Introduction
Retinoblastoma (RB) is the most common intraocular
malignancy in children. Worldwide, the incidence of RB is ~1 in
15,000, with nearly 9,000 new cases of RB reported each year
(1). In China, most children with
RB are in the late-stage of the disease because of delayed/late
diagnosis. The morbidity and mortality are significantly higher
than in developed countries (2,3).
Patients with RB are typically children <5 years old and >89%
of patients are <3 years old at the onset of RB; some present
symptoms at birth (4). At present,
the main treatments are systemic chemotherapy combined with local
treatment (1). Although the
efficacy of chemotherapy has recently improved, the long-term
systemic chemotherapy can cause severe side effects, such as bone
marrow suppression, ototoxicity, renal toxicity and other adverse
reactions that affect the child’s quality of life (5,6). These
side effects are particularly serious because children are in an
active phase of development during the treatment. Other
disadvantages include the induction of multidrug resistance induced
by the high-dose chemotherapy used to treat RB (7,8).
Therefore, finding a new method to treat RB effectively has become
a priority. Biological treatments (and in special adoptive
immunotherapy) have been shown to have great potential as an
adjunct treatment to control the disease. Adoptive immunotherapy
uses the natural ability of the immune system to recognize and
eliminate continuously arising transformed cells. This approach can
be efficiently employed for the eradication of residual cancer
cells and prevention or delay of tumor relapse (9). An example is the case of solid tumors,
where antigen-specific immunotherapy has emerged as a promising
approach (10–12). One of the key players in mediating
immune response are the dendritic cells (DCs) as they are
specialized in priming naive helper and cytotoxic T-lymphocytes and
directly trigger natural killer (NK) cell function (13). In addition, DCs can be loaded with
antigens (DC-Ag) that may increase the DC’s specificity. This in
turn enhances the targeting of killing cancer cells (14–16).
Functional T-lymphocytes can be generated in vitro from
peripheral blood mononuclear cells (PBMCs) by using specific media
formulations. A sub-population of the T-lymphocytes, differentiated
in vitro by addition of specific cytokines to the media
culture is the cytokine-induced killer (CIK) cells. The CIK cells
are major histocompatibility complex (MHC)-unrestricted with the
characteristic CD3+CD56+ phenotype and they
have been shown, in vitro and in medical practice, to be
efficient killers of tumor cells (9,17–19).
These CIK cells with the ability to attack tumor cells are
expressed on the cell surface of CD3/CD56. Some studies have shown
that CIKs co-cultured with DCs (DC-CIKs) can have better antitumor
activity on a variety of cancers (20–22).
However, until now DC-CIKs have not been reported to exhibit
cytotoxicity towards RB cells.
There are some questions about the ability of the
antigens obtained from cancer cell line lysates to generate enough
cancer cell targeting specificity to allow for DC-CIKs to be used
extensively for clinical treatment instead of a personalized
therapy. However, the ability of the antigen (Ag) loaded DC-CIK
co-culture (DC-Ag-CIK) to transactivate one another via the
reciprocal production of cytokines could promote more tumor killing
activity in vitro. This enhanced tumor killing activity
could overcome the limitation of not using a personalized therapy.
In advanced RB cases, chemotherapy is the main available treatment
that offers a chance of rescuing the eyeballs. However, the drugs
used in this treatment have a negative effect on humoral and
cellular immunity and the damage to the immune system is related to
both the dose and the duration of administration (23,24).
Clearly then an effective immunotherapy treatment against RB would
be advantageous. Thus, we evaluated the antitumor immune responses
of immunotherapy on RB cells in vitro and provide evidence
for a new method for treating RB. We used RB antigen pulsed DCs
co-cultured with CIK cells to investigate the cytotoxic effect on
the RB cell line RB-Y79. Our results showed that the Ag pulsed DC
cells promote cell proliferation and increase the CIK
differentiation in vitro by regulating the secretion of IL-6
and decreasing IL-10 cytokine levels. Also we show that Ag pulsed
DCs specifically enhanced the CIK cytotoxicity on RB cells.
Importantly, this is the first demonstration of the ability of
DC-Ag-CIKs to kill RB cells and carboplatin resistance RB cells.
Because it is specific for RB with no cytotoxic effect on normal
retina cells, this immunotherapeutic approach could represent a
safe, less toxic and effective treatment for RB.
Materials and methods
Cell lines and reagents
Human retinoblastoma cell line RB-Y79 was purchased
from American Type Culture Collection (ATCC, Rockville, MD, USA),
human retinal pigment epithelium cell line hTERT-RPE1 was purchased
from JENNIO Biological Co. (Guangzhou, China). All the cells were
cultured at 37°C in a humidified incubator under 5% CO2
in RPMI-1640 (Gibco, USA) with 10% fetal bovine serum (FBS) (Gibco,
Australia) and 1% penicillin-streptomycin solution (Gibco, USA).
All anti-human CD3-FITC, CD8-APC, CD56-PE, CD80-PE, CD83-APC,
CD86-FITC antibodies were obtained from BD Co. (BD, USA). Cytokines
recombinant mutant human tumor necrosis factor-α (TNF-α),
recombinant human interferon-γ (IFN-γ), recombinant human
interleukin-2 (IL-2), recombinant human granulocyte/macrophage
colony-stimulating factor (GM-CSF), recombinant human interleukin-4
(IL-4) and CD3 monoclonal antibody were purchased from Peprotech
(Rocky Hill, NJ, USA). Propidium iodide (PI) and
5-carboxy-fluorescein diacetate succinimidyl ester (CFSE) were
purchased from Beyotime Institute of Biotechnology (Shanghai,
China), Carboplatin was purchased from Qilu Pharmaceutical Co.
(Ji’nan, Shandong, China).
Generation of dendritic cells and CIK
cells
Blood was freshly drawn from healthy volunteers
according to our protocol accepted by the local ethics committee
(LEC). PBMCs were isolated by Ficoll/Hypaque density gradient
centrifugation, according to standard procedures (25). PBMCs at a density of
5×106 cells/ml were allowed to adhere in Alys-505
complete culture medium (Alys-505 medium supplemented with 0.5%
auto-plasma and 1% penicillin/streptomycin) for 3 h at 37°C in a
humidified 5% CO2 incubator. The adherent PBMCs were
cultured in Alys-505 complete medium with 500 U/ml GM-CSF and 1000
U/ml IL-4 to generate DCs. The medium was changed every 3 days. In
the experiment where TNF-α was tested, 1000 U/ml of TNF-α was added
to the media on day 6. The non-adherent PBMCs were cultured in
Alys-505 complete medium containing 1000 IU/ml IFN-γ, 200 ng/ml CD3
monoclonal antibody and 250 U/ml IL-2 for generating CIK cells.
After day 7 in culture, CIK cells were sub-cultured in fresh
Alys-505 complete culture medium with 200 U/ml IL-2 every 3
days.
Tumor lysates and pulsing DCs
RB-Y79 tumor lysates were generated by three rapid
freeze-thaw cycles. Briefly, confluent cultures of RB-Y79 were
detached by incubation with trypsin-EDTA 0.05% for 5 min, washed in
PBS and resuspended in PBS at a density of 1.5×107
cells/ml. The cells in suspension were frozen in liquid nitrogen
and disrupted by three freeze-thaw cycles. The cell lysis was
clarified by centrifugation for 10 min at 600 × g. The supernatant
was then collected and stored at −80°C. DCs were incubated in the
presence of the tumor lysates from the day 3 of DC maturation.
CIK and DC cells co-culture and cells
proliferation assay
CIK cells were co-cultured on day 7 with autologous
7-day DC or DC-Ag in a 3:1 ratio. All the groups were cultured at
37°C in a humidified 5% CO2 incubator and sub-cultured
every 3 days in Alys-505 complete culture medium with 200 U/ml
IL-2. Cell number was counted from day 7 to 15 in culture using
Counter Star with the software Automated Cell Counter.
Flow cytometry
DCs were phenotyped with a panel of antibodies:
CD80-PE, CD83-APC and CD86-FITC. CIK cells were phenotyped with
antibodies against CD3-FITC, CD8-APC and CD56-PE. A mouse IgG
(PE/FITC/APC) (BD) was used as a negative control in all the
assays. Briefly, 1×106 cells were incubated with the
corresponding antibodies at 4°C for 15 min and then washed with
PBS. A total of 10,000 cells were measured and analysed using
CellQuest Pro software. Dual-color flow cytometric analysis was
performed on a BD FACSCalibur.
Quantitation of cytokine production by DC
and CIK cells
On day 15, supernatants from all experimental groups
were collected. IL-6 and IL-10 levels in the media culture were
quantitatively measure by using a Cytometric Bead Array Human
Th1/Th2 Cytokine kit II (BD) according to the manufacturer’s
instructions.
Cytotoxicity assay
CIK cells, DC-CIK cells and DC-Ag-CIK cells were
harvest on day 15 and the cytotoxic activity was measured on target
cells, either RB-Y79 or hTERT-RPE1 cells. Briefly, RB-Y79 cells and
hTERT-RPE1 cells were suspended in PBS at a density of
1×105 cells/ml and stained with 2 μmol/l CFSE for 10 min
at 37°C. After washing 3 times with RPMI-1640 medium containing 10%
FBS, the CFSE-labeled RB-Y79 cells or hTERT-RPE1 cells were
co-cultured with CIK cells, DC-CIK cells, DC-Ag-CIK cells,
respectively. A 10:1, 20:1 and 40:1 effector:target cell ratio was
used. RB-Y79 or hTERT-RPE1 cells were cultured with media from the
respective effector cell group and they were used as control for
the natural death percentage. After culturing for 24 h, the cells
from all groups were collected and washed twice with PBS and then
incubated with PI (1 μg/ml) at room temperature for 10 min. Life
and dead cells were analysed using dual-color flow cytometric
analysis performed on a BD FACSCalibur. Each group was tested in
triplicate.
For testing the effect of the media on CIK
cytotoxicity, 1×105 RB-Y79 cells were co-cultured with
1×106 CIK cells using conditioned media from CIK cells,
DC medium and DC-Ag medium. The cytotoxicity was analysed in
triplicate as desribed above. In all experiments the cytotoxic
activity was defined as the percentage of dead cells after 24 h of
treatment less the natural death percentage of the respective cell
type.
Cytotoxicity assay on RB-resistant cells
(RB-R)
RB-Y79 cells in logarithmic phase were incubated
with 40 μg/ml at 37°C in a humidified 5% CO2 incubator
for 2 h. After centrifugation and washing, the medium containing
the drug was discarded. Cells were cultured then in complete
culture medium (RPMI + 10% FBS and 1% penicillin/streptomycin).
Once the culture growth into the logarithmic phase again the
Carboplatin treatment was repeated. The same procedure was repeated
for several months until generating a stable resistant cell line at
40 μg/ml carboplatin.
For testing RB-R cell resistance to carboplatin,
RB-Y79 and RB-R cells were cultured in the presence of 10, 20, 40,
50, 60, 70, 80, 90 or 100 μg/ml carboplatin respectively. After 24
h, cell numbers were counted in the culture using Counter Star with
the Automated Cell Counter software. In addition, 1×105
RB-Y79 and RB-R cells were cultured with carboplatin at 40 μg/ml,
respectively. The cytotoxicity was analysed as described above.
For the cytotoxicity assay, CIK, DC-CIK cells and
DC-Ag-CIK cells were harvest on day 15 and co-cultured with RB-Y79
and RB-R cells in a ratio 20:1 in the presence or absence of 40
μg/ml carboplatin. After culturing for 24 h, the cells from all
groups were collected and washed twice with PBS and then stained
with 2 μmol/l CFSE and PI 1 μg/ml. Viable and dead cells were
analysed using dual-color flow cytometric analysis performed on a
BD FACSCalibur. Each group was tested in triplicate.
For every experiment the cytotoxic activity was
defined as the percentage of dead cells after 24 h of treatment
less the percentage of natural death of the respective cell
type.
Results
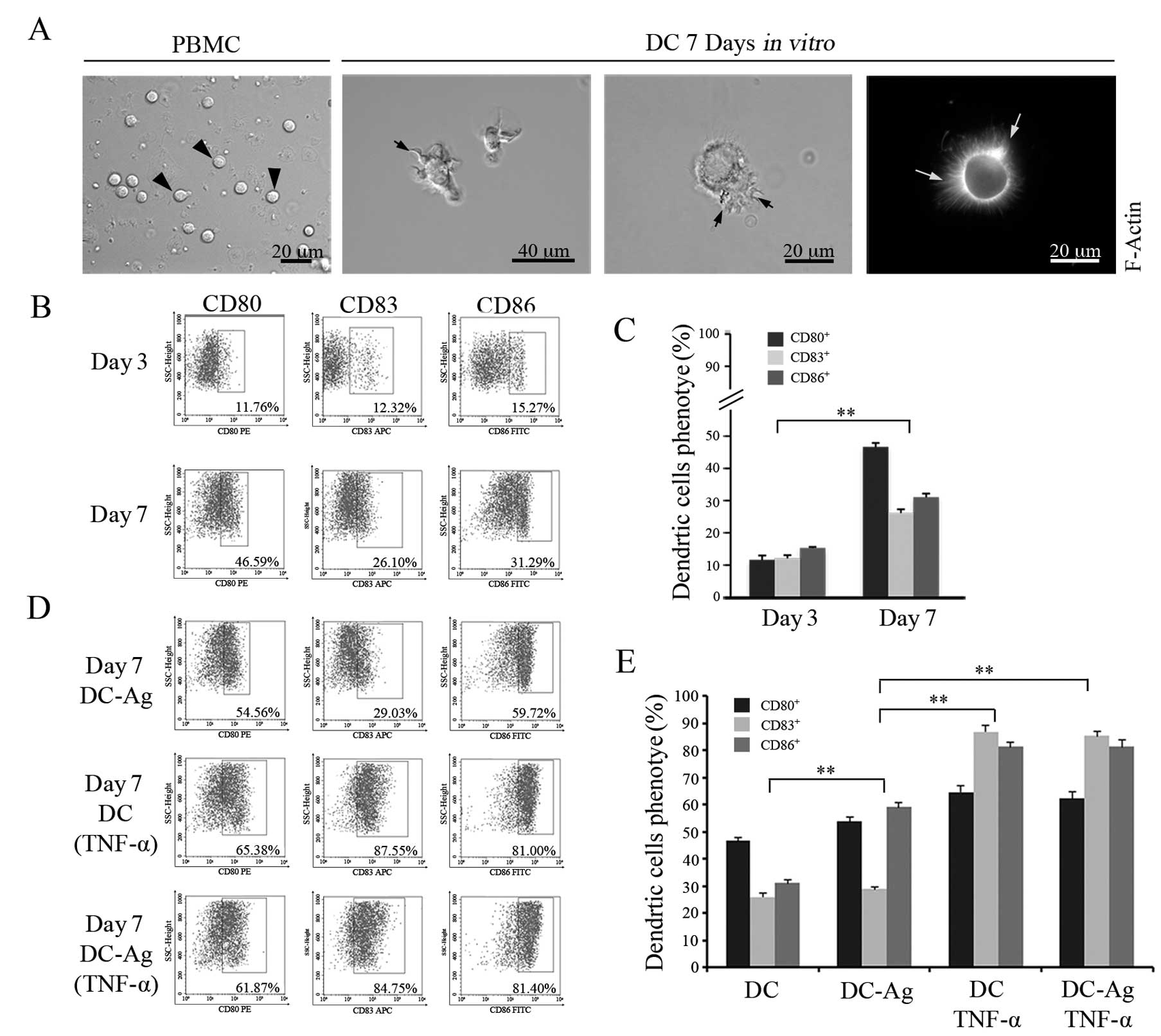
Morphological characterization and
phenotypical analysis of DC maturation in vitro
In this study mature and active DCs were generated
in vitro from peripheral blood mononuclear cells (PBMCs)
taken from a healthy volunteer. Cell differentiation in culture was
followed over time by checking cell morphology. The mature
phenotype was recognized by analyzing the expression of the
clusters of differentiation (CD80, CD83, CD86) in the cell membrane
using flow cytometry (FCM) (Fig.
1).
Freshly isolated PBMCs were spherical and small, the
cell surface was smooth and no protrusions were observed by light
microscopy (Fig. 1A, left image).
After culturing for 3 days in the presence of cytokine-enriched
media, the adherent cells became larger and oval. On day 7, cells
lost the ability to adhere to the plastic and started to grow in
suspension. An additional morphological feature of these cells was
the increment in size, irregular shaped nuclei and the formation of
numerous dendritic-like actin processes emerging from the cell body
(Fig. 1A, middle and right images)
depicting the typical DC morphology corresponding to a mature
phenotype. As expected, this change in morphology was accompanied
by an increase in the cell surface expression of the CDs (Fig. 1B). After 7 days in culture, FCM
assay detected values for the CDs significantly higher compared
with the cells on day 3 (P<0.01) [compare CD80 (11.60±1.37)%,
CD83 (12.11±0.94)% and CD86 (15.28±0.35)% on day 3 with CD80
(46.65±1.26)%, CD83 (26.14±1.16)% and CD86 (31.05±1.12)% on day 7].
The results showed that DCs developed into the mature phenotype
after 7 days (Fig. 1C).
To test the effect of tumor antigen on DC
differentiation and maturation in vitro, tumor lysed from
RB-Y79 cells (Ag) was added to the DCs from day 3 in culture. On
day 7, DC-Ag cells were harvested and the same CDs as above were
analysed by FCM. As shown in Fig. 1B
and D (top panels), the DC-Ag cells expressed higher levels of
CD80 and CD86 compared with DC cultured in cytokine enriched media
alone (without Ag) (P<0.01). However, no significant differences
were found for CD83 levels.
Because TNF-α have a positive effect on DC
differentiation and maturation (26), we tested whether the antigen had a
co-stimulatory effect after 1 day of treatment with TNF-α on DC
maturation. To examine this hypothesis we analysed CD expression
levels on cell surface by FCM (Fig.
1D, bottom panels). The results showed that the addition of
TNF-α to the culture medium on day 6 increased significantly CD80,
CD83 and CD86 expressions compared to DC without TNF-α (P<0.01),
with more obvious differences in the expression level of CD83.
TNF-α had a similar effect on DC-Ag cells (Fig. 1E) (P<0.05).
Overall, our findings showed that the antigen could
upregulate the expression of the CDs that act as co-stimulatory
molecules promoting DC differentiation. In addition, TNF-α had a
strong effect on the overall maturation of DCs.
Because TNF-α favored DC differentiation in
vitro, the following experiments were performed in the presence
of TNF-α. Thus, we can discriminate the effects on DC function
attributable to Ag that are independent of maturation.
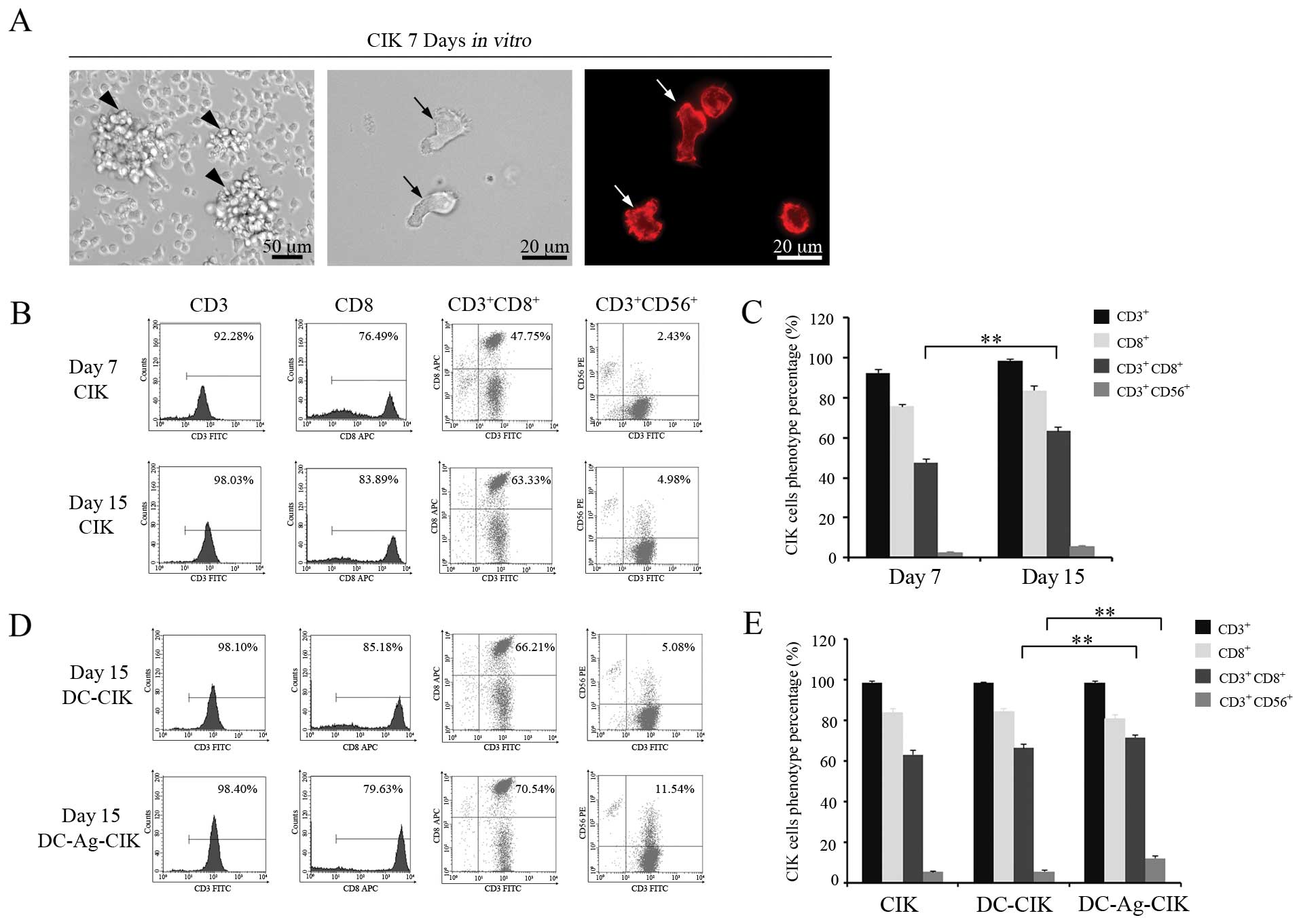
Morphologic characterization and
phenotypic analysis of CIK maturation in vitro
CIK cells are a sub-population of cytotoxic
T-lymphocytes with the characteristic
CD3+CD56+ phenotype. In order to generate CIK
cells in vitro, the non-adherent PBMCs were separated and
cultured in the presence of interferon-γ (IFN-γ), CD3 monoclonal
antibody and IL-2 among other cytokines. After the 7th day in
culture, cell morphology indicated that CIK cells had acquired the
mature phenotype (Fig. 2A). At day
7, the cell area was on average five times bigger than at day 1 in
culture (data no shown) depicting irregular shape. Multiple cells
formed clusters and gathered into cell aggregates (arrows in
Fig. 2A, left panel).
Interestingly, we noted that the actin cytoskeleton of these mature
CIK cells attained a polarized distribution (Fig. 2A, middle and right panels).
Next, we analysed the proportion of cells expressing
CD3+CD56+, CD3+CD8+,
CD3+ and CD8+ in the cell population that had
grown in the cytokine-enriched media. As shown in Fig. 2B and C, the proportion of
CD3+CD56+ and CD3+CD8+
cells increased on day 15 compared with day 7 (P<0.01).
It has been reported that the CIK cells could
interact with DCs resulting in an increment of CIK cytotoxic and
cytolytic activities against tumor cells. To examine if this
applies to our CIK in the presence of DC-Ag, we further examined
whether these DC-Ag cells could expand the proportion of mature
CD3+CD56+ CIK cells in culture. CIK and
DC-CIK or DC-Ag-CIK phenotypes were assayed with
fluorescence-activated cell sorting analyses. On day 15, the
proportion of CD3+CD56+ and
CD3+CD8+ cells were higher when the CIK cells
were co-cultured with DC-Ag cells (P<0.01). No obvious
differences were found when DC-CIK cells were compared with CIK
cells (P>0.05). There were no significant differences for the
population of CD3+ and CD8+ cells compared
between the other groups (P>0.05) (Fig. 2D and E).
The results stated above showed the
CD3+CD56+ CIK cell proportion increases in
the T-lymphocyte population when they are co-cultured with DC-Ag
cells.
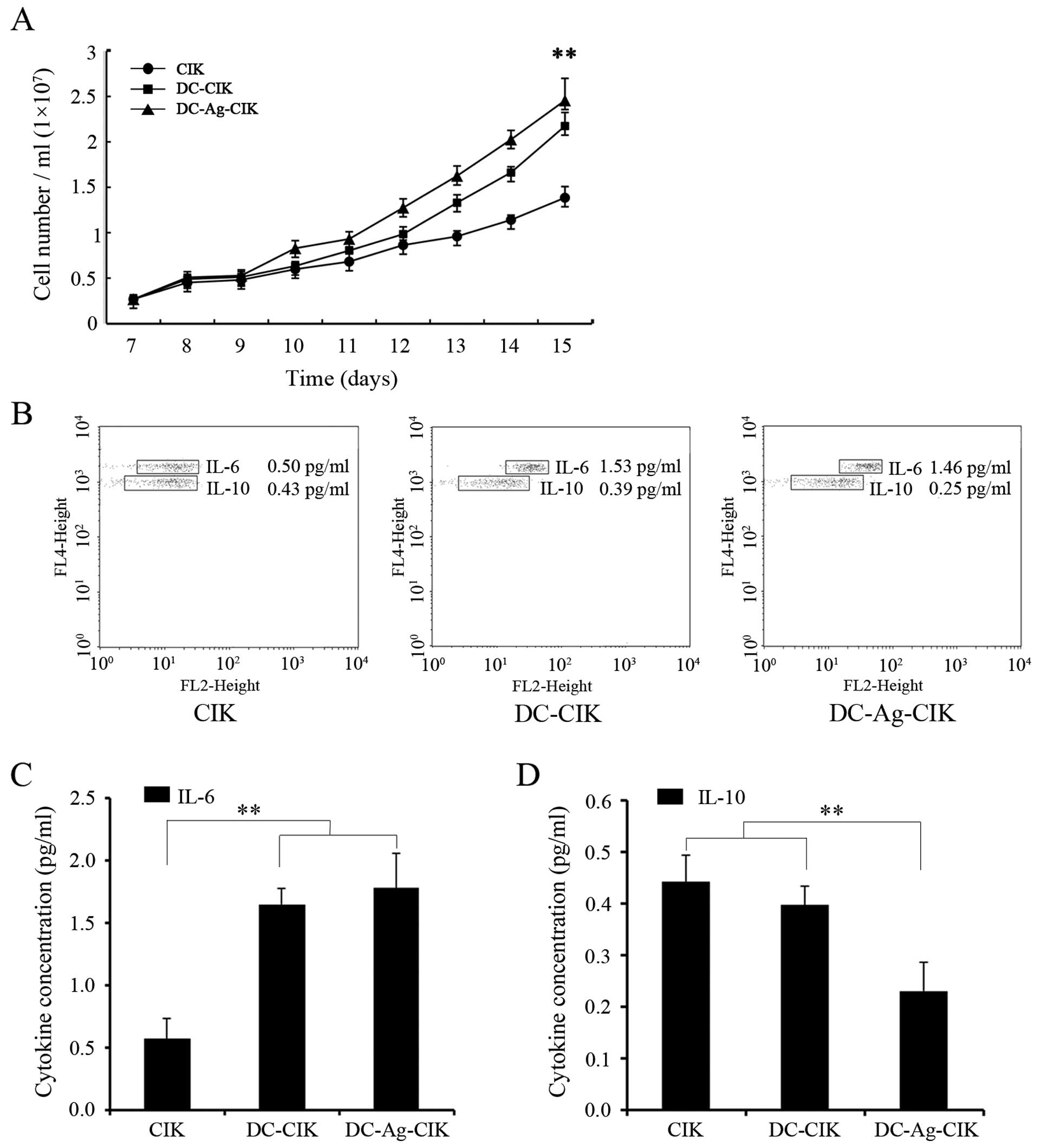
Proliferation of CIK cells and cytokine
production
We found that CIK cells in culture proliferated in a
non-exponential manner, especially from day 9 onwards (Fig. 3A). To examine whether DCs or DC-Ag
affected CIK proliferation, CIK cells were co-cultured with DCs or
DC-Ag from day 7 to 15 and the cell number per ml of medium was
quantified everyday in the different groups (Fig. 3A). After 15 days in culture the
absolute cell number had significantly (P<0.01) increased
5.36±1.58, 8.37±2.19 and 9.38±1.97-fold in CIK, DC-CIK and
DC-Ag-CIK, respectively (Fig.
3A).
To investigate a possible cause for the differences
observed on CIK cell proliferation, we determined the level of two
cytokines that have been shown to play a role in the proliferation
of these cells (IL-6 and IL-10). IL-6 was selected because it was
shown to have stimulatory effect on CIK proliferation and IL-10
because it has inhibitory effect on primary alloreactive T-cell
responses (27,28). The supernatants from CIK and DC-CIK
or DC-Ag-CIK cultures were collected on day 15 and the specific
cytokines were quantified by using a Cytometric Bead Array Human
Th1/Th2 cytokine kit. As shown in Fig.
3B and C, IL-6 levels increased 3-fold when CIK cells were
grown in the presence of DC cells or DC-Ag (1.65±0.13 pg/ml) and
(1.78±0.28 pg/ml), respectively, compared with levels of secreted
IL-6 in CIK culture alone (0.57±0.16 pg/ml) (P<0.01). In
contrast, secreted IL-10 level was two-fold lower in DC-Ag-CIK cell
co-culture (Fig. 3C) compared with
CIK or CIK co-cultured with DC cells (Fig. 3B and D). These results indicate that
CIK cells increased proliferation in the presence of DCs and DC-Ag
might be a consequence of the combination of a low level of the
inhibitory IL-10 and elevation of the stimulatory IL-6 levels in
these co-culture conditions.
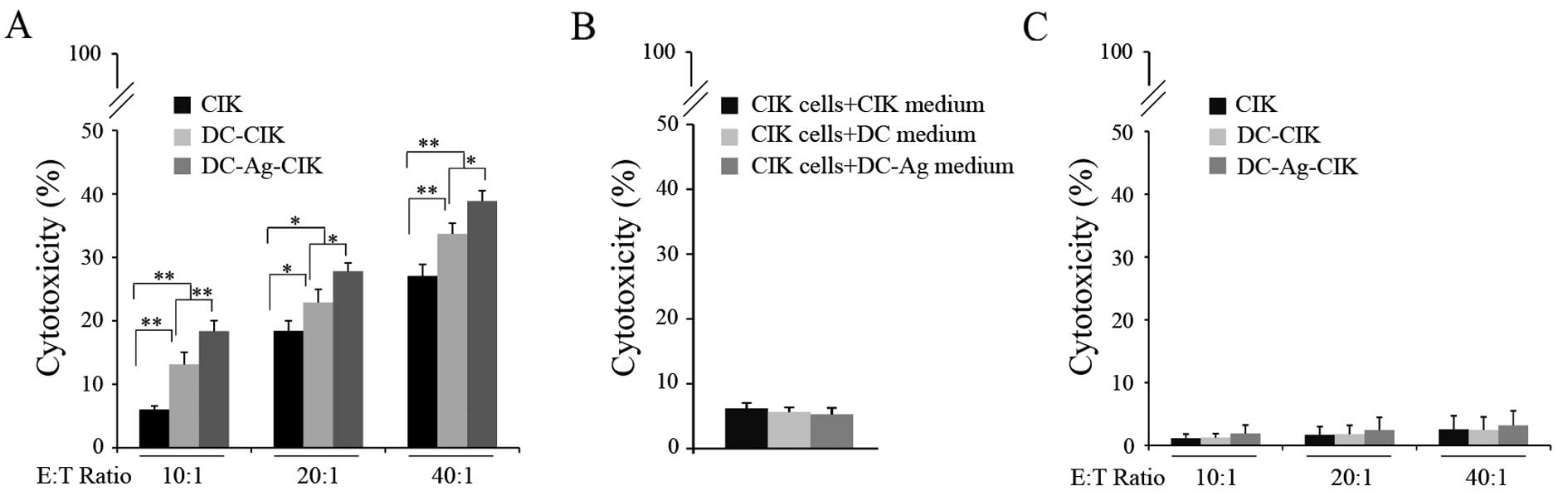
Cytotoxicity assay
Having shown that the DC-Ag cell enhanced CIK
proliferation and differentiation in vitro (Figs. 1 and 2) we next tested whether these processes
translated to an increment in CIK cytotoxic activity on RB-Y79
cells (Fig. 4).
CIK cells were co-cultured either with autologous
non-pulsed DCs or tumor lysate-pulsed DCs (DC-Ag) for 7 days
starting on culture day 7 and tested for cytotoxicity against
RB-Y79 cell line at day 14. The cytotoxicity was assayed by FCM
using specific dyes for labeling viable and dead cells. The
results, shown in Fig. 4, are
expressed as the proportion of cells that are dead relative to the
total number of cancer cells in the culture, minus the natural cell
death observed in each condition.
As shown in Fig. 4A,
CIK cells generated by the cytokine-enriched media killed ~6% of
the RB-Y79 cells after co-culturing for 24 h at a ratio of 10:1
[effector cells:target cells (E:T)]. The cytotoxicity doubled when
the CIK had been co-cultured with the DCs for 7 days. Moreover, the
CIK cytotoxicity was significantly higher when they were in the
presence of DC-Ag (P<0.001). Higher E:T ratios resulted in
significantly higher cytotoxicity (P<0.01) throughout the
experimental conditions. This observation suggests that the effects
are unlikely to result from unspecific influences, other than the
killer cells.
To rule out the possibility that the cytotoxicity
was the result of some unidentified agent present in the cell media
rather than by the CIK cell activity, the cytotoxic of the assay
was performed on RB-Y79 cells incubating the CIK with conditioned
media taken from DC or DC-Ag cultures instead of with the DCs or
DC-Ag. No significant difference was found on the CIK cytotoxic
activity on RB-Y79 cells between the 3 treatments (Fig. 4B) (P>0.05). These observations
suggested that the enhancement of cytotoxic activity on tumor cells
could be at least in part explained by the direct interaction of
CIK cells with mature DC rather than by activity of the components
we added to the culture media.
CIK cells have been described as a highly efficient
cytotoxic effector capable of lysing tumor cell targets by a
mechanism that is non-MHC restricted. Because of this, we explored
whether CIK cells (mature and activated with DC and DC-Ag) have
specific cytotoxic activity on RB cell lines or if they also
targeted normal retinal cells. We set up the cytotoxic assay as
described above and we determined the CIK cytotoxic activity on
hTERT-RPE1 cells. Surprisingly, no significant cytotoxicity was
detected on hTERT-RPE1 cells in any of the assayed groups. The CIK
cytotoxic activity on these cells was 1.13% at an E:T ratio of 10:1
(Fig. 4C).
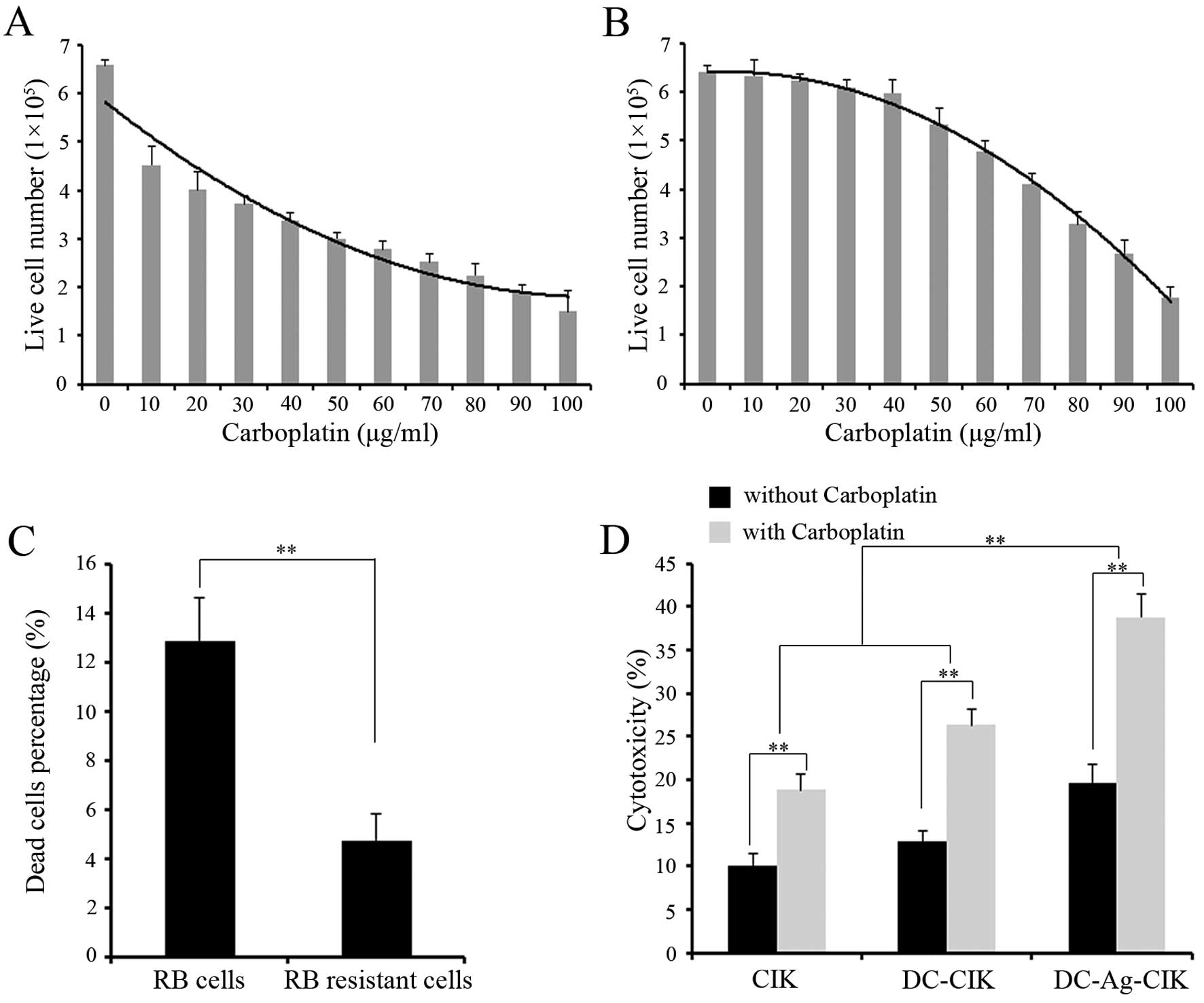
Cytotoxicity assay for
carboplatin-resistant RB cells
After establishing the RB-Y79 carboplatin-resistant
cell line, cells were tested for their ability to survive and
proliferate in a media containing carboplatin. Resistant and
sensitive RB cells were cultured with increasing dosis of
carboplatin and the cell survival was analysed after 24 h. The
result showed that the number of live RB-sensitive cells decreased
significantly as the carboplatin concentration increased. In
contrast, the number of RB-resistant living cells was not
significantly affected until a dose of 50 μg/ml of carboplatin.
However, RB-resistant survival cells gradually decreased with 50
μg/ml or higher drug concentration (Fig. 5A and B). Given this ability of the
RB-resistant cells to survive at 40 μg/ml carboplatin, we named it
RB-R cells.
In order to prove that RB-R cells are resistant to
carboplatin, 40 μg/ml of carboplatin was used on RB-sensitive cells
and RB-R cells. The result showed that the ability to survive
carboplatin was more than two-fold increased in RB-R cells compared
to the sensitive RB cells (Fig.
5C).
We have shown that CIK, DC-CIK and DC-Ag-CIK cells
had a strong cytotoxic activity on normal RB cells, we wanted to
test whether the effector cells could kill RB-R cells efficiently.
CIK, DC-CIK and DC-Ag-CIK cells were used after 7 days co-culture
on day 14 and tested for cytotoxicity against RB-R cell line at 40
μg/ml. The cytotoxicity was assayed by FCM using specific dyes for
labeling viable and dead cells. DC-Ag-CIK cells generated by the
cytokine-enriched media killed ~20% of the RB-R cells after
co-culturing for 24 h at an E:T ratio of 20:1 (Fig. 5D). The cytotoxicity of DC-Ag-CIK
cells against RB-R cells were higher than DC-CIK and CIK cells
(P<0.01), but there was no significant difference between DC-CIK
and CIK cells (P>0.05). To test the combined effect of
carboplatin, RB-R cells co-cultured with effector cells were
incubated with 40 μg/ml carboplatin for 24 h. The cytotoxicity of
DC-Ag-CIK in combination with carboplatin showed the best antitumor
activity compared with DC-CIK and CIK cells, and DC-CIK were higher
than CIK cells (P<0.01). In all groups, the effector cells that
were combined with carboplatin showed higher cytotoxicity than
effector cells alone (P<0.01).
Discussion
In recent years, there has been growing interest in
cancer immunotherapy. After reducing tumor burden, immunotherapy
can be used as an adjunctive treatment that can effectively remove
the residual tumor cells. Our study focused on the two principal
players: the T-lymphocytes and the DCs. DCs are the most powerful
antigen-presenting cells, with a capacity of antigen-presentation
that is hundreds of times more efficient than B lymphocytes and
macrophages (29). They can
activate resting T-cells and induce the generation of
antigen-specific cytotoxic T-lymphocytes, which are the initiators
of the immune response (30). On
the other hand, the CIK cells are a population of heterogeneous
T-lymphocytes generated by the in vitro differentiation of
mononuclear cells from the peripheral blood (31). CIK cells cultured with multiple
cytokines including IL-2, CD3 monoclonal antibody and IFN-γ and
those that express the CD3+CD56+ have been
shown to have a strong antitumor effect (19,32,33).
Among their advantages over other T-lymphocytes generated in
vitro from peripheral blood such as LAK/NK cells, CIK cells are
easy to generate in large numbers, readily expandable from cancer
patients (34), with minimal
graft-versus host reaction (35)
and the cytotoxic activity is not restricted to MHC (36,37).
Immunotherapy has been used for treating a variety of adult solid
tumors such as liver cancer, gastric carcinoma, melanoma, and renal
carcinoma with good efficacy (32,38–41).
For pediatric solid tumors, some studies have shown that CIK
treatment have a good antitumor activity in vitro on
lymphoma, osteosarcoma, Ewing’s family tumors and neuroblastoma
(42,43), but only few studies have been
reported on the treatment of children because of a lower incidence
of pediatric tumors and due to ethical issues.
One practical strategy for improving the
effectiveness of immunotherapy relies on the fact that enhanced
cytotoxicity is gained when CIK cells are co-culture with DCs
(44–46) and this effect is even stronger when
CIK are co-cultured with tumor antigen pulsed DCs (20,47).
Combining DCs pulsed with Ag (which exhibit unique
antigen-presenting function) with CIK cells (which have strong
antitumor activity) would probably cause a double antitumor effect.
In pediatric oncology a few tumor-associated antigens have been
identified (48). This problem is
solved in part by the use of the whole-tumor lysed as a source of
tumor-antigens for DC-loading. This would induce a stronger immune
response than that obtained by pulsing DCs with a single or perhaps
several defined tumor peptides (49). In addition, with this approach,
there is no need for revealing the nature of the tumor-associated
antigen and its epitope. Therefore, for our study we used a
retinoblastoma (RB) cell line (RB-Y79 cells) lysates as tumor
antigen to load DCs.
To develop an effective cancer therapy there are at
least three important aspects we have to achieve: one is obtaining
enough effector cell numbers, two, producing effector cells with a
high cytotoxic activity against tumor cells and three, generating
effector cells with specific cytotoxicity for the target cell.
In PBMCs the normal proportion of
CD3+CD56+ cells is low (1–5%). We have been
able to obtain up to 40% of CD3+CD56+ cells
under certain experimental conditions (data not shown). This very
high proportion will make it difficult to discern what might be
modest effects of the Ag or of co-culture with DC cells. To prevent
this, we used a cytokine-enriched media to achieve a basal level of
maturation that allowed us to detect slight differences of DC-Ag on
CIK differentiation. In fact, the CIK proportion after 15 days in
culture increased by ~300% of CD3+CD56+
proportion in the presence of the DC-Ag cells.
In our study, the DC-Ag-CIK cells were substantially
more cytotoxic against RB cells than DC-CIK and CIK cells alone.
This increment in the CIK cytotoxic activity when co-cultured with
DC pulsed with the Ag is probably due to both an increment of the
proportion of T-lymphocyte expressing
CD3+CD56+ plus a larger number of cells
actively differentiating and proliferating. The increment in cell
number could be explained by the fact that the secreted IL-6 is
higher in the co-cultured DC-Ag-CIK inducing cells to proliferate.
IL-6 is a pleiotropic cytokine secreted by DCs, T-lymphocytes and
macrophages, which has been shown to support the growth of T- and
B-lymphocytes in vitro(27).
Another possible explanation could be that IL-6 triggers IL-2
secretion which in turn can promote autocrine CIK proliferation
(50). Accordingly, a decrease in
the level of secreted IL-10 could promote proliferation. It has
been reported that IL-10 downregulates CD80 and CD86 expression at
the DC surface therefore having an inhibitory effect on
alloreactive T-cell responses (28). On the other hand, IL-10 has been
involved in decreased secretion of IL-12 and IFN-γ which are the
cytokines reported to promote lymphocyte proliferation (28). Our results are consistent with IL-6
and IL-10 playing a role in the observed proliferation of CIK
cells. However, the extent to which this may account for the net
effects seen needs to be addressed.
Regardless whether the effector cells were
co-cultured with DCs or Ag pulsed DCs or even CIK alone, there was
no significant cytotoxicity on normal retina cells. This fact can
not be explained by the presence of the Ag or the DC function
itself since the CIK themselves failed to kill the normal retina
cells. This provides preliminary evidence supporting the safety of
this approach in the clinical practice for RB. It is also important
to highlight that the enhanced cytotoxicity, the high proliferation
ratio, the increment in differentiation of the
CD3+CD56+ cells and the specificity of the
cytotoxicity on tumor cells are not due to components or cytokines
added to the culture media. Furthermore, those improve features of
CIKs are rather the direct result of the DCs and the DC-Ag cells on
the CIK maturation and function. Lin et al(51) showed in vitro that the
addition of IL-6 in the culture medium could increase
CD3+CD56+ T-lymphocytes and therefore
enhances proliferation and cytotoxicity of effector cells. In light
of this report, it would be useful to examine whether the CIK-Ag-DC
cells in this study are comparable in cytotoxic activity against RB
cells to the CIK-IL-6 cells described by Lin et al(51).
Finally, we present substantial evidence of
effective cytotoxicity of the combination of CIK with DC-Ag on
tumor RB cells. Albeit our CIK active proportion is relatively low
under our experimental conditions, once the DC cells are in the
blood stream they could still activate the naïve T-lymphocytes. In
this event, administration of the complex CIK-Ag-DC can have
advantages over other immunotherapies. In fact, it has been
reported that injected DC-CIK in the body exert their action for
≤40 days. The efficacy of this therapy has yet to be demonstrated
in animal paradigms. This study demonstrates for the first time
that an immunotherapy-based approach using CIK cells could be
effective to treat RB, mainly because it has selective effect on
carboplatin-resistant retinoblastoma cells. Thus highly efficient
immunotherapy based on DC-Ag-CIK cells is a potentially effective
and safe means of treating RB especially for patients where
traditional chemical therapy has failed.
Acknowledgements
We are grateful to Dr Cristian Acosta and Dr Yue Sun
for insightful comments on the manuscript. We thank Poten
Biomedical Co. (Shenzhen, China) for providing technical support
and Guillaume Charras for Live-ActGFP plasmid. This study was
supported by both Shenzhen Development and Reform Commission
[grants no. (2011)1680] and the Capital Clinical Features Applied
Research Plan (grants no. Z121107001012055). P.K., J.L, H.W, X.C
and T.L. are supported in part by Poten Biomedical Institute for
Cancer Immunotherapy.
References
|
1
|
Dimaras H, Kimani K, Dimba EA, et al:
Retinoblastoma. Lancet. 379:1436–1446. 2012. View Article : Google Scholar
|
|
2
|
Bai S, Ren R, Li B, et al: Delay in the
diagnosis of retinoblastoma in China. Acta Ophthalmol. 89:e72–74.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
MacCarthy A, Draper GJ, Steliarova-Foucher
E and Kingston JE: Retinoblastoma incidence and survival in
European children (1978–1997). Report from the Automated Childhood
Cancer Information System project. Eur J Cancer. 42:2092–2102.
2006.
|
|
4
|
Gallie BL, Zhao J, Vandezande K, White A
and Chan HS: Global issues and opportunities for optimized
retinoblastoma care. Pediatr Blood Cancer. 49:1083–1090. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Houston SK, Murray TG, Wolfe SQ and
Fernandes CE: Current update on retinoblastoma. Int Ophthalmol
Clin. 51:77–91. 2011. View Article : Google Scholar
|
|
6
|
Radhakrishnan V, Kashyap S, Pushker N, et
al: Outcome, pathologic findings and compliance in orbital
retinoblastoma (International Retinoblastoma Staging System stage
III) treated with neoadjuvant chemotherapy: a prospective study.
Ophthalmology. 119:1470–1477. 2012. View Article : Google Scholar
|
|
7
|
Pica A, Moeckli R, Balmer A, et al:
Preliminary experience in treatment of papillary and macular
retinoblastoma: evaluation of local control and local complications
after treatment with linear accelerator-based stereotactic
radiotherapy with micromultileaf collimator as second-line or
salvage treatment after chemotherapy. Int J Radiat Oncol Biol Phys.
81:1380–1386. 2011.
|
|
8
|
Wilson MW, Fraga CH, Rodriguez-Galindo C,
Hagedorn N, Leggas ML and Stewart C: Expression of the multi-drug
resistance proteins and the pregnane X receptor in treated and
untreated retinoblastoma. Curr Eye Res. 34:386–394. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rutella S, Iudicone P, Bonanno G, et al:
Adoptive immunotherapy with cytokine-induced killer cells generated
with a new good manufacturing practice-grade protocol. Cytotherapy.
14:841–850. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Campoli M, Ferris R, Ferrone S and Wang X:
Immunotherapy of malignant disease with tumor antigen-specific
monoclonal antibodies. Clin Cancer Res. 16:11–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leffers N, Daemen T, Helfrich W, et al:
Antigen-specific active immunotherapy for ovarian cancer. Cochrane
Database Syst Rev. CD0072872010.
|
|
12
|
Palucka K, Ueno H, Fay J and Banchereau J:
Dendritic cells and immunity against cancer. J Intern Med.
269:64–73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cella M, Sallusto F and Lanzavecchia A:
Origin, maturation and antigen presenting function of dendritic
cells. Curr Opin Immunol. 9:10–16. 1997. View Article : Google Scholar
|
|
14
|
de Vleeschouwer S, Rapp M, Sorg RV, et al:
Dendritic cell vaccination in patients with malignant gliomas:
current status and future directions. Neurosurgery. 59:988–999.
2006.PubMed/NCBI
|
|
15
|
Schnurr M, Galambos P, Scholz C, et al:
Tumor cell lysate-pulsed human dendritic cells induce a T-cell
response against pancreatic carcinoma cells: an in vitro model for
the assessment of tumor vaccines. Cancer Res. 61:6445–6450.
2001.
|
|
16
|
Wu YG, Wu GZ, Wang L, Zhang YY, Li Z and
Li DC: Tumor cell lysate-pulsed dendritic cells induce a T cell
response against colon cancer in vitro and in vivo. Med Oncol.
27:736–742. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mesiano G, Todorovic M, Gammaitoni L, et
al: Cytokine-induced killer (CIK) cells as feasible and effective
adoptive immunotherapy for the treatment of solid tumors. Expert
Opin Biol Ther. 12:673–684. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi L, Zhou Q, Wu J, et al: Efficacy of
adjuvant immunotherapy with cytokine-induced killer cells in
patients with locally advanced gastric cancer. Cancer Immunol
Immunother. 61:2251–2259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Zhu L, Wei J, et al: The effects
of cytokine-induced killer cells for the treatment of patients with
solid tumors: a clinical retrospective study. J Cancer Res Clin
Oncol. 138:1057–1062. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhan HL, Gao X, Pu XY, et al: A randomized
controlled trial of postoperative tumor lysate-pulsed dendritic
cells and cytokine-induced killer cells immunotherapy in patients
with localized and locally advanced renal cell carcinoma. Chin Med
J (Engl). 125:3771–3777. 2012.
|
|
21
|
Yang L, Ren B, Li H, et al: Enhanced
antitumor effects of DC-activated CIKs to chemotherapy treatment in
a single cohort of advanced non-small-cell lung cancer patients.
Cancer Immunol Immunother. Jun 29–2012.(Epub ahead of print).
|
|
22
|
Zhong R, Teng J, Han B and Zhong H:
Dendritic cells combining with cytokine-induced killer cells
synergize chemotherapy in patients with late-stage non-small cell
lung cancer. Cancer Immunol Immunother. 60:1497–1502. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Komada Y, Zhang SL, Zhou YW, et al:
Cellular immunosuppression in children with acute lymphoblastic
leukemia: effect of consolidation chemotherapy. Cancer Immunol
Immunother. 35:271–276. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mackall CL: T-cell immunodeficiency
following cytotoxic antineoplastic therapy: a review. Stem Cells.
18:10–18. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmidt-Wolf IG, Lefterova P, Mehta BA, et
al: Phenotypic characterization and identification of effector
cells involved in tumor cell recognition of cytokine-induced killer
cells. Exp Hematol. 21:1673–1679. 1993.PubMed/NCBI
|
|
26
|
Pletinckx K, Stijlemans B, Pavlovic V, et
al: Similar inflammatory DC maturation signatures induced by TNF or
Trypanosoma brucei antigens instruct default Th2-cell responses.
Eur J Immunol. 41:3479–3494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Puri RK and Leland P: Systemic
administration of recombinant interleukin-6 in mice induces
proliferation of lymphoid cells in vivo. Lymphokine Cytokine Res.
11:133–139. 1992.PubMed/NCBI
|
|
28
|
Buelens C, Willems F, Delvaux A, et al:
Interleukin-10 differentially regulates B7-1 (CD80) and B7-2 (CD86)
expression on human peripheral blood dendritic cells. Eur J
Immunol. 25:2668–2672. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kulkarni AB, Mullbacher A and Blanden RV:
Functional analysis of macrophages, B cells and splenic dendritic
cells as antigen-presenting cells in West Nile virus-specific
murine T lymphocyte proliferation. Immunol Cell Biol. 69:71–80.
1991. View Article : Google Scholar
|
|
30
|
Hasel T, Yoshimura R, Wada S and Chargui
J: Dendritic cells, generated in vitro, are immunocompetent and
very useful in the induction of specific cytotoxic T lymphocyte
activity. Transplant Proc. 33:3814–3815. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu PH and Negrin RS: A novel population of
expanded human CD3+CD56+ cells derived from T
cells with potent in vivo antitumor activity in mice with severe
combined immunodeficiency. J Immunol. 153:1687–1696.
1994.PubMed/NCBI
|
|
32
|
Wang FS, Liu MX, Zhang B, et al: Antitumor
activities of human autologous cytokine-induced killer (CIK) cells
against hepatocellular carcinoma cells in vitro and in vivo. World
J Gastroenterol. 8:464–468. 2002.PubMed/NCBI
|
|
33
|
Ma Y, Zhang Z, Tang L, et al:
Cytokine-induced killer cells in the treatment of patients with
solid carcinomas: a systematic review and pooled analysis.
Cytotherapy. 14:483–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alvarnas JC, Linn YC, Hope EG and Negrin
RS: Expansion of cytotoxic CD3+ CD56+ cells
from peripheral blood progenitor cells of patients undergoing
autologous hematopoietic cell transplantation. Biol Blood Marrow
Transplant. 7:216–222. 2001.PubMed/NCBI
|
|
35
|
Baker J, Verneris MR, Ito M, Shizuru JA
and Negrin RS: Expansion of cytolytic CD8(+) natural killer T cells
with limited capacity for graft-versus-host disease induction due
to interferon gamma production. Blood. 97:2923–2931. 2001.
|
|
36
|
Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume
KG and Weissman IL: Use of a SCID mouse/human lymphoma model to
evaluate cytokine-induced killer cells with potent antitumor cell
activity. J Exp Med. 174:139–149. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leemhuis T, Wells S, Scheffold C, Edinger
M and Negrin RS: A phase I trial of autologous cytokine-induced
killer cells for the treatment of relapsed Hodgkin disease and
non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 11:181–187.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang YS, Yuan FJ, Jia GF, et al: CIK
cells from patients with HCC possess strong cytotoxicity to
multidrug-resistant cell line Bel-7402/R. World J Gastroenterol.
11:3339–3345. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang JT, Shen YP, Wu CP, et al:
Increasing the frequency of CIK cells adoptive immunotherapy may
decrease risk of death in gastric cancer patients. World J
Gastroenterol. 16:6155–6162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen J, Huang X, Huang G, Chen Y, Chen L
and Song H: Preconditioning chemotherapy with cisplatin enhances
the antitumor activity of cytokine-induced killer cells in a murine
melanoma model. Cancer Biother Radiopharm. 27:210–220. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Su X, Zhang L, Jin L, et al: Immunotherapy
with cytokine-induced killer cells in metastatic renal cell
carcinoma. Cancer Biother Radiopharm. 25:465–470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hongeng S, Petvises S, Worapongpaiboon S,
Rerkamnuaychoke B, Pakakasama S and Jootar S: Generation of
CD3+ CD56+ cytokine-induced killer cells and
their in vitro cytotoxicity against pediatric cancer cells. Int J
Hematol. 77:175–179. 2003.
|
|
43
|
Verneris MR, Arshi A, Edinger M, et al:
Low levels of Her2/neu expressed by Ewing’s family tumor cell lines
can redirect cytokine-induced killer cells. Clin Cancer Res.
11:4561–4570. 2005.PubMed/NCBI
|
|
44
|
Wang QJ, Wang H, Pan K, et al: Comparative
study on anti-tumor immune response of autologous cytokine-induced
killer (CIK) cells, dendritic cells-CIK (DC-CIK) and
semi-allogeneic DC-CIK. Chin J Cancer. 29:641–648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shi SB, Ma TH, Li CH and Tang XY: Effect
of maintenance therapy with dendritic cells: cytokine-induced
killer cells in patients with advanced non-small cell lung cancer.
Tumori. 98:314–319. 2012.PubMed/NCBI
|
|
46
|
Yang XJ, Huang JA, Lei W, Zhu YB and Zhang
XG: Antitumor effects of cocultured dendritic cells and
cytokine-induced killer cells on lung cancer in vitro and in vivo.
Ai Zheng. 25:1329–1333. 2006.PubMed/NCBI
|
|
47
|
Tan G, Zhang X, Feng H, Luo H and Wang Z:
The therapeutic effect of cytokine-induced killer cells on
pancreatic cancer enhanced by dendritic cells pulsed with K-ras
mutant peptide. Clin Dev Immunol. 2011:6493592011.PubMed/NCBI
|
|
48
|
Coughlin CM, Vance BA, Grupp SA and
Vonderheide RH: RNA-transfected CD40-activated B cells induce
functional T-cell responses against viral and tumor antigen
targets: implications for pediatric immunotherapy. Blood.
103:2046–2054. 2004. View Article : Google Scholar
|
|
49
|
Yufeng D, Guocheng Z, Dongliang X, et al:
Whole-tumor-antigen-pulsed dendritic cells elicit cytotoxic T-cell
response against pediatric nasopharyngeal carcinoma in vitro. Med
Oncol. 26:78–85. 2009. View Article : Google Scholar
|
|
50
|
Holsti MA and Raulet DH: IL-6 and IL-1
synergize to stimulate IL-2 production and proliferation of
peripheral T cells. J Immunol. 143:2514–2519. 1989.PubMed/NCBI
|
|
51
|
Lin G, Wang J, Lao X, et al: Interleukin-6
inhibits regulatory T cells and improves the proliferation and
cytotoxic activity of cytokine-induced killer cells. J Immunother.
35:337–343. 2012. View Article : Google Scholar : PubMed/NCBI
|