Introduction
Lung cancer, which is strongly associated with
tobacco use, is a leading cause of cancer-related mortality
worldwide (1). Despite the progress
of therapeutic modalities, the prognosis of patients with lung
cancer remains poor (2,3).
Over the past decade, epidermal growth factor
receptor (EGFR)-specific tyrosine kinase inhibitors (TKIs), which
are molecular-targeted drugs, have been reported to be effective
for the treatment of non-small cell lung cancer with EGFR mutation
(4,5). This mutation is frequently found in
never smokers and cases of adenocarcinoma (6), whereas it is uncommon in patients with
a smoking history of >13–25 pack-years or in those who have not
stopped smoking cigarettes <10–25 years ago (7–9).
Although smoking is a risk factor for lung cancer,
pulmonary emphysema (10) and
pulmonary obstructive disorders (11) are also independent risk factors.
Patients with interstitial lung disease (ILD), such as combined
pulmonary fibrosis and emphysema (CPFE), which is an emerging
entity that is strongly associated with tobacco use (12), frequently develop lung cancer
(13,14). As shown in a previous study, all
lung cancer patients with ILD were reported to be smokers (15). These reports suggest that the
presence of emphysema and/or ILD may negatively correlate with the
development of EGFR-mutant lung cancer, which is relatively
uncommon in smokers (6). However,
the prevalence of these underlying diseases in smokers with
EGFR-mutant lung cancer remains unclear.
The present study was conducted to examine the
correlation between the EGFR mutation status and the prevalence of
underlying lung disease in smokers with lung cancer.
Patients and methods
Patients
The records for all consecutive patients with
non-small cell or non-squamous cell lung cancer who underwent
surgical resection at the Ibarakihigashi National Hospital
(Ibaraki, Japan) from January, 2007 through December, 2010 were
retrospectively investigated. According to previous studies
(8,9), it was ensured that only those patients
who had a smoking history of ≥15 pack-years were selected from
these records. Patient characteristics such as smoking intensity
and tumor site were also evaluated. This study was approved by the
Institutional Human Ethics Committee of the Ibaraki-higashi
National Hospital.
Radiographic analysis
For each patient, a helical computed tomography (CT)
scanner was utilized (slice thickness, 2.5–10 mm). In the majority
of patients, the slice thickness was ≤5.0 mm (window level, −600;
window width, 1,500). All measurements were independently performed
by a board-certified radiologist and a board-certified
pulmonologist, who were both blinded to the clinical data. CT
images at three levels were evaluated: the top of the aortic arch,
the tracheal carina, and 2 cm above the highest hemidiaphragm. In
each slice, right and left images were assessed separately, and
therefore six images in total were evaluated for each patient. The
severity of emphysematous changes was scored visually according to
the scoring system described by Goddard et al(16). Emphysematous changes were defined as
the areas of low attenuation and vascular disruption (16,17).
Based on the percentage of emphysematous area in the evaluated
lung, each image was classified as normal (score 0), <25%
affected (score 1), <50% affected (score 2), <75% affected
(score 3), or ≥75% affected (score 4). Scores obtained by the two
reviewers were summed for each image. The maximum possible scores
were eight in one image, 16 in one slice, and 48 in the whole
lung.
Interstitial changes were defined as the presence of
ground-grass opacities, consolidation, reticular shadows,
honeycombing and/or traction bronchiectasis or bronchiolectasis, as
described in previous studies (12,15).
If one or more of these findings were observed, the patients were
evaluated as having ILD.
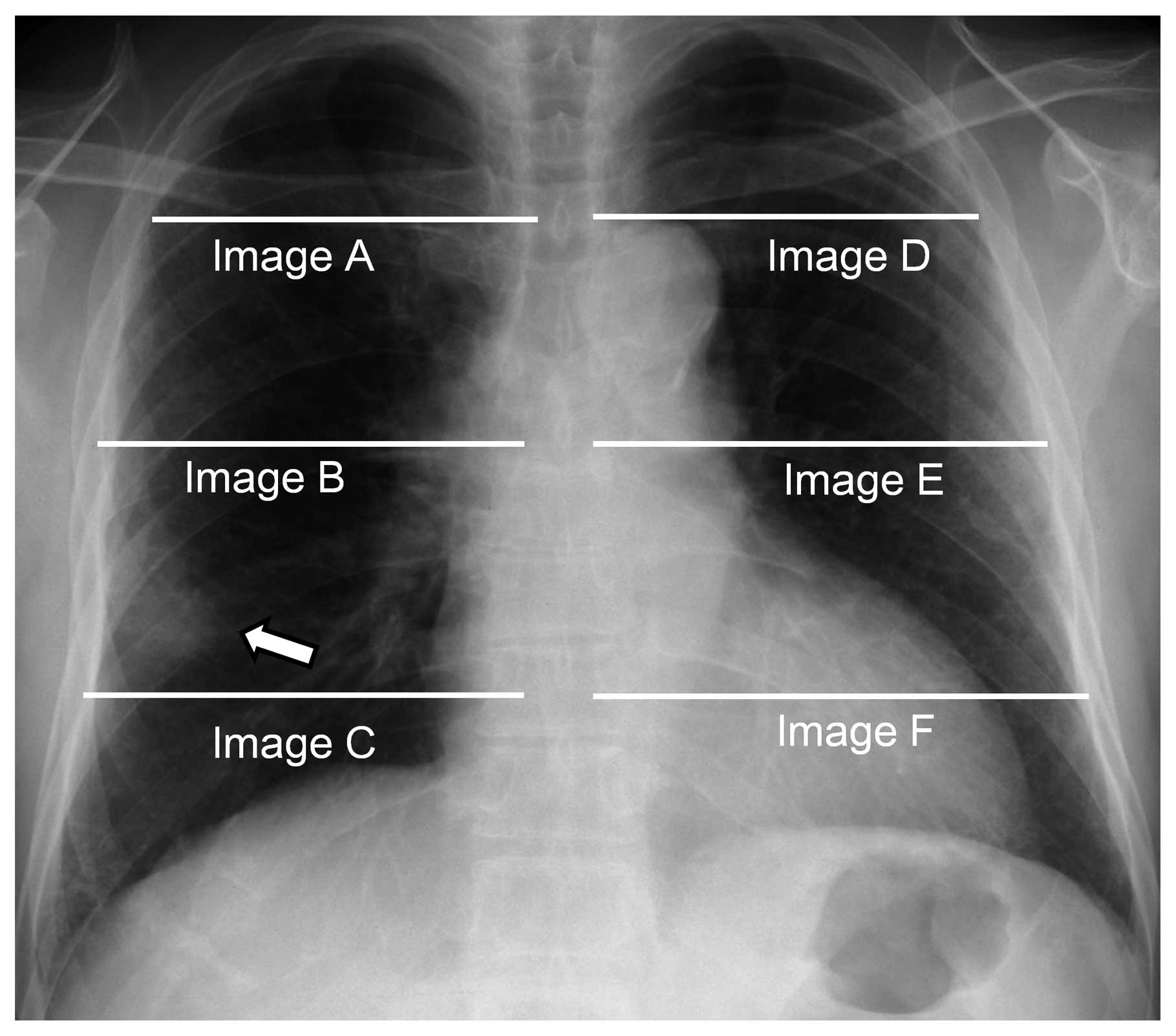
In addition, we focused on the ipsilateral image of
the lung nearest to the tumor among the six images (defined as
‘ipsilateral image’) as shown in Fig.
1, for the purpose of assessing pulmonary status in the area
surrounding the tumor.
Pulmonary function analysis
Pulmonary function testing was performed by trained
technicians according to the criteria of the American Thoracic
Society (18). In all patients, no
bronchodilator was used since pulmonary function was examined for
the purpose of evaluating tolerability to surgical resection. The
diagnosis of chronic obstructive pulmonary disease (COPD) was based
on the examination of forced expiratory volume (FEV) in 1 sec
(FEV1) according to the following formula:
(FEV1)/forced vital capacity (FVC) ≤70%.
Molecular analysis
All patients provided written informed consent for
the comprehensive use of the results of molecular analysis. Genomic
DNA samples were isolated from fresh-frozen or paraffin-embedded
tissues obtained mainly by surgical resection. All clinical samples
were independently examined using the peptide nucleic acid-locked
nucleic acid polymerase chain reaction clamp method for the
detection of EGFR mutations. Testing was performed at the Saitama
Medical University (Saitama) or the Mitsubishi Chemical Medience
Corporation (Tokyo, Japan) (19).
Statistical analysis
Fisher's exact test were used for the comparison of
categorical variables such as gender, pathology, tumor site and
pathological stage. The Mann-Whitney U test was performed for
continuous variables, including age, pulmonary function variables,
and emphysema score. P-values <0.05 were considered to indicate
statistically significant differences. All analyses were performed
using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA,
USA).
Results
Patient characteristics
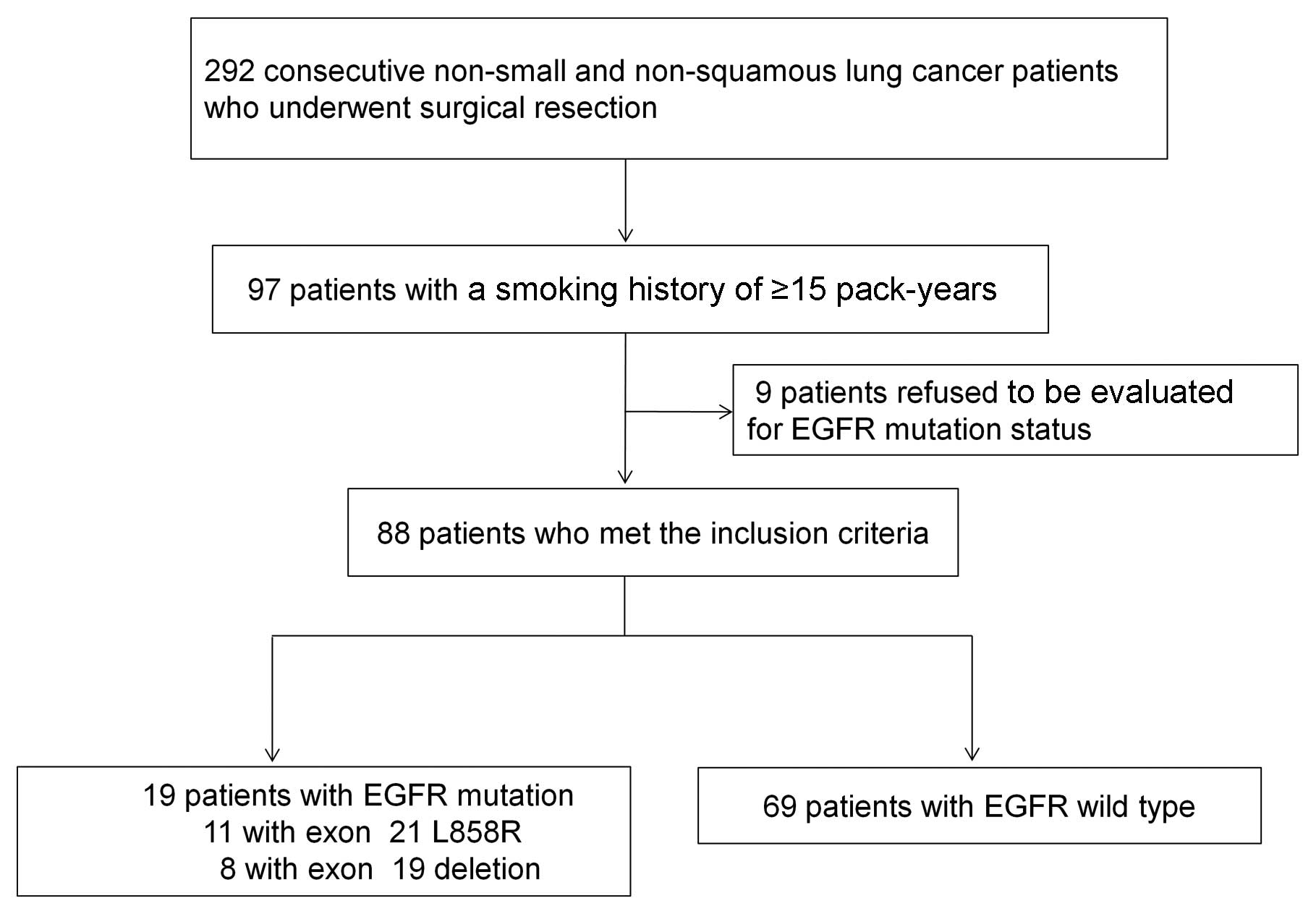
The study profile is shown in Fig. 2. Of the 292 consecutive patients
with non-small cell or non-squamous cell lung cancer who underwent
surgical resection, 97 had a smoking history of ≥15 pack-years. Of
these, nine refused to be examined for EGFR mutation status. Of the
remaining 88 patients who met the inclusion criteria, 19 (21.6%)
were found positive for the EGFR mutation (exon 21 L858R, n=11;
exon 19 deletion, n=8).
The 88 patients were divided into two groups
according to EGFR mutation status: the mutation-positive group (19
patients) and the wild-type group (69 patients). The
characteristics of the patients in these two groups are presented
in Table I. Almost all the patients
in both groups were males and were diagnosed with adenocarcinoma.
The lung tumor sites and smoking status in the mutation-positive
group were similar to those in the wild-type group (P=0.58 and
0.80). Patients in the mutation-positive group tended to have lower
smoking intensity than those in the wild-type group, although this
difference was not significant (P=0.07). In terms of disease stage
and tumor size, the results in the mutation-positive group were
similar to those in the wild-type group (P=1.00 and 0.60).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| EGFR mutation
status | |
|---|
|
| |
|---|
| Characteristics | Positive (n=19) | Wild-type (n=69) | aP-value |
|---|
| Gender |
| Male/female | 19/0 | 63/6 | 0.34 |
| Age | | | |
| Years (median) | 72 (46–87) | 73 (54–81) | 0.97 |
| Pathology |
| Adenocarcinoma | 18 | 62 | |
| Non-small cell
lung cancer (not otherwise specified or combined) | 1 | 7 | 1.00 |
| Primary site (upper
lobe/middle or lingular/lower) | 10/3/6 | 46/6/17 | 0.58 |
| Smoking
history |
|
Current/former | 8/11 | 33/36 | 0.80 |
| Smoking intensity,
duration |
| Pack-years,
median | 38 (15–68) | 40 (15–144) | 0.07 |
| Pathological
stage |
| I and II | 15 | 52 | 1.00 |
| III and IV | 4 | 17 | |
| Tumor size (median,
mm) | 25 (15–65) | 27 (10–100) | 0.60 |
Radiographic analysis
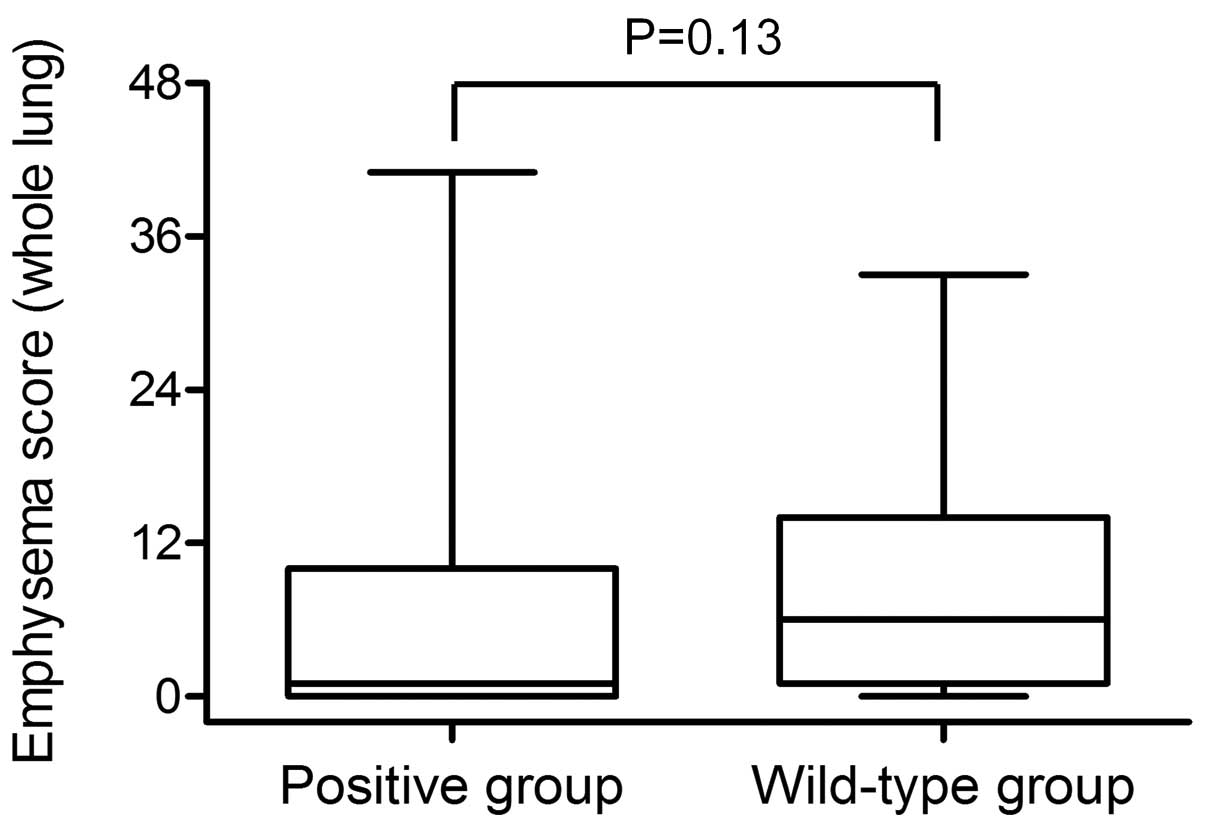
The median emphysema score for the whole lung was
one (range, 0–41) in the mutation-positive group and six (range,
0–33) in the wild-type group (Fig.
3). Emphysema scores tended to be lower in the
mutation-positive group than in the wild-type group (P=0.13).
Interstitial changes were observed in 19 patients
(mutation-positive group, n=1; wild-type group, n=18). Interstitial
changes also tended to be less common in the mutation-positive
group compared to the wild-type group (P=0.06).
When we focused on the ipsilateral image, the
percentage of patients with emphysematous changes was 26.3% in the
mutation-positive group and 58.0% in the wild-type group (Table II). Emphysematous changes in the
ipsilateral image were less common in the mutation-positive group
than in the wild-type group (P=0.02). Interstitial changes in the
ipsilateral image was observed in ten patients. All ten patients
were from the wild-type group (P=0.11). Therefore, the percentage
of patients who did not present with emphysematous nor interstitial
changes in the ipsilateral image was 73.7% in the mutation-positive
group and 36.2% in the wild-type group (P=0.005). The majority of
patients in the mutation-positive group had a radiographically
normal lung in the ipsilateral image.
 | Table IIEGFR mutation status and underlying
lung disease in the ipsilateral image of the lung nearest to the
tumor. |
Table II
EGFR mutation status and underlying
lung disease in the ipsilateral image of the lung nearest to the
tumor.
| EGFR mutation
status | |
|---|
|
| |
|---|
| Underlying lung
disease | Positive 19 cases
(%) | Wild-type 69 cases
(%) | P-value |
|---|
| Emphysematous
changes | | | |
| Present | 5 (26.3) | 40 (58.0) | 0.02a |
| Absent | 14 (73.7) | 29 (42.0) | |
| Emphysema
score | | | |
| 0 | 14 (73.7) | 29 (42.0) | |
| >0 to ≤2 | 1 (5.3) | 22 (31.9) | |
| >2 to ≤4 | 3 (15.8) | 13 (18.8) | |
| >4 to ≤6 | 0 (0.0) | 3 (4.3) | |
| >6 to ≤8 | 1 (5.3) | 2 (2.9) | |
| Interstitial
changes | | | |
| Present | 0 (0.0) | 10 (14.5) | 0.11 |
| Absent | 19 (100.0) | 59 (85.5) | |
| Emphysematous
and/or interstitial changes | | | |
| Present | 5 (26.3) | 44 (63.8) | 0.005a |
| Absent | 14 (73.7) | 25 (36.2) | |
Pulmonary function analysis
Median vital capacity (VC)/predicted VC (% VC) was
106.7% (range, 69.8–140.2%) in the mutation-positive group and
101.8% (67–152.9%) in the wild-type group. The difference between
the groups for this parameter was not significant (P=0.95). In
FEV1% testing, similar results were observed for both
groups (mutation-positive group: 69.9%, range, 39.1–93.7; wild-type
group: 71.5%, range, 44.6–95.0; P=0.94). The percentage of patients
who met the diagnostic criteria of COPD was 52.6% in the
mutation-positive group and 46.3% in the wild-type group. The
difference between the groups regarding this parameter was not
significant (P=0.80). When the analysis was limited to patients
with no interstitial changes, no significant difference was
observed between the two groups in terms of % VC and
FEV1% (P=0.40 and 0.43).
Discussion
In this study, EGFR-mutant lung cancer was commonly
observed in the areas where emphysematous and interstitial chnages
were absent, even in smokers. This finding suggests that
EGFR-mutant lung cancer develops in areas unaffected by smoking,
even in smokers.
EGFR mutation is frequently detected in cases of
adenocarcinoma as well as in non-smokers (4,5,20).
Although the EGFR mutation has been reported to be relatively rare
in non-smokers with environmental exposure to tobacco (21), the prevalence of underlying lung
disease in non-smokers is low compared to that in smokers. In the
present study, we examined smokers with a smoking history of ≥15
pack-years; this history was found to negatively correlate with the
presence of EGFR mutation, and as shown by our results, EGFR-mutant
lung cancer was commonly observed in areas where emphysematous and
interstitial changes were absent. To the best of our knowledge,
only one previous study on the correlation of the EGFR mutation
status with underlying lung diseases, such as emphysema and
pulmonary fibrosis has been published [Usui et al(22)]. In their study, the frequency of
EGFR mutation was reported low in patients with emphysema and
pulmonary fibrosis compared to those without. Although the results
of that study seem consistent with those in the current study,
their results included both never smokers and smokers (never
smokers, 37.4%; smokers, 62.6%). Furthermore, lung cancer was at an
advanced clinical stage in the majority of patients (stage IIIB and
IV, 68.2%) and only the presence of emphysema and/or fibrosis in
the whole lung was roughly evaluated in that study (22). In the present study, the detailed
radiographic evaluation focused on the six images from the whole
lung, and the results revealed that emphysematous changes in the
image nearest to the tumor were less common in the
mutation-positive group. Therefore, EGFR-mutant lung cancer may
develop in areas that appear radiographically normal, even in
smokers.
The precise mechanism accounting for the results of
this study remains unknown. However, it is generally accepted that
the presence of emphysema is related to the development of lung
cancer (10,23–25).
In addition, patients with ILD such as CPFE, which is strongly
associated with tobacco use, have been reported to develop lung
cancer more frequently than those with emphysema alone (12–14).
Recent reports indicate that patients with underlying lung diseases
are susceptible to smoking-related inflammation, which may
ultimately contribute to lung cancer development (13,22,23,26,27).
Although the mechanisms underlying this association have not been
clearly identified, the results of the present study may indicate a
clinical possibility: EGFR-mutant lung cancer may develop in areas
less affected by tobacco use and the mechanism of carcinogenesis in
EGFR-mutant lung cancer in smokers may resemble that in
non-smokers. In the present study, EGFR-mutant lung cancer in
smokers was commonly observed in the areas where emphysematous and
interstitial changes were absent compared to wild-type lung cancer.
Of note, although the degree of emphysematous changes in the whole
lung was not significantly different between the two groups
(P=0.13), the difference became clearer when the assessment of
emphysematous changes focused on the ipsilateral image (P=0.02).
These results suggest that the absence of emphysematous and
interstitial changes in the ipsilateral image, not in the whole
lung, is correlated with the development of EGFR-mutant lung
cancer.
The results of this study may provide two important
implications for clinical practice. First, EGFR-TKIs may be
effective for EGFR-mutant lung cancer even in smokers. Previous
studies have reported no differences in progression-free and
overall survival rates between smokers and non-smokers with
EGFR-mutant lung cancer (22,28–30).
However, detailed information regarding smoking intensity was not
provided in those reports. Lung cancer in smokers has been
associated with more widespread chromosomal abnormalities than in
non-smokers (31). Therefore,
various genetic alterations in patients with lung cancer may be
associated with resistance to EGFR-TKIs. For example, EGFR-mutant
lung cancer other than adenocarcinoma has been reported to exhibit
a poor response to EGFR-TKIs, indicating that multiple factors
other than EGFR signaling may play an important role in
carcinogenesis and tumor growth (32,33).
Considering that EGFR-mutant lung cancer was commonly observed in
the areas where emphysematous and interstitial changes were absent
even in smokers, these tumors may bear a genetic resemblance to
those in non-smokers. Therefore, these tumors, similar to the ones
found in non-smokers, may respond well to EGFR-TKI treatment,
although, the fatal adverse effects of ILD should be carefully
monitored in smokers (34). Second,
smokers without emphysematous and interstitial changes may have a
high possibility of developing EGFR-mutant lung cancer compared to
those with such changes. The present study revealed that EGFR
mutation was found in 5/45 (11.1%) patients with emphysematous
changes, 0/10 (0%) patients with interstitial changes and 14/39
(35.9%) patients without these underlying diseases in the
ipsilateral image (Table III).
Although a number of studies have revealed that the prevalence of
EGFR mutation in smokers ranges from 8.4 to 15.6% (8,28,30),
the prevalence of EGFR mutation in smokers without emphysematous
and interstitial changes seems much higher. Therefore, our results
indicate the importance of evaluating the EGFR mutation status in
patients with no emphysematous nor interstitial changes in the
ipsilateral lung near the tumor, regardless of smoking history.
 | Table IIIPrevalence of EGFR mutation in
patients with underlying lung disease detected in the ipsilateral
image of the lung nearest to the tumor. |
Table III
Prevalence of EGFR mutation in
patients with underlying lung disease detected in the ipsilateral
image of the lung nearest to the tumor.
| Underlying lung
disease | No. of patients
with EGFR mutation | Total no. of
patients | % |
|---|
| Emphysematous
changes | 5 | 45 | 11.1 |
| Interstitial
changes | 0 | 10 | 0 |
| None | 14 | 39 | 35.9 |
The present study had several limitations. First,
the study was carried out at a single institution and was a
retrospective study with a small sample size. In addition, the
slice thickness of the CT images in the majority of patients was
≤5.0 mm; therefore, the results do not reflect the evaluation of
the high-resolution CT images and thus, the prevalence of
underlying lung disease could be underestimated. Finally, the
evaluation of emphysema was conducted visually and
semiquantitatively, not automatically or densitometrically.
However, a systemic review and meta-analysis by Smith et
al(25) reported a significant
association of lung cancer risk with emphysema detected by visual
evaluation rather than by automated methods.
In conclusion, our results revealed that EGFR-mutant
lung cancer was commonly observed in the areas where emphysematous
and interstitial changes were absent, even in smokers. EGFR-mutant
lung cancer definitely exists in smokers and may develop in areas
that appear radiographically normal. It would be important to
evaluate the EGFR mutation status in patients with no emphysematous
or interstitial changes in the ipsilateral lung near the tumor,
regardless of smoking history. The results of this study should be
confirmed in a future prospective study.
Acknowledgements
We thank Ms. Kimiko Sakurai for providing
secretarial assistance.
References
|
1
|
Wynder EL and Graham EA: Landmark article
May 27, 1950: Tobacco Smoking as a possible etiologic factor in
bronchiogenic carcinoma. A study of six hundred and eighty-four
proved cases. By Ernest L. Wynder and Evarts A. Graham. JAMA.
253:2986–2994. 1985. View Article : Google Scholar
|
|
2
|
Schiller JH, Harrington D, Belani CP, et
al: Comparison of four chemotherapy regimens for advanced
non-small-cell lung cancer. N Engl J Med. 346:92–98. 2002.
View Article : Google Scholar
|
|
3
|
Ohe Y, Ohashi Y, Kubota K, et al:
Randomized phase III study of cisplatin plus irinotecan versus
carboplatin plus paclitaxel, cisplatin plus gemcitabine, and
cisplatin plus vinorelbine for advanced non-small-cell lung cancer:
Four-Arm Cooperative Study in Japan. Ann Oncol. 18:317–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paez JG, Janne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kosaka T, Yatabe Y, Endoh H, Kuwano H,
Takahashi T and Mitsudomi T: Mutations of the epidermal growth
factor receptor gene in lung cancer: biological and clinical
implications. Cancer Res. 64:8919–8923. 2004. View Article : Google Scholar
|
|
7
|
Lee YJ, Shim HS, Kang YA, et al: Dose
effect of cigarette smoking on frequency and spectrum of epidermal
growth factor receptor gene mutations in Korean patients with
non-small cell lung cancer. J Cancer Res Clin Oncol. 136:1937–1944.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pham D, Kris MG, Riely GJ, et al: Use of
cigarette-smoking history to estimate the likelihood of mutations
in epidermal growth factor receptor gene exons 19 and 21 in lung
adenocarcinomas. J Clin Oncol. 24:1700–1704. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jida M, Toyooka S, Mitsudomi T, et al:
Usefulness of cumulative smoking dose for identifying the EGFR
mutation and patients with non-small-cell lung cancer for gefitinib
treatment. Cancer Sci. 100:1931–1934. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilson DO, Weissfeld JL, Balkan A, et al:
Association of radiographic emphysema and airflow obstruction with
lung cancer. Am J Respir Crit Care Med. 178:738–744. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maldonado F, Bartholmai BJ, Swensen SJ,
Midthun DE, Decker PA and Jett JR: Are airflow obstruction and
radiographic evidence of emphysema risk factors for lung cancer? A
nested case-control study using quantitative emphysema analysis.
Chest. 138:1295–1302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cottin V, Nunes H, Brillet PY, et al:
Combined pulmonary fibrosis and emphysema: a distinct
underrecognised entity. Eur Respir J. 26:586–593. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Usui K, Tanai C, Tanaka Y, Noda H and
Ishihara T: The prevalence of pulmonary fibrosis combined with
emphysema in patients with lung cancer. Respirology. 16:326–331.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kitaguchi Y, Fujimoto K, Hanaoka M,
Kawakami S, Honda T and Kubo K: Clinical characteristics of
combined pulmonary fibrosis and emphysema. Respirology. 15:265–271.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kenmotsu H, Naito T, Kimura M, et al: The
risk of cytotoxic chemotherapy-related exacerbation of interstitial
lung disease with lung cancer. J Thorac Oncol. 6:1242–1246. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goddard PR, Nicholson EM, Laszlo G and
Watt I: Computed tomography in pulmonary emphysema. Clin Radiol.
33:379–387. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bergin C, Muller N, Nichols DM, et al: The
diagnosis of emphysema. A computed tomographic-pathologic
correlation. Am Rev Respir Dis. 133:541–546. 1986.PubMed/NCBI
|
|
18
|
Ferguson GT, Enright PL, Buist AS and
Higgins MW: Office spirometry for lung health assessment in adults:
A consensus statement from the National Lung Health Education
Program. Chest. 117:1146–1161. 2000. View Article : Google Scholar
|
|
19
|
Nagai Y, Miyazawa H, Huqun, et al: Genetic
heterogeneity of the epidermal growth factor receptor in non-small
cell lung cancer cell lines revealed by a rapid and sensitive
detection system, the peptide nucleic acid-locked nucleic acid PCR
clamp. Cancer Res. 65:7276–7282. 2005. View Article : Google Scholar
|
|
20
|
Takano T, Ohe Y, Sakamoto H, et al:
Epidermal growth factor receptor gene mutations and increased copy
numbers predict gefitinib sensitivity in patients with recurrent
non-small-cell lung cancer. J Clin Oncol. 23:6829–6837. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee YJ, Cho BC, Jee SH, et al: Impact of
environmental tobacco smoke on the incidence of mutations in
epidermal growth factor receptor gene in never-smoker patients with
non-small-cell lung cancer. J Clin Oncol. 28:487–492. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Usui K, Ushijima T, Tanaka Y, et al: The
frequency of epidermal growth factor receptor mutation of nonsmall
cell lung cancer according to the underlying pulmonary diseases.
Pulm Med. 2011:2901322011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gullon JA, Suarez I, Medina A, Rubinos G,
Fernandez R and Gonzalez I: Role of emphysema and airway
obstruction in prognosis of lung cancer. Lung Cancer. 71:182–185.
2011. View Article : Google Scholar
|
|
24
|
de Torres JP, Bastarrika G, Wisnivesky JP,
et al: Assessing the relationship between lung cancer risk and
emphysema detected on low-dose CT of the chest. Chest.
132:1932–1938. 2007.PubMed/NCBI
|
|
25
|
Smith BM, Pinto L, Ezer N, Sverzellati N,
Muro S and Schwartzman K: Emphysema detected on computed tomography
and risk of lung cancer: a systematic review and meta-analysis.
Lung Cancer. 77:58–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamada N, Yamaya M, Okinaga S, et al:
Microsatellite polymorphism in the heme oxygenase-1 gene promoter
is associated with susceptibility to emphysema. Am J Hum Genet.
66:187–195. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kikuchi A, Yamaya M, Suzuki S, et al:
Association of susceptibility to the development of lung
adenocarcinoma with the heme oxygenase-1 gene promoter
polymorphism. Hum Genet. 116:354–360. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
D'Angelo SP, Pietanza MC, Johnson ML, et
al: Incidence of EGFR exon 19 deletions and L858R in tumor
specimens from men and cigarette smokers with lung adenocarcinomas.
J Clin Oncol. 29:2066–2070. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morita S, Okamoto I, Kobayashi K, et al:
Combined survival analysis of prospective clinical trials of
gefitinib for non-small cell lung cancer with EGFR mutations. Clin
Cancer Res. 15:4493–4498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rosell R, Moran T, Queralt C, et al:
Screening for epidermal growth factor receptor mutations in lung
cancer. N Engl J Med. 361:958–967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sanchez-Cespedes M, Ahrendt SA, Piantadosi
S, et al: Chromosomal alterations in lung adenocarcinoma from
smokers and nonsmokers. Cancer Res. 61:1309–1313. 2001.PubMed/NCBI
|
|
32
|
Shukuya T, Takahashi T, Kaira R, et al:
Efficacy of gefitinib for non-adenocarcinoma non-small-cell lung
cancer patients harboring epidermal growth factor receptor
mutations: a pooled analysis of published reports. Cancer Sci.
102:1032–1037. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Castillo L, Etienne-Grimaldi MC, Fischel
JL, Formento P, Magne N and Milano G: Pharmacological background of
EGFR targeting. Ann Oncol. 15:1007–1012. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kudoh S, Kato H, Nishiwaki Y, et al:
Interstitial lung disease in Japanese patients with lung cancer: a
cohort and nested case-control study. Am J Respir Crit Care Med.
177:1348–1357. 2008. View Article : Google Scholar : PubMed/NCBI
|