Introduction
It has been established that primary tumors and
metastases are composed of subsets of diverse cell types (1–3), and
the existence within tumors of a small fraction of cells
representing cancer stem cells responsible for tumor recurrence
after therapy has been postulated (4). On the other hand, in numerous
biochemical and biological studies it is often assumed that the
cells of established cell lines cultured in vitro represent
relatively homogeneous cell populations. Cells of different lines
are studied and the differences among them are correlated with the
degree of malignancy (5–10).
The aims of the present study were: i) to examine
the diversity of morpho-physiological cell features, which have
often been correlated with cell invasiveness and malignancy
(11–19) in two rat prostate carcinoma cell
lines applying cell cloning; ii) to check the expression of
connexin 43 and transcription factor Snail, involved in
epithelial-to-mesenchymal transition (EMT) and cell invasiveness in
morpho-physiologically different clones (20–23);
and iii) to examine the effects of caffeine, theophyline and
papaverine, recently reported to have anti-metastatic activity
in vivo(24–27) upon proportions of the particular
types of cell clones. Experiments were carried out on two rat
prostate carcinoma cell lines of the Dunning R-3327 system,
differing in their capacity to produce metastases. The AT-2 cell
line is characterized by moderate metastatic potential
(approximately 5–20%) whereas the malignant MAT-LyLu cell line has
high metastatic potential (over 90%) (5–7). These
cell lines belong to a series of over 20 lines separated from the
original Dunning R-3327 cell line, isolated from spontaneous rat
prostate carcinoma in 1963 (28,29).
Materials and methods
Cell culture
Cells of the AT-2 and MAT-LyLu cell lines were
propagated in a standard humidified incubator at 37°C in 5%
CO2/95% air atmosphere. Plastic culture dishes (plates
and Petri dishes) were purchased from Falcon. Cells were grown in
RPMI-1640 medium (Lonza) supplemented with 10% heat-inactivated
fetal calf serum (Gibco BRL, Middlesex, UK) and a 1% antibiotic
solution at a final concentration of 100 IU penicillin, 100 μg
streptomycin, and 0.25 μg amphotericin B per ml (Gibco BRL).
In cell cloning experiments, cells were seeded at a
density of 300 cells per 6-cm Petri dish (6 cm in diameter). After
2, 3, and 4 days the cells and their clones were observed under an
inverted phase contrast microscope (objective, ×20), and the
particular types of clones were counted. Microphotographs were
captured with a Leica DMI6000B inverted microscope with a DFC360FX
CCD camera. For observations of cells incubated for longer than 5
days, particular clones were separated and transferred to 6-well
plates. Cell viability was tested with trypan blue exclusion tests.
Cell viability exceeded 90% in all experiments. Cells for the
preparation of cell suspensions for seeding were counted using a
Bürker hemocytometer.
Immunocytochemistry and fluorescence
microscopy
Cells were plated on coverslips and cultured in
RPMI-1640 medium for 72 h. After washing in PBS, the cells were
fixed for 10 min in 4% paraformaldehyde (PFA in PBS), and washed
three times in PBS. The cells were then incubated for 10 min in
0.1% Triton X-100 permabilization solution, and washed three times
in PBS. Cells were incubated in a blocking solution of 3% BSA in
PBS for 20 min, double-stained for vinculin (Sigma) and F-actin
(phalloidin conjugated with TRITC; Sigma) labeled with Alexa
488-conjugated goat anti-mouse IgG (Molecular Probes) and
counterstained with Hoechst 333246 (Sigma). The cell staining was
visualized using a Leica DMI6000B inverted microscope, and images
were captured with a DFC360FX CCD camera.
Western blot analysis
Proteins from the cells were extracted with RIPA
lysis buffer (150 mM NaCl, 10 mM Tris pH 7.5, 1% NP4O, 1%
deoxycholate, 0.1% SDS, protease inhibitor cocktail) (Roche).
Proteins from total cell lysates were resolved on 10% SDS-PAGE gel,
transferred to nitrocellulose membranes and blocked in 5% non-fat
milk in PBS/Tween-20. Blots were exposed to the primary rabbit
polyclonal anti-Snail (1:500, Abcam), rabbit monoclonal anti-Cx43
(1:3000, Abcam), mouse monoclonal anti-α-tubulin (1:3000, Sigma)
and monoclonal mouse anti-β-catenin (1:3000, Santa Cruz) antibodies
followed by detection of the antibodies using HRP-labeled secondary
antibodies (1:3000, Invitrogen) and SuperSignal West Pico Substrate
(Pierce, Rockford, IL). Visualization of the secondary antibody was
performed using a chemiluminescence detection procedure according
to the manufacturer's protocol (Microchemi). Tubulin was used as a
loading control.
Time lapse-monitoring of the movement of
individual cells
Cell movement was observed with a Leica DMI6000B
inverted microscope with IMC contrast optics, and equipped with a
digital DFC360FX CCD camera, at 37°C and in a 5% CO2
atmosphere. AT-2 clones were seeded into 6-well plates at a density
of 4×102 cells/cm2 and incubated in RPMI-1640
medium supplemented with 10% FBS and antibiotics for 24 h before
recording. The cell trajectories were constructed from 97
subsequent centroid positions recorded over 480 min at 5 min time
intervals under a magnification of ×20. The cell trajectories were
presented in circular diagrams with the starting point of each
trajectory situated at the plot center (30–33).
The Hiro program written by W. Czapla was used for analysis of
parameters characterizing cell locomotion, as previously described
by Miekus et al(34). For
each data point measured, at least 50 cells were analyzed.
Results
When cells grow to high densities in vitro,
differences in cell size and shape are observed among groups of
cells located in different regions of the single-cell culture
vessel (35). In our experiments,
several discrete cell phenotypes were noted within the same
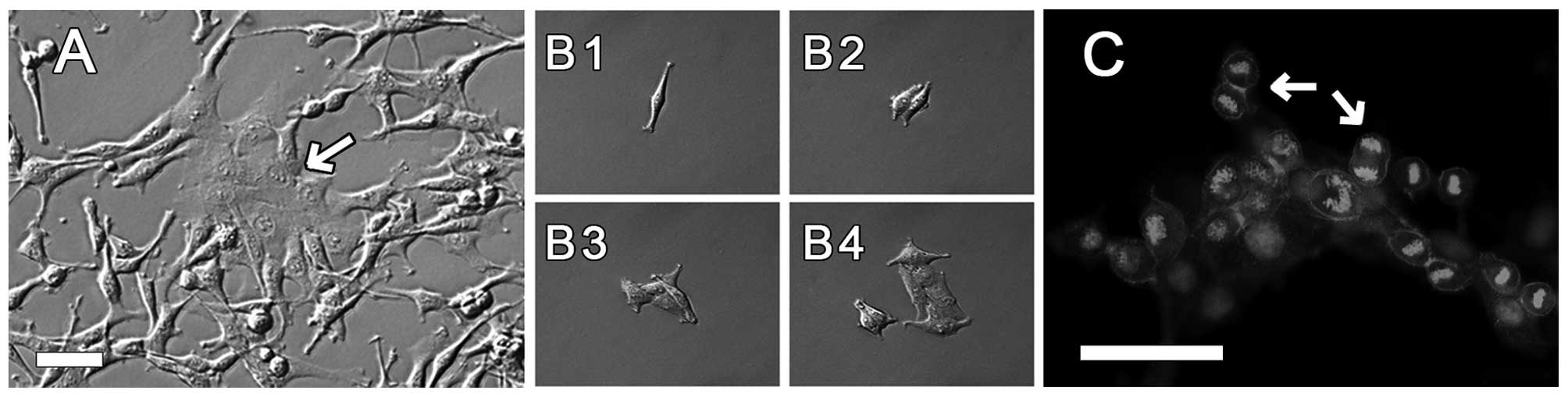
cultures. Fig. 1A shows an example
of local groups of cells differing in morphology in AT-2 rat
prostate cells grown in a single culture. It is possible to
distinguish flat and well-spread epithelial-like cells among the
dominant elongated, spindle-like cells. It is difficult to analyze
such local differences in crowded cell cultures. Therefore, we
observed cell clones originating from single cells. Fig. 1B1-B4 shows the development of a cell
clone from one cell as monitored for 3 days with time-lapse
photography. We noted (also in further experiments) that cell
divisions within clones occurred synchronously, in particular in
compact clones composed of cells (Fig.
1C). In some cases, as observed with time-lapse photography,
almost all cells in clones composed of 8–32 cells were
simultaneously undergoing mitosis.
In order to obtain further information on the
morphological heterogeneity of AT-2 and MAT-LyLu rat prostate
cancer cell lines in subsequent experiments, we examined clones
grown under identical cell culture conditions. It was difficult to
discern morphological differences among single cells immediately
after seeding. After the first 2–3 cell divisions, when small
clones composed of 4–8 cells appeared, the diversity of cells and
clones became distinguishable. After 3–6 days in culture, clones
originating from single cells clearly differed among themselves in
respect to cell characteristics which are known to correlate with
neoplastic growth and/or invasiveness. Analysis of cell morphology
in the different clones limited to their description as epithelial
or spindle-like (mesenchymal-like) appeared insufficient.
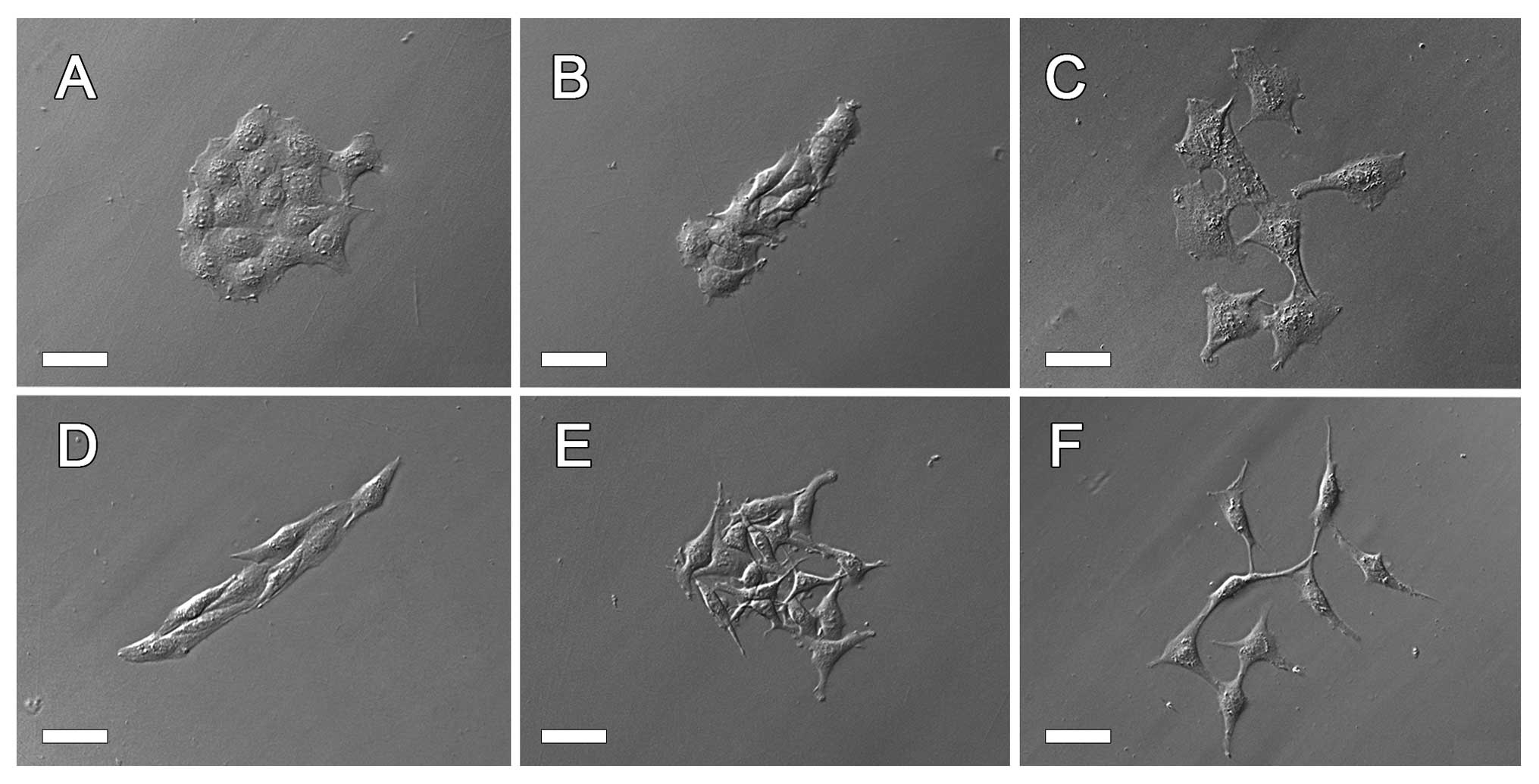
We observed three characteristic
morpho-physiological features of cells and their clones: i) cell
shape within clones: epithelial-like (unpolarized, well-spread) vs.
mesenchymal-like (spindle-like, elongated); ii) mode of cell
multi-layering by formation of compact bumps (knots, bulges, domes)
of flattened, non-polarized cells or by loosely packed layers of
elongated cells crossing one another with their filopodia; and iii)
clone organization, i.e. compact vs. dispersed with single cells
emigrating rapidly from the clone. Three different types of
epithelial-like cell clones were distinguished as well as the
corresponding three clonal types of mesenchymal-like cells. The
morphology of these six diverse types are shown in Fig. 2. The well-delineated compact clones
of unpolarized, epithelial-like cells (designated as E1) or
elongated, polarized, less spread and usually arranged in parallel
mesenchymal-like cells (designated as M1) are shown in Fig. 2A and D. In these clones the cells
grew in monolayers and exhibited contact inhibition of movement. E2
and M2 were designated as clones which were composed of
well-delineated groups of epithelial-like (E2) or mesenchymal-like
(M2) cells showing lack of contact inhibition and growing in
multi-layers (Fig. 2B and E). E3
and M3 were colonies formed by cells of epithelial-like (E3) or
mesenchymal-like (M3) morphology which immediately after divisions
separated from one another and actively migrated as single cells
(Fig. 2C and F). Clones E2 and M2
appeared more frequently on days 4–6, when the majority of
individual clones contained >20 cells. Epithelial-like cells in
E2 clones formed rather regular, multi-layered domes (bulbs),
whereas elongated cells randomly crossed one another with their
filopodial extensions in M2 clones (Figs. 3A and B; 4B and C). These cell features were
observed in compact clones originating from both AT-2 and MAT-LyLu
cell lines in spite of their differences in malignancy, although
compact clones were less frequent in the highly malignant MAT-LyLu
cell line.
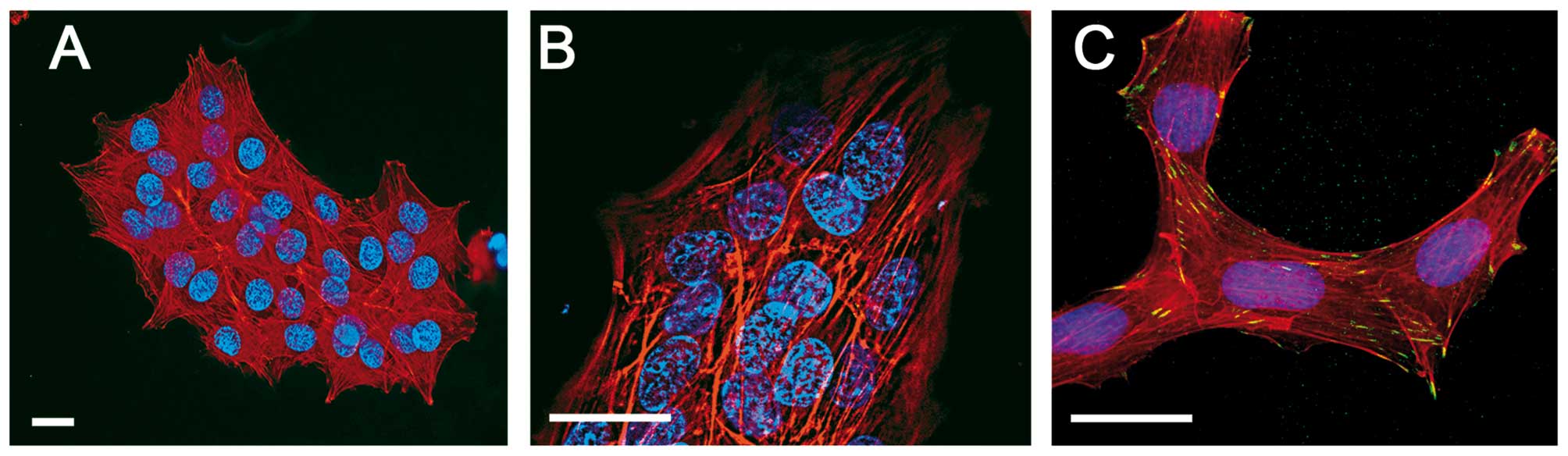
The morphology of E1, E2 and M1, M2 clones was
additionally examined after fixation and staining of nuclei with
Hoechst, the actin cytoskeleton with TRITC phalloidin, and vinculin
green with Alexa 488 (Fig. 3). This
facilitated discrimination of cell multi-layering in the E2 and M2
clones. The visualization of the actin cytoskeleton revealed that
in compact clones stress fibers were present even in cells of the
upper layers and that vinculin occurs in cell-to-cell contacts
(Fig. 3B and C).
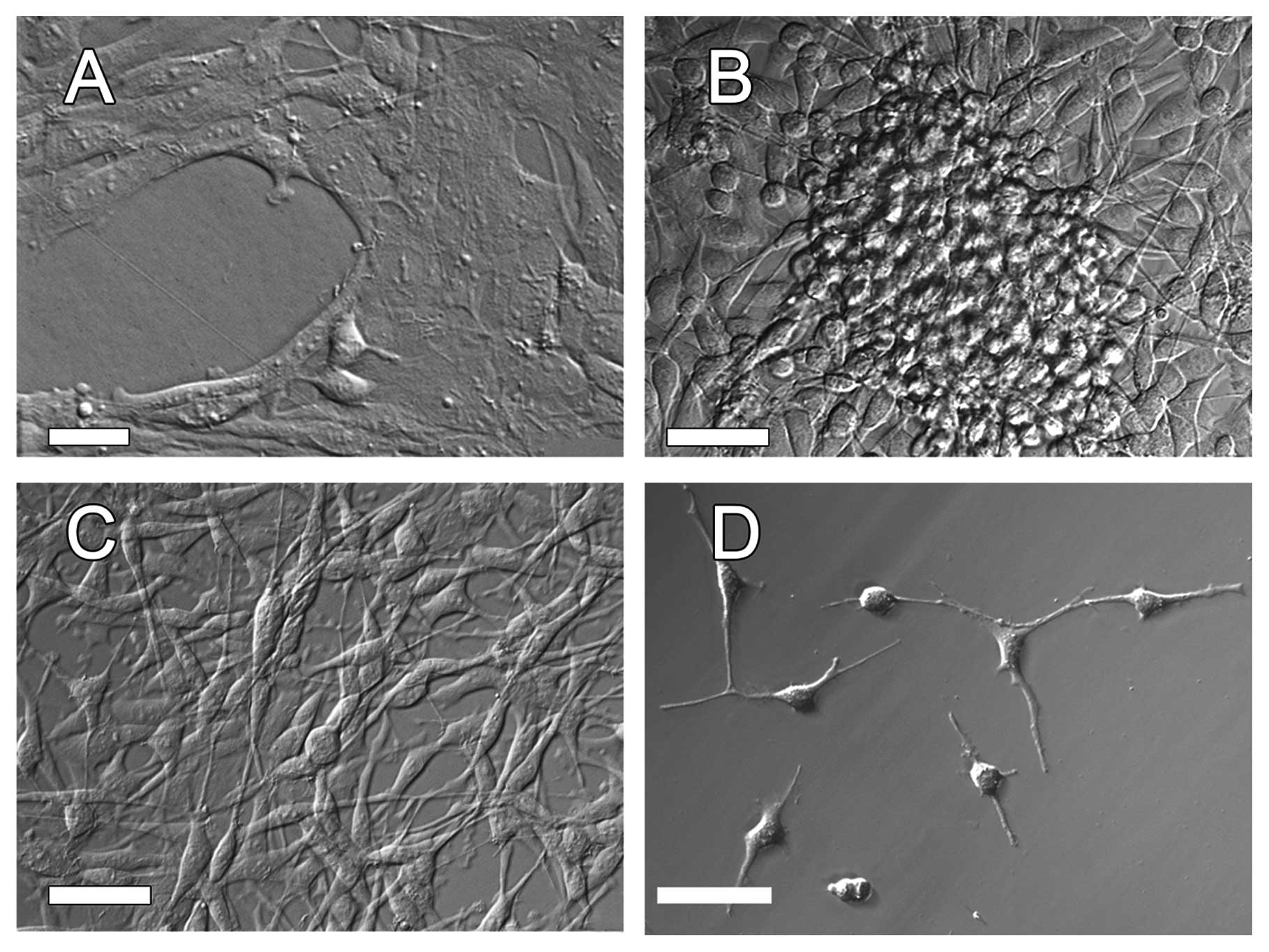
The morpho-physiological features of the cells
forming particular types of clones was preserved when the clones
continued to grow in separation from one another as shown in
Fig. 4A-C. This made it possible to
analyze the selected protein expression using western blotting.
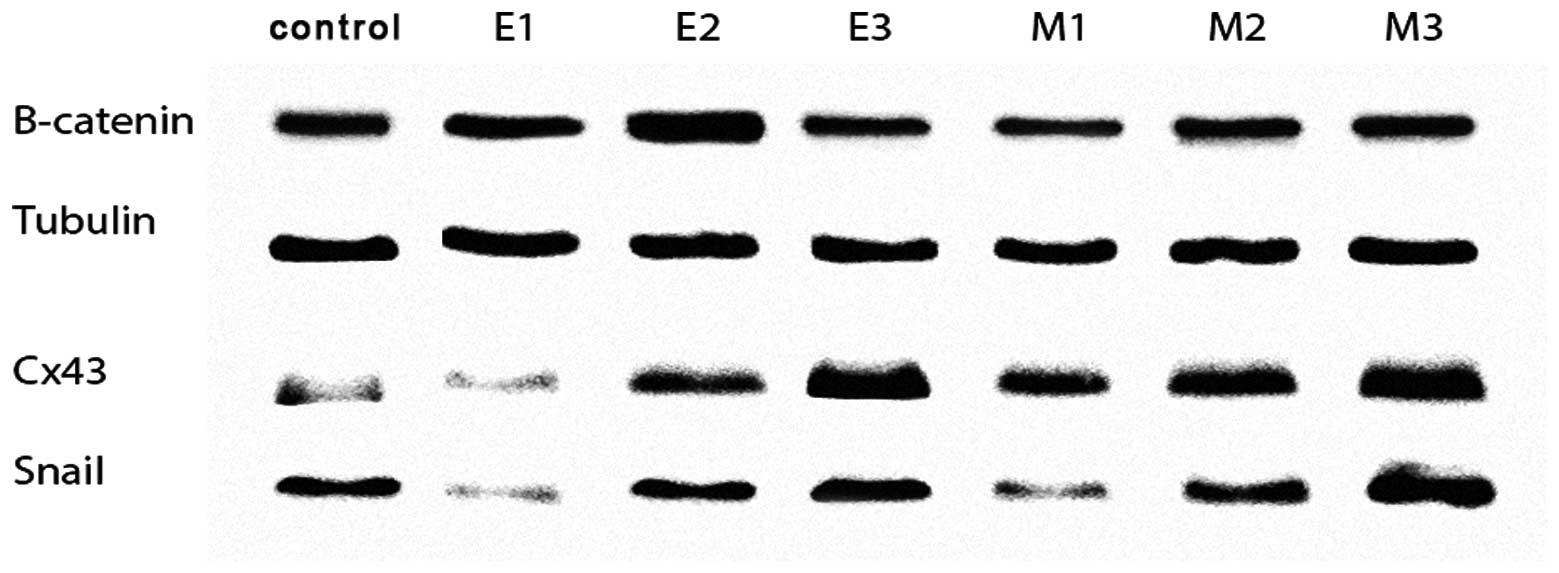
Recently, the expression of connexin 43 and transcription factor
Snail in cancer cells has been postulated to be involved in cancer
cell invasiveness (18–21,23).
Western blot analyses of tubulin, β-catenin, connexin 43 and Snail
expression in six diverse morpho-physiological types of clones of
single AT-2 cells are illustrated in Fig. 5. High expression levels of connexin
43 and Snail were found in dispersed clones, in which cells rapidly
separated from one another and showed active translocation, and in
multi-layered clones, independent of whether they exhibited
epithelial-like or mesenchymal-like morphologies. The increased
expression of connexin 43 was also observed in compact, monolayered
clones of mesenchymal-like cells but not in compact epithelial-like
clones. The expression of β-catenin was similar in all clone
types.
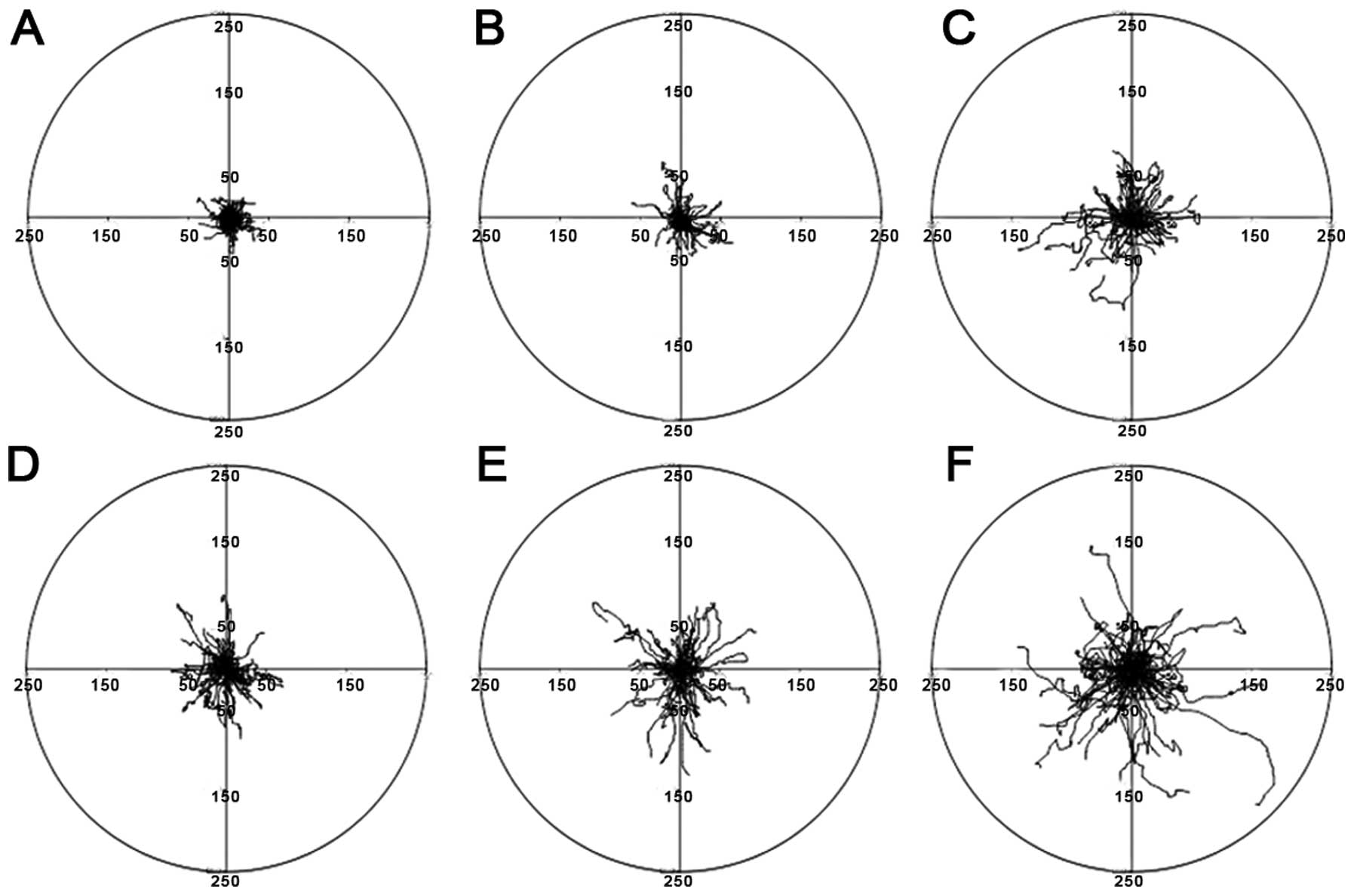
Time-lapse analysis of the motility of the single
cells separated from various clones confirmed that mesenchymal-like
cells from clones of corresponding morphology exhibited greatly
increased motility. In particular, the greatest final displacements
and lengths of cell trajectories of cells recorded 24 h after
isolation from mesenchymal-like M3 and M2 clones as wells as from
E3 epithelial-like clones are shown in circular diagrams in
Fig. 6.
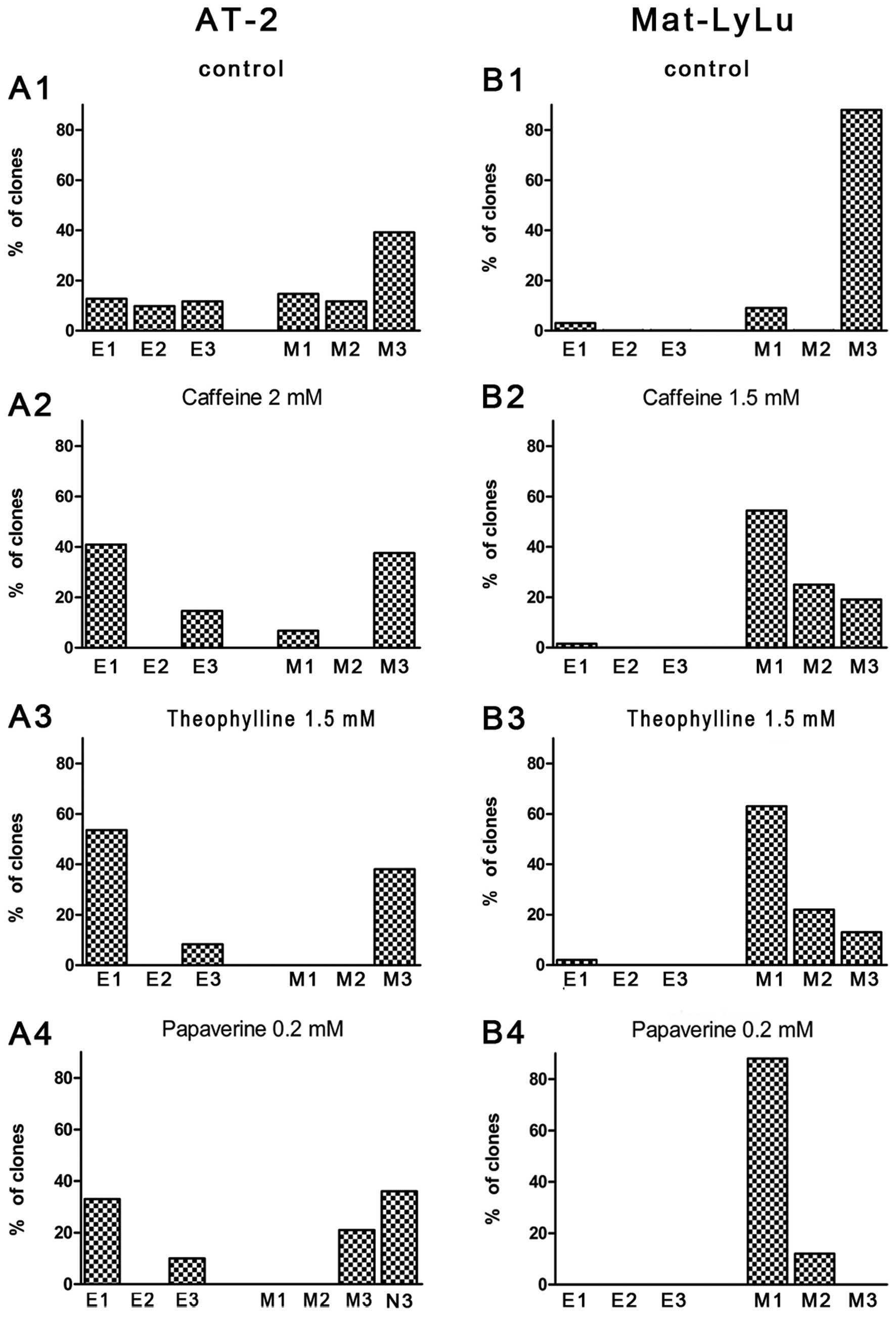
The distribution of different clones in cultures of
AT-2 and MAT-LyLu cells revealed that in the AT-2 cell line more
E1-3 clone types occurred whereas in the MAT-LyLu cell line
(characterized by a high metastatic potential) M3 clone types
dominated (Fig. 7A1 and B1). Under
control conditions after 72 h in culture, >90% of clones from
both cell lines contained >20 cells. These results permitted
further examination of the effects of caffeine, theophylline and
papaverine, recently reported to influence the growth of tumors
in vivo(24–27), upon growth and the proportions of
cell clones with defined morphology in the rat prostate cancer cell
lines. In preliminary experiments, we observed the effects of the
tested substances on cell viability (data not shown). In subsequent
experiments, concentrations were chosen which did not alter cell
viability >3% after 4 days of culture. The effects of a 2-mM
concentration of caffeine, 2-mM and 1.5-mM concentrations of
theophylline, and a 0.2-mM concentration of papaverine upon the
proportion of various types of clones in AT-2 and MAT-LyLu cell
lines grown for 3 days are shown in Fig. 7A2-A4 and B2-B4. These concentrations
appeared to clearly influence the clonal growth of cells of the
examined cell lines. Caffeine and theophylline had similar effects
except that theophylline appeared to have a greater toxic effect on
the MAT-LyLu than on AT-2 cells and a 2-mM concentration inhibited
the formation of MAT-LyLu clones. Therefore, a 1.5-mM concentration
was used in this case. Papaverine, in addition to inducing changes
in the proportion of various types of clones, caused visible
changes in the morphology of the AT-2 cells. Numerous AT-2 cells
produced very long filopodia-like or dendrite-like extensions
resembling those of neuronal cells (Figs. 4D and 7A4).
In AT-2 cells, a clear decrease in the proportion of
spindle-like cells (M) and E3 and M3 clones in relation to the
compact clones (E1 and M1) was visible, and cell overlapping, at
least within the first few days of culture, disappeared. These
effects occurred in parallel with the cell growth retardation in
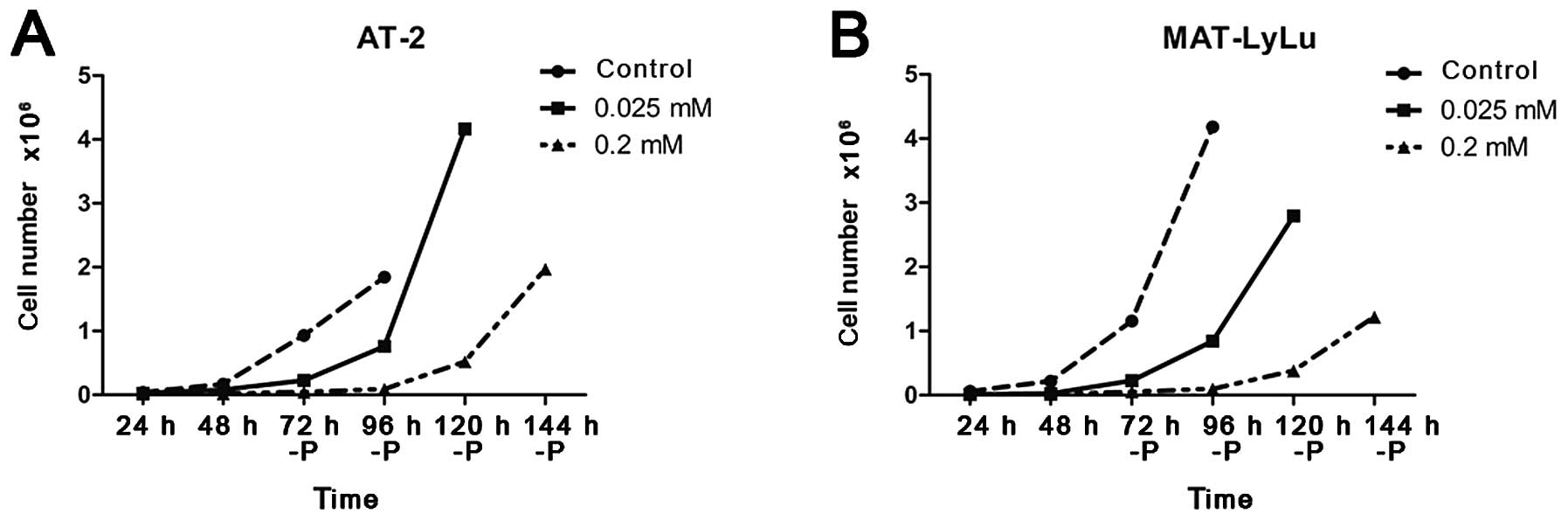
the presence of 2 mM caffeine and theophylline. Cell numbers in the
single clones continuously increased although much more slowly than
that under control conditions. After 72 h, the number of single
clones counted was usually 8 cells whereas under control conditions
this number was >20. The retardation of cell growth, strongest
in the presence of 0.2 mM papaverine, was reversible (Fig. 8). Experiments in which the number of
cells in the culture was estimated could not discriminate between
the fast growth of only a subset of cells, or rather slight
acceleration of growth of all cells present in the culture vessel.
We could not, therefore, resolve whether the arborized cells with
strongly modified morphology could revert to normal morphology and
undertake growth or if these cells were rapidly overgrown by cells
with spindle-like morphology. This aspect requires separate
experiments.
Discussion
Permanent neoplastic cell lines often serve as
models in the research of the biology of cancer cells. The Dunning
series of rat prostate cancer cell lines have a common origin from
a single tumor but are characterized by a different capacity to
produce metastases when tested in vivo. The AT-2 cell line
is moderately malignant (producing less than 20% metastases)
whereas the highly malignant MAT-LyLu cell line has the capacity to
metastasize in more than 90% of cases (5–7). Our
experiments revealed that both of these cell lines are
heterogeneous, but the clones of single cells from the AT-2 line
displayed a greater heterogeneity than the clones from the MAT-LyLu
cell line.
Clonal analysis showed that the clones differed in
regards to morpho-physiological cell features commonly correlated
with neoplastic cell characteristics including invasiveness and
capacity to metastasize. Typical features correlated with
malignancy include: i) low stability of cell-to-cell contact,
release from contact inhibition of growth and movement, resulting
in cell multi-layering and random growth (12,14,36);
and ii) the transition from unpolarized, flat epithelial-like cell
shapes to spindle-like, elongated mesenchymal-like shapes of
invasive cells of high tumor-producing capacity (11,36,37),
which is usually described in the contemporary literature as EMT
(16–19,22).
The presence of these cell features was examined in clones of cells
of the AT-2 and MAT-LyLu rat prostate adenocarcinoma cell lines.
The main observation is that the clones of single cells from one
line showed diverse expression of features commonly considered as
characteristic for cancer cells. Apart from clones showing typical
characteristics of malignant cells (designed as M2 and M3 clone
types), clones resembling normal epithelial or mesenchymal cells
were also present (i.e. E1 and M1 clones). These are compact clones
of cells showing contact inhibition of movement and growth in one
cell layer. In clones of the MAT-LyLu cell line, cells exhibiting
typical features of highly malignant cells dominated. They grew and
when dispersed, actively moved and separated from one another after
division, assuming a spindle-like morphology or forming
multi-layers of cells released from contact inhibition, crossing
one another. The reported higher capacity of the MAT-LyLu cell line
to form metastases in vivo than the AT-2 cell line (5–7) may be
associated with a much higher proportion of M2 and M3 type clones
in the former. The cells isolated from dispersed mesenchymal-like
clones (M3) showed the highest motility when compared with cells
separated from the other clonal types. This is in accordance with
the common postulate that highly invasive cells are characterized
by high motile activity. In the AT-2 cell line, cell clones of the
malignant morpho-physiological phenotype were present, although in
a much lower proportion.
Markedly, in some cell clones of epithelial-like
morphology, cells maintaining an epithelial-like shape (typically
half-moon-like) could separate from one another and disperse
showing active motility (cells from A3 clones). These results
suggest that apart from the need to compare the properties among
cells of different established cell lines it is advantageous to
take into account the heterogeneity of cells within these cell
lines. This may also include the molecular study of various
proteins, the expression of which is postulated to be associated
with cell malignancy, invasiveness and EMT. Recently, it was
reported that the increased expression of connexin 43 and
transcription factor Snail is associated with increased motility,
invasiveness and EMT in prostate cancer cells (18–21,23).
Our western blot analysis revealed that in
accordance with these suggestions in the clones of single cells
from the AT-2 line, a concomitant increase in the expression of
connexin 43 and Snail was observed in dispersed clones of cells
rapidly separated from one another showing active fast
translocation, and in multi-layered clones, independent of whether
the cells had epithelial-like or mesenchymal-like morphology. The
increased expression of connexin 43 was also observed in compact,
monolayered clones of mesenchymal-like cells but not in compact
epithelial-like clones. The expression of β-catenin was similar in
all clonal types. These results support the conclusion that the
anti-proliferative activity of potential anticancer drugs should
consider the effects of the tested compounds on particular cell
types and their clones present within the established cancer cell
lines propagated in vitro.
We examined the effect of three substances,
caffeine, theophylline and papaverine, recently found to have
limited anticancer activity in prostate cancer (24–27).
In the AT-2 cell line, these agents decreased the proportion of
clones having features attributed to malignancy at concentrations
which reversibly retarded cell growth but did not inhibit cell
multiplication and formation of clones (37). The results of observations performed
in vitro correspond to reports showing moderate anticancer
activity of caffeine and theophylline in vivo. In addition,
papaverine strongly modified the shape of cells in some subsets of
clones causing cell arborization and formation of long dendrite- or
axon-like protrusions. Similar modifications of cell shape in the
presence of papaverine were described in a fraction of mouse
neuroblastoma cell culture (38).
We suggest that differences in cell heterogeneity within cell lines
apart from the differences among cell lines should be taken into
account in experiments in which the effects of anticancer agents
are examined using established cancer cell lines in vitro.
Changes in the proportion of different clones should be considered
in studies of the effects of agents modifying cell features. The
presentation of cases in regards to the transition of morphology of
single clones appears insufficient. Moreover, the promotion and
progression in cancer cell lines expanded in vitro should be
investigated with methods of single-cell cloning taking into
account initial cell diversity.
Time-lapse recording of the development of clones
from single cells revealed extensive synchronization of cell
divisions maintained for a few cell generations in compact clones.
This can provide a convenient model for research into the
dependence of cell features and activity upon cell progression
through phases of the cell cycle.
Our results emphasize the need for clonal analysis
of cancer cell reactions to various factors, including anticancer
agents, when experiments are carried out using established cancer
cell lines in vitro. The diversity of cells within the
propagated in vitro established cell lines may be associated
with, and correspond to, the heterogeneity and genetic instability
of cells within tumors in vivo and their primary cultures
(1–3,11,39).
This implies that in addition to the differences among established
cancer cell lines, differences concerning cells within cell lines
should not be neglected when conducting research on the biology of
cancer cells carried out under cell culture conditions.
Acknowledgements
This work ws financially supported by the Polish
National Science Centre (grant 2011/01/B/NZ3/00004).
References
|
1
|
Wang N, Wilkin A, Böcking A and Tribukait
B: Evaluation of tumor heterogeneity of prostate carcinoma by flow
and image DNA cytometry and histopathological grading. Anal Cell
Physiol. 20:49–62. 2000.PubMed/NCBI
|
|
2
|
Singh RK and Talmadge JE: The evolution of
diversity within tumors and metastases. Selected Aspects of Cancer
Progression: Metastasis, Apoptosis and Immune Response. Kaiser HE
and Nasir A: Springer Science-Business Media BV; Dordrecht: pp.
59–90. 2008
|
|
3
|
Marusyk A and Polyak K: Tumor
heterogeneity: causes and consequences. Biochim Biophys Acta.
1805:105–117. 2010.PubMed/NCBI
|
|
4
|
Dirks P: Cancer stem cells. Invitation to
a second round. Nature. 466:40–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carter HB and Coffey DS: Cell surface
charge in predicting metastatic potential of aspirated cells from
the Dunning rat prostate adenocarcinoma model. J Urol. 140:173–175.
1988.PubMed/NCBI
|
|
6
|
Carter HB, Partin AW and Coffey DS: Cell
surface charge in predicting metastatic potential in an animal
model of prostate cancer: flow cytometric quantification of cell
surface charge. J Urol. 142:1338–1341. 1989.PubMed/NCBI
|
|
7
|
Fraser SP, Ding Y, Liu A, Foster CS and
Djamgoz MBA: Tetrodotoxin suppress morphological enhancement of the
metastatic MAT-LyLu rat prostate cell line. Cell Tissue Res.
295:505–512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bironaite D, Nesland JM, Dalen H, Risberg
B and Bryne M: N-Glycans influence the in vitro adhesive and
invasive behaviour of three metastatic cell lines. Tumor Biol.
200:165–175. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Djamgoz MBA, Mycielska M, Madeja Z, Fraser
SP and Korohoda W: Directional movement of rat prostate cancer
cells in direct-current electric field. Involvement of voltage
gated Na+ channel activity J Cell Sci. 114:2697–2705.
2001.PubMed/NCBI
|
|
10
|
Pokorna E, Zicha D, Chaloupkova A,
Matousková E and Vesely P: Two dynamic morphotypes of sarcoma
cells, asymmetric stellate and triangle with leading lamella, are
related to malignancy. Folia Biol. 49:33–39. 2003.PubMed/NCBI
|
|
11
|
Sanford KK, Likely GD and Earle WR: The
development of variations in transplantability and morphology
within a clone of mouse fibroblasts transformed to
sarcoma-producing cells in vitro. J Natl Cancer Inst. 15:215–237.
1954.PubMed/NCBI
|
|
12
|
Abercrombie M and Ambrose EJ: The surface
properties of cancer cells: a review. Cancer Res. 22:332–345.
1962.
|
|
13
|
Moscona A, Trowell QA and Willmer EN:
Methods. (Chap. 2). Cell and Tissues in Culture. 1. Willmer EN:
Academic Press; London: pp. 1–98. 1965
|
|
14
|
Burger MM: The significance of surface
structure changes for growth control under crowded conditions.
Growth Control in Cell Cultures. Wolstenhome GEW and Knight J:
Churchill Livingstone; London: pp. 45–62. 1971
|
|
15
|
Wyckoff JB, Segall JE and Condeelis JS:
The collection of the motile population of cells from a living
tumor. Cancer Res. 60:5401–5404. 2000.PubMed/NCBI
|
|
16
|
Thiery JP: Epithelial-mesenchymal
transitions in development and pathogenesis. Curr Opin Cell Biol.
15:740–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Savagner P: The epithelial-mesenchymal
transition (EMT) phenomenon. Ann Oncol. 21(Suppl 7): vii89–vii92.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Graham TR, Zhau H, Odera-Marah VA,
Osunkoya AO, Kimbrok S, Tighiouart M, Liu T, Simons JW and O'Regan
RM: Insulin-like growth factor-I-dependent up-regulation of ZEB1
drives epithelial-to-mesenchymal transition in human prostate
cancer cells. Cancer Res. 68:2479–2488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Klarmann GJ, Hurt EM, Matheus LA, Zhang X,
Duhagon MA, Mistree T, Thomas SB and Farrar WL: Invasive prostate
cancer cells are tumor initiating cells that have a stem cell-like
genomic signature. Clin Exp Metastasis. 26:433–446. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tate AW, Lung T, Radhakrishnan A, Lim SD,
Lin X and Edlund M: Changes in gap junctional connexin isoforms
during prostate cancer progression. Prostate. 66:19–31. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kanczuga-Koda L, Sulkowski S, Lenczewski
A, Koda M, Wincewicz A, Baltaziak M and Sulkowska M: Increased
expression of connexins 26 and 43 in lymph node metastases of
breast cancer. J Clin Pathol. 59:429–433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baritaki S, Chapman A, Yeung K, Spandidos
DA, Palladino M and Bonavida B: Inhibition of epithelial to
mesenchymal transition in metastatic prostate cancer cells by the
novel proteasome inhibitor, NPI-0052: pivotal roles of Snail
repression and RKIP induction. Oncogene. 28:3573–3585. 2009.
View Article : Google Scholar
|
|
23
|
Czyz J, Szpak K and Madeja Z: The role of
connexins in prostate cancer promotion and progression. Nat Rev
Urol. 9:274–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lentini A, Mattioli P, Nicolini L,
Pietrini A, Abbruzzese A and Beninati S: Anti-invasive effects of
theophylline on experimental B16–F10 melanoma lung metastasis.
Cancer J. 10:274–278. 1997.PubMed/NCBI
|
|
25
|
Spangler JG: Bone biology and physiology:
implications for novel osteoblastic osteosarcoma treatments. Med
Hypotheses. 70:281–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Holick CN, Smith SG, Giovannucci E and
Michaud DS: Coffee, tea, caffeine intake, and risk of adult glioma
in three prospective cohort studies. Cancer Epidemiol Biomarkers
Prev. 19:39–47. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wilson KM, Kasperzyk JL, Rider JR,
Kenfield S, Van Dam RM, Stampfer MJ, Giovannucci E and Mucci LA:
Coffee consumption and prostate risk and progression in the health
professionals follow up study. J Natl Cancer Inst. 103:876–884.
2011. View Article : Google Scholar
|
|
28
|
Isaacs JT: The R-3327 system of rat
prostatic cancers. Urol Oncol. 2:115–116. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramaekers FC, Verhagen AP, Isaacs JT,
Feitz WF, Moesker O, Schaart G, Schalken JA and Voijs GP:
Intermediate filament expression and the progression of prostatic
cancer as studied in the Dunning R-3327 rat prostatic carcinoma
system. Prostate. 14:323–339. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Erickson CA and Nuccitelli R: Embryonic
fibroblast motility and orientation can be influenced by
physiological electric fields. J Cell Biol. 98:296–307. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Korohoda W and Madeja Z: Contact of
sarcoma cells with aligned fibroblasts accelerated their
displacement: computer-assisted analysis of tumour cell locomotion
in co-culture. Biochem Cell Biol. 75:263–276. 1997. View Article : Google Scholar
|
|
32
|
Waligorska A, Wianecka-Skoczen M, Nowak P
and Korohoda W: Some difficulties in research into cell motile
activity under isotropic conditions. Folia Biol. 55:9–16. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Daniel-Wojcik A, Misztal K, Bechyne I,
Sroka J, Miekus K, Madeja Z and Czyz J: Cell motility affects the
intensity of gap junctional coupling in prostate carcinoma and
melanoma cell populations. Int J Oncol. 33:309–315. 2009.PubMed/NCBI
|
|
34
|
Miekus K, Czernik M, Sroka J, Czyz J and
Madeja Z: Contact stimulation of prostate cancer cell migration:
the role of gap junctional coupling and migration stimulated by
heterotypic cell-to-cell contacts in determination of the
metastatic phenotype of Dunning rat prostate cancer cells. Biol
Cell. 97:893–903. 2005. View Article : Google Scholar
|
|
35
|
Burke JM, Skumasz CMB, Irwing PE and McKay
BS: Phenotypic heterogeneity of retinoid pigment epithelial cells
in vitro and in situ. Exp Eye Res. 62:63–73. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Forrester JA: Microelectrophoresis of
normal and polyoma virus transformed hamster kidney fibroblasts.
Cell Electrophoresis. Ambrose EJ: J. & A. Churchill Ltd;
London: pp. 115–124. 1965
|
|
37
|
Korohoda W and Czyz J: Efficacy of the
Frame and Hu mathematical model for the quantitative analysis of
agents influencing growth of chick embryo fibroblasts. Folia
Histochem Cytobiol. 32:113–118. 1994.PubMed/NCBI
|
|
38
|
Prasad KN and Sheppard JR: Inhibitor of
cyclic-nucleotide phosphodiesterase tumor cells induce
morphological differentiation of mouse neuroblastoma cell culture.
Exp Cell Res. 73:436–440. 1972. View Article : Google Scholar
|
|
39
|
Weiss L and Subjeck JR: Electrical
heterogeneity of the surfaces of Ehrlich ascites tumor cells. Ann
NY Acad Sci. 238:352–361. 1974. View Article : Google Scholar : PubMed/NCBI
|