Introduction
Acute myeloid leukemia (AML) is a heterogeneous
disease with variable clinical outcomes. Despite the fact that the
outcome of AML in young patients has substantially improved, only
45–55% of children with AML are long-term survivors using intensive
chemotherapy protocols (1,2). Diverse poor prognostic factors are
associated with pediatric AML (3,4).
Studies into new prognostic factors and a more thorough
understanding of it will allow for improved therapeutic strategies
to enhance overall patient survival.
Hsp27, a member of the small heat shock protein
family, has been found to be overexpressed in several types of
human carcinomas arising from prostate, breast, gastric, ovarian,
bladder and pancreas (5–10). Its expression correlates with TNM
stage (11), and is associated with
tumor location, poor overall survival and unfavorable prognosis
(12,13). Hsp27 may protect tumor cells from
chemotherapy and result in aggressively-growing and
therapy-resistant tumors (14,15).
Knockdown of Hsp27 expression induces apoptosis via Bax activation
in a PI3K dependent mechanism in renal epithelial cells and
decreases clonogenic survival in HCT116 human colon cancer cells
(16,17). Although numerous studies regarding
Hsp27 have been performed on malignant cells from a solid tumor,
little is known about the role and the expression of Hsp27 in
pediatric acute leukemia (AL).
In this study, the expression of Hsp27 in various
pediatric AL [acute lymphoblastic leukemia (ALL), M1, M2, M3, M4,
M5 and M6] bone marrow mononuclear cells (BMMCs), the relationship
between expression of Hsp27 and M4/M5 subtype clinical status and
its influence on the chemoresistance and apoptosis of leukemia
cells to anticancer drugs, were investigated collectively. We
demonstrated that Hsp27 may be a promising therapeutic target for
pediatric AML-M4/M5.
Materials and methods
Patients
Ninety-four children with newly diagnosed AL were
studied. Diagnosis was based on May-Grunwald Giemsa stained bone
marrow smears and cytochemistry according to the
French-American-British (FAB) Group criteria (18). Complete remission (CR), refractory
and bone marrow (BM) relapse were defined according to the National
Cancer Institute (19). All AML
patients were treated by an anthracycline and cytarabine-based
induction chemotherapy regimen. One patient received hematopoietic
stem cell transplantation (HSCT) after induction therapy. Other
patients in CR after induction chemotherapy received post-induction
therapy with chemotherapy alone. This study was approved by the
research Ethics Committee at the Central South University, and
written informed consent was obtained from all patients.
Cell culture
The human Jurkat T leukemia cells, Burkitt’s
lymphoma cell Raji, the M2 AML cell line HL-60, the
BCR-ABL+ erythroleukemia cell line K562, the acute
monocytic leukemia cell line THP-1 (Xiangya School of Medicine Type
Culture Collection, China), and the M4 AML cell line OCI/AML-3
(JENNIO Biological Technology, Guangzhou, China) were cultured in
RPMI-1640 or DMEM medium with 10% heat-inactivated fetal bovine
serum (FBS), in 5% CO2 and 95% air.
Cell separation
For the BMMCs, bone marrow samples were obtained
from the patients with AML or ALL. The diagnoses of AML and ALL
were based on morphology and flow cytometric analysis of
immunophenotype. The BMMCs were isolated by Ficoll density gradient
centrifugation (20,21).
Gene transfection and RNAi
Hsp27 siRNA was transfected into cells using
X-tremeGENE siRNA Reagent (Roche Applied Science) according to the
manufacturer’s instructions. In general, the transfection
efficiency was >80%.
Cell viability assay
Cell viability was assessed by the MTT assay. Cells
were plated in 96-well flat bottom tissue culture plates at a
density of ~5×105 cells/well. After the treatment of
cells, 20 μl of 5 mg/ml MTT (in PBS) was added to each well, and
was continually incubated for 4 h at 37°C. The formazan granules
obtained in cells were then dissolved in 150 μl dimethyl sulfoxide
(DMSO). The absorbance values were detected at a wavelength of 490
nm by a 96-well multiscanner autoreader (Thermo Fisher Scientific,
USA). The experiments were performed 3 times. Cell viability of MTT
(%) = (OD of treated cells/OD of control cells) × 100% (20,21).
Reverse transcription PCR (RT-PCR)
Total RNA was isolated from leukemia cells using the
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s protocol. RNA concentration and purity were measured
with a spectrophotometer at A260 and A260/280, respectively. RNA
was reverse-transcribed into cDNA using a Primescript™ RT reagent
kit (Invitrogen) according to the manufacturer’s instructions. The
sequences of primers used were: for β-actin; forward,
5′-TCCTTCCTGGGCATGGAGTC-3′ and reverse,
5′-GTAACGCAACTAAGTCATAGTC-3′. For Hsp27; forward,
5′-CCTCTTCGATCAAGCTTCG-3′ and reverse, 5′-AGCGGAGCTGAACCACTGA-3′.
β-actin was used as an internal control to evaluate the relative
expressions of Hsp27. The conditions for polymerase chain reaction
(PCR) to Hsp27 were: denaturation at 94°C for 2 min, followed by 30
cycles of 94°C for 30 sec, 56°C for 30 sec (β-actin, 50°C for 30
sec), 72°C for 30 sec, then by a 5-min elongation at 72°C. The PCR
products were analyzed with 1.0% agarose gel electrophoresis and
were EB stained, photographed and scanned using Band Leader
software for grey scale semi-quantitative analysis.
Western blot analysis
Western blot analysis was used for analyses of
expression of Hsp27, cleaved PARP, cleaved caspase-3 and actin.
Antibodies against Hsp27, cleaved PARP, cleaved caspase-3 and actin
were purchased from Cell Signaling Technology (Danvers, MA, USA).
In brief, leukemia cells with different treatments were collected
and lysed. The whole cell lysate was separated by 12% SDS-PAGE and
electrophoretically transferred onto polyvinylidene difluoride
(PVDF) blotting membrane (Beyotime, Beijing, China). The membrane
was blocked with 5% non-fat dry milk in TBST (50 mM Tris pH 7.5,
100 mM NaCl, 0.15% Tween-20), incubated with the primary antibodies
(at various dilutions) for 12 h at 4°C, and washed three times with
TBST for 10 min. The membranes were then incubated for 12 h at 4°C
with different secondary antibodies and detected with ECL reagent
(Pierce, Rockford, IL, USA) after three washes with TBST for 10
min. Membranes were exposed to X-ray film and the expressions of
the targeted proteins were quantified by detecting the specific
band recorded on X-ray film. BandScan 5.0 system was used to
quantify and analyze each specific band obtained after western blot
analysis.
Apoptosis assays
Apoptosis in cells was assessed using the FITC
Annexin V Apoptosis Detection kit [Annexin V-FITC, propidium iodide
(PI) solution and Annexin V binding buffer]. This assay involves
staining cells with Annexin V-FITC (a phospholipid-binding protein
that binds to disrupted cell membranes) in combination with PI (a
vital dye that binds to DNA penetrating into apoptotic cells). Flow
cytometric analysis (FACS) was performed to determine the
percentage of cells that were undergoing apoptosis (Annexin V+/PI)
(21).
Statistical analysis
Data are expressed as the means ± SEM. Significance
of differences between groups was determined by two-tailed
Student’s t-test or Mann-Whitney U test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Expression of Hsp27 in pediatric AL
patients and leukemia cell lines
We studied 94 patients aged 1–15 years with newly
diagnosed AL at the Department of Pediatrics of Xiangya Hospital
from January 2007 to December 2011. There were 54 males and 40
females. Median age was 5 years (range, 1–13 years). According to
FAB classification, 32 patients were identified as ALL, 4 as M1, 22
as M2, 7 as M3, 12 as M4, 15 as M5 and 2 as M6. At time of
diagnosis, median white blood cell (WBC) count was
32.3×109/l (range, 0.6–335.4×109/l), median
hemoglobin level was 67 g/l (range, 33–121 g/l), and median
platelet count was 29.5×109/l (range,
6–157×109/l).
Firstly, we determined Hsp27 mRNA and protein
expressions in bone marrow samples from 7 newly diagnosed AL
samples, including 1 patient with ALL and 6 patients with subtypes
of AML (M1, M2, M3, M4, M5 and M6). As shown in Fig. 1A, Hsp27 showed markedly higher
expressions in M4/M5 subtypes compared with other types.
Additionally, the relative Hsp27 mRNA level was determined from the
bone marrow samples of the 94 newly diagnosed patients by RT-PCR
analysis. Upregulated Hsp27 expression was also found in BMMCs
derived from patients with M4/M5 subtypes. Conversely, these levels
were lower in BMMCs derived from ALL, M1, M2, M3 and M6 (Fig. 1B).
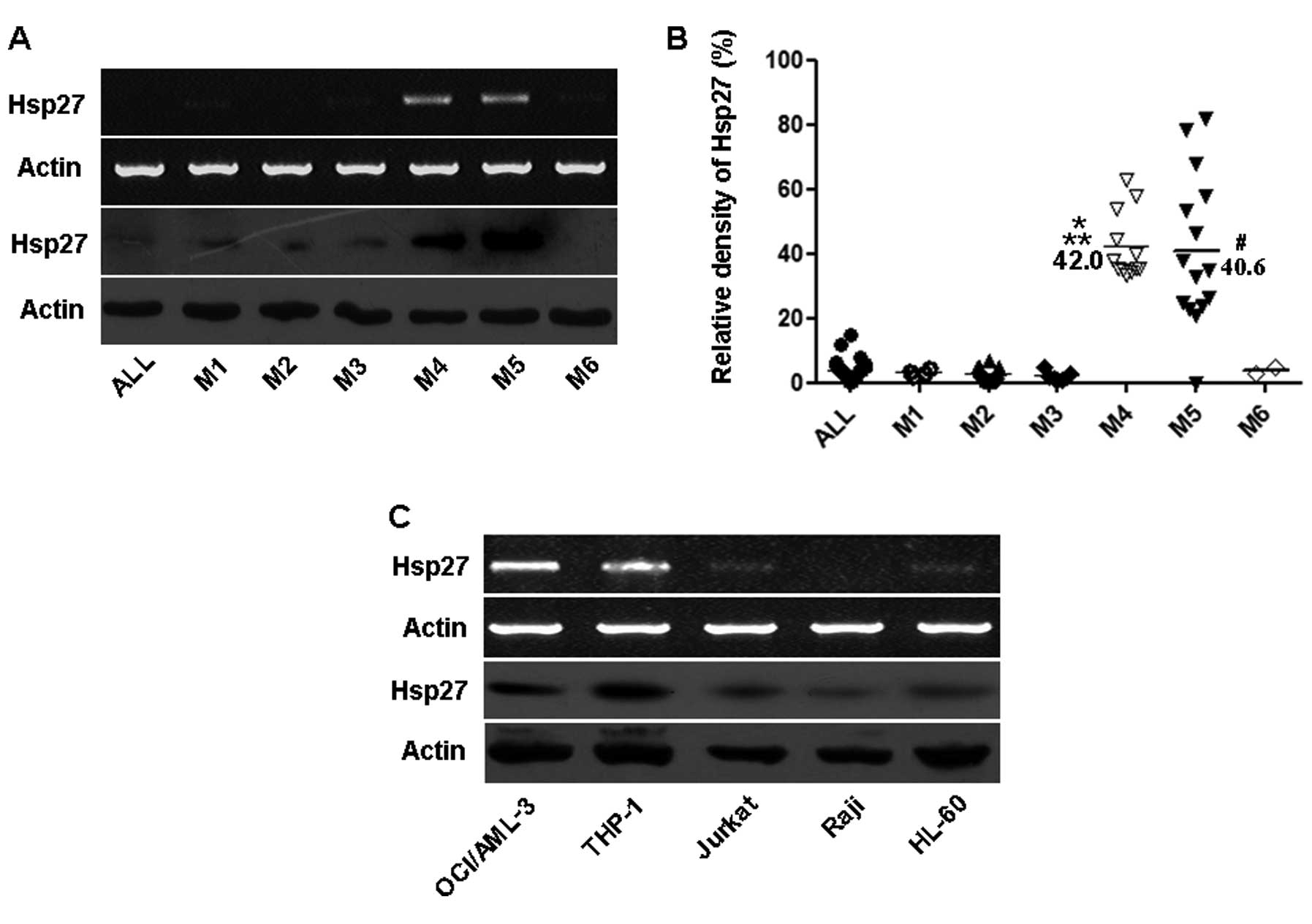 | Figure 1Expression of Hsp27 in pediatric AL
patients and leukemia cell lines. (A) Expression of Hsp27 in 7
patients. RT-PCR and western blot analysis of Hsp27 in 7 patients
from pediatric ALL and different subtypes of AML as indicated.
Actin was used as a loading control. (B) Relative expression levels
of Hsp27 in childhood AL. Total mRNA was extracted from patient
BMMCs, and Hsp27 level was determined by the relative optical
intensity of the bands by RT-PCR analysis. Each dot represents the
relative Hsp27 levels in each individual sample.
*P<0.05 vs. ALL, M1, M2, M3 and M6;
#P<0.05 vs. ALL, M1, M2, M3 and M6;
**P>0.05 vs. M5. (C) Expression of Hsp27 in 5
leukemia cell lines. Total mRNA and protein were extracted from
different leukemia cell lines. Hsp27 levels were determined by
RT-PCR and western blot analysis. Actin was used as a loading
control. ALL, acute lymphoblastic leukemia; M1–M6, the
classification of FAB to AML; AML, acute myeloid leukemia. |
Moreover, we determined the levels of Hsp27 in five
leukemia cell lines (OCI/AML-3, THP-1, Jurkat, Raji and HL-60) by
RT-PCR and western blot analysis. Levels of Hsp27 were high in
OCI/AML-3 and THP-1 leukemia cell lines, whereas Hsp27 expression
was noticeably lower in Jurkat, Raji and HL-60 cell lines (Fig. 1C). These date suggest that Hsp27
plays a potential contributory role in the pathogenesis of
pediatric AML-M4/M5.
Correlation between expression of Hsp27
and clinical status in pediatric M4/M5 subtypes
To further evaluate the clinical relevance between
Hsp27 and pediatric AML-M4/M5, we investigated Hsp27 expression in
BMMCs obtained from 27 patients, including 12 patients with M4 and
15 patients with M5. The main characteristics of the 27 patients
(15 males and 12 females) are presented in Table I. Median age was 9 years (range,
1–13 years). At time of diagnosis, median WBC count was
23.3×109/l (range, 2.2–219.7×109/l).
Twenty-two of the 27 patients (81%) were in complete remission with
1 or 2 induction chemotherapy. Median relative Hsp27 mRNA
expression level was 0.355 (range, 0.0–0.817). The survival outcome
results revealed that 12/27 patients (44%) were in complete
remission after ~12 months of chemotherapy. A total of 14 patients
died (51%). Relapse and refractory leukemia were the commonest
cause of mortality (n=10), followed by infection (n=2) and
hemorrhage (n=2). All AML patients were treated by an anthracycline
and cytarabine-based chemotherapy regimen. One patient received
HSCT following induction therapy. The induction chemotherapy
regimen information is summarized in Table II.
 | Table ICharacteristics of the 27 AML-M4/M5
patients. |
Table I
Characteristics of the 27 AML-M4/M5
patients.
| Subtype | No. | Age (years) | Gender | Initial leukocyte
count (×109/l) | BM evaluation after 1
or 2 induction chemotherapy | Relative Hsp27 mRNA
expressiona | Survival outcome |
|---|
| M4 | 1 | 5 | F | 97.8 | CR | 0.627 | Deceased
(relapse) |
| 2 | 3 | F | 14.2 | CR | 0.377 | CR |
| 3 | 13 | F | 23.3 | CR | 0.355 | Deceased
(infection) |
| 4 | 13 | F | 64.7 | IR (BM blasts,
37.5%) | 0.537 | Deceased
(refractory) |
| 5 | 12 | M | 78.3 | CR | 0.579 | Deceased
(relapse) |
| 6 | 4 | F | 13.5 | CR | 0.347 | CR |
| 7 | 2 | M | 27.9 | CR | 0.354 | CR |
| 8 | 8 | M | 20.3 | IR (BM blasts,
23%) | 0.333 | Deceased
(refractory) |
| 9 | 9 | M | 8.2 | CR | 0.441 | CR |
| 10 | 9 | M | 37.3 | CR | 0.399 | CR |
| 11 | 13 | F | 33.6 | IR (BM blasts,
15%) | 0.341 | Deceased
(refractory) |
| 12 | 2 | M | 6.0 | CR | 0.353 | Deceased
(hemorrhage) |
| M5 | 13 | 12 | F | 5.4 | CR | 0.380 | Deceased
(hemorrhage) |
| 14 | 1 | M | 42.5 | CR | 0.236 | CR |
| 15 | 2 | M | 8.8 | CR | 0.346 | HSCT |
| 16 | 11 | F | 10.8 | CR | 0.328 | CR |
| 17 | 11 | F | 11.1 | CR | 0.263 | Deceased
(refractory) |
| 18 | 13 | M | 31.5 | IR (BM blasts,
8%) | 0.465 | CR |
| 19 | 13 | M | 2.2 | CR | 0 | Deceased
(infection) |
| 20 | 2 | M | 2.4 | CR | 0.248 | CR |
| 21 | 4 | M | 4.6 | CR | 0.227 | CR |
| 22 | 8 | M | 219.7 | CR | 0.817 | Deceased
(relapse) |
| 23 | 7 | F | 145.7 | IR (BM blasts,
55.5%) | 0.781 | Deceased
(refractory) |
| 24 | 4 | M | 4.2 | CR | 0.208 | CR |
| 25 | 10 | F | 132.0 | CR | 0.680 | Deceased
(relapse) |
| 26 | 10 | M | 86.3 | CR | 0.578 | Deceased
(relapse) |
| 27 | 4 | F | 56.0 | CR | 0.533 | CR |
 | Table IIInduction chemotherapy regimens of
AML-M4/M5 patients. |
Table II
Induction chemotherapy regimens of
AML-M4/M5 patients.
| Regimen | Induction
chemotherapy |
|---|
| Regimen 1 | Daunorubicin 40
mg/m2/day from Days 1 to 3 |
| M4 (3 patients
aged <10 years) | Cytarabine 200
mg/m2/day from Days 1 to 7 |
| M5 (2 patients
aged <9 years) | |
| Regimen 2 | Idarubicin 10
mg/m2/day from Days 1 to 3 |
| M4 (3 patients
aged >10 years) | Cytarabine 200
mg/m2/day from Days 1 to 7 |
| M5 (5 patients
aged >9 years and 2 patients aged <9 years) | |
| Regimen 3 | Homoharringtonine 3
mg/m2/day from Days 1 to 7 |
| M4 (2 patients
aged <10 years) | Daunorubicin 40
mg/m2/day from Days 1 to 3 |
| M5 (1 patient aged
>9 years) | Cytarabine 200
mg/m2/day from Days 1 to 7 |
| Regimen 4 | Daunorubicin 40
mg/m2/day from Days 1 to 3 |
| M4 (2 patients
aged <10 years and 1 patient aged >10 years) | Cytarabine 200
mg/m2/day from Days 1 to 7 |
| M5 (2 patients
aged <9 years and 1 patient aged >9 years) | Yituobogan 100
mg/m2/day from Days 5 to 7 |
| Regimen 5 | Homoharringtonine 3
mg/m2/day from Days 1 to 7 |
| M4 (1 patient aged
<10 years) | Cytarabine 200
mg/m2/day from Days 1 to 7 |
| M5 (2 patients
aged <9 years) | Yituobogan 100
mg/m2/day from Days 1 to 3 |
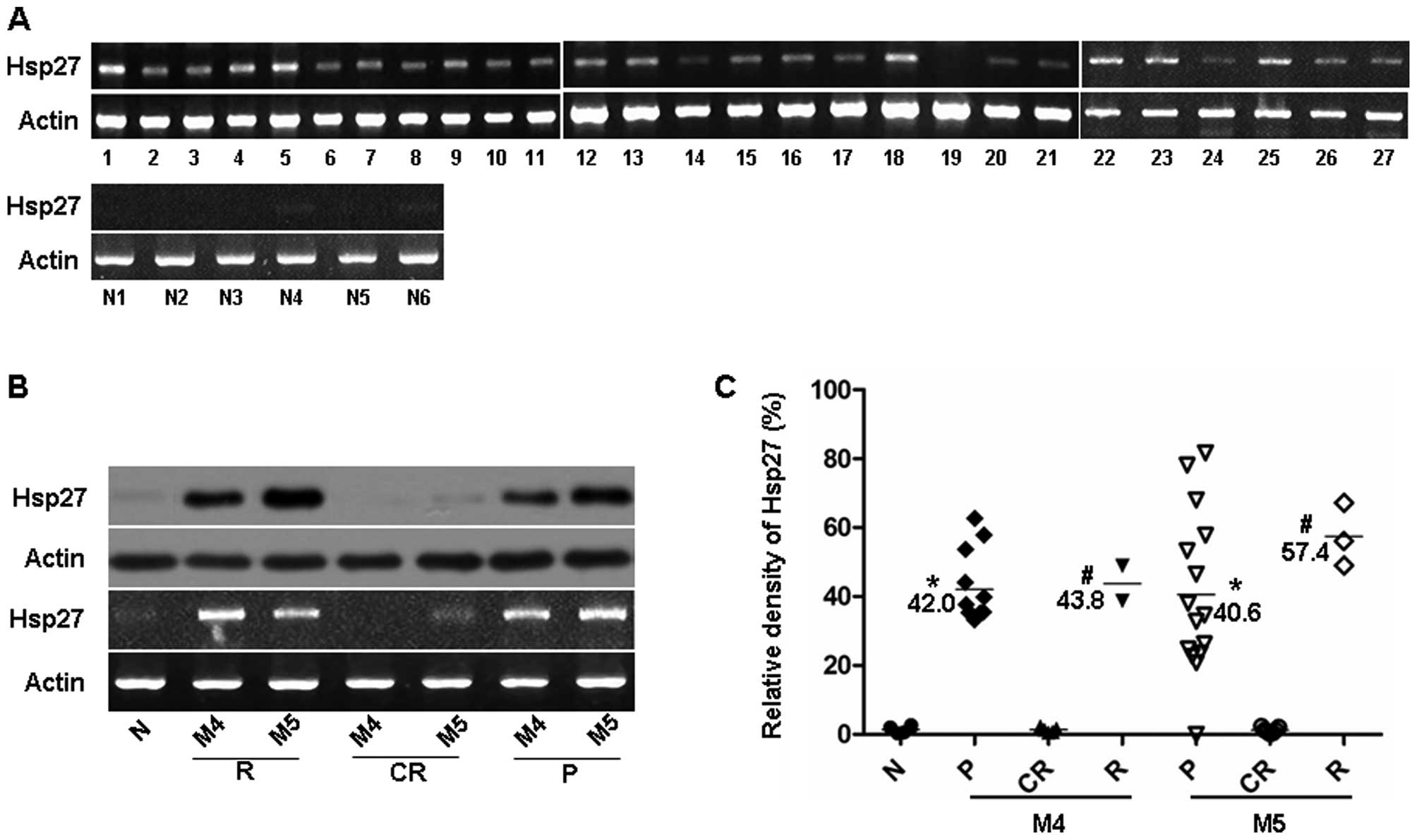
We firstly detected Hsp27 expression in 27 newly
diagnosed M4/M5 patients and 6 normal healthy subjects by RT-PCR
analysis (Fig. 2A). The relative
Hsp27 mRNA levels are presented in Table I. There was a trend towards a higher
incidence of relapse or refractory leukemia in the Hsp27
high-expression patients. There was also a poor prognosis in the
patients with high WBC and older age at diagnosis (Table III). Moreover, we investigated the
expression level of Hsp27 by RT-PCR and western blot analysis in
BMMCs obtained from 2 patients with relapse at different clinical
status, including 1 patient with M4 and 1 patient with M5. High
expression levels of Hsp27 were found in BMMCs derived from
patients with primary and relapse leukemia compared with the
patients with complete remission, or the normal healthy subject (1
patient) (Fig. 2B).
 | Table IIIResults of various variables of the
patients divided by the BM evaluation. |
Table III
Results of various variables of the
patients divided by the BM evaluation.
| CR group
(n=12) | Relapse/refractory
group (n=10) | P-valuea |
|---|
| Hsp27 | 0.3505
(0.208–0.533)b | 0.5785
(0.263–0.817) | 0.012 |
| WBCs
(x109/l) at diagnosis | 13.85 (2.4–56) | 82.3
(11.1–219.7) | 0.003 |
| Hb (g/l) at
diagnosis | 59.5 (37–83) | 65. 5 (32–82) | 0.574 |
| Platelets
(x109/l) at diagnosis | 26 (7–62) | 22 (5–79) | 0.489 |
| Age at diagnosis
(years) | 4 (1–13) | 10 (5–13) | 0.016 |
Furthermore, we investigated the relative Hsp27 mRNA
expression levels in the 27 pediatric M4/M5 patients at different
clinical status. Higher levels of Hsp27 expression were found in
BMMCs derived from patients with primary (n=27) and relapse
leukemia (n=5) (Fig. 2C). By
contrast, Hsp27 was not detectable in BMMCs derived from patients
with complete remission (n=12), or normal healthy subjects (n=6)
(Fig. 2C). These data indicate that
Hsp27 correlates well with the clinical status in pediatric M4/M5
subtypes.
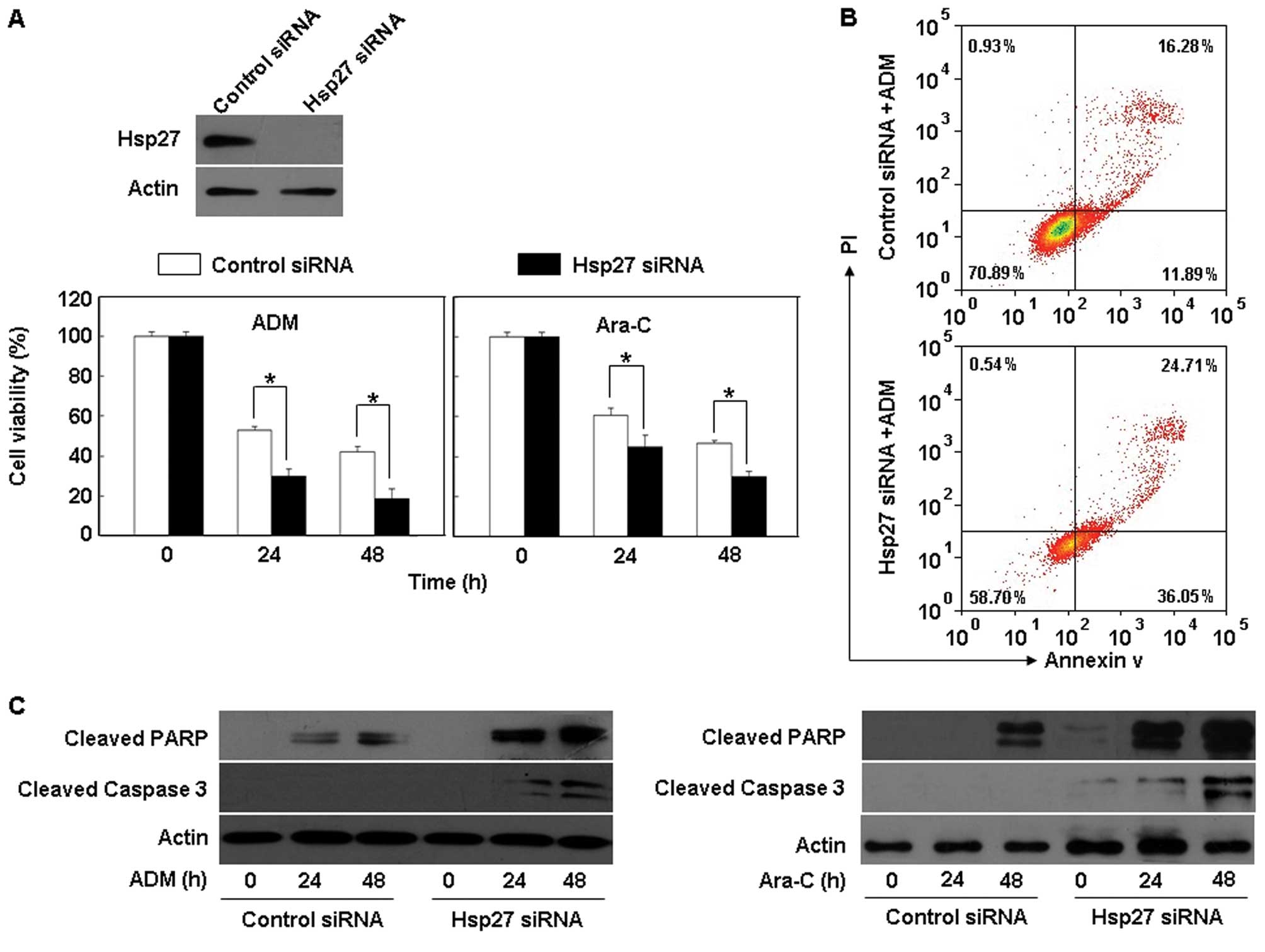
Knockdown of Hsp27 expression enhances
sensitivity of THP-1 cells to chemotherapeutic drugs
Resistance to chemotherapy is a major impediment to
the successful treatment of AML. Hsp27 is considered to play a role
in the development of cancer and to modulate tumor response to
cytotoxic therapy (22). Whether
Hsp27 regulates leukemia cell chemosensitivity has yet to be
clarified. In this study, we treated THP-1 cells harboring
different status of Hsp27 with several widely used chemotherapeutic
drugs in clinic leukemia such as adriamycin (ADM; 1 μg/ml) and
cytosine arabinoside (Ara-C; 0.2 μM) (20,21).
We showed that inactivation of Hsp27 by small interfering RNA
(siRNA) significantly enhanced the cytotoxicity of anticancer drugs
in THP-1 cells, as compared with the control groups (Fig. 3A). Furthermore, knockdown of Hsp27
expression significantly augmented the anticancer drug-induced
apoptosis, as indicated by increases in Annexin V staining and in
the amounts of cleaved caspase-3 and PARP (Fig. 3B and C), supporting a potential
prosurvival role for Hsp27 in THP-1 leukemia cells exposed to
chemotherapy.
Discussion
Hsp27 is emerging as a promising therapeutic target
for the treatment of various types of cancer. High levels of Hsp27
have been observed in a number of cancer cells (23,24),
compared to normal cells, in which expression is undetectable or
relatively low (25). Moreover, its
aberrant expression in cancer is associated with aggressive tumor
behavior, increased resistance to chemotherapy, and poor prognosis
for the patients (12–14). In our previous investigation, we
demonstrated that the prototypical damage-associated molecular
pattern molecules (DAMPs), HMGB1 and S100A8, were abundantly
expressed in newly diagnosed pediatric AL and contributed to
chemotherapy resistance (20,21).
Hsp27, another characterized DAMP (26), and the role of Hsp27 in pediatric
leukemia has yet to be clearly addressed.
AML represents 20% of all AL cases in children and
adolescents. Clinical outcome of patients with AML remains poor
with a long-term survival of 30–50% in pediatric patients (27,28).
The identification of prognostic factors and aberrant signaling
pathways in AML is important for the development of new molecular
therapies and might improve risk-adapted therapeutic strategies for
AML patients. In this study, we found that Hsp27 was abundantly
expressed in newly diagnosed pediatric AML-M4/M5 and may be a new
target in leukemia therapy.
In childhood AML, MLL gene rearrangements at
chromosome band 11q23 are mainly restricted to the FAB M4 and M5
subtypes, which confer a poor prognosis (29). FAB classification was previously
reported to be of prognostic value; M1, M2, M3 and M4Eo are
associated with a longer remission duration time than M4 and M5
(30). Also, high WBC count and
older age at diagnosis have been established as risk factors for
mortality (31,32). In the present study, we found that
Hsp27 was abundantly expressed in pediatric AML-M4/M5, but was
barely expressed in other types. High expression of Hsp27
correlated well with WBC count and age at diagnosis. There was a
trend towards a higher incidence of relapse or refractory leukemia
in the Hsp27 high-expression pediatric AML-M4/M5 patients.
Meanwhile, Hsp27 expression also positively correlated well with
clinical status in pediatric AML-M4/M5. We found that Hsp27
expression was significantly higher in the active phase (such as in
the primary and relapse phase) and returned to normal in complete
remission. This suggested that Hsp27 was associated with an
unfavorable prognosis and reduced overall survival in pediatric
M4/M5.
Tumor recurrence as a result of resistance to
chemotherapeutic drugs remains a formidable problem in managing
leukemia patients. Although various mechanisms of drug resistance
have thus far been proposed, an exact mechanism remains to be
established (33). Several studies
demonstrated that the overexpression of Hsp27 appears to be
correlated with increased resistance to chemotherapeutic
drug-induced apoptosis in cancer cells (34,35).
It associates with components of the extrinsic and intrinsic
apoptotic pathway, inhibiting the execution of apoptosis and is
emerging as an antiapoptotic factor (36,37).
In this study, we found that depletion of Hsp27 expression in THP-1
cells by RNA interference increased the chemotherapy sensitivity
and the apoptosis of leukemia cells to these anticancer drugs. This
clearly suggests a strong effect of Hsp27 on chemoresistance of
THP-1 leukemia cells.
In conclusion, in the present study we showed that
Hsp27 was overexpressed in pediatric AML-M4/M5, and functioned as
an unfavorable prognosis factor. Knockdown of Hsp27 expression by
RNA interference increased leukemia cell sensitivity to
anti-drug-induced apoptosis. These results support the theory that
Hsp27 plays an important role in the tumorigenesis of pediatric
AML-M4/M5 and may be exploited as a new target for enhancing the
efficacy of chemotherapeutic drugs against leukemia.
Acknowledgements
This study was supported by grants from The National
Natural Science Foundation of China (81270616 to Y.Y. and 31171328
to L.C.).
References
|
1
|
Woods WG: Curing childhood acute myeloid
leukemia (AML) at the half-way point: promises to keep and miles to
go before we sleep. Pediatr Blood Cancer. 46:565–569. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Creutzig U, Zimmermann M, Reinhardt D,
Dworzak M, Stary J and Lehrnbecher T: Early deaths and
treatment-related mortality in children undergoing therapy for
acute myeloid leukemia: analysis of the multicenter clinical trials
AML-BFM93 and AML-BFM 98. J Clin Oncol. 22:4384–4393. 2004.
View Article : Google Scholar
|
|
3
|
Pui CH, Carroll WL, Meshinchi S and Arceci
RJ: Biology, risk stratification, and therapy of pediatric acute
leukemias: an update. J Clin Oncol. 29:551–565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rubnitz JE and Inaba H: Childhood acute
myeloid leukemia. Br J Haematol. 159:259–276. 2012. View Article : Google Scholar
|
|
5
|
Rocchi P, Beraldi E, Ettinger S, Fazli L,
Vessella RL, Nelson C and Gleave M: Increased Hsp27 after androgen
ablation facilitates androgen-independent progression in prostate
cancer via signal transducers and activators of transcription
3-mediated suppression of apoptosis. Cancer Res. 65:11083–11093.
2005. View Article : Google Scholar
|
|
6
|
Love S and King RJ: A 27 kDa heat shock
protein that has anomalous prognostic powers in early and advanced
breast cancer. Br J Cancer. 69:743–748. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang Q, Ye J, Chen W, et al: Heat shock
protein 27 is overexpressed in tumor tissues and increased in sera
of patients with gastric adenocarcinoma. Clin Chem Lab Med.
48:263–269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Langdon SP, Rabiasz GJ, Hirst GL, King RJ,
Hawkins RA, Smyth JF and Miller WR: Expression of the heat shock
protein Hsp27 in human ovarian cancer. Clin Cancer Res.
1:1603–1609. 1995.PubMed/NCBI
|
|
9
|
Lebret T, Watson RW, Molinié V, O’Neill A,
Gabriel C, Fitzpatrick JM and Botto H: Heat shock proteins Hsp27,
Hsp60, Hsp70, and Hsp90: expression in bladder carcinoma. Cancer.
98:970–977. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Melle C, Ernst G, Escher N, et al: Protein
profiling of microdissected pancreas carcinoma and identification
of Hsp27 as a potential serum marker. Clin Chem. 53:629–635. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu Z, Zhi J, Peng X, Zhong X and Xu A:
Clinical significance of Hsp27 expression in colorectal cancer. Mol
Med Rep. 3:953–958. 2010.PubMed/NCBI
|
|
12
|
Tweedle EM, Khattak I, Ang CW, et al: Low
molecular weight heat shock protein HSP27 is a prognostic indicator
in rectal cancer but not colon cancer. Gut. 59:1501–1510. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang F, Zhang P, Shi C, Yang Y and Qin H:
Immunohistochemical detection of Hsp27 and hnRNP K as prognostic
and predictive biomarkers for colorectal cancer. Med Oncol.
29:1780–1788. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang YX, Xiao ZQ, Chen ZC, et al: Proteome
analysis of multidrug resistance in vincristine-resistant human
gastric cancer cell line SGC7901/VCR. Proteomics. 6:2009–2021.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Andrieu C, Taieb D, Baylot V, et al: Heat
shock protein 27 confers resistance to androgen ablation and
chemotherapy in prostate cancer cells through eIF4E. Oncogene.
29:1883–1896. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Havasi A, Li Z, Wang Z, et al: Hsp27
inhibits Bax activation and apoptosis via a phosphatidylinositol
3-kinase-dependent mechanism. J Biol Chem. 283:12305–12313. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O’Callaghan-Sunol C, Gabai VL and Sherman
MY: Hsp27 modulates p53 signaling and suppresses cellular
senescence. Cancer Res. 67:11779–11788. 2007.PubMed/NCBI
|
|
18
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DA, Gralnick HR and Sultan C: Proposed revised
criteria for the classification of acute myeloid leukemia: a report
of the French-American-British Cooperative Group. Ann Intern Med.
103:620–625. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheson BD, Cassileth PA, Head DR, et al:
Report of the National Cancer Institute sponsored workshop on
definitions of diagnosis and response in acute myeloid leukemia:
review. J Clin Oncol. 8:813–819. 1990.PubMed/NCBI
|
|
20
|
Yang L, Yu Y, Kang R, et al: Up-regulated
autophagy by endogenous high mobility group box-1 promotes
chemoresistance in leukemia cells. Leuk Lymphoma. 53:315–322. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang L, Yang M, Zhang H, et al:
S100A8-targeting siRNA enhances arsenic trioxide-induced myeloid
leukemia cell death by downregulating autophagy. Int J Mol Med.
29:65–72. 2012.PubMed/NCBI
|
|
22
|
Garrido C, Ottavi P, Fromentin A, Hammann
A, Arrigo AP, Chauffert B and Mehlen P: Hsp27 as a mediator of
confluence-dependent resistance to cell death induced by anticancer
drugs. Cancer Res. 57:2661–2267. 1997.PubMed/NCBI
|
|
23
|
Williams K, Chubb C, Huberman E and
Giometti CS: Analysis of differential protein expression in normal
and neoplastic human breast epithelial cell lines. Electrophoresis.
19:333–343. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Myung JK, Afjehi-Sadat L,
Felizardo-Cabatic M, Slavc I and Lubec G: Expressional patterns of
chaperones in ten human tumor cell lines. Proteome Sci. 2:82004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ciocca DR, Oesterreich S, Chamness GC,
McGuire WL and Fuqua SA: Biological and clinical implications of
heat shock protein 27,000 (Hsp27): a review. J Natl Cancer Inst.
85:1558–1570. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen GY and Nuñez G: Sterile inflammation:
sensing and reacting to damage. Nat Rev Immunol. 10:826–837. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaspers GJ and Creutzig U: Pediatric acute
myeloid leukemia: international progress and future directions.
Leukemia. 19:2025–2029. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Molgaard-Hansen L, Glosli H, Jahnukainen
K, et al: Quality of health in survivors of childhood acute myeloid
leukemia treated with chemotherapy only: A NOPHO-AML study. Pediatr
Blood Cancer. 57:1222–1229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jo A, Tsukimoto I, Ishii E, Asou N, et al:
Age-associated difference in gene expression of paediatric acute
myelomonocytic lineage leukaemia (FAB M4 and M5 subtypes) and its
correlation with prognosis. Br J Haematol. 144:917–929. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ravindranath Y, Chang M, Steuber CP, et
al: Pediatric Oncology Group (POG) studies of acute myeloid
leukemia (AML): a review of four consecutive childhood AML trials
conducted between 1981 and 2000. Leukemia. 19:2101–2116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Estey E and Dohner H: Acute myeloid
leukaemia. Lancet. 368:1894–1907. 2006. View Article : Google Scholar
|
|
32
|
Dutcher JP, Schiffer CA and Wiernik PH:
Hyperleukocytosis in adult acute nonlymphocytic leukemia: impact on
remission rate and duration, and survival. J Clin Oncol.
5:1364–1372. 1987.PubMed/NCBI
|
|
33
|
Ross DD: Novel mechanisms of drug
resistance in leukemia. Leukemia. 14:467–473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mori-Iwamoto S, Kuramitsu Y, Ryozawa S, et
al: Proteomics finding heat shock protein 27 as a biomarker for
resistance of pancreatic cancer cells to gemcitabine. Int J Oncol.
31:1345–1350. 2007.PubMed/NCBI
|
|
35
|
Choi DH, Ha JS, Lee WH, et al: Heat shock
protein 27 is associated with irinotecan resistance in human
colorectal cancer cells. FEBS Lett. 581:1649–1656. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lanneau D, Brunet M, Frisan E, Solary E,
Fontenay M and Garrido C: Heat shock proteins: essential proteins
for apoptosis regulation. J Cel Mol Med. 12:743–761. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Voss OH, Batra S, Kolattukudy SJ,
Gonzalez-Mejia ME, Smith JB and Doseff AI: Binding of caspase-3
prodomain to heat shock protein 27 regulates monocyte apoptosis by
inhibiting caspase-3 proteolytic activation. J Biol Chem.
282:25088–25099. 2007. View Article : Google Scholar : PubMed/NCBI
|