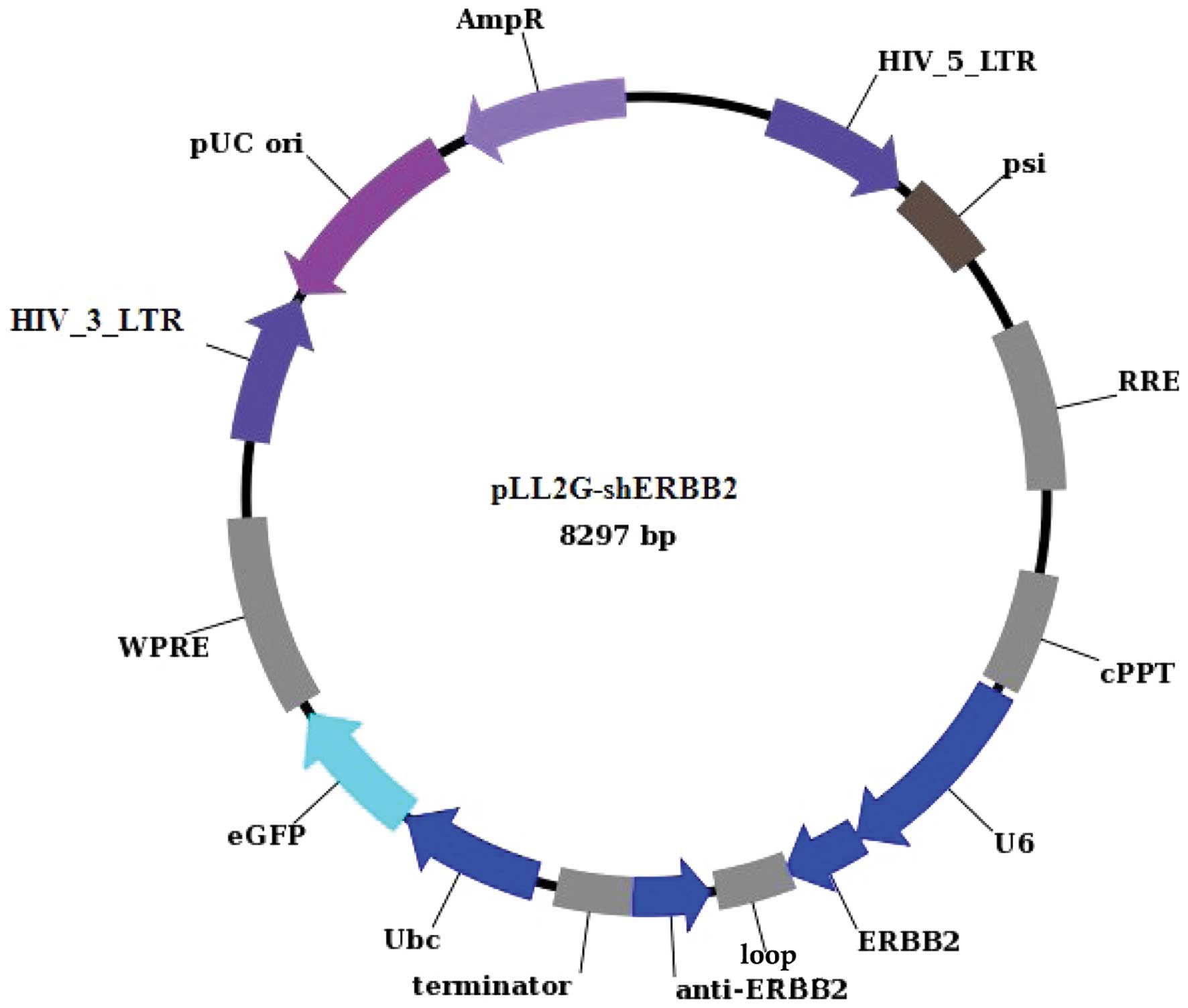
In the present study, we constructed a lentiviral
vector mediating RNAi targeting ErbB-2 (pLL2G-shERBB2). The
efficiency of pLL2G-shERBB2 plasmids in interfering with ErbB-2
expression was confirmed by western blotting. We used lentiviral
infection to knock down ErbB-2 in BT474 cells resulting in reduced
expression. After downregulating the expression of ErbB-2, we
detected the changes in TACC1 expression at the mRNA and protein
levels by real-time PCR and western blotting, respectively. Our
results demonstrated changes in the sensitivity of the cells to
chemotherapeutic drugs after silencing of the ErbB-2 gene.
Materials and methods
Cell lines and cell culture
BT474 [ERBB2(+), TACC1(−)] and MCF-7 [ERBB2(−),
TACC1(+)] (22) cancer cells were
obtained from the Type Culture Collection of the Chinese Academy of
Sciences, Shanghai, China. The cells were maintained in RPMI-1640
(Gibco, Carlsbad, CA, USA) supplemented with 10% FBS (fetal bovine
serum) (v/v) and penicillin (100 U/ml)/streptomycin (100 mg/ml) at
37°C in 5% CO2, 95% air.
Construction and production of lentiviral
vectors
We designed and cloned an shRNA template into a
lentiviral vector. A self-inactivating lentiviral vector containing
a GFP reporter and a U6 promoter upstream of the cloning
restriction sites (HpaI and XhoI) was used. The
introduction of oligonucleotides encoding shRNAs between these
restriction sites enables the production of the shRNA in
vitro. Four coding regions corresponding to targeted human
ERBB2 starting at different positions were used. We constructed
four shRNA-ERBB2 lentiviral vectors, namely pLL2G-shERBB2-1,
pLL2G-shERBB2-2, pLL2G-shERBB2-3 and pLL2G-shERBB2-4, respectively
(Table I). Briefly,
oligonucleotides were annealed, digested and inserted between the
HpaI and XhoI restriction sites of the plasmid
vector. Some mutations were introduced in the sense sequence of the
hairpin structure to facilitate sequence and avoid destruction by
bacteria during amplification in the bacterial host. Correct
insertions of shRNA cassettes were confirmed by restriction mapping
and direct DNA sequencing. We constructed a vector, pLL2G-shERBB2
(Fig. 1), co-infected with
recombinant lentiviral vectors into 293T cells using Lipofectamine
2000 (Invitrogen, Carlsbad, CA, USA). To detect the interference
effects of the target, the expression of ErbB-2 mRNA and protein
was determined using real-time PCR and western blotting,
respectively. Recombinant and control lentiviral vectors were
produced by co-transfecting 293T cells with the lentiviral
expression plasmid and packaging plasmids (pLV/helper-SL3,
pLV/helper-SL4 and pLV/helper-SL5). Infectious lentiviral vectors
were harvested at 48 h post-infection, centrifuged to remove cell
debris, and filtered through 0.45-μm cellulose acetate filters. The
infectious titer was determined by hole-by-dilution titer assay.
The virus titers produced were ~4.9×107 TU/ml.
 | Table IFour coding regions corresponding to
targeted human ErbB-2. |
Table I
Four coding regions corresponding to
targeted human ErbB-2.
| Oligo name | Oligo sequence |
|---|
| shERBB2-1 |
5′-TTGTCAGTATCCAGGCTTTGTACTCGAGTACAAAGCCTGGATACTGACATTTTTC-3′ |
|
5′-TCGAGAAAAATGTCAGTATCCAGGCTTTGTACTCGAGTACAAAGCCTGGATACTGACAA-3′ |
| shERBB2-2 |
5′-TGATCACAGGTTACCTATACATCTCGAGATGTATAGGTAACCTGTGATCTTTTTC-3′ |
|
5′-TCGAGAAAAAGATCACAGGTTACCTATACATCTCGAGATGTATAGGTAACCTGTGATCA-3′ |
| shERBB2-3 |
5′-TGAATATGTGAACCAGCCAGATCTCGAGATCTGGCTGGTTCACATATTCTTTTTC-3′ |
|
5′-TCGAGAAAAAGAATATGTGAACCAGCCAGATCTCGAGATCTGGCTGGTTCACATATTCA-3′ |
| shERBB2-4 |
5′-TGAAGTACACGATGCGGAGACTCTCGAGAGTCTCCGCATCGTGTACTTCTTTTTC-3′ |
|
5′-TCGAGAAAAAGAAGTACACGATGCGGAGACTCTCGAGAGTCTCCGCATCGTGTACTTCA-3′ |
| Negative
sh-control |
5′-TGCGCGCTTTGTAGGATTCGCTCGAGCGAATCCTACAAAGCGCGCTTTTTC-3′ |
|
5′-TCGAGAAAAAGCGCGCTTTGTAGGATTCGCTCGAGCGAATCCTACAAAGCGCGCA-3′ |
Lentiviral infection
Cells grown in 6-well culture plates (500,000
cells/well) were treated as required. When the cells were ~80%
confluent in complete RPMI-1640 medium, they were infected with the
lentiviral constructs. BT474 cells infected with
lentiviral-mediated Lenti-shERBB2-eGFP were used as the study
cells; cells infected with the negative shRNA (Lenti-sh-control)
were used as the negative controls, and cells without infection
were considered as the blank controls.
Quantification by real-time PCR
Total RNA was isolated using RNAiso (Takara, Japan).
The concentration and purity of RNA were determined using a
spectrophotometer. Reverse transcriptase was used to create cDNA
for further analyses. Reverse transcriptase and quantitative
real-time polymerase chain reaction (RT-PCR) assays were carried
out using SYBR Premix Ex Taq II (Perfect Real-Time) (Takara) and
real-time PCR amplification equipment. Reverse transcriptase and
real-time PCR was carried out according to the instructions
provided in the kit. The PCR primers (synthesized by Sangon
Biotech, Shanghai, China) used to detect ErbB-2, TACC1 and GAPDH
were as follows: ErbB-2, sense strand 5′-CATGTCATCGTCCTCCAGCAG-3′,
and antisense strand 5′-TTGACTCTGAATGTCGGCCAA-3′, with a product
length of 161 bp; TACC1, sense strand 5′-TGGTCAGAAATCAGCTGGTGC-3′,
and antisense strand 5′-GCACAGTGGACCCGTCTTCTT-3′, with a product
length of 230 bp; GAPDH, sense strand 5′-CTGCACCACCAACTGCTTAG-3′,
and antisense strand 5′-TGAAGTCAGAGGAGACCACC-3′, with a product
length of 135 bp. The real-time PCR procedure consisted of two
steps: one cycle at 95°C for 10 sec, followed by 40 cycles at 95°C
for 5 sec and 60°C for 20 sec. The expression of ErbB-2 was
determined by normalization of the threshold cycle of these genes
to that of the control housekeeping gene (GAPDH). Data were
analyzed using the comparative ΔΔCT method.
Western blot analysis
Whole-cell proteins in various breast cancer cell
lines were isolated. The lysates were centrifuged, and the
supernatant was collected and stored at −80°C according to the
manufacturer’s instructions. Total protein (10 μl) was loaded per
well, separated by 10–15% SDS-PAGE, and transferred to
polyvinylidene difluoride membranes at 60 V for 1 h at 4°C. The
membranes were blocked and incubated with primary antibodies
(Bioworld Co., USA; diluted 1:1,000 in TBS-A). The membranes were
rinsed thrice with 0.1% Tween 20-PBS for 30 min. The secondary
antibodies (Abcam Co., Cambridge, UK; diluted 1:1,200 in TBS-A)
were used with Peroxidase-conjugated Affinipure goat anti-mouse IgG
(1:8,000) and Peroxidase-conjugated Affinipure goat anti-rabbit IgG
(1:8,000) for 1 h at room temperature. The blotted membranes were
washed three times with 0.1% Tween 20-PBS for 15 min and three
times with PBS for 15 min. The immunoblots were detected using an
electrochemiluminescence kit and exposed to the Vilber Fusion FX5
automatic gel imaging analysis system (Vilber, Marne La Vallée,
France).
Cell counting kit-8 (CCK-8) assay
The drug sensitivity assay was carried out using the
mitochondrial reduction activity assay. According to the protocol
of the CCK-8 assay kit (Dojindo, Kumamoto, Japan), cells grown in
96-well culture plates (8,000 cells/well) were treated as required.
The experimental group consisted of cells infected with the
lentivirus and the control group consisted of non-infected cells.
After a 24-h culture, docetaxel (Aventis Pharma, Guildford, UK) was
added at the following concentrations: 0.01, 0.05, 0.25, 0.5, 0.75,
1, 5 and 25 μg/ml. Next, cells in each well were incubated with 10
μl of CCK-8 at 37°C for 4 h. The optical density (OD) for each well
was measured at 450 nm using a microplate reader (Bio-Rad Model
550; Bio-Rad Laboratories, Hercules, CA, USA). CCK-8 experiments
were repeated three times on different days. The means and standard
deviations of the optical density (OD) of the replicates were
calculated for each well. The cell inhibitory rate was calculated
according to the following equation: Cell inhibitory rate = [1 −
(OD experiment − OD blank)/(OD control − OD blank)] × 100%. The 50%
inhibition concentration (IC50) of the drug was
determined by chartography.
Statistical analysis
For all measurements as needed, the statistical
significance between groups was assessed by one-way ANOVA based on
the homogeneity of variance test (SPSS 13.0, SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
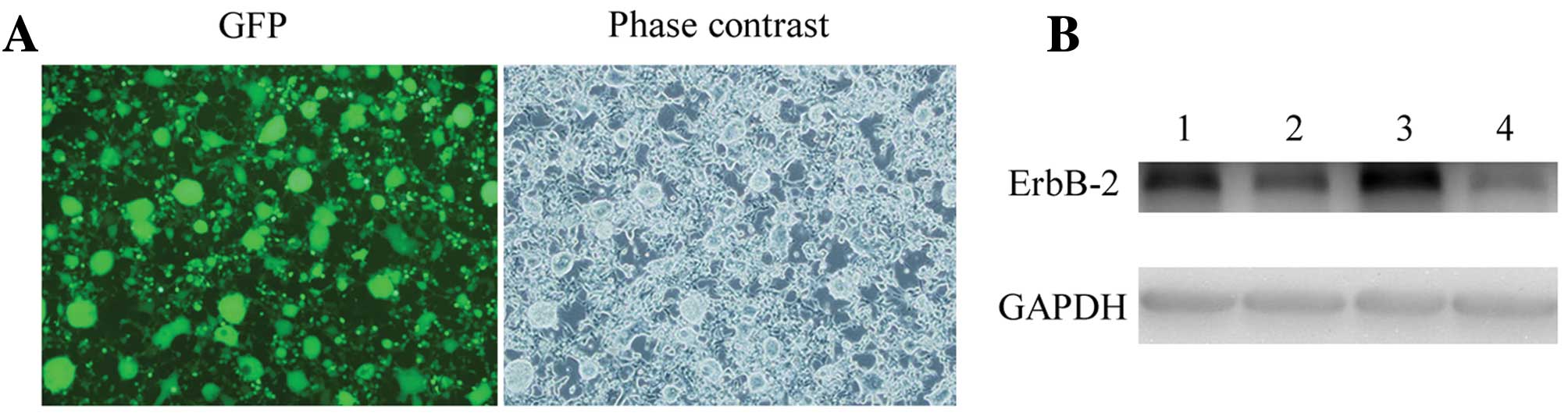
Selection of the most effective
ErbB2-specific shRNA expression vector
Four plasmids containing shERBB2 (pLL2G-shERBB2)
were coinfected into 293T cells, respectively. GFP expression in
the 293T cells was observed under a fluorescence microscope 48 h
after infection with pLL2G-shERBB2. According to the results of the
western blotting assay, pLL2G-shERBB2-4 was the most effective
lentiviral vector and, thus, was used in the subsequent research
(Fig. 2).
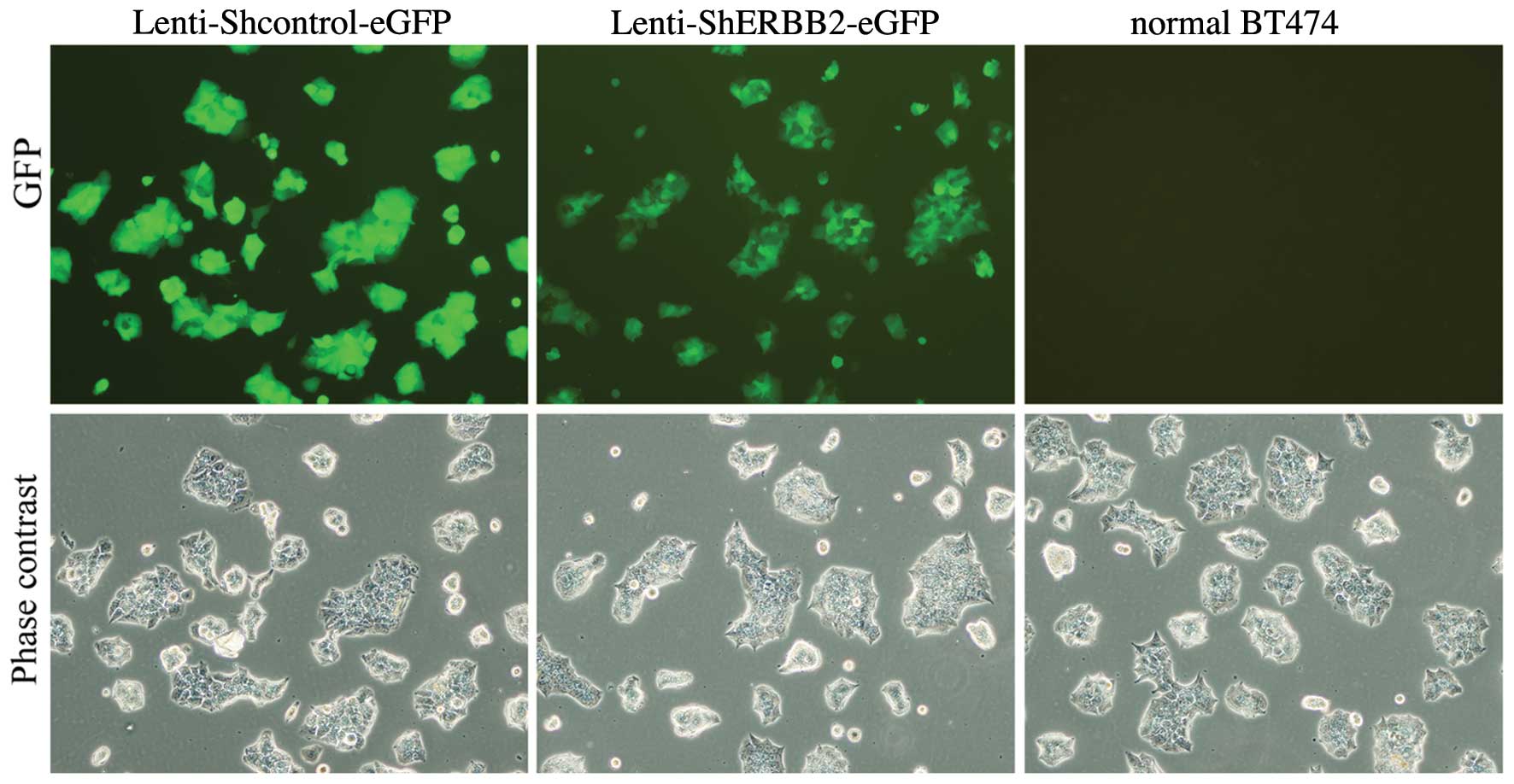
Infection efficiency of the viral
delivery in vitro
BT474 cells were infected with the lentiviral vector
encoding GFP, resulting in GFP expression in the majority of
cultured cells. The infection efficiency was assayed by
fluorescence microscopy after cells were infected by the lentivirus
for 48 h. A robust infection efficiency of 79% was observed
(Fig. 3). The fluorescence
gradually weakened and the infected cells began to undergo
apoptosis on the fifth day resulting in the gradual decline of the
infection rate. BT474 cells that were infected with
Lenti-shERBB2-eGFP and non-infected cells co-cultured showed no
synergistic growth inhibition effect when treated using the same
protocols. We observed that the proliferation of BT474 cells was
significantly inhibited after infection with Lenti-shERBB2-eGFP
when compared with the Lenti-shcontrol-eGFP and normal BT474 cells
(P<0.05). Real-time PCR and western blot analysis showed that
the mRNA and protein expression levels of ErbB-2 were lower in the
cells infected with Lenti-shERBB2-eGFP than in the cells infected
with the Lenti-shcontrol and the normal BT474 cells (P<0.05)
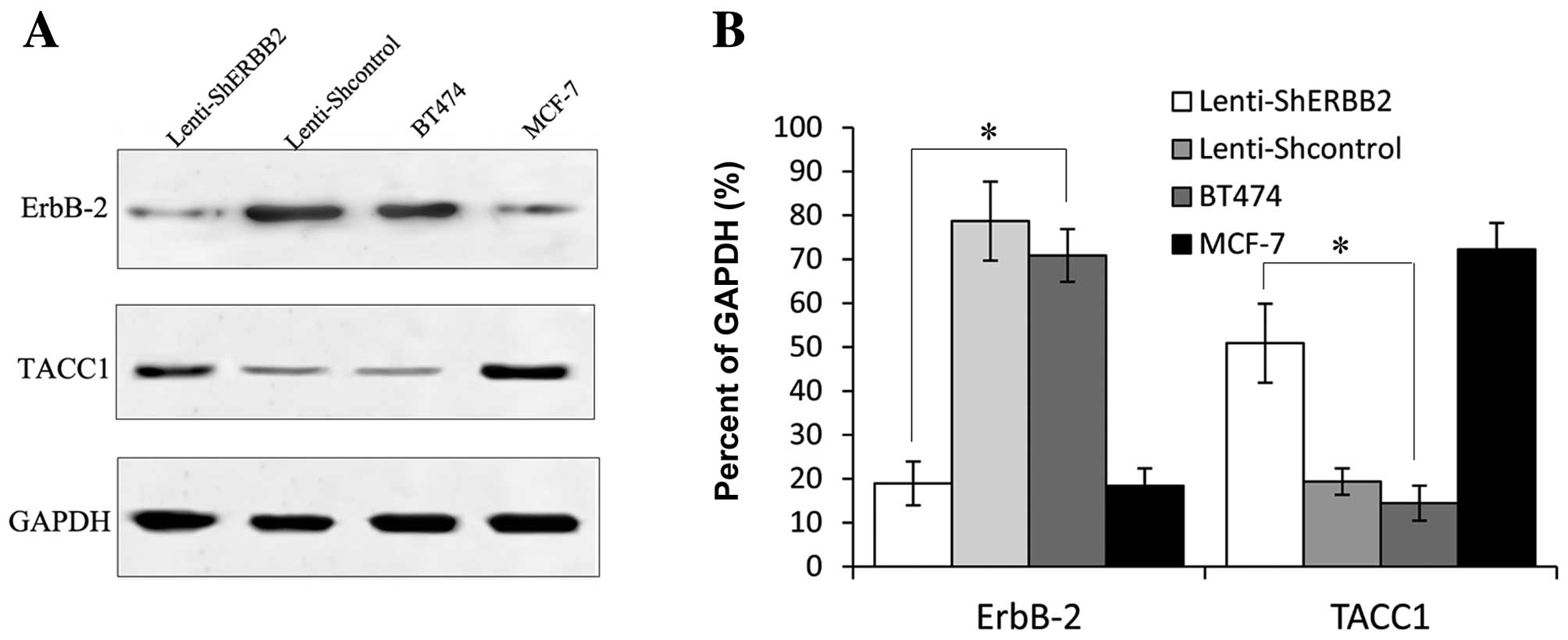
(Figs. 4 and 5).
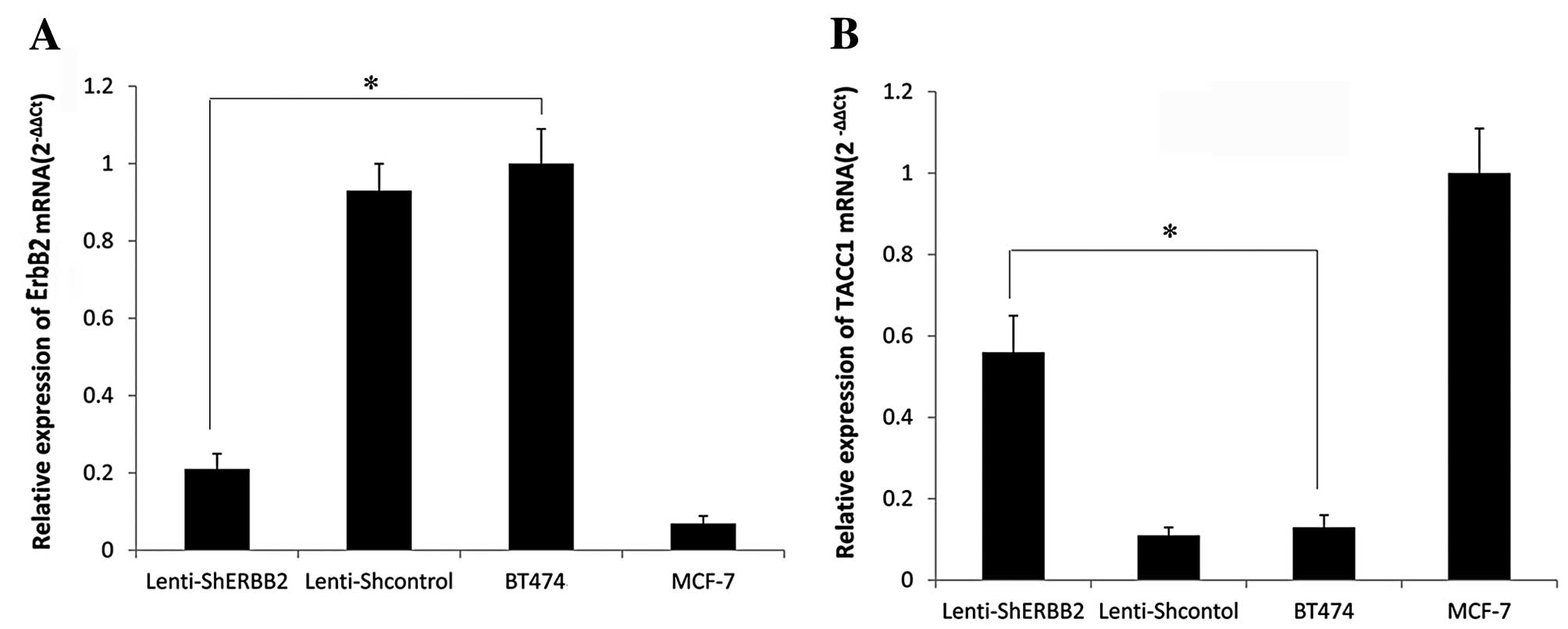
mRNA expression of ErbB-2 and TACC1
Following lentiviral infection of BT474 cells, the
mRNA expression of ErbB-2 and TACC1 was examined by real-time PCR.
A significant difference was noted between the silenced and control
cells (P<0.05), a reduction in the expression of ErbB-2 was
noted. The expression of ErbB-2 mRNA in the BT474 cells was
successfully knocked down. On the other hand, the expression of
ErbB-2 mRNA showed no significant difference between the blank
control group and the negative control-shRNA group (P>0.05). As
shown in Fig. 4B, the expression of
TACC1 mRNA was affected following ErbB-2 knockdown, with a
different expression level noted in the ErbB-2-silenced cells when
compared to the control cells. The expression of TACC1 in the
ErbB-2-positive cells (BT474) was expressed at a low level.
However, after interference of the expression of ErbB-2, expression
of TACC1 was upregulated. The result of real-time PCR showed that
silencing of the ErbB-2 gene markedly increased the levels of TACC1
mRNA (P<0.05) (Fig. 4).
ErbB-2 and TACC1 protein expression
The protein expression of ErbB-2 and TACC1 was
evaluated by western blotting. The gray scale of the stained area
was measured under identical conditions. The average optical
density for ErbB-2 protein expression in the ErbB2-shRNA-infected
cells was lower when compared with the value in the blank control
and the negative control-shRNA cells, respectively. This indicated
marked reduction in ErbB-2 protein following ErbB2-shRNA infection;
the observed differences were significant (P<0.05) (Fig. 5). Consistent with the above mRNA
result, the average optical density for TACC1 protein expression in
the BT474, MCF-7, Lenti-shcontrol and Lenti-shERBB-2 cells showed
significant differences between the cell groups (P<0.05)
(Fig. 5). TACC1 was overexpressed
when the ErbB-2 protein was markedly inhibited by ErbB2-shRNA in
BT474 cells. The results were in accordance with the mRNA levels
(Fig. 4B).
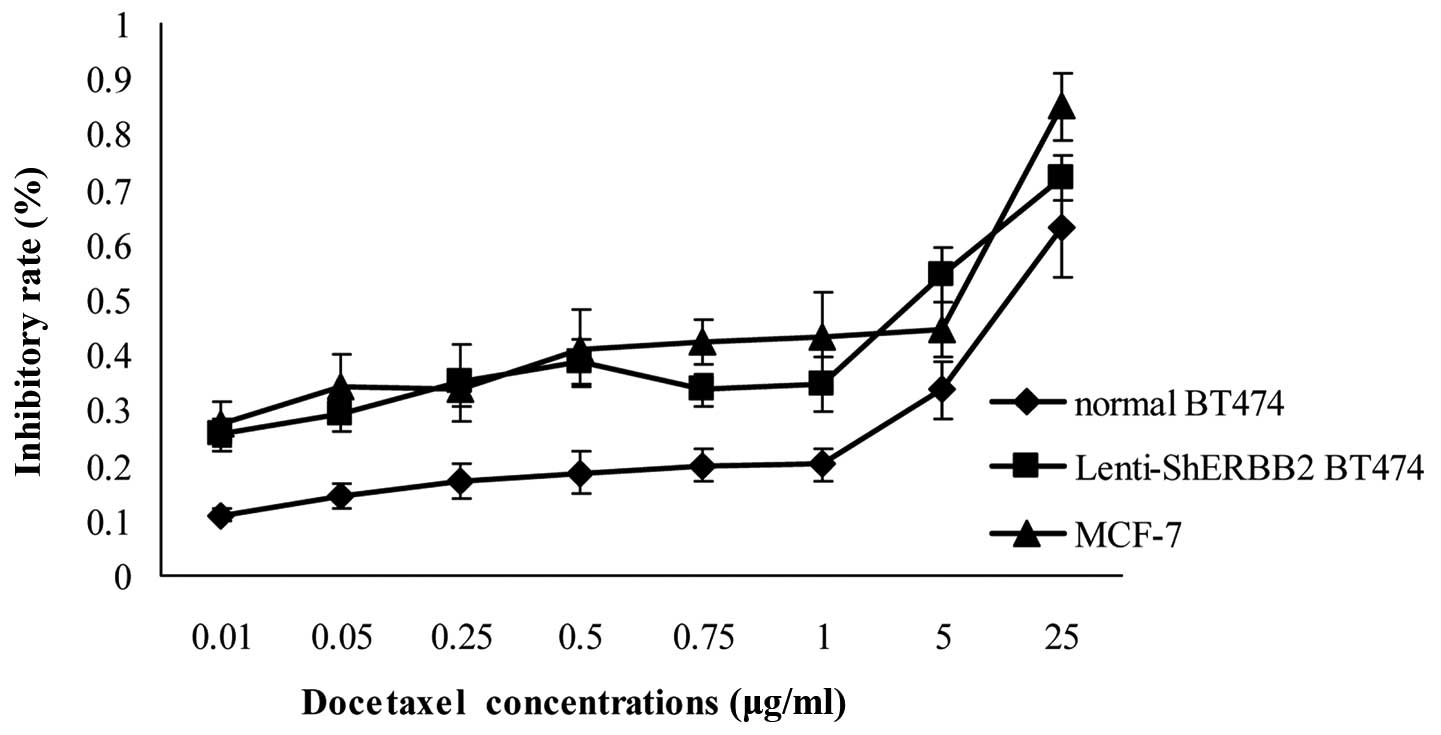
Effects of Lenti-shERBB2-eGFP on cell
sensitivity to docetaxel treatment
Breast cancer cells that overexpress ErbB-2 are more
resistant to chemotherapeutic agents such as paclitaxel (Taxol) and
docetaxel (Taxotere) than cells that do not overexpress ErbB-2
(23). After inhibiting ErbB-2
expression using Lenti-shERBB2-eGFP infection, the IC50
(index of chemotherapy sensitivity to docetaxel) of the
Lenti-shERBB2-infected cells was 2.77±0.04 μg/ml. The
IC50 of the BT474 cells was 30.01±0.13 μg/ml, and this
value for the MCF-7 cells was 1.15±0.03 μg/ml. That is, after
ErbB-2 was inhibited, the breast cancer cell sensitivity to
chemotherapy improved 10-fold. The result indicated that the
supression of ErbB-2 expression inhibits the proliferation of BT474
cells and increases the cell sensitivity to docetaxel treatment;
this difference was statistically significant (Fig. 6).
Discussion
This study confirmed the knockdown of ErbB-2 in
BT474 cells by lentiviral infection and that TACC1 exhibited higher
expression when ErbB-2 was expressed at a low level.
Lenti-shERBB2-eGFP obviously inhibited the expression of ErbB-2
mRNA and protein in BT474 breast cancer cells and also increased
the chemotherapy sensitivities to docetaxel in vitro. We
also observed that infection with the lentivirus inhibited BT474
cell growth and promoted apoptosis.
ErbB receptors, in particular ErbB-2, play an
important role in carcinoma formation, and dysfunction promotes
tumorigenesis. Downregulation of ErbB-2 was found to result in an
increase in cells in the G1 phase and the induction of apoptosis
(24). Overexpression of the ErbB-2
gene has frequently been observed in human tumors, including those
of the breast, stomach, lung and oral cavity (25–27).
Capable of stable and highly specific silencing of gene expression,
shRNAs have been extensively applied to silence abnormal gene
expression in the treatment of cancer. Given that ErbB-2 was found
to be overexpressed in the BT474 cell line (28), we examined the functional
consequence of ErbB-2 silencing in BT474 cells. In the present
study, after Lenti-shERBB2 was infected into the BT474 breast
cancer cells, ErbB-2 expression was significantly reduced at the
mRNA and protein levels. Since ErbB family-mediated signaling plays
a critical role in cell growth, survival, adhesion and motility
(8), our data suggest that the
ErbB-2 gene is a feasible RNAi target for gene silencing. The data
described here provide proof that siRNA can be used therapeutically
for human cancer. ErbB-2 as well as TACC1 may be referred to as
novel cancer therapeutic targets.
It has been documented that TACC1 is downregulated
in ErbB-2-positive breast cancer cells and tissues (22). Yet, few studies have examined the
functional relationship of TACC1 and ErbB-2 in breast cancer cells.
In this study, we investigated the relationship between ErbB-2 and
TACC1, specifically in BT474 cells. The expression of TACC1 was
examined following Lenti-shERBB2-mediated silencing of the ErbB-2
gene in BT474 cells. We found that silencing of ErbB-2 induced
changes in TACC1 expression. Although TACC1 and ErbB-2 both
contribute to the progression of certain types of tumors, our data
suggest that TACC1 expression may be strictly associated with
ErbB-2 amplification in BT474 cells.
In addition to the regulation of expression, TACC1
may be regulated at multiple levels, such as mutations, loss of
heterozygosity, promoter methylation, or activation of alternative
signaling pathways. Several studies have shown synergistic effects
of TACC1 with other genes during tumorigenesis (17,20,21).
On the other hand, there is also evidence that TACC1 is involved in
drug resistance to chemotherapy and tumor progression (29). Consistent with this hypothesis,
research has found that TACC1 overexpression appears to promote
paclitaxel-induced cell death (30). It is possible that TACC1 may promote
chemosensitivity, resulting from the ability of TACC1 to activate
Ras, PI3K and PKB (29,31), whereas the chemosensitivity of
TACC1-overexpressing cells may be due to the interaction of TACC1
with microtubules and the mitotic apparatus. In these signaling
pathways, TACC1 has been described as transforming (11) and, yet, is downregulated in
anthracyclin-treated mammary tumors (14). TACC1 expression results in an
increase in both ERK and PKB phosphorylation (32) in mammary tissues. Knuefermann et
al(33) delineated a pathway
that involves HER2/PI-3K/Akt in mediating multidrug resistance in
human breast cancer cells. They found that infection of ErbB-2 in
MCF7 breast cancer cells that express HER3 resulted in a
phosphoinoside-3 kinase (PI-3K)-dependent activation of Akt, and
was associated with an increased resistance of cells to multiple
chemotherapeutic agents, including paclitaxel. Regarding
PI-3K-dependent Akt activation, while the latter is associated with
cell resistance, following the selective inhibition of PI-3K or
Akt, cell sensitivity to chemotherapy significantly increased
(29). There appears to be a
certain correlation between ErbB-2 and TACC1 in PI-3K/Akt-related
chemotherapy resistance. If true, overexpression of TACC1 may serve
as a useful marker for chemosensitive tumors.
In summary, we demonstrated that ErbB-2 was
effectively silenced in BT474 cells via lentiviral infection,
providing evidence that the use of lentiviral shRNA can sensitize
docetaxel-resistant ErbB-2-overexpressing breast cancer cells to
the drug by repressing ErbB-2 expression. This in turn may have
important implications for the development of a novel therapy that
combines chemotherapy and gene therapy. In addition to new
diagnostic and prognostic markers, TACC1 may be a novel target for
breast cancer therapy. Future studies are required to examine the
molecular and biological relationship of ErbB-2 and TACC1 in breast
cancer cells in greater detail.
Acknowledgements
This study was supported by the Shandong Natural
Science Foundation (2009HW024; Y2006C23); the Shandong Excellent
Young Scientist Research Award Fund Project (2006BSB14114;
BS2010YY013) and the Shandong Tackle Key Problems in Science and
Technology (2010GSF10245).
References
|
1
|
Tai W, Qin B and Cheng K: Inhibition of
breast cancer cell growth and invasiveness by dual silencing of
HER-2 and VEGF. Mol Pharm. 7:543–556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartsch R, Wenzel C, Zielinski CC and
Steger GG: HER-2-positive breast cancer: hope beyond trastuzumab.
BioDrugs. 21:69–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Engel RH and Kaklamani VG: HER2-positive
breast cancer: current and future treatment strategies. Drugs.
67:1329–1341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dos Santos ML, Gimenes KP, Silva WA Jr and
Nagai MA: Transcriptome changes induced by docetaxel in human
mammary cell lines expressing different levels of ERBB2. Int J Mol
Med. 23:733–743. 2009.
|
|
5
|
Yang G, Cai KQ, Thompson-Lanza JA, Bast RC
Jr and Liu J: Inhibition of breast and ovarian tumor growth through
multiple signaling pathways by using retrovirus-mediated small
interfering RNA against Her-2/neu gene expression. J Biol Chem.
279:4339–4345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Faltus T, Yuan J, Zimmer B, et al:
Silencing of the HER2/neu gene by siRNA inhibits proliferation and
induces apoptosis in HER2/neu-overexpressing breast cancer cells.
Neoplasia. 6:786–795. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choudhury A, Charo J, Parapuram SK, et al:
Small interfering RNA (siRNA) inhibits the expression of the
Her2/neu gene, upregulates HLA class I and induces apoptosis of
Her2/neu positive tumor cell lines. Int J Cancer. 108:71–77. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Menard S, Casalini P, Campiglio M, Pupa SM
and Tagliabue E: Role of HER2/neu in tumor progression and therapy.
Cell Mol Life Sci. 61:2965–2978. 2004.PubMed/NCBI
|
|
9
|
Coronado Martin PJ, Fasero Laiz M, Garcia
Santos J, Ramirez Mena M and Vidart Aragon JA: Overexpression and
prognostic value of p53 and HER2/neu proteins in benign ovarian
tissue and in ovarian cancer. Med Clin. 128:1–6. 2007.(In
Spanish).
|
|
10
|
Still IH, Vince P and Cowell JK: The third
member of the transforming acidic coiled coil-containing gene
family, TACC3, maps in 4p16, close to translocation breakpoints in
multiple myeloma, and is upregulated in various cancer cell lines.
Genomics. 58:165–170. 1999. View Article : Google Scholar
|
|
11
|
Still IH, Hamilton M, Vince P, Wolfman A
and Cowell JK: Cloning of TACC1, an embryonically expressed,
potentially transforming coiled coil containing gene, from the 8p11
breast cancer amplicon. Oncogene. 18:4032–4038. 1999. View Article : Google Scholar
|
|
12
|
Ray ME, Yang ZQ, Albertson D, et al:
Genomic and expression analysis of the 8p11-12 amplicon in human
breast cancer cell lines. Cancer Res. 64:40–47. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gergely F, Karlsson C, Still I, Cowell J,
Kilmartin J and Raff JW: The TACC domain identifies a family of
centrosomal proteins that can interact with microtubules. Proc Natl
Acad Sci USA. 97:14352–14357. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Conte N, Charafe-Jauffret E, Delaval B, et
al: Carcinogenesis and translational controls: TACC1 is
down-regulated in human cancers and associates with mRNA
regulators. Oncogene. 21:5619–5630. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rhodes DR, Barrette TR, Rubin MA, Ghosh D
and Chinnaiyan AM: Meta-analysis of microarrays: interstudy
validation of gene expression profiles reveals pathway
dysregulation in prostate cancer. Cancer Res. 62:4427–4433.
2002.PubMed/NCBI
|
|
16
|
Line A, Slucka Z, Stengrevics A, Li G and
Rees RC: Altered splicing pattern of TACC1 mRNA in gastric cancer.
Cancer Genet Cytogenet. 139:78–83. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Conte N, Delaval B, Ginestier C, et al:
TACC1-chTOG-Aurora A protein complex in breast cancer. Oncogene.
22:8102–8116. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nguyen HG, Chinnappan D, Urano T and Ravid
K: Mechanism of Aurora-B degradation and its dependency on intact
KEN and A-boxes: identification of an aneuploidy-promoting
property. Mol Cell Biol. 25:4977–4992. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghayad SE, Vendrell JA, Bieche I, et al:
Identification of TACC1, NOV, and PTTG1 as new candidate genes
associated with endocrine therapy resistance in breast cancer. J
Mol Endocrinol. 42:87–103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ryser S, Dizin E, Jefford CE, et al:
Distinct roles of BARD1 isoforms in mitosis: full-length BARD1
mediates Aurora B degradation, cancer-associated BARD1beta
scaffolds Aurora B and BRCA2. Cancer Res. 69:1125–1134. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Devilard E, Bladou F, Ramuz O, et al:
FGFR1 and WT1 are markers of human prostate cancer progression. BMC
Cancer. 6:2722006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wilson KS, Roberts H, Leek R, Harris AL
and Geradts J: Differential gene expression patterns in
HER2/neu-positive and -negative breast cancer cell lines and
tissues. Am J Pathol. 161:1171–1185. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ueno NT, Yu D and Hung MC:
Chemosensitization of HER-2/neu-overexpressing human breast cancer
cells to paclitaxel (Taxol) by adenovirus type 5 E1A. Oncogene.
15:953–960. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng LS, Zha Z, Xi JJ, Jiang B, Liu J and
Yao XB: Downregulation of HER2 by adenovirus-mediated RNA
interference and its inhibitory effect on growth of SKBR3 breast
cancer cell. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 23:691–695.
2007.(In Chinese).
|
|
25
|
Verri E, Guglielmini P, Puntoni M, et al:
HER2/neu oncoprotein overexpression in epithelial ovarian cancer:
evaluation of its prevalence and prognostic significance. Clinical
study. Oncology. 68:154–161. 2005. View Article : Google Scholar
|
|
26
|
Nalwoga H, Odida M and Wabinga H: c-erbB-2
oncoprotein over-expression in breast cancer and its relationship
to histology and grade in a Ugandan population. East Afr Med J.
83:411–415. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cornolti G, Ungari M, Morassi ML, et al:
Amplification and overexpression of HER2/neu gene and HER2/neu
protein in salivary duct carcinoma of the parotid gland. Arch
Otolaryngol Head Neck Surg. 133:1031–1036. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoon S, Lee MY, Park SW, et al:
Up-regulation of acetyl-CoA carboxylase alpha and fatty acid
synthase by human epidermal growth factor receptor 2 at the
translational level in breast cancer cells. J Biol Chem.
282:26122–26131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cully M, Shiu J, Piekorz RP, Muller WJ,
Done SJ and Mak TW: Transforming acidic coiled coil 1 promotes
transformation and mammary tumorigenesis. Cancer Res.
65:10363–10370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee MJ, Gergely F, Jeffers K, Peak-Chew SY
and Raff JW: Msps/XMAP215 interacts with the centrosomal protein
D-TACC to regulate microtubule behaviour. Nat Cell Biol. 3:643–649.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Marmor MD, Skaria KB and Yarden Y: Signal
transduction and oncogenesis by ErbB/HER receptors. Int J Radiat
Oncol Biol Phys. 58:903–913. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gabillard JC, Ulisse S, Baldini E, et al:
Aurora-C interacts with and phosphorylates the transforming acidic
coiled-coil 1 protein. Biochem Biophys Res Commun. 408:647–653.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Knuefermann C, Lu Y, Liu B, et al:
HER2/PI-3K/Akt activation leads to a multidrug resistance in human
breast adenocarcinoma cells. Oncogene. 22:3205–3212. 2003.
View Article : Google Scholar : PubMed/NCBI
|