Introduction
Childhood and adolescent acute myeloid leukemia
(AML) is one of the most challenging types of childhood cancer to
successfully treat (1). The relapse rate remains
unacceptably high, with a 5-year event-free survival (EFS) of
approximately 50% (2). In addition,
successful treatment can only be achieved using highly intensive
chemotherapy that results in relatively high rates of
treatment-related mortality and significant side-effects (3). Although extensive efforts are being
made to eliminate these issues, an efficient and effective method
of treating AML has yet to be developed. Novel therapeutic
strategies are urgently required to improve the prognosis of this
disease.
The cancer stem cells (CSCs) theory postulates the
origin of cancer from a new perspective. CSCs are a small subset of
cancer cells that possess stem cell-like properties, such as, the
ability to self-renew via asymmetric division and to produce
differentiated progeny. These cells generally remain in a quiescent
state (4) and comprise a small
minority of the total tumor population. They have an extensive
capacity to proliferate, differentiate, and self-renew, enabling
them to repopulate recipients after transplantation (5). This small population of cells within a
cancer is responsible for drug resistance and the recurrence of
cancer (6). Hence, the specific
targeting of CSCs therapeutically must be explored. To date, the
possible existence of CSCs has been shown in leukemia (7,8) and in
certain solid tumors (9,10). CSCs have also been identified in
immortalized cell lines (5),
long-term cultured cancer cells (10,11),
and patient tumor samples (5),
using the side population (SP) technique.
Extensive research has focused on leukemia stem
cells (LSCs) in the pursuit of new ideas for targeted therapy.
Therefore, sorting, identifying and enriching LSCs has become
particularly important in leukemia treatment research. Side
population (SP) cells, as defined by Hoechst 33342 exclusion in
flow cytometry, represent only a small fraction of the whole cell
population (12); their properties
occupy an important position in several investigations (13,14).
Previous studies have shown that CSCs can be identified by an SP
phenotype based on fluorescence-activated flow cytometry. SP cells
have been found not only in patient tumor samples but also in
immortalized cell lines and long-term cultured cancer cells
(15,16); they have demonstrated the capacity
to function as stem cells in the tissues from which they were
isolated and may be able to transdifferentiate (17). SP cells share the most relevant
features of LSCs, i.e., the self-renewal potential and quiescent
status, and they contain relatively high concentrations of tumor
stem cell indicators (18).
Concurrent studies have shown that SP cells in human cancer have
various origins, including acute myelogenous leukemia,
neuroblastoma, and glioma (10,11,19).
These studies have also suggested that SP cells may be a source of
cancer stem cells (CSCs). SP cells can be sorted using flow
cytometry, which is a suitable application for LSC sorting
(20).
The human acute monocytic leukemia cell line THP-1,
which was originally established from an infant diagnosed with AML
(21), provides an experimental
model for functional, preclinical therapeutics and target
identification studies of AML. In this study, we identified cancer
SP cells by isolating them in the THP-1 cell line. In SP and NSP
cells, we evaluated the cell cycle, the capacity for self-renewal,
the presence of leukocyte surface antigens, and the expression of
the multidrug resistance gene and the apoptosis gene. The aim of
this study was to enrich the LSC subpopulation in the THP-1 cell
line with arabinosylcytosine (Ara-C) and to study the relationship
between SP cells and LSCs.
Materials and methods
Cell line and culture
The human acute monocytic leukemia cell line THP-1
(Shanghai Institute of Cell Biology) was cultured in RPMI-1640
medium (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine
serum (FBS) (Hyclone), 100 U/ml penicillin-streptomycin (Invitrogen
Life Technologies, Grand Island, NY, USA) at 37°C under a 5%
CO2 atmosphere.
SP cell analysis using fluorescence
microscopy
The SP cells were suspended at 1×106
cells/ml in pre-warmed RPMI-1640 medium containing 2% FBS, 100 U/ml
penicillin-streptomycin G, 100 μg/ml streptomycin and 10 mmol/l
HEPES buffer. These cells were then incubated at 37°C for 90 min
with 5 μg/ml Hoechst 33342 (Sigma-Aldrich, St. Louis, MO, USA),
protected from light, either alone or in the presence of 50 μmol/l
verapamil (Sigma-Aldrich). The cells were placed immediately on ice
and were then washed and resuspended in cold phosphate-buffered
saline (PBS) containing 1% FBS. Fluorescence microscopy (Leica,
Germany) was employed to detect the morphology of SP cells among
the THP-1 cells. The cells that were not stained or colored light
blue were identified as SP cells.
SP cell analysis and sorting using flow
cytometry
Following incubation with Hoechst 33342 at 4°C, 1
μg/ml propidium iodide (PI) (BD Pharmingen, San Diego, CA, USA) was
added to label the dead cells, and the mixture was then filtered
through a 40-μm cell strainer (BD Falcon) to obtain a single-cell
suspension. Cell analyses and purification were performed using
MoFlo carrying a triple-laser (DakoCytomation, Fort Collins, CO,
USA). Hoechst 33342 was excited with the UV laser at 350 nm, and
fluorescence emission was measured with 405/BP30 (Hoechst blue) and
570/BP20 (Hoechst red) optical filters. PI labeling was measured
through the 630/BP30 filter for the discrimination of dead cells.
SP and NSP cells in each well were isolated (22).
Cell surface immunophenotyping
Immunophenotyping was conducted using conjugated
monoclonal human antibodies reactive to CD34 and CD38 (BD
Pharmingen). The staining was performed in the dark at 4°C for 30
min. Isotype control antibodies and live unstained cells were used
to establish gating parameters for positive cells. The percentage
of the positive cells was obtained via the CellQuest software (BD
Pharmingen).
Cell cycle analysis
SP and NSP cells were harvested and then washed
twice with PBS. The supernatant was discarded and the pellets were
dissolved with 1 ml of 70% cold ethanol. After incubating at 4°C
for at least 12 h, cells were stained with 50 g/ml PI supplemented
with 50 g/ml RNase and then incubated in the dark at 21°C for 30
min. The samples were profiled for DNA content by flow cytometry
(BD Biosciences), and 10,000 events were recorded for each sample.
The percentages of cells in the G0/G1, S and
G2/M phases were obtained by CellQuest software.
Cell proliferation assay
The cells were grown in a 96-well plate, and the
relative cell number was determined using a Cell Counting Kit-8
(Dojindo, Kumamoto, Japan) according to the manufacturer’s
protocol. The SP and NSP cells were plated at a density of
1×104 cells/well in 96-well plates for 0–9 days. After
10 μl CCK-8 solution was added to each well, cells were incubated
for a further 4 h at 37°C, and the absorbance was measured at 450
nm using an automated ELISA reader (BioTek, Winooski, VT, USA).
Validation of gene expression by
qPCR
Quantitative-PCR (qPCR) analysis was performed
according to the manufacturer’s protocol. First, total RNA was
extracted from cells with an RNAprep pure Cell kit (Tiangen Biotech
Co., Ltd., Beijing, China). One microgram of total RNA was reverse
transcribed into cDNA with l μl M-MuLV RT (200 μg/μl) using a
Single-Strand cDNA Synthesis Kit (Stratagene, La Jolla, CA, USA)
and analyzed using an ABI 7900 (Applied Biosystems, Foster City,
CA, USA). Specific primers for qPCR of GAPDH (housekeeping gene)
and the additional genes of interest were designed using
Assay-by-Design primer design software (Applied Biosystems) or were
purchased as Assays-on-Demand from Applied Biosystems. The primers
for these genes are shown in Table
I. The relative amounts of product were calculated using the
comparative CT (2−ΔΔCt) method.
 | Table IPrimer sequences for different
genes. |
Table I
Primer sequences for different
genes.
| Gene | Primer sequences
(5′-3′) |
|---|
| GAPDH | F:
ACCACAGTCCATGCCATCAC |
| R:
TCCACCACCCTGTTGCTGTA |
| ABCG2 | F:
GTCTAAGCAGGGACGAACAATC |
| R:
GCCAATAAGGTGAGGCTATCAA |
| ABCB1 | F:
TGGTGTTTGGAGAAATGACAGAT |
| R:
GAAACCTGAATGTAAGCAGCAAC |
| Bcl-2 | F:
GTGGATGACTGAATACCTGAACC |
| R:
AGACAGCCAGGAGAAATCAAAC |
| Bax | F:
GGTTGTCGCCCTTTTCTACTT |
| R:
GTGAGGAGGCTTGAGGAGTCT |
In vivo tumor formation
All animal studies were performed in compliance with
the Guidelines for the Care and Use of the Laboratory Animals in
Henan Province, China. Naïve male 6–8-week-old NOD/SCID mice were
obtained from Beijing HFK Bioscience Co. (Beijing, China), kept
under specific pathogen-free (SPF) conditions and used as tumor
transplant recipients. Mice were housed five per cage. Thirty mice
were randomly divided into six groups, five mice per group. Growing
cells, sorted from the SP cells (1×103, 1×104
and 1×105 per mouse) and NSP cells (1×104,
1×105 and 1×106 per mouse) diluted in PBS,
were mixed with 50 ml Matrigel (BD Biosciences) and injected
intravenously via the tail vein. Three to four weeks later, human
AML engraftment (hCD45+/CD33+ cells) was
assessed in the peripheral blood and bone marrow by tail bleed and
aspiration of the femur, respectively.
Enrichment of LSCs in an SP of THP-1 with
Ara-C
THP-1 cells (1×108 cells/ml) were
incubated for 24 h at 37°C under a 5% CO2 atmosphere
with four different concentrations of Ara-C: 10, 100, 1,000 and
2,000 μg/ml. The cells were then harvested and washed twice with
PBS. The proportion of SP cells was detected, respectively, for
each Ara-C concentration.
Statistical analysis
Statistical analyses were performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). Data are presented as the means ±
SD. Differences were determined using the Student’s t-test or
Fisher’s exact test and one-way analysis of variance (ANOVA)
followed by Scheffe’s post hoc test, as indicated in the
text. P<0.05 was considered to indicate a statistically
significant difference. All experiments were performed in
triplicate.
Results
Prevalence of SP cells in THP-1
cells
The SP cells overexpressed the multidrug-resistant
proteins that allowed them to efflux various drugs and xenobiotics,
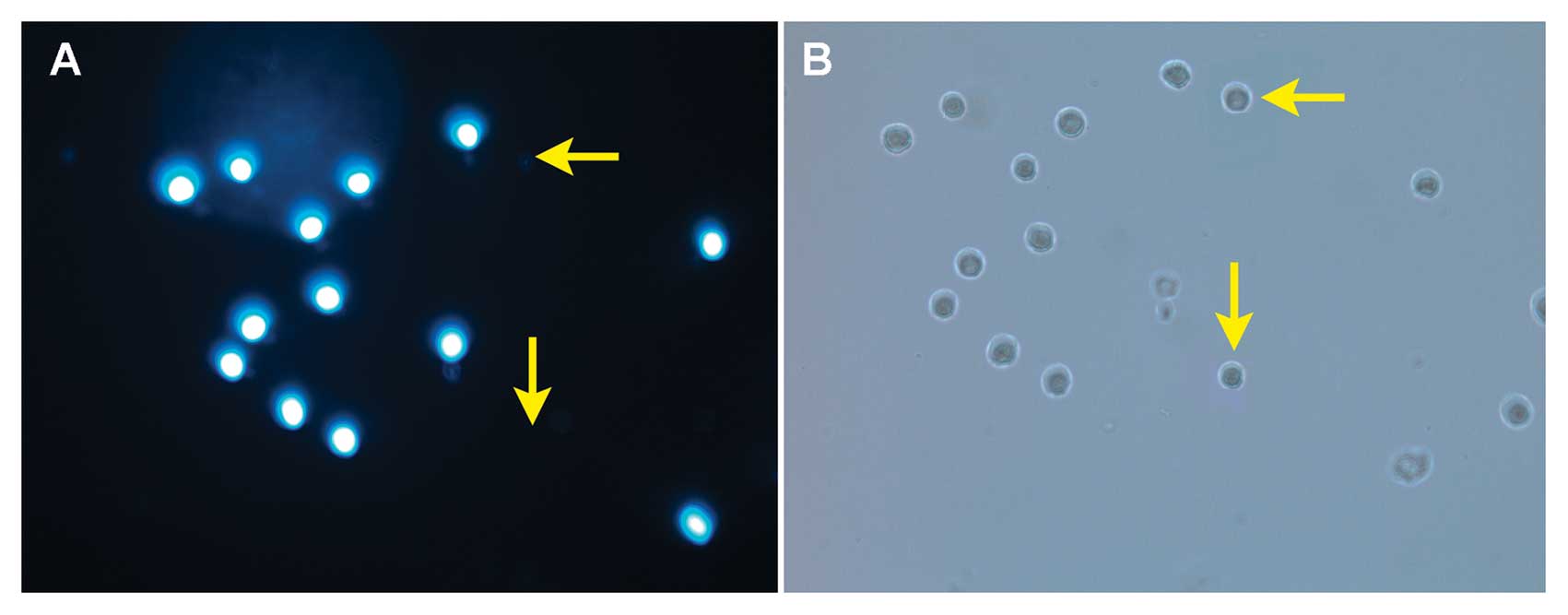
as well as the Hoechst 33342 dye (23). As shown in Fig. 1, fluorescence microscopy with
Hoechst 33342 staining demonstrated that the SP cells were present
among the THP-1 cells. Whereas >90% of the THP-1 cells were
stained intense blue, a small population of the cells remained
unstained (Fig. 1A, arrow). The
micrographs of THP-1 SP and NSP cells were observed under visible
light. There was no significant difference between SP and NSP cells
in morphology (Fig. 1B, arrow).
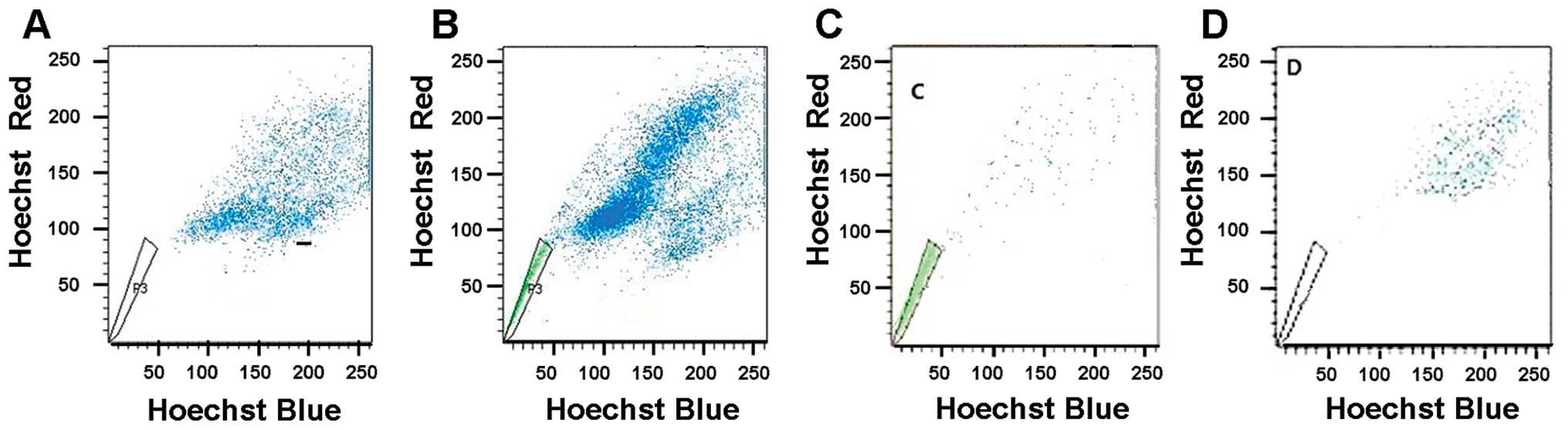
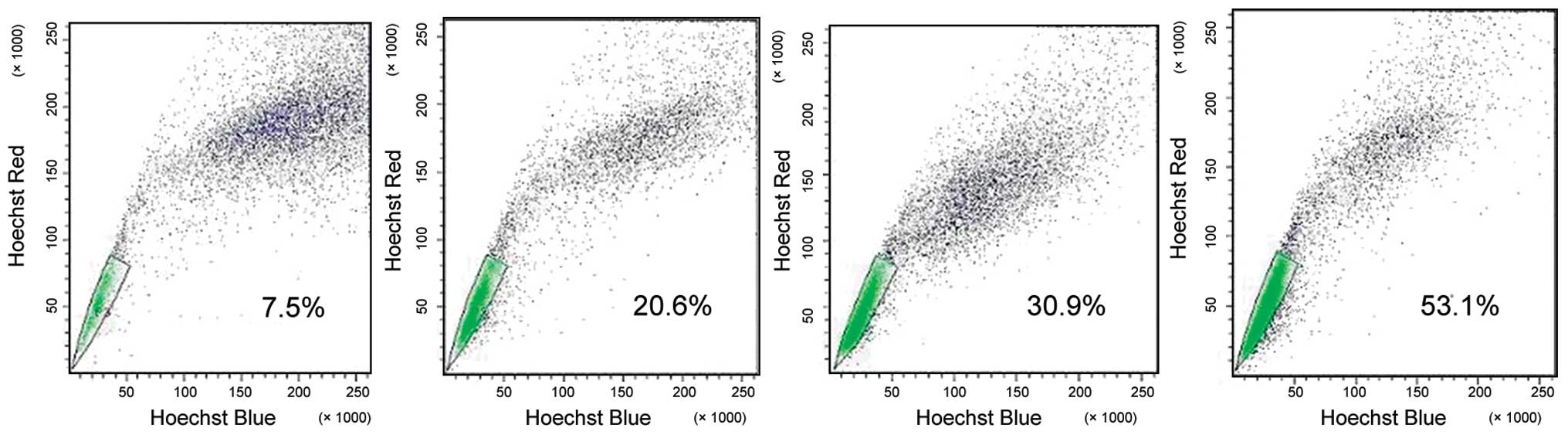
Flow cytometry analysis with Hoechst 33342 staining
demonstrated that the dimly stained Hoechst 33342 cells on the
corner of the plot were gated as the SP population and represented
a percentage of 1.81±0.99% of total THP-1 cells (Fig. 2A). Since the SP profile was blocked
by staining in the presence of verapamil, a calcium channel
blocker, the SP cell frequency was practically eliminated in the
THP-1 cells stained with Hoechst 33342 and verapamil (Fig. 2B). To determine the sensitivity of
our system, the SP and NSP cells in THP-1 cells were sorted and the
purity was tested separately. The results showed that the cells of
SP tube were still concentrated in the THP-1 SP region and the
cells of NSP tube were still concentrated in the subregion of the
main group of cells prior to sorting. Purity levels were
96.75±1.55% and 97.03±1.87%, respectively (Fig. 2C and D).
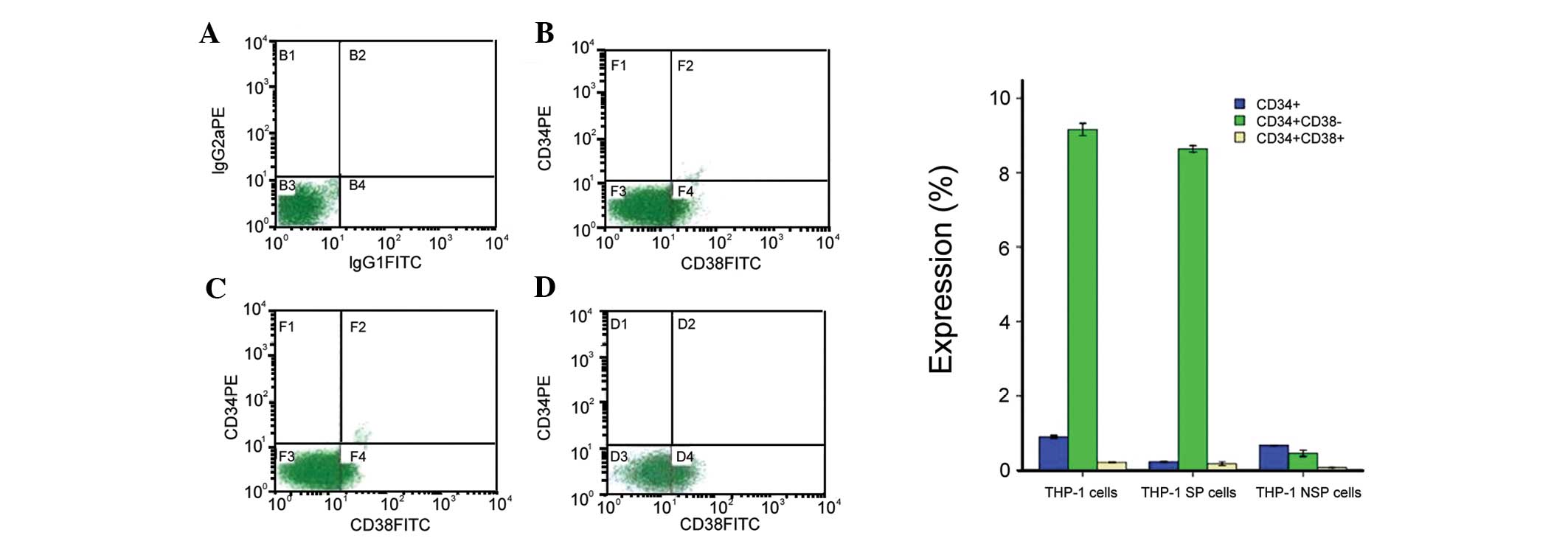
Expression of cell surface markers in SP
and NSP cells
To further define the cells within the SP fraction,
we used two cell-surface markers (CD34 and CD38) associated with
LSCs (24). The analysis of SP and
NSP cells revealed that these cells differ significantly in the
expression of cell surface markers. The overall percentage of cells
positive for CD34 was significantly lower in the NSP compared with
the SP cells in all cell lines examined (Fig. 3).
Although the expression of
CD34+/CD38− in the SP fraction (Fig. 3C) was small, it was statistically
significant and substantially higher than that in unsorted THP-1
cells (Fig. 3B) and NSP cells
(Fig. 3D). The percentage of
CD34+ and CD38− expression in SP cells was
8.68±0.20%, markedly higher than that in NSP cells (0.16±0.08%)
(P<0.05) (Fig. 3).
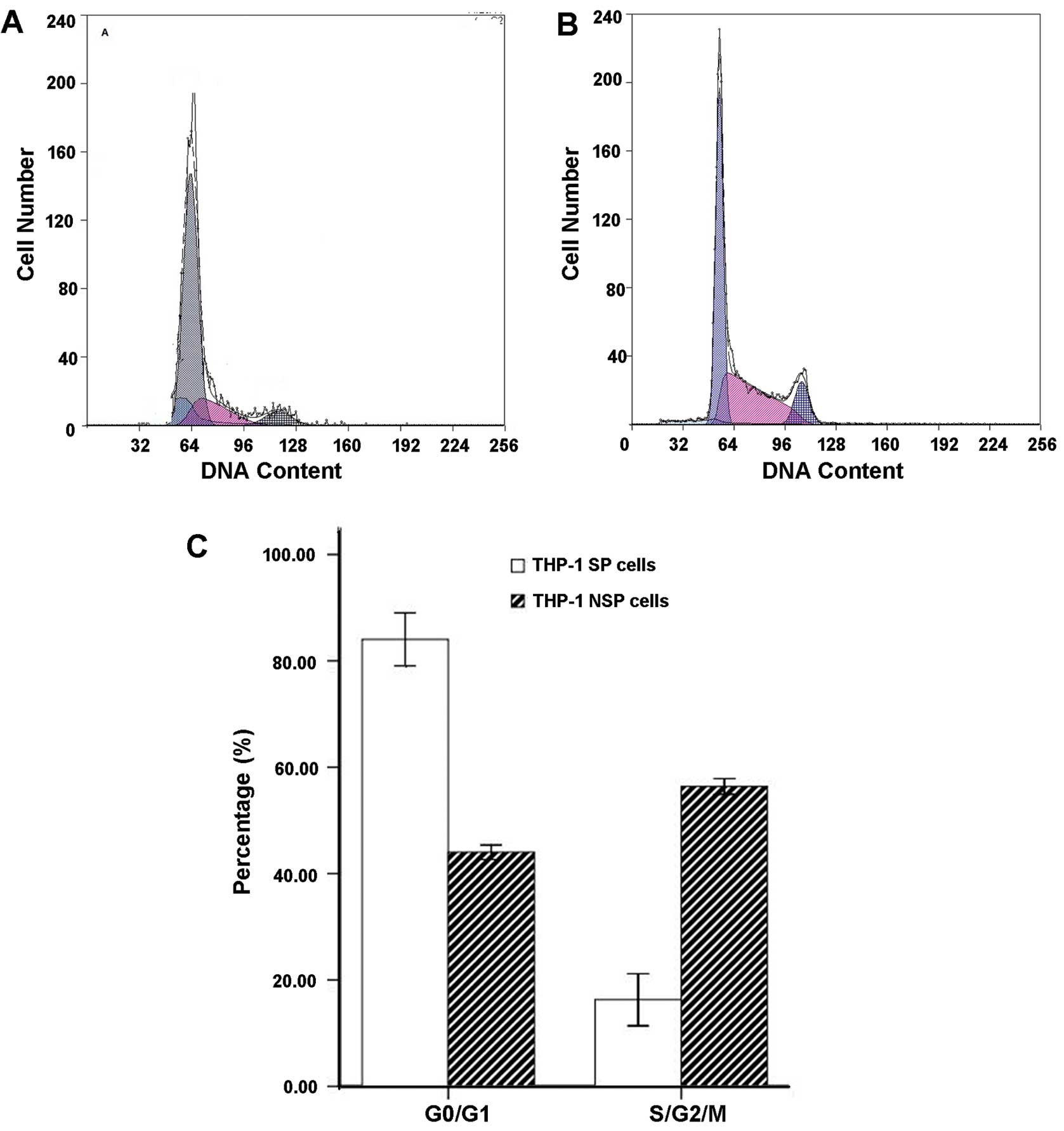
Cell cycle in THP-1 SP and NSP cells
The G0/G1 phase cells in the
THP-1 SP subpopulation accounted for ~84.04±4.98% of the total
cells and more than NSP cells (44.02±1.35%).
THP-1 SP cells resulted in blockage of the cell
cycle from the G0/G1 phase to the S phase.
Compared with NSP cells, the ratio of G0/G1
phase cells significantly increased, and the ratio of S-phase cells
significantly decreased in SP cells (P<0.05) (Fig. 4).
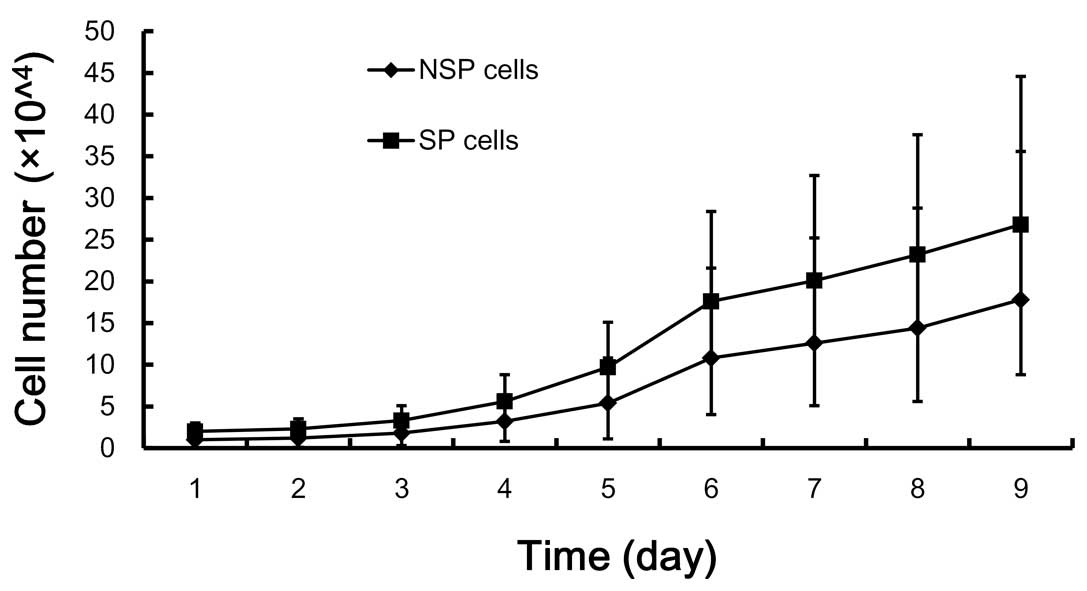
In vivo growth characteristics of SP and
NSP cells
The growth characteristics of the SP subpopulation
were consistent with the predicted behavior of primitive precursor
cells, including a high proliferative rate and self-renewal
capacity. The proliferation of SP cells was significantly higher
than that of NSP cells (P<0.05) (Fig. 5).
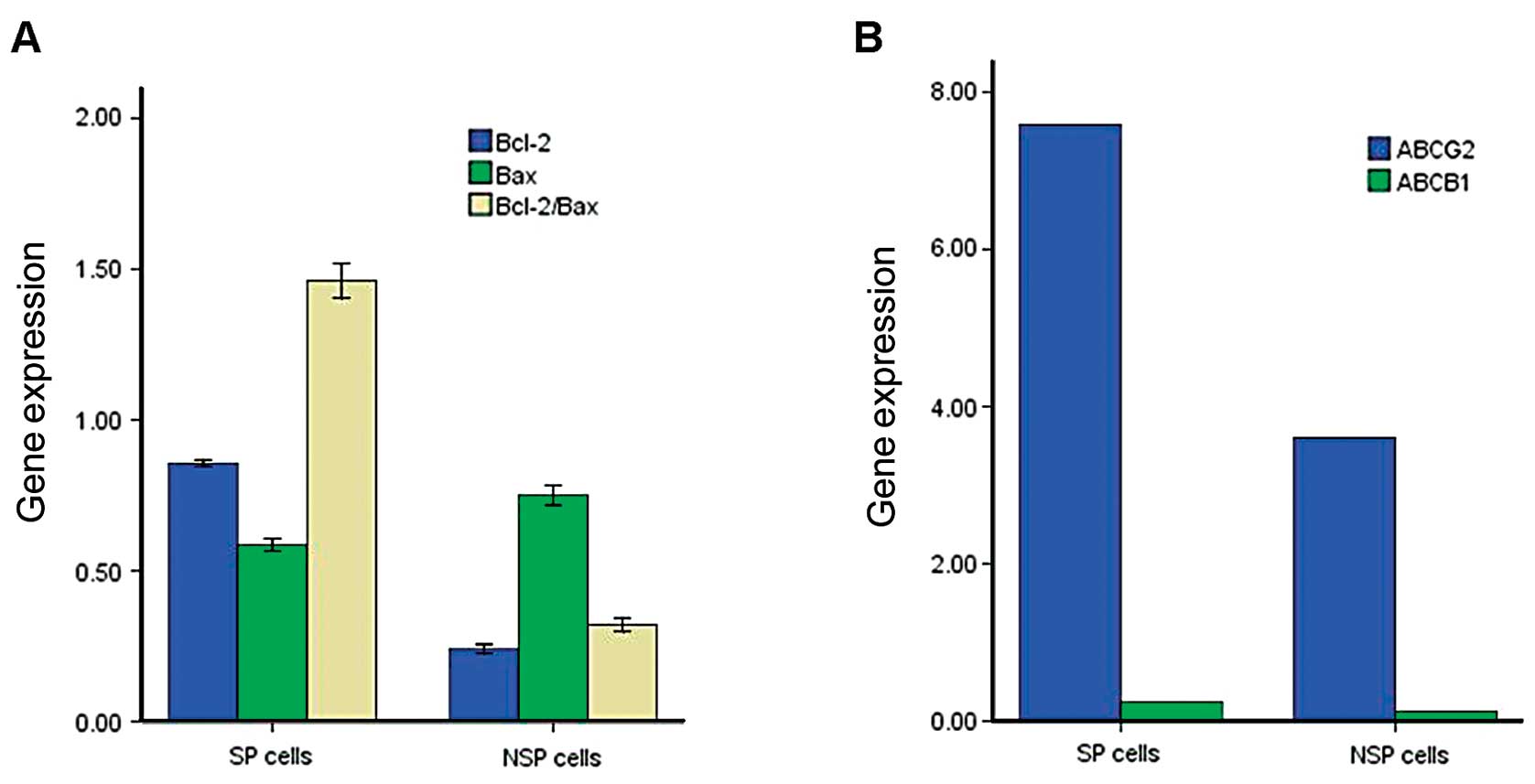
Analysis of gene expression in SP and NSP
cells
We isolated RNA from sorted SP and NSP cells of the
leukemia cell line THP-1 and used quantitative real-time RT-PCR
amplification analysis to quantify the relative expression of the
ABC transporter gene, the ABCB1 and ABCG2 gene
product currently believed to be most closely associated with the
SP phenotype (25). All SP
fractions expressed higher levels of the ABCB1 and
ABCG2 transporter gene than did the NSP fractions (Fig. 6A). We also measured the expression
levels of two other cell apoptosis genes, Bcl-2 and
Bax; the former gene was clearly expressed at higher
concentrations in SP compared with the NSP, whereas the latter gene
was not. However, the Bcl-2 and Bax values were
significantly higher than in the NSP (P<0.05) (Fig. 6B).
THP-1 SP cells exhibit higher
tumorigenicity than NSP cells
To determine whether the SP cells we identified in
the THP-1 cell line might also be more tumorigenic, we performed
xenograft experiments in vivo. Three or 4 weeks after THP-1
SP cells were transplanted intravenously via the tail vein, human
AML engraftment (hCD45+/CD33+) was assessed
in the peripheral blood and bone marrow by tail bleed and
aspiration of the femur, respectively. The results indicated that
the systemic disseminated leukemia model had been established
successfully by injecting 1×103 THP-1 SP cells in
NOD/SCID mice (Fig. 6A). The SP
cells isolated from the THP-1 cells were more tumorigenic than the
NSP cells. NSP cells require a quantity of at least
1×106 to establish a tumor model. Statistically, there
were significant differences in the incidence of leukemia among
different groups (P<0.05). The H&E strain of histologic
sections revealed that almost all the leukemia xenotransplants
successfully induced leukemia in the mice (Fig. 7B and C).
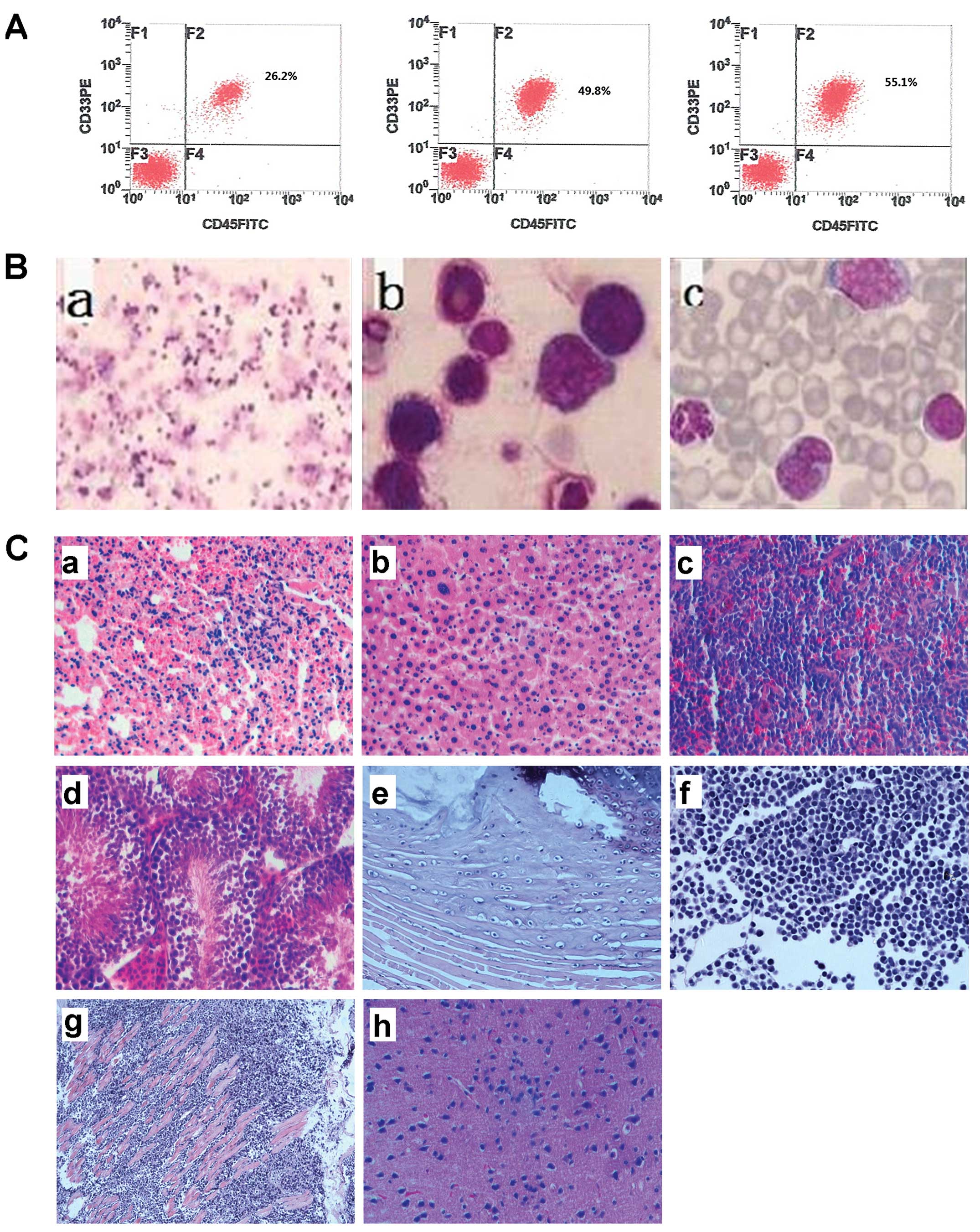 | Figure 7Identification of human THP-1
xenotransplant leukemia models. (A) Human AML engraftment
(hCD45+CD33+ cells) was assessed in the
peripheral blood and bone marrow using flow cytometry. (B) Wright’s
staining of bone marrow of NOD/SCID mice injected with SP cells.
(a) magnification, ×100, (b) magnification, ×1,000 and (c)
peripheral blood (magnification, ×1,000). (C) Pathological
examination of NOD/SCID mice injected with SP cells. Representative
H&E staining sections of organs and tissues (H&E
magnification, ×200). AML cells can be found in (a) lung, (b)
liver, (c) spleen, (d) didymus, (e) paravertebral muscles, (f)
lymph node, and (g) musculi faciales. (h) Cerebral edema was
observed. |
SP increases in the THP-1 cell line
It has been reported that CSCs can resist apoptosis
when they are exposed to apoptosis inducement factors (26). Considering this characteristic of
CSCs, we formulated a hypothesis that apoptosis resistance of CSCs
can be used to increase the proportion of SP cells. In an apoptosis
inducement model, the proportion of SP cells may increase in
surviving cells. In this study, SP cells were co-cultured with
different concentrations of Ara-C, the proportion of SP cells
increased significantly, and the proportion of SP cells increased
with the Ara-C concentration (Fig.
8).
Discussion
Using the side population (SP) technique, Zheng
et al(27) reported the SP
rate in acute promyelocytic leukemia NB4 cells to be less than 1%.
In addition, SP cells possess intrinsic stem cell properties and
express some of the characteristic stem cell genes. In the present
study, analysis by fluorescence microscopy and flow cytometry
demonstrated the presence of SP cells, and we were able to identify
a small SP component (1.81±0.99%) of cancer cells from the THP-1
leukemia cell line. The SP cells were practically non-existent in
the presence of Hoechst 33342 and verapamil, a calcium channel
blocker. The percentages of SP cells detected were similar to those
in most previous reports: 0.8–1.9% in human multiple myeloma cell
line RPMI-8226 and NCI-H929 (28),
0.47–4% in an adult T-cell leukemia/lymphoma cell line (29), and 0.5–29.9% in human acute myeloid
leukemia (AML) (8), but less than
the 4–37% noted in neuroblastoma cell lines (15). Furthermore, the SP cells and the
majority of non-SP (NSP) cells were indistinguishable
morphologically. Therefore, the isolation and identification of
cancer stem cells (CSCs) remains very difficult (8). To the best of our knowledge, this is
the first described isolation of cancer stem-like cells from the
THP-1 cell line.
To date, AML leukemia stem cells (LSCs) are the most
well-studied CSCs population (30).
AML is typically a disease of stem progenitor cell origin. No
special markers have been successfully developed to identify those
cells in different tumors; different tumors have different CSCs
markers (one or more). Blair et al(31) demonstrated that only a small
quantity of a defined subset of cells were consistently clonogenic
and that all AML LSC subtypes possess the same cell-surface markers
(32). As determined by
immunophenotyping, SP cells share some phenotypic characteristics
with bone marrow, such as the absence of mature hematopoietic
lineage markers CD34 and expression of CD38. Bonnet et al
have identified LSCs in human AML as a common immunophenotype
(CD34+/CD38−) and have demonstrated their
self-renewal potential (1). We
therefore examined the expression of CD34/CD38 in SP cells, NSP
cells, and unsorted THP-1 cells. Our results showed that CD34/CD38
were both expressed at low levels on the membranes of SP and NSP
cells. The percentages of CD34+ and
CD34+/CD38− cells in the SP were higher than
those in the NSP and among common THP-1 cells (P<0.05). These
results suggest that the presence of LSCs in THP-1 cells is rare
and that the number of LSCs is quite limited; however, LSCs may be
more prevalent in the SP.
LSCs, similar to their normal HSC counterparts,
exhibit a range of characteristics that enable their long-term
survival. Some of these characteristics also facilitate their
escape from the cytotoxic effects of chemotherapy; for example,
LSCs are primarily present in a quiescent phase of the cell cycle
(33). In the present study, the
cell cycles of two cell subsets, SP and NSP of the THP-1 cell line,
were analyzed by flow cytometry. A majority of the SP cells
remained in the G0/G1 phase, and the NSP
cells remained in the S or G2 or M phases. The
proliferation of SP cells was significantly higher than that of NSP
cells (P<0.05).
A study by Hope et al(34) showed that AML originates from a
hierarchy of LSC classes that differ in self-renewal capacity.
Clarke et al(35) reported
that SP cells produced two to seven times more colonies than NSP
cells. In support of their putative stem cell nature, only the SP
cells possessed the ability to produce colonies with both
myoepithelial and luminal epithelial cell types. In the present
study, the cell growth curves showed that the growth rate of SP
cells was significantly faster than that of NSP cells; the cells
continued to proliferate and the plateau period was not evident.
This observation may indicate that SP cells contain more LSCs, and,
therefore, cell proliferation is accelerated.
SPs are small subpopulations of cells with enriched
stem cell activity that show a distinct ‘low’ Hoechst 33342
dye-staining pattern. The SP phenotype is mediated by the
ATP-binding cassette (ABC) family of transporter proteins. Various
types of ABC transporters have been shown to contribute to drug
resistance in numerous types of cancer by pumping drugs out of
cells (25,36). Notably, some ABC transporters are
expressed by several types of stem cells. One of the major
mediators seems to be ABCG2 or BCRP (37), which was initially identified in
drug-selected MCF7 breast cancer cells and was later found to
efflux multiple chemotherapeutic drugs and xenobiotics (38). Other supporting evidence shows that
SP cells preferentially express ABCG2 (13,39).
SP cells also express other ABC transporters such as MDR-1 (i.e.,
ABCB1 or P-glycoprotein), suggesting that these latter molecules
may also be involved in mediating the SP phenotype (40). We therefore isolated RNA from sorted
SP and NSP cells of the THP-1 leukemia cell lines, and we used a
real-time RT-PCR assay to quantify the relative expression of the
ABCG2 and ABCB1 transporters. The mRNA expression levels of the two
genes of SP cells were higher than those among NSP cells.
Independent of whether THP-1SP cells are indeed a tumor ‘stem cell’
population, their high expression of drug efflux transporter genes
and their associated high capacity to efflux lipophilic drugs may
have a significant effect on treatment outcome (5).
Stem cell resistance to apoptosis through
complicated mechanisms (41,42),
such as the regulation of Bcl-2 or Bax (43,44),
has been proven experimentally. Compared with SP cells, NSP cells
in tumors are more susceptible to apoptosis inducement or
chemotherapy. We also measured the expression levels of two other
apoptosis regulation genes, Bcl-2 and Bax; the former
gene was clearly expressed at higher concentrations in SP cells
compared with NSP cells, whereas the latter gene was not. There was
no difference between SP and NSP cells in Bax expression,
but the Bcl-2/Bax values of the SP cells were significantly
higher than those of the NSP cells. These results suggest that
apoptosis resistance may aid in screening the markers of CSCs.
It is believed that only the CSCs, but not the
majority of their remaining descendants, are responsible for
tumorigenesis, progression, metastasis, and relapse following
treatment (45). Repopulation in
recipients after transplantation is the most important
characteristic of CSCs. Purified SP cells from certain cell lines
were more tumorigenic than the corresponding NSP cells. To evaluate
the tumorigenic ability of THP-1 SP cells in vivo, NOD/SCID
mice were injected with different subpopulations and different
quantities of THP-1 cells intravenously by tail vein, and the
incidence of leukemia was compared among the groups. As we
anticipated, the systemic disseminated leukemia model was
established successfully by injecting 1×103 THP-1 SP
cells. Unsorted THP-1 cells require a quantity of at least
1×106 to establish a tumor model.
Isolation and identification of the SP cells is
helpful in studying the difference between the CSCs and
non-tumorigenic cells in certain aspects. However, the proportion
of LSCs is quite limited, and the isolation and identification of
CSCs is very difficult. CSCs have been reported to resist apoptosis
when they are exposed to apoptosis inducement factors (26). This characteristic of CSCs led us to
formulate a hypothesis that apoptosis resistance by CSCs can be
used to increase the proportion of SP cells. In an apoptosis
inducement model, the proportion of SP cells may increase in
surviving cells. Cytarabine (Ara-C) is commonly used for the
treatment of acute leukemia. Incorporation of Ara-C into DNA is a
key event in the killing of proliferating leukemic cells, but it is
relatively ineffective against LSCs, which retain a quiescent
status (46). In this study, we
used Ara-C to kill common proliferating THP-1 cells. Following
co-culture with Ara-C, the proportion of SP cells increased
significantly and with Ara-C concentration.
In our study, all the representative LSC markers
were significantly increased in SP cells compared with NSP cells;
therefore, our results suggest that isolated SP cells could
characterize the properties of LSCs. The proportion of SP cells may
be increased after they are co-cultured with Ara-C, and this
technique can be applied to the study of LSCs.
References
|
1
|
Woods WG: Curing childhood acute myeloid
leukemia (AML) at the half-way point: promises to keep and miles to
go before we sleep. Pediatr Blood Cancer. 46:565–569. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gorman MF, Ji L, Ko RH, et al: Outcome for
children treated for relapsed or refractory acute myelogenous
leukemia (rAML): a Therapeutic Advances in Childhood Leukemia
(TACL) Consortium study. Pediatr Blood Cancer. 55:421–429. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaspers GJ and Zwaan CM: Pediatric acute
myeloid leukemia: towards high-quality cure of all patients.
Haematologica. 92:1519–1532. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benchaouir R, Rameau P, Decraene C, et al:
Evidence for a resident subset of cells with SP phenotype in the
C2C12 myogenic line: a tool to explore muscle stem cell biology.
Exp Cell Res. 294:254–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schatton T, Murphy GF, Frank NY, et al:
Identification of cells initiating human melanomas. Nature.
451:345–349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lapidot T, Sirard C, Vormoor J, et al: A
cell initiating human acute myeloid leukaemia after transplantation
into SCID mice. Nature. 367:645–648. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kondo T, Setoguchi T and Taga T:
Persistence of a small subpopulation of cancer stem-like cells in
the C6 glioma cell line. Proc Natl Acad Sci USA. 101:781–786. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hirschmann-Jax C, Foster AE, Wulf GG, et
al: A distinct ‘side population’ of cells with high drug efflux
capacity in human tumor cells. Proc Natl Acad Sci USA.
101:14228–14233. 2004.
|
|
12
|
Goodell MA, Brose K, Paradis G, Conner AS
and Mulligan RC: Isolation and functional properties of murine
hematopoietic stem cells that are replicating in vivo. J Exp Med.
183:1797–1806. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shimano K, Satake M, Okaya A, et al:
Hepatic oval cells have the side population phenotype defined by
expression of ATP-binding cassette transporter ABCG2/BCRP1. Am J
Pathol. 163:3–9. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Falciatori I, Borsellino G, Haliassos N,
et al: Identification and enrichment of spermatogonial stem cells
displaying side-population phenotype in immature mouse testis.
FASEB J. 18:376–378. 2004.PubMed/NCBI
|
|
15
|
Hu C, Li H, Li J, et al: Analysis of ABCG2
expression and side population identifies intrinsic drug efflux in
the HCC cell line MHCC-97L and its modulation by Akt signaling.
Carcinogenesis. 29:2289–2297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Guo LP, Chen LZ, Zeng YX and Lu
SH: Identification of cancer stem cell-like side population cells
in human nasopharyngeal carcinoma cell line. Cancer Res.
67:3716–3724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haraguchi N, Utsunomiya T, Inoue H, et al:
Characterization of a side population of cancer cells from human
gastrointestinal system. Stem Cells. 24:506–513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Setoguchi T, Taga T and Kondo T: Cancer
stem cells persist in many cancer cell lines. Cell Cycle.
3:414–415. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feuring-Buske M and Hogge DE: Hoechst
33342 efflux identifies a subpopulation of cytogenetically normal
CD34(+)CD38(−) progenitor cells from patients with acute myeloid
leukemia. Blood. 97:3882–3889. 2001.PubMed/NCBI
|
|
20
|
Guo Y, Follo M, Geiger K, Lubbert M and
Engelhardt M: Side-population cells from different precursor
compartments. J Hematother Stem Cell Res. 12:71–82. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsuchiya S, Yamabe M, Yamaguchi Y,
Kobayashi Y, Konno T and Tada K: Establishment and characterization
of a human acute monocytic leukemia cell line (THP-1). Int J
Cancer. 26:171–176. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao Q, Geng L, Kvalheim G, Gaudernack G
and Suo Z: Identification of cancer stem-like side population cells
in ovarian cancer cell line OVCAR-3. Ultrastruct Pathol.
33:175–181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishikawa T and Nakagawa H: Human ABC
transporter ABCG2 in cancer chemotherapy and pharmacogenomics. J
Exp Ther Oncol. 8:5–24. 2009.PubMed/NCBI
|
|
24
|
Rombouts WJ, Martens AC and Ploemacher RE:
Identification of variables determining the engraftment potential
of human acute myeloid leukemia in the immunodeficient NOD/SCID
human chimera model. Leukemia. 14:889–897. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jonker JW, Freeman J, Bolscher E, et al:
Contribution of the ABC transporters Bcrp1 and Mdr1a/1b to the side
population phenotype in mammary gland and bone marrow of mice. Stem
Cells. 23:1059–1065. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Raguz S and Yague E: Resistance to
chemotherapy: new treatments and novel insights into an old
problem. Br J Cancer. 99:387–391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng X, Seshire A, Ruster B, et al:
Arsenic but not all-trans retinoic acid overcomes the aberrant stem
cell capacity of PML/RARalpha-positive leukemic stem cells.
Haematologica. 92:323–331. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsui W, Wang Q, Barber JP, et al:
Clonogenic multiple myeloma progenitors, stem cell properties, and
drug resistance. Cancer Res. 68:190–197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kayo H, Yamazaki H, Nishida H, Dang NH and
Morimoto C: Stem cell properties and the side population cells as a
target for interferon-α in adult T-cell leukemia/lymphoma. Biochem
Biophys Res Commun. 364:808–814. 2007.PubMed/NCBI
|
|
30
|
Wang JC and Dick JE: Cancer stem cells:
lessons from leukemia. Trends Cell Biol. 15:494–501. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Blair A, Hogge DE, Ailles LE, Lansdorp PM
and Sutherland HJ: Lack of expression of Thy-1 (CD90) on acute
myeloid leukemia cells with long-term proliferative ability in
vitro and in vivo. Blood. 89:3104–3112. 1997.PubMed/NCBI
|
|
32
|
Blair A and Sutherland HJ: Primitive acute
myeloid leukemia cells with long-term proliferative ability in
vitro and in vivo lack surface expression of c-kit (CD117). Exp
Hematol. 28:660–671. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Naka K, Hoshii T and Hirao A: Novel
therapeutic approach to eradicate tyrosine kinase inhibitor
resistant chronic myeloid leukemia stem cells. Cancer Sci.
101:1577–1581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hope KJ, Jin L and Dick JE: Acute myeloid
leukemia originates from a hierarchy of leukemic stem cell classes
that differ in self-renewal capacity. Nat Immunol. 5:738–743. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Clarke RB: Isolation and characterization
of human mammary stem cells. Cell Prolif. 38:375–386. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang XB: Molecular mechanism of
ATP-dependent solute transport by multidrug resistance-associated
protein 1. Methods Mol Biol. 596:223–249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou S, Schuetz JD, Bunting KD, et al: The
ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem
cells and is a molecular determinant of the side-population
phenotype. Nat Med. 7:1028–1034. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Doyle LA and Ross DD: Multidrug resistance
mediated by the breast cancer resistance protein BCRP (ABCG2).
Oncogene. 22:7340–7358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Summer R, Kotton DN, Sun X, Ma B,
Fitzsimmons K and Fine A: Side population cells and Bcrp1
expression in lung. Am J Physiol Lung Cell Mol Physiol.
285:L97–L104. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bunting KD, Zhou S, Lu T and Sorrentino
BP: Enforced P-glycoprotein pump function in murine bone marrow
cells results in expansion of side population stem cells in vitro
and repopulating cells in vivo. Blood. 96:902–909. 2000.PubMed/NCBI
|
|
41
|
Taipale J and Beachy PA: The Hedgehog and
Wnt signalling pathways in cancer. Nature. 411:349–354. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
van Stijn A, van der Pol MA, Kok A, et al:
Differences between the CD34+ and CD34− blast
compartments in apoptosis resistance in acute myeloid leukemia.
Haematologica. 88:497–508. 2003.
|
|
43
|
Chipuk JE, Moldoveanu T, Llambi F, Parsons
MJ and Green DR: The BCL-2 family reunion. Mol Cell. 37:299–310.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang QL, Niu Q, Niu PY, et al: Bax gene
silencing: a potential intervention in aluminum-induced neural cell
death. J Biol Regul Homeost Agents. 24:7–17. 2010.PubMed/NCBI
|
|
45
|
Komuro H, Saihara R, Shinya M, et al:
Identification of side population cells (stem-like cell population)
in pediatric solid tumor cell lines. J Pediatr Surg. 42:2040–2045.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Misaghian N, Ligresti G, Steelman LS, et
al: Targeting the leukemic stem cell: the Holy Grail of leukemia
therapy. Leukemia. 23:25–42. 2009. View Article : Google Scholar : PubMed/NCBI
|