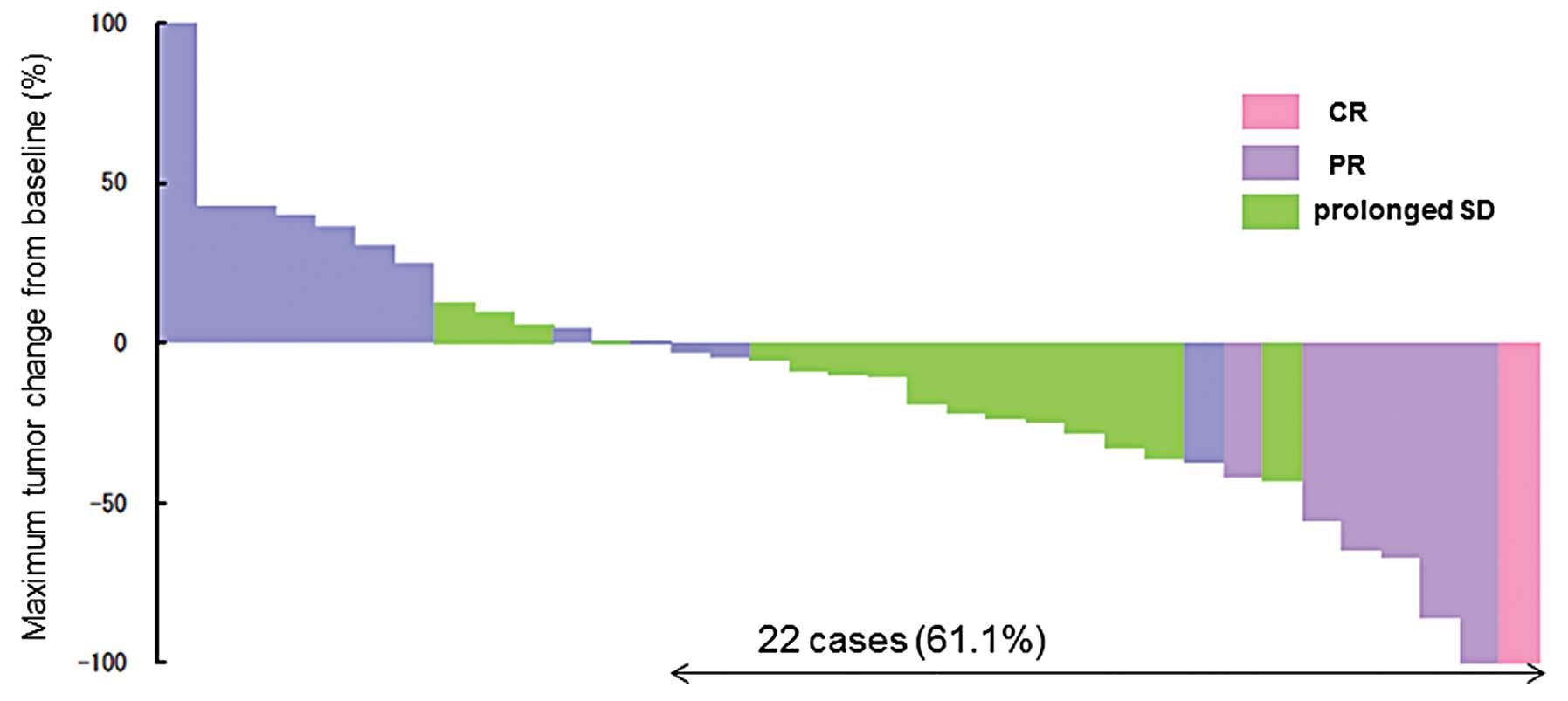
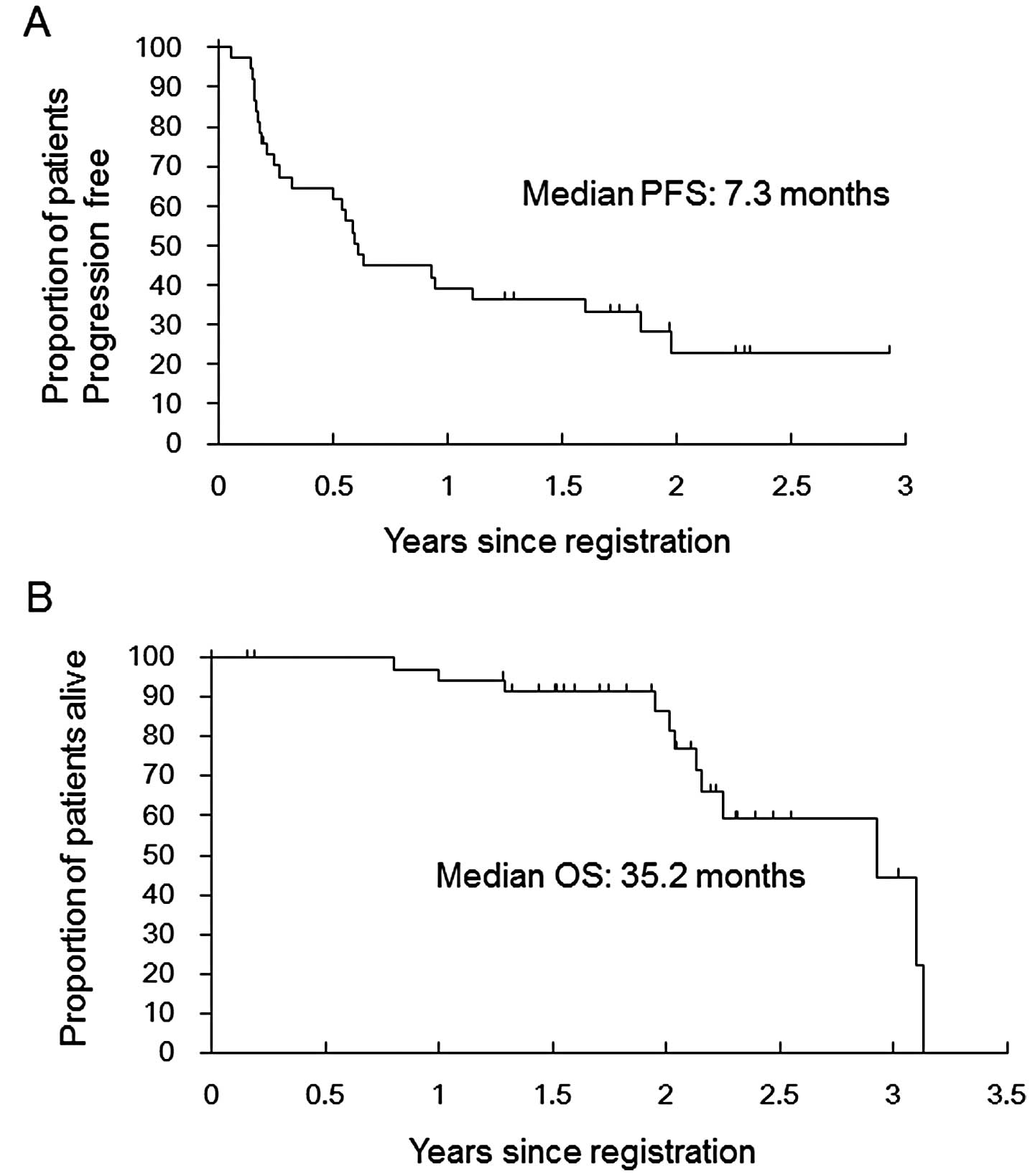
|
1
|
Jonat W, Kaufmann M, Sauerbrei W, Blamey
R, Cuzick J, Namer M, Fogelman I, de Haes JC, de Matteis A, Stewart
A, Eiermann W, Szakolczai I, Palmer M, Schumacher M, Geberth M and
Lisboa B; Zoladex Early Breast Cancer Research Association Study.
Goserelin versus cyclophosphamide, methotrexate, and fluorouracil
as adjuvant therapy in premenopausal patients with node-positive
breast cancer: the ‘Zoladex’ Early Breast Cancer Research
Association study. J Clin Oncol. 20:4628–4635. 2002.
|
|
2
|
Kaufmann M, Jonat W, Blamey R, Cuzick J,
Namer M, Fogelman I, de Haes JC, Schumacher M and Sauerbrei W;
Zoladex Early Breast Cancer Research Association (ZEBRA) Trialists'
Group. Survival analyses from the ZEBRA study: goserelin (Zoladex)
versus CMF in premenopausal women with node-positive breast cancer.
Eur J Cancer. 39:1711–1717. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ackerman GE, Smith ME, Mendelson CR,
MacDonald PC and Simpson ER: Aromatization of androstenedione by
human adipose tissue stromal cells in monolayer culture. J Clin
Endocrinol Metab. 53:412–417. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jonat W, Kaufmann M, Blamey RW, Howell A,
Collins JP, Coates A, Eiermann W, Jänicke F, Njordenskold B and
Forbes JF: A randomized study to compare the effect of the
luteinising hormone releasing hormone (LH-RH) analogue goserelin
with or without tamoxifen in pre- and perimenopausal patients with
advanced breast cancer. Eur J Cancer. 31A:137–142. 1995. View Article : Google Scholar
|
|
5
|
Forward DP, Cheung KL, Jackson L and
Robertson JF: Clinical and endocrine data for goserelin plus
anastrozole as second-line endocrine therapy for premenopausal
advanced breast cancer. Br J Cancer. 90:590–594. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. J Natl Cancer
Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Japan Breast Cancer Society. General Rules
for Clinical and Pathological Recording of Breast Cancer. 14th
edition. Kanehara Publications; Tokyo: 2000, (In Japanese).
|
|
8
|
National Cancer Institute. Common
Terminology Criteria for Adverse Events version 3.0 (CTCAE).
http://ctep.cancer.gov.
|
|
9
|
Bachelot T, Bourgier C, Cropet C,
Ray-Coquard I, Ferrero JM, Freyer G, Abadie-Lacourtoisie S, Eymard
JC, Debled M, Spaëth D, Legouffe E, Allouache D, El Kouri C and
Pujade-Lauraine E: Randomized phase II trial of everolimus in
combination with tamoxifen in patients with hormone
receptor-positive, human epidermal growth factor receptor
2-negative metastatic breast cancer with prior exposure to
aromatase inhibitors: a GINECO study. J Clin Oncol. 30:2718–2724.
2012. View Article : Google Scholar
|
|
10
|
Smith IE and Dowsett M: Aromatase
inhibitors in breast cancer. N Engl J Med. 348:2431–2442. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smith IE, Dowsett M, Yap YS, Walsh G,
Lønning PE, Santen RJ and Hayes D: Adjuvant aromatase inhibitors
for early breast cancer after chemotherapy-induced amenorrhoea:
caution and suggested guidelines. J Clin Oncol. 24:2444–2447. 2006.
View Article : Google Scholar
|
|
12
|
Carlson RW, Theriault R, Schurman CM,
Rivera E, Chung CT, Phan SC, Arun B, Dice K, Chiv VY, Green M and
Valero V: Phase II trial of anastrozole plus goserelin in the
treatment of hormone receptor-positive, metastatic carcinoma of the
breast in premenopausal women. J Clin Oncol. 28:3917–3921. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Michaud JB, Jones KL and Buzdar AU:
Combination endocrine therapy in the management of breast cancer.
Oncologist. 6:538–546. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nabholtz JM, Buzdar A, Pollak M, Harwin W,
Burton G, Mangalik A, Steinberg M, Webster A and von Euler M:
Anastrozole is superior to tamoxifen as first-line therapy for
advanced breast cancer in postmenopausal women: results of a North
American multicenter randomized trial. J Clin Oncol. 18:3758–3767.
2000.PubMed/NCBI
|
|
15
|
Paridaens RJ, Dirix LY, Beex LV, Nooij M,
Cameron DA, Cufer T, Piccart MJ, Bogaerts J and Therasse P: Phase
III study comparing exemestane with tamoxifen as first-line
hormonal treatment of metastatic breast cancer in postmenopausal
women: the European Organisation for Research and Treatment of
Cancer Breast Cancer Cooperative Group. J Clin Oncol. 26:4883–4890.
2008. View Article : Google Scholar
|
|
16
|
Mouridsen H, Gershanovich M, Sun Y,
Pérez-Carrión R, Boni C, Monnier A, Apffelstaedt J, Smith R,
Sleeboom HP, Jänicke F, Pluzanska A, Dank M, Becquart D, Bapsy PP,
Salminen E, Snyder R, Lassus M, Verbeek JA, Staffler B,
Chaudri-Ross HA and Dugan M: Superior efficacy of letrozole versus
tamoxifen as first-line therapy for postmenopausal women with
advanced breast cancer: results of a phase III study of the
International Letrozole Breast Cancer Group. J Clin Oncol.
19:2596–2606. 2001.
|
|
17
|
Osborne CK, Pippen J, Jones SE, Parker LM,
Ellis M, Come S, Gertler SZ, May JT, Burton G, Dimery I, Webster A,
Morris C, Elledge R and Buzdar A: Double-blind, randomized trial
comparing the efficacy and tolerability of fulvestrant versus
anastrozole in postmenopausal women with advanced breast cancer
progressing on prior endocrine therapy: results of a North American
trial. J Clin Oncol. 20:3386–3395. 2002. View Article : Google Scholar
|
|
18
|
Kaufmann M, Bajetta E, Dirix LY, Fein LE,
Jones SE, Zilembo N, Dugardyn JL, Nasurdi C, Mennel RG, Cervek J,
Fowst C, Polli A, di Salle E, Arkhipov A, Piscitelli G, Miller LL
and Massimini G: Exemestane is superior to megestrol acetate after
tamoxifen failure in postmenopausal women with advanced breast
cancer: results of a phase III randomized double-blind trial. J
Clin Oncol. 18:1399–1411. 2000.
|
|
19
|
Yao S, Xu B, Li Q, Zhang P, Yuan P, Wang
J, Ma F and Fan Y: Goserelin plus letrozole as first- or
second-line hormonal treatment in premenopausal patients with
advanced breast cancer. Endocr J. 58:509–516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gnant M, Mlineritsch B, Schippinger W,
Luschin-Ebengreuth G, Pöstlberger S, Menzel C, Jakesz R, Seifert M,
Hubalek M, Bjelic-Radisic V, Samonigg H, Tausch C, Eidtmann H,
Steger G, Kwasny W, Dubsky P, Fridrik M, Fitzal F, Stierer M,
Rücklinger E and Greil R; ABCSG-12 Trial Investigators and Marth C.
Endocrine therapy plus zoledronic acid in premenopausal breast
cancer. N Engl J Med. 360:679–691. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Torrisi R, Bagnardi V, Rotmensz N, Scarano
E, Iorfida M, Veronesi P, Luini A, Viale G, Santoro A, Colleoni M
and Goldhirsch A: Letrozole plus GnRH analogue as preoperative and
adjuvant therapy in premenopausal women with ER positive locally
advanced breast cancer. Breast Cancer Res Treat. 126:431–441. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Masuda N, Sagara Y, Kinoshita T, Iwata H,
Nakamura S, Yanagita Y, Nishimura R, Iwase H, Kamigaki S, Takei H
and Noguchi S: Neoadjuvant anastrozole versus tamoxifen in patients
receiving goserelin for premenopausal breast cancer (STAGE): a
double-blind, randomised phase 3 trial. Lancet Oncol. 13:345–352.
2012. View Article : Google Scholar
|