Introduction
Breast cancer is a leading cause of death worldwide
and represents the primary cause of mortality among women in Brazil
(1). Breast tumors are
conventionally classified based on prognostic factors, including
histological type and grade, proliferation index and angiolymphatic
invasion. The St. Gallen Consensus, the American Society of
Clinical Oncology (ASCO) and the College of American Pathologists
(CAP) also state the evaluation of estrogen receptor (ER),
progesterone receptor (PR) and human epidermal growth factor
receptor 2 (HER2) status for the prognosis and recommendation of
adjuvant therapy (2–4).
Although well-established as prognostic and
diagnostic tools, information provided by classical pathological
evaluation still fails to predict, with accuracy, the patient’s
clinical progression. Thus, the genetic and transcriptional
diversity of tumor cells are receiving considerable attention, as
they may represent the primary cause of unpredictable tumor
behavior and the failure of certain currently used treatments. In
their pioneering study, Perou et al(5), identified a correlation between
histopathological findings and the gene expression profile of
various types of breast tumor, correlating classic
immunohistochemistry (IHC) and cDNA microarrays. Theirs and
subsequent studies (6–8) defined novel molecular subtypes of
breast tumors, including luminal A, luminal B, HER2, basal and,
more recently, the claudin-low subtype (9).
Subsequently, using an experimental approach similar
to that used in previous studies, Kao et al(10) applied molecular profile
classification to known breast cancer cell lines. Many of the cell
lines investigated (MCF-7 or MDA-MB-231) were obtained from
metastatic tumors, and are frequently used as breast cancer models.
However, metastasis-derived cells have already undergone crucial
stages in tumor progression, including the development of invasive
capability, cellular adhesion to other organism sites and
adaptation to a new environment. Therefore, although widely used,
these cell lines do not represent the cells present in primary
tumors.
The use of breast cancer cell lines derived from
primary tumors as in vitro models has rarely been reported
and may offer relevant data regarding this type of cancer,
increasing the knowledge provided by metastasis-derived cell
research. To further understand breast cancer in its initial
stages, we investigated the MACL-1 and MGSO-3 breast cancer cell
lines previously derived from primary human tumors in our
laboratory (11). Correa et
al characterized these cells lines as authentic tumor and
immortalized cell lines through serial passages, loss of contact
inhibition, telomerase activity (to confirm immortalization),
ability to assemble colonies on agar plates and formation of tumors
in immunodeficient mice (11).
Moreover, these cells present the differential expression of genes
and surface molecules, such as MUC1 and GAPDH (12), and resistance to γ-irradiation
(13).
To gain better understanding of these cell lines,
this study evaluated the phenotypic markers from the MACL-1 and
MGSO-3 cell lines in comparison to primary tumors and xenograft
implants in immunodeficient mice, developed from these cell lines
using IHC. Additionally, copy number alterations (CNAs) were
evaluated using array comparative genomic hybridization (aCGH).
These findings render MACL-1 and MGSO-3 the first characterized
breast cancer cell lines to potentially be used for comparative
research with other established breast cancer cell lines.
Materials and methods
Cell culture
The MACL-1 and MGSO-3 cell lines were previously
derived from breast tumor tissue in our laboratory [Correa et
al(11)]. The cells were grown
in Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich, St.
Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS;
Sigma-Aldrich) and penicillin/streptomycin (100 U/ml; Life
Technologies, Carlsbad, CA, USA) at 37°C in an atmosphere of 5%
CO2.
Xenotransplants
Pathogen-free
BALB/c.Cg-Foxn1nu/ AnNTacUnib mice (age,
6–8 weeks) were housed in filter-top cages, and sterile water and
food were provided ad libitum. The manipulations were
conducted aseptically inside a laminar flow hood. One million
MACL-1 and MGSO-3 cells were diluted in phosphate buffer and
injected subcutaneously between the scapulae of each animal, as
described in our previous study (11). The mice were examined for tumor
growth every 3 days. When the tumors reached 10 mm in size, the
mice were sacrificed and the tumor was dissected for histological
examination. Animal experiments were approved by the Animal Use
Ethics Committee of the Federal University of Minas Gerais (Belo
Horizonte, Brazil).
Histopathological analysis
Primary tumors were obtained from 2 breast cancer
samples obtained from 2 patients (patients 1 and 2) who had
presented at Santa Casa de Misericórdia Hospital in Belo Horizonte,
Brazil. Samples were routinely processed, embedded in paraffin and
4-μm-thick sections were cut and stained with hematoxylin and eosin
(H&E) to evaluate tumor morphology and grade. To evaluate tumor
xenografts, the animals were sacrificed and the tumors were excised
and fixed in 4% buffered formaldehyde for 24–48 h. Tumor fragments
were then rinsed with phosphate buffer, dehydrated in a series of
graded ethanol washes and embedded in paraffin. To compare the
MACL-1 and MGSO-3 cells grown in vitro with tumors grown
in vivo and primary tumors, the cells were cultured in
chamber slides (Lab-TekII, Thermo Fisher Scientific Inc., Waltham,
MA, USA). Subsequent to attaining confluence, the cells were fixed
with buffered formalin for 1–2 min, washed with phosphate buffer,
and stored in this solution until immunohistochemical staining was
performed. This study was approved by the institutional Human
Ethics Committee (ETIC 03120203000).
Immunohistochemical analysis
Immunohistochemical analysis was performed using the
antibodies shown in Table
I(2,3,5,14–16).
Sections were deparaffinized using xylene and rehydrated in a
series of decreasing concentrations of ethanol solutions.
Heat-induced epitope retrieval was then carried out in citrate
buffer (sodium citrate, 10 mM; pH 6.0) in a pressure cooker for 4
min at full pressure. Subsequent to cooling, endogenous peroxidase
was blocked using a 3% hydrogen peroxide solution for 20 min. The
slides were then washed with phosphate buffer solution (10 mM; pH
7.4) and incubated with primary antibodies for 20–30 min or
overnight at 4°C and washed 3 times with phosphate buffer. The
slides were subsequently incubated using the Advance HRP (Dako,
Carpinteria, CA, USA) or MACH 4 Universal HRP-Polymer (Biocare
Medical, Concord, CA, USA) detection systems, according to the
respective manufacturer’s instructions. The slides were washed 3
times with phosphate buffer and the colored reaction product was
developed using 3,3-diaminobenzidine tetrahydrochloride (DAB; Dako)
as a substrate for 1 min, while nuclear contrast was achieved using
Harris hematoxylin counterstaining. Paraffin sections from the
original primary tumors and xenografts were examined using the same
procedure. ER and PR staining were evaluated using the Allred
scoring system (2). HER2 staining
was evaluated as recommended by the CAP/ASCO guidelines (3). Ki-67 was evaluated as the percentage
of staining. Qualitative analyses (positivity/negativity) were
carried out for the remaining antibodies in the absence of any
current official recommendations. Negative controls were obtained
by omitting primary antibodies. Heat-induced epitope retrieval was
omitted for cultured cells and sections stained for HER2 (clone
CB11).
 | Table IPrimary antibodies, clones, dilution
ratios and sources used for immunohistochemical staining. |
Table I
Primary antibodies, clones, dilution
ratios and sources used for immunohistochemical staining.
| Antibodies | Clone | Dilution | Source |
|---|
| Estrogen receptor
(ERa) | 6F11 | 1:100 | Neomarkers |
| Estrogen receptor
(ERb) | SP1 | 1:100 | Neomarkers |
| Progesterone
receptor (PRa) | PgR 312 | 1:200 | Novocastra |
| Progesterone
receptor (PRb) | PgR 636 | 1:400 | Dako |
| HER2a | CB11 | 1:200 | Novocastra |
| HER2b | Rabbit
polyclonal | 1:2,000 | Dako |
| Ki-67 | MIB-1 | 1:800 | Dako |
| CD44 | F10-44-2 | 1:40 | Novocastra |
| CD24 | SN3 | 1:50 | Neomarkers |
| CD133 | Rabbit
polyclonal | 1:100 | Abcam |
| Cytokeratin 5
(CK5) | XM26 | 1:300 | Neomarkers |
| EGFR | EGFR-25 | 1:100 | Novocastra |
Clonogenic assay
Cell survival was measured using clonogenic assay
(17). Briefly, 900 cells were
seeded in 10-cm2 plates and incubated for 10 days.
Colonies were stained using a mixture of 6.0% glutaraldehyde and
0.5% crystal violet, and then rinsed with water. Colonies with
>50 cells were counted as survivors. Surviving fractions were
normalized by the plating efficiency of MDA-MB-231 cells.
Statistical analysis was carried out using GraphPad Prism 5
software (GraphPad Software, Inc., La Jolla, CA, USA) using one-way
ANOVA and Duncan’s post-test. P<0.05 was considered to indicate
a statistically significant difference.
aCGH
Genomic DNA from MACL-1 and MGSO-3 cell lineages was
obtained using SDS/proteinase K digestion, followed by
phenol/chloroform extraction and ethanol precipitation (18) and treatment with 20 μg/ml RNase A
(Sigma-Aldrich). CNAs were evaluated in the MACL-1 and MGSO-3 cell
lines using the high-resolution SurePrint G3 Human CGH Microarray
kit, 4×180K (Agilent Technologies, Santa Clara, CA, USA). A female
genomic DNA control sample (Promega, Fitchburg, WI, USA) was used
as the reference. Test and reference DNA were fluorescently labeled
using the Agilent Genomic DNA Enzymatic Labeling kit (Agilent
Technologies). Experiments were performed in duplicate by swapping
dyes between the test and control samples to reduce analytic errors
resulting from labeling and hybridization. Subsequent to slide
scanning (Agilent DNA Scanner, at 5-μm resolution), image data were
extracted and normalized using Feature Extraction 10.1.1.1 software
(Agilent Technologies). The array-based CGH data were analyzed
using the Nexus Copy Number software version 6.0 (BioDiscovery,
Hawthorne, CA, USA) with a FASST2 segmentation algorithm,
responsible for the detection of statistically significant CNAs, a
sensitivity threshold of 1.00E-6, 3 consecutive probes, and a
log2 ≤-0.13 and ≥+0.3 for the determination of a loss or
gain region, respectively.
Results and Discussion
Immunohistochemistry
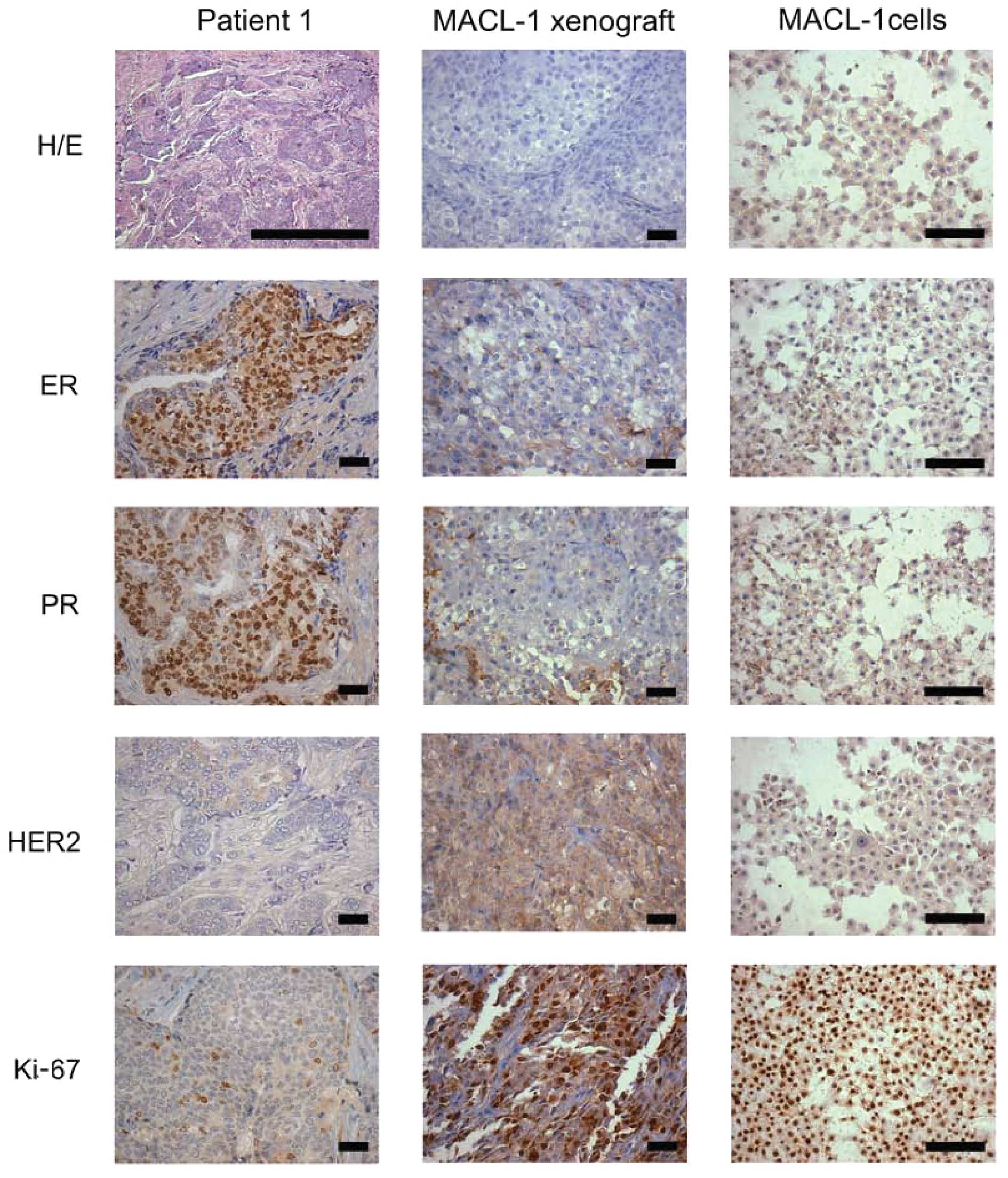
The breast tumor sample from patient 1 exhibited an
invasive ductal carcinoma morphology that may be sub-classified as
a luminal A subtype carcinoma (ER/PR-positive and HER2-negative)
(Fig. 1). Tumors associated with
this subtype are known to be less aggressive and have improved
prognosis in patients (6).
Moreover, the tumor from patient 1 had a low mitotic grade
(<25%), as demonstrated using H&E-stained slides and Ki-67
staining.
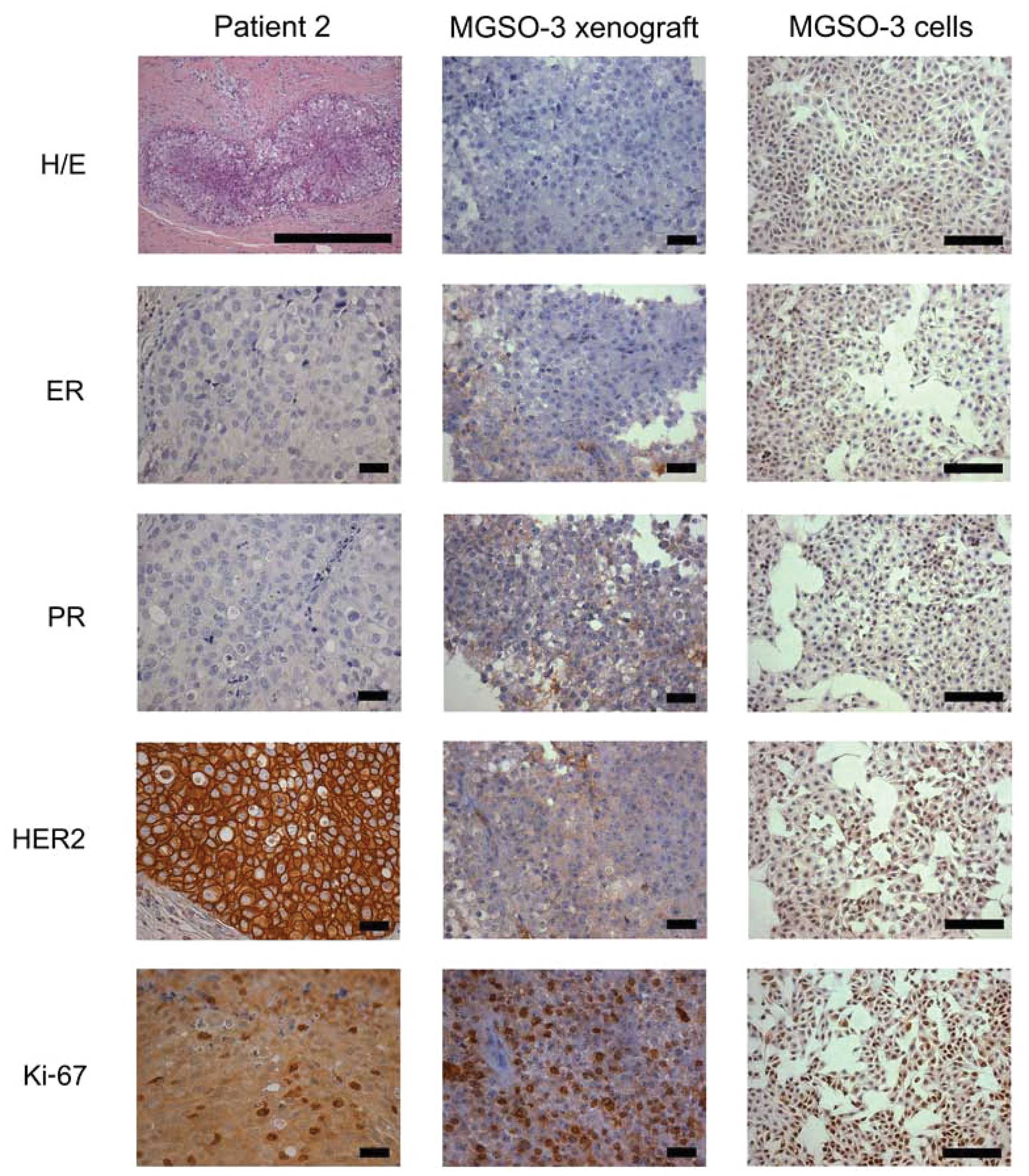
Conversely, the breast tumor sample from patient 2
was considered to be a ductal carcinoma in situ (DCIS) and
presented with a HER2 subtype profile, given that the tumor was
negative for ER/PR staining and showed strong HER2 staining (3+)
(Fig. 2). Breast tumors of the HER2
subtype have a worse prognosis and comprise some of the most
aggressive tumors (6).
Additionally, this primary tumor had a high mitotic index
(>25%), as demonstrated by H&E and Ki-67-stained slides.
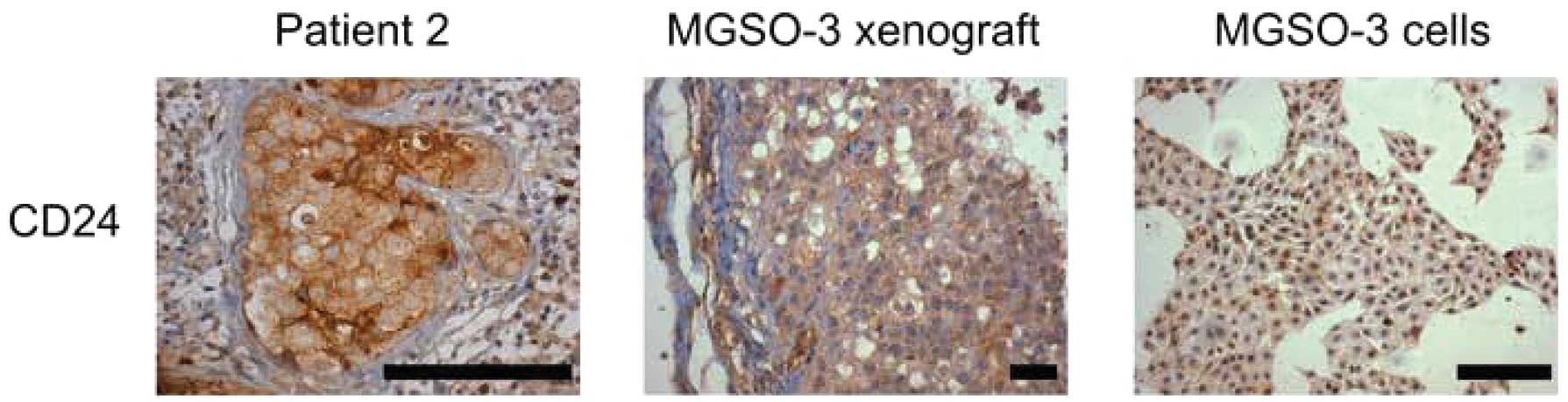
The tumor sample from patient 2 also showed marked
CD24 staining, although MGSO-3 cultured cells and xenografts from
these cells were not stained using this marker (Fig. 3). CD24 is a mucin-like adhesion
molecule expressed at multiple stages of B-cell development. This
protein increases metastatic potential in tumors since it is a
ligand of P-selectin, an adhesion receptor of endothelial cells and
platelets (19), and has been
implicated as an indicator of worse survival prognosis in breast
cancer patients (20). Reports that
breast cancer stem cells have the CD44+/CD24−
phenotype, as shown in the study by Al Hajj et al(15), are inconsistent with the metastatic
role of CD24. Nonetheless, the metastasis process is biologically
distinct from that of tumor growth in cancer stem cells, explaining
the presence or absence of this marker at diverse stages of breast
cancer progression (21).
CD24 staining of the primary tumor of patient 2 may
be indicative of a carcinoma that, albeit non-invasive, is
associated with tumor progression of a more aggressive phenotype,
corresponding to its HER2 subtype classification.
Despite displaying a high mitotic index, the MACL-1
and MGSO-3 cells and their derivative tumor xenografts in the
immunodeficient mice did not display ER, PR or HER2 staining. The
basal phenotype (Ck5 and EGFR) and the breast cancer stem cell
markers (CD44, CD24 and CD133) were absent in the primary tumor and
cultured cells of patient 1, as well as the xenografts derived from
the 2 cell lines (Table II).
 | Table IIImmunohistochemical profiles of the
primary tumors of the patients (patients 1 and 2), cultured cell
lines (MACL-1 and MGSO-3) and cell line-derived tumor
xenografts. |
Table II
Immunohistochemical profiles of the
primary tumors of the patients (patients 1 and 2), cultured cell
lines (MACL-1 and MGSO-3) and cell line-derived tumor
xenografts.
| Patient | Cultured cell
line | Tumor
xenograft |
|---|
|
|
|
|
|---|
| Antibodies | 1 | 2 | MACL-1 | MGSO-3 | MACL-1 | MGSO-3 |
|---|
| ER | + | − | − | − | − | − |
| PR | + | − | − | − | − | − |
| HER2 | − | + | − | − | − | − |
| Ki-67 | + | + | + | + | + | + |
| CD44 | − | − | − | − | − | − |
| CD24 | − | + | − | − | − | − |
| CD133 | − | − | − | − | − | − |
| CK5 | − | − | − | − | − | − |
| EGFR | − | − | − | − | − | − |
The in vitro establishment of cells derived
from primary tumors is a rare event, occurring in relatively few
attempts (22) and may thus require
selection for an ‘in vitro establishment’ phenotype
(23). It is possible that, as a
result of adaptation to a new environment, MACL-1 and MGSO-3 cells
shifted to a more appropriate expression pattern for cell culture
conditions. Changes in primary tumor markers in the corresponding
cultured cell lines have been reported by Brozova et
al(24) in breast cancer and by
Strojnik et al(25) in
glioblastoma. Differences in the aCGH profiles of breast cancer
(26) and the methylation patterns
of multiple types of cancer (27)
have also been reported in studies comparing cell lines to their
respective primary tumors.
Furthermore, the successful transplantation of
MACL-1 and MGSO-3 cells into nude mice is noteworthy, since only
7–20% of these implants are successfully accomplished (28). Specifically, the development of
in vivo xenografts of tumor cells allows for the testing of
novel therapeutic approaches and the study of local invasion and
interaction with stroma (28).
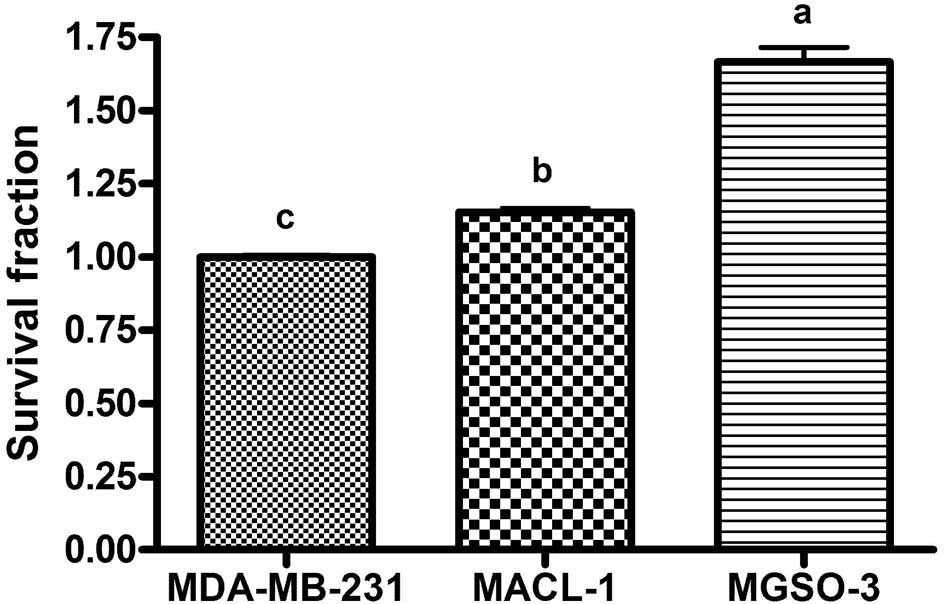
Clonogenic assay
Clonogenic or clonogenic survival assay evaluates
the competence of cells to generate a significant number of
daughter cells on culture plates after a certain period of time or
treatment. The MGSO-3 cell line demonstrated the highest capacity
to form colonies after 10 days of incubation, followed by the
MACL-1 and MDA-MB-231 lines (Fig.
4). Similar data has been previously reported by Correa et
al(11), describing the greater
proliferative capability of MGSO-3 when compared to MACL-1 cells
using a cell doubling time assessment. Additionally, MGSO-3 tumor
xenografts in immunodeficient mice were reported to grow more
rapidly compared to MACL-1 tumors (11), and the 2 cell lines demonstrated
competence to form tumor-like colonies in soft agar. In a
subsequent experiment, MGSO-3 cell lines formed the largest and
most numerous colonies that were compatible with xenotransplant and
culture growth features (11).
aCGH
Subsequent to slide scanning and data extraction
using the Feature Extraction software, aCGH data were analyzed
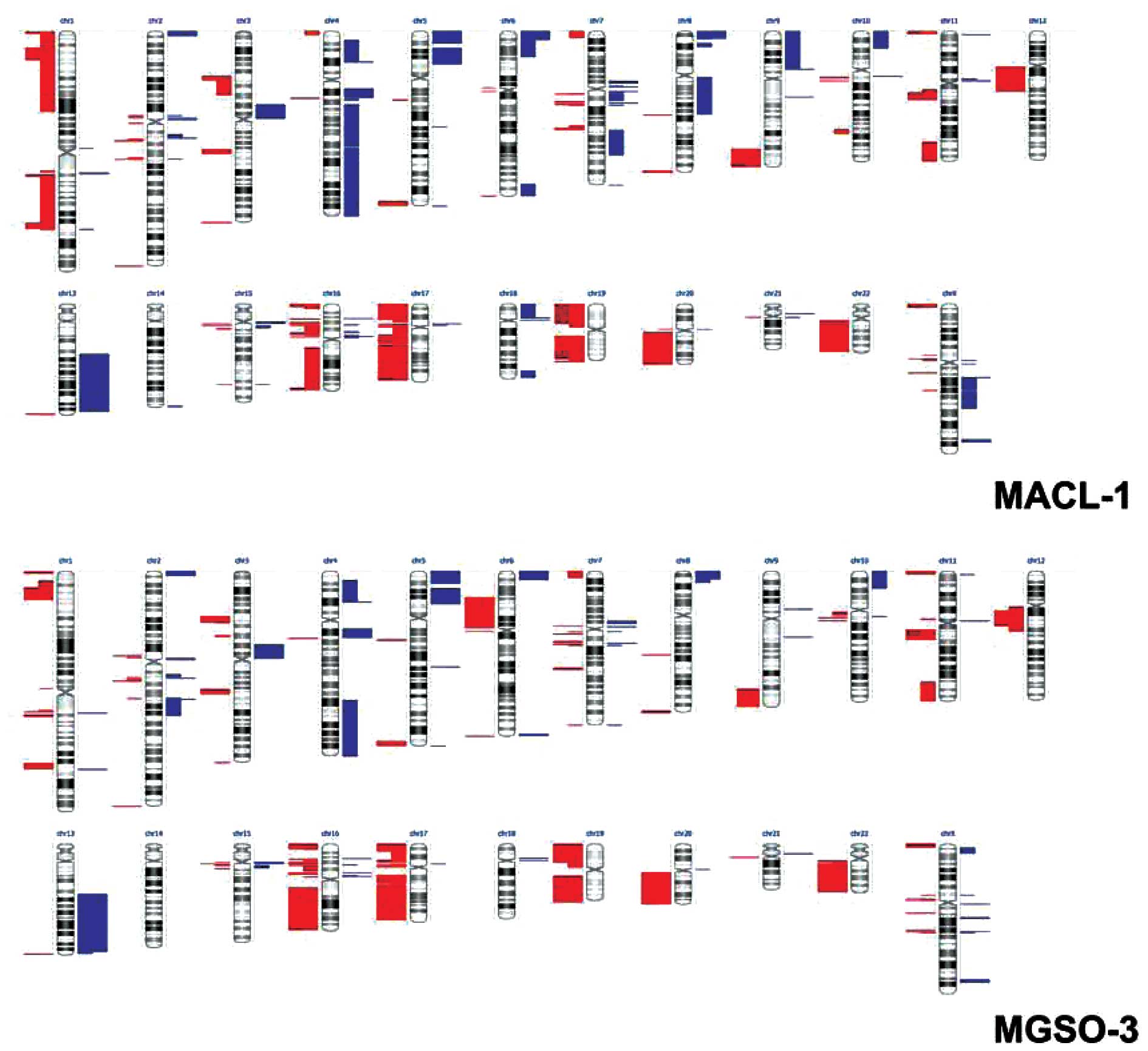
using the Nexus Copy Number software. Fig. 5 displays a whole-genome image
derived from the analysis and depicts the extensive chromosomal
alterations present in the MACL-1 and MGSO-3 cells, a number of
them detected on the 2 dye swapped replicates (represented by
double-length bars).
The total CNAs attributed to the MACL-1 and MGSO-3
cell lines were 172.5±30.4 and 166.5±12, respectively. However,
when only alterations present on the two replicates and with a
p-value <0.05 are considered, 25 and 33 copy number alterations
arise for MACL-1 and MGSO-3, respectively.
The complexity of MACL-1 and MGSO-3 genomes has
already been observed by our group when we attempted to explore the
karyotype profile of these cells through G-banding or DAPI staining
(data not shown). This impairment was promptly demonstrated through
our aCGH data, which confirmed extremely complex alterations
hindering chromosomal mapping through those techniques. MACL-1 and
MGSO-3 cell lines displayed common alterations, such as loss of
considerable portions of chromosomes 17, 19 and 22 (Table III). Losses on chromosome 17 took
place on the following regions: 17q12, 17q12-q21.2, 17q21.2,
17q21.31-q23.1, 17q23.1-q24.1, 17q24.1-q25.2 and 7q25.2-q25.3
(Table III). The MACL-1 and
MGSO-3 cells showed significant losses on 17q21.31. Kim et
al(29) showed that losses on
this region are related to prostate cancer and 17q21.31 is known to
be completely lost in the PC3 cell line (30). Another important alteration is
associated with the 17q12-q21.2 region, where the HER-2
(ERBB2) gene is located. The loss of this region could
explain the lack of HER-2 expression in MGSO-3 cells and derived
xenotransplants from this cell line in nude mice.
 | Table IIIMain altered genomic regions on
MACL-1 and MGSO-3 cell lines, present on the 2 dye swap replicates,
with a p-value <0.05. |
Table III
Main altered genomic regions on
MACL-1 and MGSO-3 cell lines, present on the 2 dye swap replicates,
with a p-value <0.05.
| Region | Event | Cytoband | Cell line |
|---|
|
chr17:0-16531500 | Loss | p13.3-p11.2 | MACL-1 |
|
chr17:31891535-33317141 | Loss | q12 | MACL-1 |
|
chr17:33661605-36347121 | Loss | q12 | MACL-1 and
MGSO-3 |
|
chr17:36548604-38591831 | Loss | q12-q21.2 | MACL-1 and
MGSO-3 |
|
chr17:38784700-40869210 | Loss | q21.2 | MACL-1 and
MGSO-3 |
|
chr17:42143048-57671531 | Loss | q21.31-q23.1 | MACL-1 and
MGSO-3 |
|
chr17:57775091-63421974 | Loss | q23.1-q24.1 | MACL-1 and
MGSO-3 |
|
chr17:63665720-75057558 | Loss | q24.1-q25.2 | MACL-1 and
MGSO-3 |
|
chr17:75269931-78653589 | Loss | q25.2-q25.3 | MACL-1 and
MGSO-3 |
|
chr19:32964337-47953667 | Loss | q13.11-q13.32 | MACL-1 and
MGSO-3 |
|
chr19:48122394-60078783 | Loss | q13.33-q13.43 | MGSO-3 |
|
chr22:17274835-18691763 | Loss | q11.1-q11.21 | MACL-1 and
MGSO-3 |
|
chr22:20247200-49565875 | Loss | q11.21-q13.33 | MACL-1 and
MGSO-3 |
For chromosome 19 the affected regions were:
19q13.11-q13.32 in the MACL-1 and MGSO-3 cell lines and
19q13.33-q13.43 in the MGSO-3 cell line (Table III). Loss on 19q13.33–13.43 is a
rare finding in human tumors, although it has been described in
ovarian cancer cells and gliomas (31,32).
The loss of heterozigosity on chromosomes in these types of tumor
suggests the location of a tumor suppressor gene, but none has yet
been found (31,33,34).
Table III also
shows alterations on chromosome 22: 22q11.1-q11.21 and
22q11.21-q13.33. Although our previously karyotyping data showed an
apparent intact chromosome 22 (data not shown), Table III shows that chromosome 22
exhibited alterations, which are frequently observed in breast
carcinomas (35–38). Previous studies have show frequent
allelic loss in this region, but similar to 19q13, a tumor
suppressor gene has yet to be confirmed (39). A gene described as important for
this region is SMARCB1, also termed IN1. IN1 is considered a tumor
suppressor gene and was originally identified in malignant rhabdoid
tumors of infancy, and subsequently in medullary carcinomas,
sarcomas, myoepithelial carcinomas and chondrosarcomas (40).
Overall the biological processes involved in MACL-1
and MGSO-3 CNAs showed alterations in genes that are engaged in
several activities including gene transcription and regulation,
cell cycle, signal transduction and metabolic processes. As
expected there does not appear to be a concise bias toward a
particular biological process.
In conclusion, MACL-1 and MGSO-3 cell lines changed
their protein expression profile possibly due to a selection
pressure for a more fitted phenotype on cell culture conditions.
This phenotypic shift was conserved in tumor xenografts in
immunodeficient mice. Despite carrying extensive chromosomal
imbalances, these cells maintained a high proliferative ability. To
the best of our knowledge, MACL-1 and MGSO-3 are the only Brazilian
breast cancer cell lines that could be used for comparative studies
with other known breast cancer cell lines.
Acknowledgements
This study was supported by the National Institutes
of Health (NIH; grant no. 1R03TW008709) and by grants from the
Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG),
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)
and Conselho Nacional de Desenvolvimento Científico e Tecnológico
(CNPq). The authors are thankful for the financial support provided
by Pró-Reitoria de Pesquisa da Universidade Federal de Minas
Gerais.
References
|
1
|
INCA. Estimativa 2012: Incidência de
câncer no Brasil. Instituto Nacional de Câncer José Alencar Gomes
da Silva, Coordenação Geral de Ações Estratégicas, Coordenação de
Prevenção e Vigilância; Rio de Janeiro. pp. 1882011
|
|
2
|
Hammond ME, Hayes DF, Dowsett M, Allred
DC, Hagerty KL, Badve S, et al: American Society of Clinical
Oncology/College of American Pathologists guideline recommendations
for immunohistochemical testing of estrogen and progesterone
receptors in breast cancer (unabridged version). Arch Pathol Lab
Med. 134:e48–e72. 2010.
|
|
3
|
Wolff AC, Hammond ME, Schwartz JN, Hagerty
KL, Allred DC, Cote RJ, et al: American Society of Clinical
Oncology/College of American Pathologists guideline recommendations
for human epidermal growth factor receptor 2 testing in breast
cancer. Arch Pathol Lab Med. 131:18–43. 2007.
|
|
4
|
Goldhirsch A, Ingle JN, Gelber RD, Coates
AS, Thurlimann B and Senn HJ: Thresholds for therapies: highlights
of the St Gallen International Expert Consensus on the primary
therapy of early breast cancer. Ann Oncol. 20:1319–1329. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perou CM, Sorlie T, Eisen MB, van de RM,
Jeffrey SS, Rees CA, et al: Molecular portraits of human breast
tumours. Nature. 406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sorlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, et al: Gene expression patterns of breast
carcinomas distinguish tumor subclasses with clinical implications.
Proc Natl Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sorlie T, Perou CM, Fan C, Geisler S, Aas
T, Nobel A, et al: Gene expression profiles do not consistently
predict the clinical treatment response in locally advanced breast
cancer. Mol Cancer Ther. 5:2914–2918. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sorlie T, Tibshirani R, Parker J, Hastie
T, Marron JS, Nobel A, et al: Repeated observation of breast tumor
subtypes in independent gene expression data sets. Proc Natl Acad
Sci USA. 100:8418–8423. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prat A, Parker JS, Karginova O, Fan C,
Livasy C, Herschkowitz JI, et al: Phenotypic and molecular
characterization of the claudin-low intrinsic subtype of breast
cancer. Breast Cancer Res. 12:R682010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kao J, Salari K, Bocanegra M, Choi YL,
Girard L, Gandhi J, et al: Molecular profiling of breast cancer
cell lines defines relevant tumor models and provides a resource
for cancer gene discovery. PLoS One. 4:e61462009.PubMed/NCBI
|
|
11
|
Correa CR, Bertollo CM and Goes AM:
Establishment and characterization of MACL-1 and MGSO-3 cell lines
derived from human primary breast cancer. Oncol Res. 17:473–482.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Correa CR, Bertollo CM, Zouain CS and Goes
AM: Glyceraldehyde-3-phosphate dehydrogenase as a surface
associated antigen on human breast cancer cell lines MACL-1 and
MGSO-3. Oncol Rep. 24:677–685. 2010.PubMed/NCBI
|
|
13
|
Bertollo CM, Correa CR, Gomes DA,
Souza-Fagundes EM and Goes AM: Effect of radiation treatment on
newly established human breast cancer cell lines MACL-1 and MGSO-3.
Tumour Biol. 31:189–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yerushalmi R, Woods R, Ravdin PM, Hayes MM
and Gelmon KA: Ki67 in breast cancer: prognostic and predictive
potential. Lancet Oncol. 11:174–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Al Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003.
|
|
16
|
Wright MH, Calcagno AM, Salcido CD,
Carlson MD, Ambudkar SV and Varticovski L: Brca1 breast tumors
contain distinct CD44+/CD24− and
CD133+ cells with cancer stem cell characteristics.
Breast Cancer Res. 10:R102008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Franken NA, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sambrook J and Russel DW: Isolation of
High-molecular-weight DNA from mammalian cells using proteinase K
and phenol. Molecular Cloning: A Laboratory Manual. Cold Spring
Harbor Laboratory Press; Cold Spring Harbor NY: 2001, PubMed/NCBI
|
|
19
|
Lim SC: CD24 and human carcinoma: tumor
biological aspects. Biomed Pharmacother. 59(Suppl 2): S351–S354.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baumann P, Cremers N, Kroese F, Orend G,
Chiquet-Ehrismann R, Uede T, et al: CD24 expression causes the
acquisition of multiple cellular properties associated with tumor
growth and metastasis. Cancer Res. 65:10783–10793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kristiansen G, Sammar M and Altevogt P:
Tumour biological aspects of CD24, a mucin-like adhesion molecule.
J Mol Histol. 35:255–262. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
O’Hare MJ: Breast cancer. Human Cancer in
Primary Culture. A Handbook. Masters JRW: Kluwer Academic
Publishers; London: pp. 271–286. 1991
|
|
23
|
Kim JB, O’Hare MJ and Stein R: Models of
breast cancer: is merging human and animal models the future?
Breast Cancer Res. 6:22–30. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brozova M, Kleibl Z, Netikova I, Sevcik J,
Scholzova E, Brezinova J, et al: Establishment, growth and in vivo
differentiation of a new clonal human cell line, EM-G3, derived
from breast cancer progenitors. Breast Cancer Res Treat.
103:247–257. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Strojnik T, Kavalar R, Barone TA and
Plunkett RJ: Experimental model and immunohistochemical comparison
of U87 human glioblastoma cell xenografts on the chicken
chorioallantoic membrane and in rat brains. Anticancer Res.
30:4851–4860. 2010.PubMed/NCBI
|
|
26
|
Tsuji K, Kawauchi S, Saito S, Furuya T,
Ikemoto K, Nakao M, et al: Breast cancer cell lines carry cell
line-specific genomic alterations that are distinct from
aberrations in breast cancer tissues: comparison of the CGH
profiles between cancer cell lines and primary cancer tissues. BMC
Cancer. 10:152010. View Article : Google Scholar
|
|
27
|
Smiraglia DJ, Rush LJ, Fruhwald MC, Dai Z,
Held WA, Costello JF, et al: Excessive CpG island hypermethylation
in cancer cell lines versus primary human malignancies. Hum Mol
Genet. 10:1413–1419. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vargo-Gogola T and Rosen JM: Modelling
breast cancer: one size does not fit all. Nat Rev Cancer.
7:659–672. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim JH, Dhanasekaran SM, Mehra R, Tomlins
SA, Gu W, Yu J, et al: Integrative analysis of genomic aberrations
associated with prostate cancer progression. Cancer Res.
67:8229–8239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Clark J, Edwards S, Feber A, Flohr P, John
M, Giddings I, et al: Genome-wide screening for complete genetic
loss in prostate cancer by comparative hybridization onto cDNA
microarrays. Oncogene. 22:1247–1252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mora J, Cheung NK, Chen L, Qin J and
Gerald W: Loss of heterozygosity at 19q13.3 is associated with
locally aggressive neuroblastoma. Clin Cancer Res. 7:1358–1361.
2001.PubMed/NCBI
|
|
32
|
Barbashina V, Salazar P, Holland EC,
Rosenblum MK and Ladanyi M: Allelic losses at 1p36 and 19q13 in
gliomas: correlation with histologic classification, definition of
a 150-kb minimal deleted region on 1p36, and evaluation of CAMTA1
as a candidate tumor suppressor gene. Clin Cancer Res.
11:1119–1128. 2005.
|
|
33
|
Chou D, Miyashita T, Mohrenweiser HW, Ueki
K, Kastury K, Druck T, et al: The BAX gene maps to the glioma
candidate region at 19q13.3, but is not altered in human gliomas.
Cancer Genet Cytogenet. 88:136–140. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smith JS, Tachibana I, Pohl U, Lee HK,
Thanarajasingam U, Portier BP, et al: A transcript map of the
chromosome 19q-arm glioma tumor suppressor region. Genomics.
64:44–50. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Iida A, Kurose K, Isobe R, Akiyama F,
Sakamoto G, Yoshimoto M, et al: Mapping of a new target region of
allelic loss to a 2-cM interval at 22q13.1 in primary breast
cancer. Genes Chromosomes Cancer. 21:108–112. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bieche I and Lidereau R: Genetic
alterations in breast cancer. Genes Chromosomes Cancer. 14:227–251.
1995. View Article : Google Scholar
|
|
37
|
Sato T, Tanigami A, Yamakawa K, Akiyama F,
Kasumi F, Sakamoto G and Nakamura Y: Allelotype of breast cancer:
cumulative allele losses promote tumor progression in primary
breast cancer. Cancer Res. 50:7184–7189. 1990.PubMed/NCBI
|
|
38
|
Allione F, Eisinger F, Parc P, Noguchi T,
Sobol H and Birnbaum D: Loss of heterozygosity at loci from
chromosome arm 22Q in human sporadic breast carcinomas. Int J
Cancer. 75:181–186. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Benetkiewicz M, Piotrowski A, Díaz De,
Ståhl T, Jankowski M, Bala D, Hoffman J, et al: Chromosome 22
array-CGH profiling of breast cancer delimited minimal common
regions of genomic imbalances and revealed frequent intra-tumoral
genetic heterogeneity. Int J Oncol. 29:935–945. 2006.
|
|
40
|
Hollmann TJ and Hornick JL: INI1-deficient
tumors: diagnostic features and molecular genetics. Am J Surg
Pathol. 35:e47–e63. 2011. View Article : Google Scholar : PubMed/NCBI
|