Introduction
A relationship between obesity and breast cancer
risk has been proposed based on epidemiological data, a positive
association with increasing body mass index being found
particularly in postmenopausal women (1–4).
Although the underlying mechanisms have yet to be fully clarified,
increased concentrations of circulating sex hormones are likely to
contribute at least in part (5). In
addition, circulating levels of an adipokine leptin, which is
secreted mainly from adipose tissue and limits food intake and
increases energy expenditure (6),
was recently suggested to have a role independent of obesity
indices in breast tumorigenesis (7). In estrogen receptor (ER)-positive
breast cancer cells, leptin has been demonstrated to stimulate
aromatase expression and cell proliferation, and both in
ER-positive and -negative breast cancer cells, leptin induced
transactivation of ErbB tyrosine kinase receptors, such as the
epidermal growth factor receptor (EGFR) and ErbB-2 (HER2/Neu),
resulting in the induction of cell proliferation and increased
survival (8–10).
To investigate the effects of obesity on mammary
carcinogenesis, a number of animal models, featuring inherited
obesity or feeding of a high fat/calorie diet, were employed. Fatty
Zucker (fa/fa) rats, which have autosomal recessive mutation
in the leptin receptor gene (11),
develop hyperinsulinemia, but blood glucose remains at normal
levels (12). In addition, they
demonstrate significantly increased serum triglyceride, total
cholesterol and leptin levels (12,13).
Lean Zucker (+/fa and +/+) rats, by contrast, exhibit normal
appearing metabolic functions and have been utilized as controls in
chemically-induced mammary carcinogenesis investigations (14–17).
In a previous study, the latency period and/or the incidence of
mammary carcinomas were reported to be shorter and greater,
respectively, in female fa/fa than +/fa and +/+ rats
treated with 7,12-dimethylbenz(a)anthracene (DMBA) (15,17).
However, in another study, female Zucker (fa/fa) rats
treated with N-methyl-N-nitrosourea (MNU) showed a
lower incidence of mammary carcinomas compared to lean Zucker
controls (+/fa and +/+) (14). A number of factors may contribute to
the discrepancy between the DMBA- and MNU-treated rats, and it
remains unclear which obesity-associated internal parameters, such
as hyperinsulinemia, hyperleptinemia or hyperlipidemia,
fundamentally affect mammary carcinogenesis.
We recently compared serum biochemical parameters
between lean Zucker (+/fa) and (+/+) rats in combination
with or without an obesity-inducing 10% corn oil diet, to clarify
whether lean Zucker (+/fa) rats might also be more sensitive
to the high fat diet than the +/+ controls (18). Serum leptin concentrations were
higher in the (+/fa) case at 7 weeks of age (~140 pg/ml as
compared to ~80 pg/ml in +/+; P<0.01), although the difference
was significantly smaller at 12 weeks of age, and serum
concentrations of other parameters including insulin, triglycerides
and total cholesterol were similar between the two genotypes. In
addition, both +/fa and +/+ rats fed basal diet mixed with
10% corn oil showed higher serum leptin levels than those fed basal
diet alone, but no other parameters examined were altered by the
obesity-inducing diet.
In the present study, to clarify the effects of
hereditary and dietary hyperleptinemia on mammary carcinogenesis,
lean Zucker (+/fa) rats with and without 10% corn oil
feeding were utilized in a DMBA-induced mammary carcinogenesis
model along with control lean Zucker (+/+) rats. In the present
study, latency period and growth rates of mammary carcinomas were
assessed by regular palpation, and at the termination,
histopathological, immunohistochemical and western blot analyses
were performed to determine expression profiles of estrogen- and
intracellular signaling cascade-related proteins in the mammary
carcinomas, as well as serum biochemistry for obesity-associated
parameters. The data demonstrated +/fa rats to indeed be
more susceptible to DMBA-induced mammary carcinogenesis than +/+
controls, with hyperleptinemia appearing to be partly associated
with tumor growth as well as with susceptibility to tumorigenesis
and a more aggressive phenotype in an estrogen-independent
manner.
Materials and methods
Chemicals and animals
DMBA was purchased from Sigma Chemical (St. Louis,
MO, USA) and dissolved in sesame oil at 10 mg/ml prior to
administration. A total of 100 female Zucker rats (lean phenotype)
at 5 weeks of age were purchased from Charles River Japan
(Kanagawa, Japan) and acclimated for 1 week prior to genotyping by
the method of Phillips et al(19). Throughout the acclimatization and
experimental periods, the animals were housed at a maximum of 3 or
4 per plastic cage with white wood chips (Sankyo Laboratory
Service, Tokyo, Japan) for bedding and transferred to clean cages
with fresh bedding twice a week in a standard air-conditioned
animal room (24±1°C, 55±5% relative humidity, 12 h light and dark
cycle). All animals had free access to basal diet (CRF-1; Oriental
Yeast Co., Tokyo, Japan) and tap water until the start of the
experiment.
Experimental protocol
Sixty-six +/fa and 32 +/+ rats at 7 weeks of
age received an intragastric administration of DMBA (50 mg/kg body
weight) by gavage, and the animals of each genotype were then
divided into basal diet (CRF-1; 357 kcal/100 g) and 10% corn oil
diet (CRF-1-based, Oriental Yeast; 414 kcal/100 g) groups. The
present dose level of DMBA at 50 mg/kg body weight was selected
based on our previous experiments, in which palpable mammary tumors
were induced at adequate incidences for detection of endogenous and
exogenous tumor promoting and/or inhibitory factors in
Sprague-Dawley (20) and F344 rats
(21). The dietary concentration of
corn oil at 10% was selected based on the previously reported
effective concentrations of linoleic acid for promotion of rat
mammary tumor development (22).
General conditions and mortality were checked daily and body weight
was measured once a week during the experimental period. The
amounts of supplied and residual diet were weighted weekly in order
to calculate the average daily food intake per week. Following DMBA
administration, a veterinary scientist (T.I.) palpated cervix,
thorax and abdomen of awake rats to detect mammary tumors once
weekly. The length, width and height of each tumor were measured
using a caliper and tumor volumes were calculated as follows:
Volume = (length) × (width) × (height) × π/6.
For endpoints for this study, the rats were
sacrificed when demonstrating over 20% decrease in body weight
excluding total tumor weight and/or when symptoms of poor physical
condition, such as decrease in locomotor activity, were found.
Volume of mammary tumors was not considered important in this
regard, since change in tumor volume was a key item for evaluation
of the effects of rat genotype and corn oil diet. All remaining
rats were sacrificed at 32 weeks following DMBA administration. The
present study design was approved by the Animal Care and
Utilization Committee of the National Institute of Health
Sciences.
Necropsy and histopathology
At the end of the experimental period, blood samples
were collected from the abdominal aorta of all surviving animals
under ether anesthesia. Serum was separated and maintained at −80°C
until use. Following euthanasia by exsanguination under ether
anesthesia, animals were subjected to necropsy. Whole skins with
mammary glands and tumors were removed, and the sizes of all
mammary tumors were recorded. Tumor volumes were calculated in the
same manner as for palpable tumors. Sections of frozen tissue of
randomly selected mammary tumors of rats in all groups were
prepared with liquid nitrogen and stored at −80°C until use. The
remaining tumor and mammary tissues were fixed in 10% neutral
buffered formalin, processed routinely to paraffin-embedded
sections at 4–5 μm, and stained with hematoxylin and eosin
(H&E) for histopathological analysis. Animals that died or that
were sacrificed on becoming moribund were similarly necropsied and
included for the sequential palpable tumor and postmortem
analyses.
Immunohistochemistry
Primary antisera for the leptin receptor (goat
polyclonal; Neuromics Antibodies, Edina, MN, USA; 1:1,000
dilution), smooth muscle actin (mouse clone 1A4; Dako, Glostrup,
Denmark; 1:200), leptin (rabbit polyclonal; Santa Cruz
Biotechnology, Santa Cruz, CA, USA; 1:200), ER α (mouse clone 6F11;
Novocastra, Newcastle, UK; 1:50 or 1:500), ER β (rabbit polyclonal;
Affinity BioReagents, Rockford, IL, USA; 1:100) and aromatase
(rabbit polyclonal; Abcam, Cambridge, MA, USA; 1:500), were
utilized for immunohistochemistry. Analyzed mammary tumors were
selected from all groups, on the basis of the genotype, diet and
phenotypes. Paraffin sections 5, 5, 10 and 10 carcinomas from the
+/+-basal diet, +/+-corn oil diet, +/fa-basal diet and
+/fa-corn oil diet groups, respectively, were used for
leptin receptor, leptin, smooth muscle actin and ER α, and frozen
sections of 3, 4, 4 and 5 each were for ER β and aromatase. Antigen
retrieval for paraffin sections was carried out in an autoclave for
10 min at 121°C in 10 mM citrate buffer (pH 6.0) for leptin
receptor, smooth muscle actin and ER α. The
streptavidin-biotin-peroxidase complex method (StreptABComplex/HRP;
Dako) was used to determine the expression and localization of each
antigen, and sections were lightly counterstained with hematoxylin
for microscopic examination. Negative controls without primary
antibody reactions were set for each antigen using serial sections.
The positivities for ER α in over 1,000 mammary adenocarcinoma
cells were assessed on each paraffin section to give percentage
values.
Western blot analysis
Twelve mammary tumors and four normal mammary tissue
samples of the +/+-basal diet, +/+-corn oil diet and
+/fa-basal diet groups were homogenized in extraction buffer
(50 mM Tris-HCl pH 7.4, 3 mM EDTA, 100 mM NaCl, 1% Tween-20, 10 mM
sodium orthovanadate, 1 mM PMSF, 10 μg/ml leupeptin, 20 μg/ml
aprotinin) and centrifuged at 14,000 g for 20 min. Equal amounts of
protein samples (50 μg) from collected supernatants were subjected
to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) on 5–20%
gradient acrylamide gels (ATTO, Tokyo, Japan), and the separated
proteins were transferred to polyvinylidene difluoride membranes
(Whatman, Sanford, ME, USA). Immunoblotting was performed using
rabbit polyclonal antibodies against ER β (Affinity BioReagents),
aromatase (Abcam), signal transducer and activator of transcription
(STAT)3 and phospho-STAT3 (Thy705) (Cell Signaling Technology,
Danvers, MA, USA), extracellular signal-regulated kinase (ERK)1/2
and phospho-ERK1/2 (R&D Systems, Minneapolis, MN, USA) or
monoclonal antibodies against β-actin (mouse clone AC-15; Sigma),
followed by exposure to peroxidase-labeled anti-rabbit or mouse
polyclonal goat antibodies (Dako) and development of signals with
TMB 3,3′,5,5′ tetramethylbenzidine (ATTO). Semi-quantitative
analyses were performed using Scion Image (alpha4.0.3.0; Scion,
Frederick, MD, USA).
Serum biochemistry
Concentrations of serum leptin, adiponectin, insulin
and insulin-like growth factor (IGF)-I were determined for randomly
selected almost half and one third of samples from +/+- and
+/fa-groups, respectively, using rat/mouse enzyme
immunoassay kits from Yanaihara Institute (Shizuoka, Japan),
Adipogen (Incheon, Korea), Mercodia (Uppsala, Sweden) and R&D
Systems, respectively. Other serum biochemical parameters including
triglyceride, total cholesterol and glucose were measured for all
samples except for those lost due to sampling error at SRL (Tokyo,
Japan).
Statistical analysis
The survival rates and incidence of palpable or
histopathologically defined mammary tumors were analyzed for
inter-group differences by the Fisher’s exact probability test.
Data for body weights and multiplicity, volume and latency of
mammary tumors, ER α-positivity in mammary adenocarcinoma sections,
serum biochemistry and western blot analysis data were examined
with the Student’s or the Welch’s t-test following the F-test.
Significance was inferred at the 5, 1 and 0.1% levels.
Results
Survival rates, body weights and food
intake
At the end of the experiment, survival rates were
94% (15/16), 81% (13/16), 85% (28/33) and 85% (28/33) in the
+/+-basal diet, +/+-corn oil diet, +/fa-basal diet and
+/fa-corn oil diet groups, respectively, with no significant
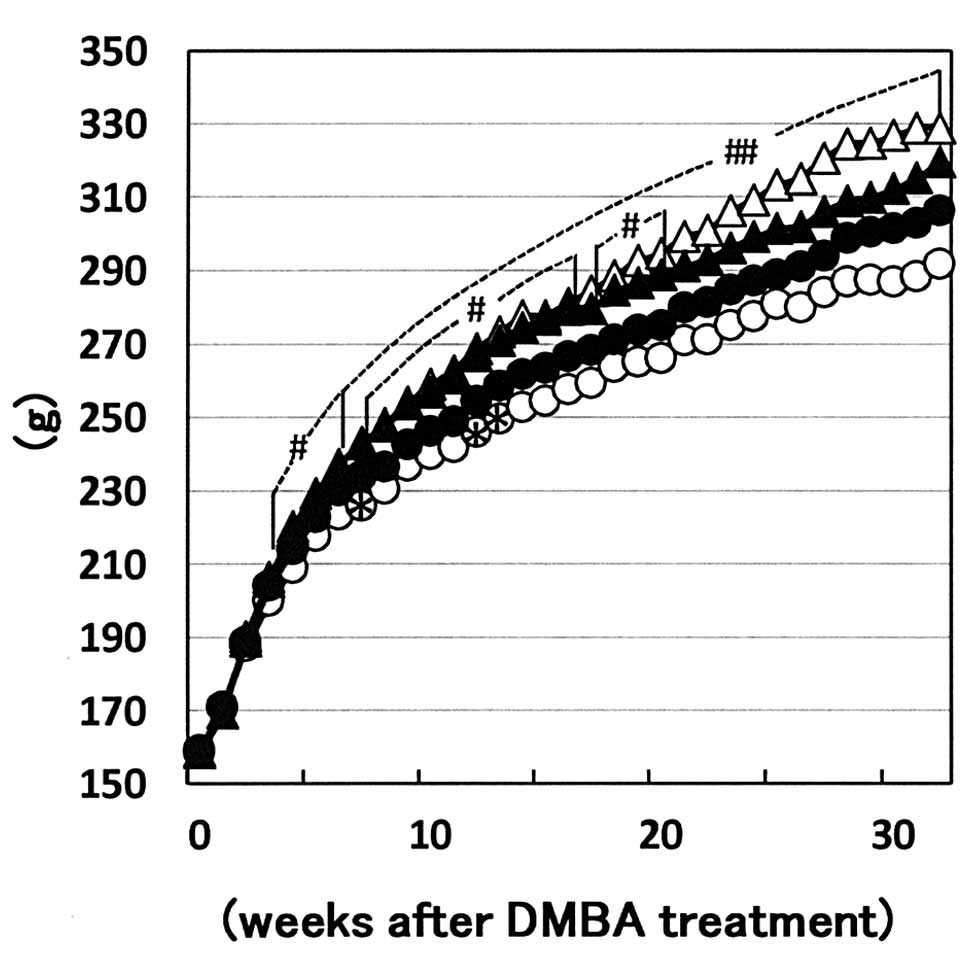
variation among the groups. Body weight curves of each group are
shown in Fig. 1. Values of the
+/+-corn oil diet group were higher than those of the +/+-basal
diet group from week 4 to the end of the experiment. In addition,
the body weights of the +/fa-corn oil diet group were higher
than those of the +/fa-basal diet group from week 8 to 20.
The differences between +/+ and +/fa of both the basal and
the corn oil diet groups were markedly smaller than those between
the basal diet and corn oil diet groups in each genotype. Average
food intake of the +/+-basal diet, +/+-corn oil diet,
+/fa-basal diet and +/fa-corn oil diet groups were
11.8–14.2, 9.2–12.8, 11.4–14.3 and 9.7–12.6 g/rat/day,
respectively, and those of the corn oil groups showed a tendency
for decrease as compared to those of the basal diet groups in both
genotypes.
Sequential changes in palpable mammary
carcinomas
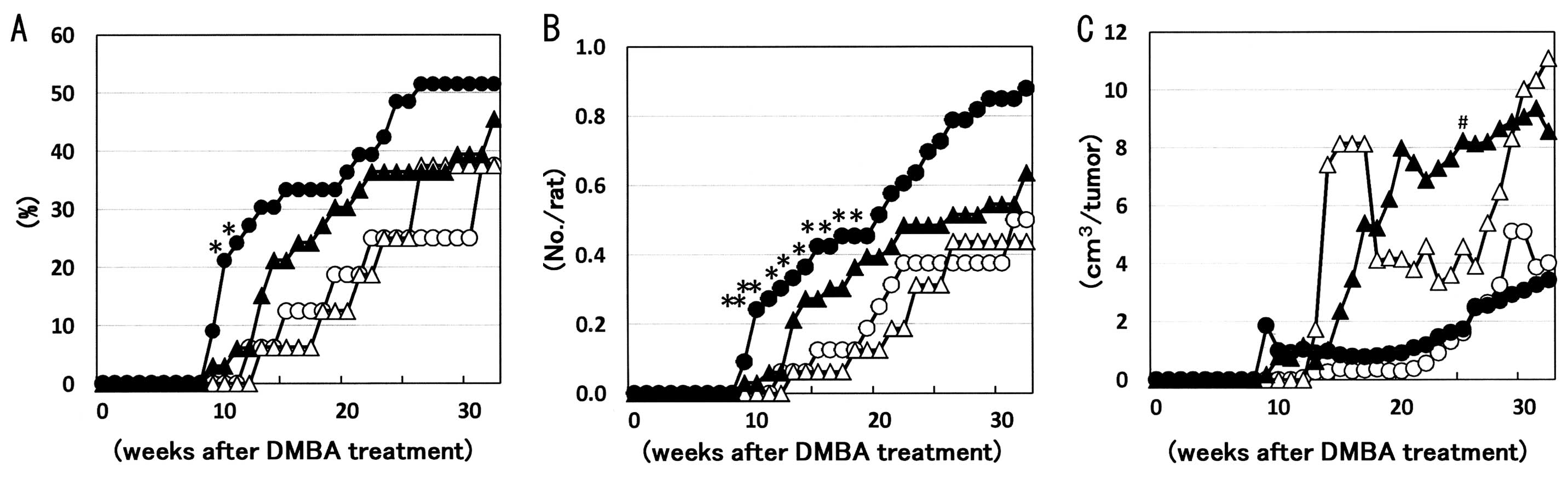
The minimum latency periods of palpable mammary
carcinomas, which were histopathologically defined postmortem, were
8 weeks following DMBA administration in both the +/fa-basal
and +/fa-corn oil diet groups, considerably shorter than the
11–12 weeks in the +/+-basal and +/+-corn oil diet groups (Fig. 2A). Incidence and multiplicity of
palpable mammary carcinomas were increased or showed a tendency for
increase in the early stages in +/fa-basal and
+/fa-corn oil diet groups as compared to their
+/+-counterparts, whereas their volume showed a tendency for
increase in the corn oil diet groups of both +/+ and +/fa as
compared to the basal diet groups (Fig.
2B and C).
Final incidence, multiplicity and volume
of mammary tumors
Incidence, multiplicity and volume findings for
histopathologically defined mammary tumors are summarized in
Table I. Histopathologically,
mammary tumors could be classified as adenocarcinomas and benign
lesions, such as adenomas, fibroadenomas and fibromas. Incidence
and multiplicity of mammary carcinomas showed a tendency for
increase (~1.5-fold) in the +/fa-basal diet group as
compared with +/+-controls, but no influence on the genotype was
noted in the corn oil diet groups. Furthermore, the corn oil diet
showed no apparent effect on the incidence and multiplicity of
mammary carcinomas in each genotype. Incidence and multiplicity of
adenomas, fibroadenomas and fibromas were similar among the groups.
On the other hand, volume of mammary carcinomas as well as
fibroadenomas showed a tendency for increase by the corn oil diet
with both +/+ and +/fa genotypes (Table II).
 | Table IFinal incidence and multiplicity data
for mammary tumors. |
Table I
Final incidence and multiplicity data
for mammary tumors.
| +/+ genotype | +/fa
genotype |
|---|
|
|
|
|---|
| Basal diet
(n=16) | Corn oil diet
(n=16) | Basal diet
(n=33) | Corn oil diet
(33) |
|---|
|
|
|
|
|
|---|
| Incidence (%) | Multiplicity
(No./rat) | Incidence (%) | Multiplicity
(No./rat) | Incidence (%) | Multiplicity
(No./rat) | Incidence (%) | Multiplicity
(No./rat) |
|---|
| Carcinoma | 7 (44) | 0.88±1.41a | 8 (50) | 0.69±0.79 | 20 (61) | 1.30±1.49 | 19 (58) | 0.94±1.27 |
| Adenoma | 1 (6) | 0.06±0.25 | 1 (6) | 0.06±0.25 | 0 | - | 1 (3) | 0.03±0.17 |
| Fibroadenoma | 4 (25) | 0.25±0.45 | 3 (19) | 0.25±0.58 | 7 (21) | 0.30±0.64 | 5 (15) | 0.24±0.61 |
| Fibroma | 0 | - | 0 | - | 2 (6) | 0.06±0.24 | 0 | - |
 | Table IIFinal volumes of mammary tumors. |
Table II
Final volumes of mammary tumors.
| +/+ genotype | +/fa
genotype |
|---|
|
|
|
|---|
| Basal diet
(n=16) | Corn oil diet
(n=16) | Basal diet
(n=33) | Corn oil diet
(n=33) |
|---|
|
|
|
|
|
|---|
| No. of tumors | Volume
(cm3/tumor) | No. of tumors | Volume
(cm3/tumor) | No. of tumors | Volume
(cm3/tumor) | No. of tumors | Volume
(cm3/tumor) |
|---|
| Carcinoma | 14 | 2.06±4.14 | 11 | 6.72±8.89 | 43 | 2.92±6.29 | 31 | 6.86±12.14 |
| Adenoma | 1 | 0.01 | 1 | 0.21 | 0 | - | 1 | 0.11 |
| Fibroadenoma | 4 | 0.26±0.23 | 4 | 18.80±31.95 | 10 | 1.49±2.54 | 8 | 4.97±13.34 |
| Fibroma | 0 | - | 0 | - | 2 | 35.56±50.21 | 0 | - |
Histopathology, immunohistochemistry and
western blot analysis of mammary carcinomas
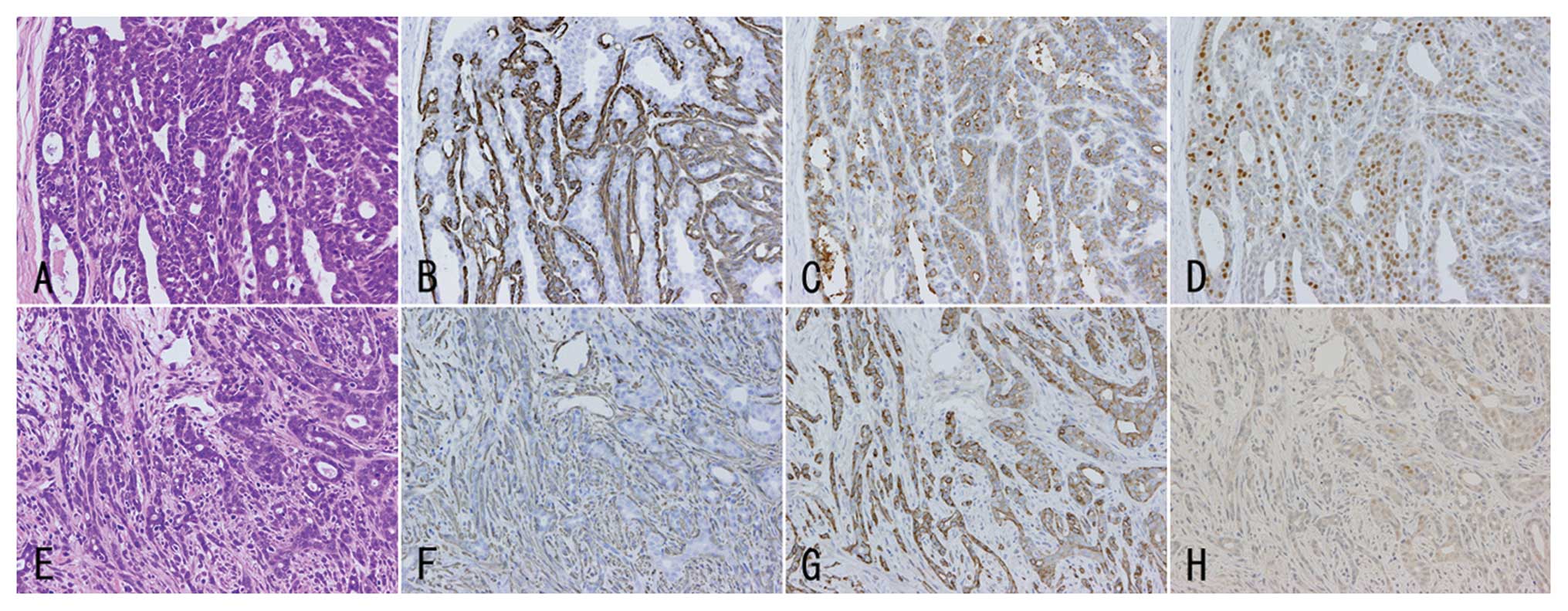
Mammary adenocarcinomas found in the present
experiment were mainly well differentiated without distinct nuclear
atypia; however, some carcinomas showed moderately/poorly
differentiated phenotypes with nuclear atypia (Fig. 3A and E). Well differentiated
carcinomas showed papillotubular structures with cribriform
patterns, and the tubules were generally well demarcated with α
smooth muscle actin-positive myoepithelial cells (Fig. 3B). On the other hand,
moderately/poorly differentiated carcinomas showed distinct
invasion with small cord/glandular or scattering patterns mainly in
the peripheral portion, and interstitial cell proliferation was
prominent (Fig. 3F). The
distribution of the sub-classified mammary carcinomas based on the
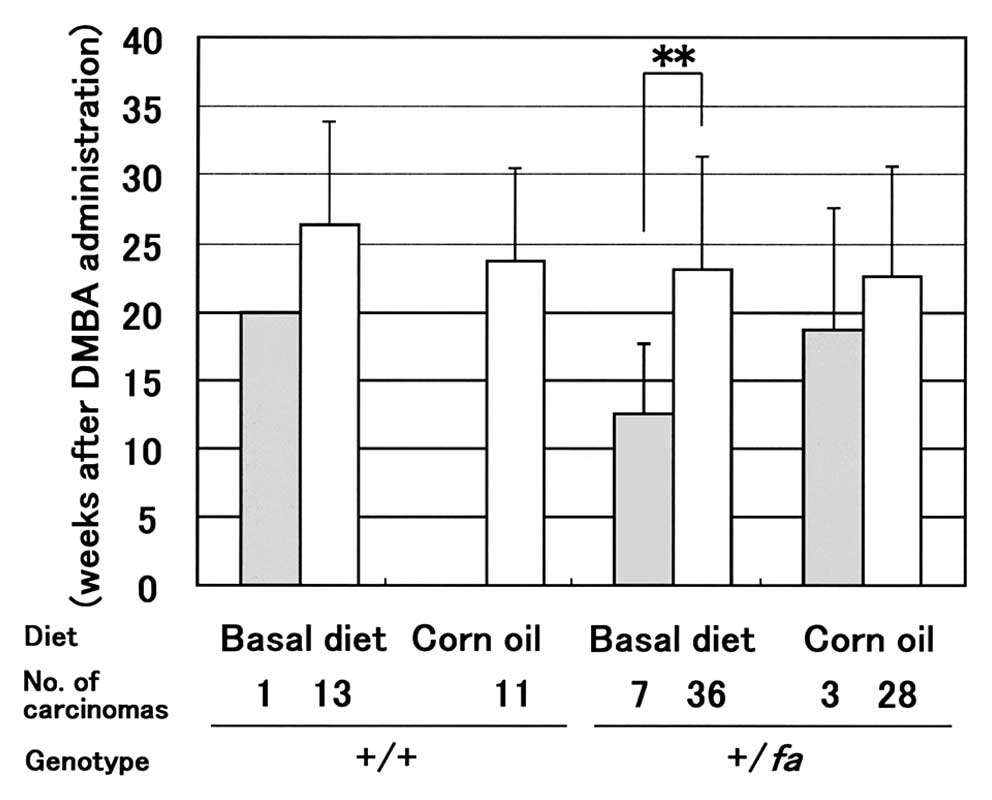
morphological phenotypes among the groups is summarized in Table III. Incidence and multiplicity of
moderately/poorly differentiated carcinomas with atypia showed a
tendency for increase in the +/fa-basal diet
and+/fa-corn oil diet groups as compared with their
+/+-counterparts (Table III). The
latency period of moderately/poorly differentiated carcinomas with
atypia was shorter than that of well differentiated carcinomas
without distinct atypia in the +/fa-basal diet group
(Fig. 4).
 | Table IIIDistribution of sub-classified
mammary carcinomas based on the morphological phenotypes among the
groups. |
Table III
Distribution of sub-classified
mammary carcinomas based on the morphological phenotypes among the
groups.
| +/+ genotype | +/fa
genotype |
|---|
|
|
|
|---|
| Basal diet
(n=16) | Corn oil diet
(n=16) | Basal diet
(n=33) | Corn oil diet
(n=33) |
|---|
|
|
|
|
|
|---|
| Incidence (%) | Multiplicity
(No./rat) | Incidence (%) | Multiplicity
(No./rat) | Incidence (%) | Multiplicity
(No./rat) | Incidence (%) | Multiplicity
(No./rat) |
|---|
| Moderately/poorly
differentiated carcinoma with atypia | 1 (6) | 0.06±0.25a | 0 | - | 6 (18) | 0.21±0.48 | 3 (9) | 0.09±0.29 |
| Well-differentiated
carcinoma without distinct atypia | 7 (44) | 0.81±1.22 | 8 (50) | 0.69±0.79 | 16 (48) | 1.09±1.49 | 17 (52) | 0.85±1.28 |
To clarify expression profiles of leptin- and
estrogen-related proteins in the well differentiated carcinomas
without distinct atypia and moderately/poorly differentiated
carcinomas with atypia, immunohistochemical and immunoblot analyses
were performed. Mammary carcinomas of both phenotypes showed
various expression intensities for leptin receptors (Fig. 3C and G) and leptin (data not shown),
whereas the cases with atypia showed lower ER α-positivities than
those without distinct atypia in the +/fa-basal diet and
+/fa-corn oil diet groups (Table IV, Fig.
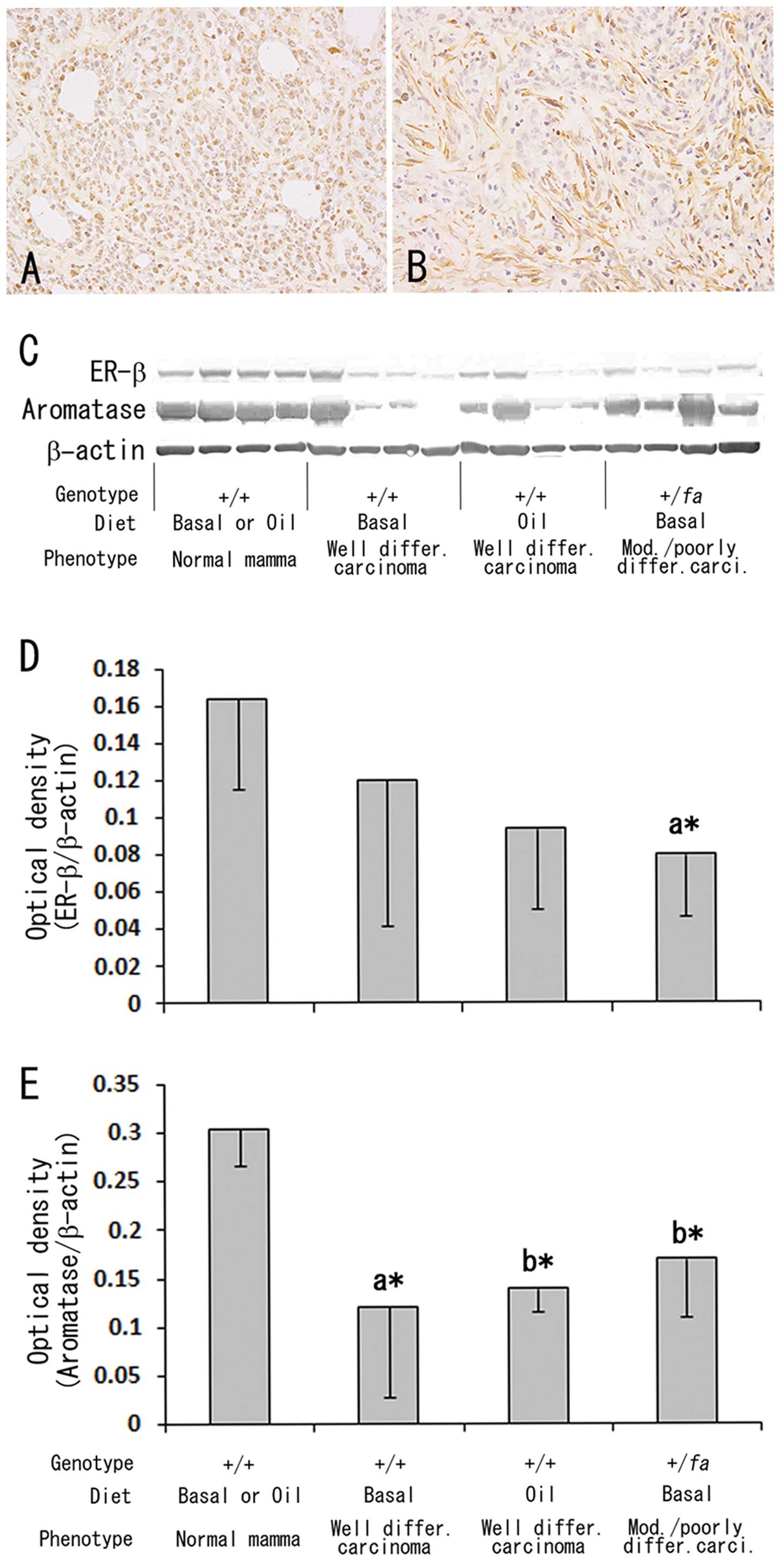
3D and H). For ER β- and aromatase-immunohistochemistry, frozen
sections of 1, 0, 3 and 3 moderately/poorly differentiated
carcinomas from the +/+-basal diet, +/+-corn oil diet,
+/fa-basal diet and +/fa-corn oil diet groups, respectively,
and 2, 4, 1 and 2 well differentiated carcinomas each were used
(Fig. 5A and B). Although no
apparent differences in the positive intensities or positive cell
ratio for ER β and aromatase were found among the combinations with
two phenotypes and two diets in the immunohistochemistry,
immunoblot analyses revealed a decrease in ER β expression levels
in moderately/poorly differentiated carcinomas (Fig. 5C and D) and decreased expression
levels of aromatase in mammary carcinomas regardless of their
phenotypes and diets as compared to the normal mammary tissue
(Fig. 5C and E).
 | Table IVEstrogen receptor (ER) α-positivity
in sub-classified mammary carcinomas based on the morphological
phenotypes. |
Table IV
Estrogen receptor (ER) α-positivity
in sub-classified mammary carcinomas based on the morphological
phenotypes.
| +/+ genotype | +/fa
genotype |
|---|
|
|
|
|---|
| Basal diet | Corn oil diet | Basal diet | Corn oil diet |
|---|
|
|
|
|
|
|---|
| No. of carcinomas
examined | ER α- positivity
(%) | No. of carcinomas
examined | ER α- positivity
(%) | No. of carcinomas
examined | ER α- positivity
(%) | No. of carcinomas
examined | ER α- positivity
(%) |
|---|
| Moderately/poorly
differentiated carcinoma with atypia | 1 | 1.0 | 0 | - | 7 | 8.9±3.9b | 3 | 14.1±11.2 |
| Well-differentiated
carcinoma without distinct atypia | 5 | 28.2±16.6a | 6 | 24.2±14.2 | 6 | 36.4±15.0 | 10 | 28.9±11.4 |
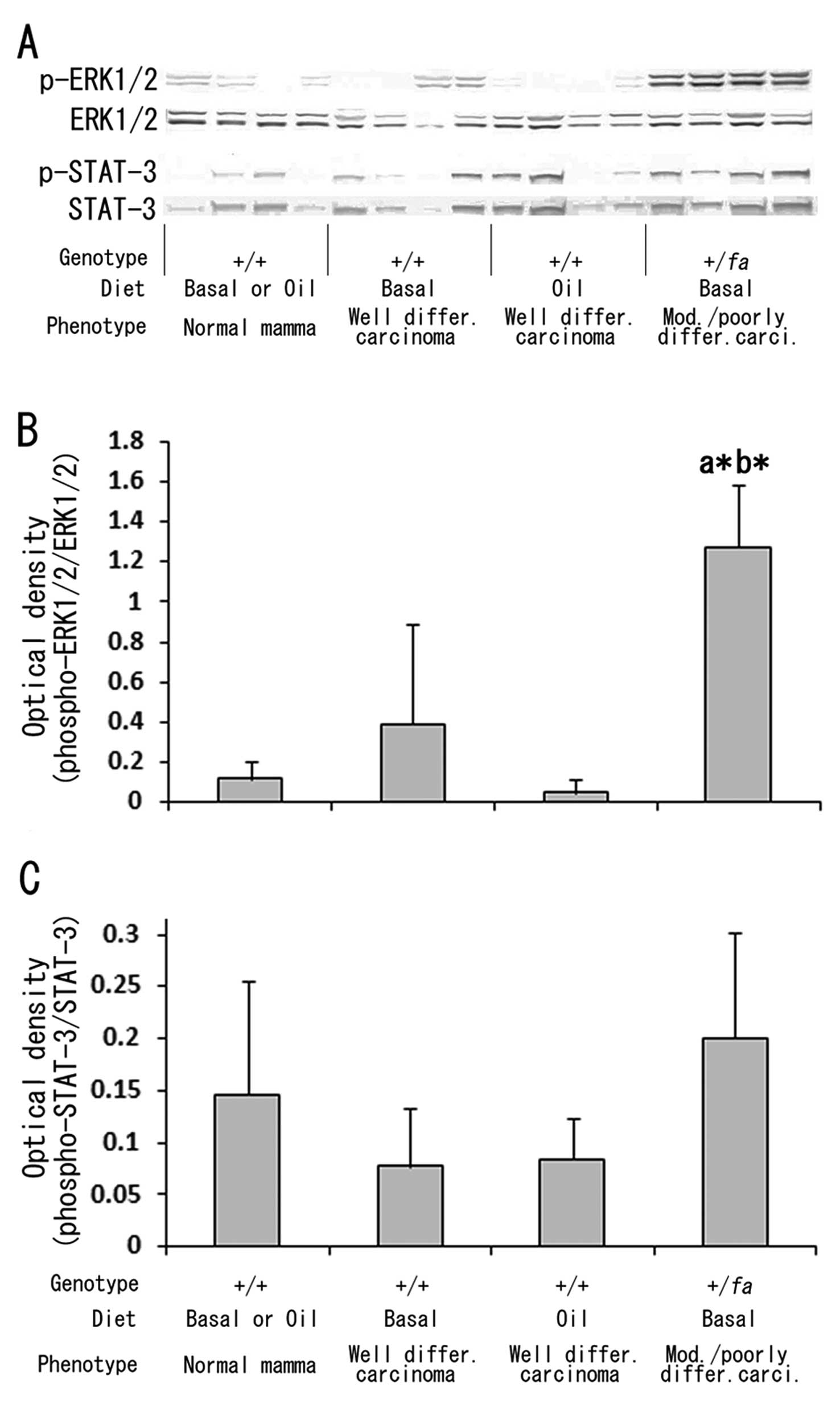
To examine the relation of intracellular signaling
cascades responsive to extracellular stimuli, such as growth
factors or cytokines, with the mammary carcinoma phenotypes,
immunoblot analyses for phosphorylation levels of ERK1/2 and STAT3
were performed, and activation of the ERK1/2 signaling pathway but
not STAT3 was demonstrated in moderately/poorly
differentiated carcinomas with atypia as compared to normal mammary
tissue and well differentiated carcinomas without distinct atypia
(Fig. 6). No influence of corn oil
diet was found with regard to either ERK1/2 or STAT3 activation
(Fig. 6).
Serum biochemistry
Data for serum levels of triglycerides, total
cholesterol and glucose at terminal sacrifice are summarized in
Table V. Although triglyceride and
total cholesterol levels declined or showed a tendency for decline
and glucose levels were elevated by the corn oil diet in the
+/fa genotype, no apparent change in these three parameters
was observed in +/+ controls. No obvious differences in these
parameters were found between the genotypes. Serum leptin levels in
the +/fa-basal diet and the +/fa-corn oil diet groups
were comparable to those in the +/+-counterparts, whereas corn oil
diet elevated serum leptin levels in the +/fa genotype with
a similar tendency for elevation in the +/+ genotype (Table V). Serum adiponectin levels in the
+/fa-basal diet group were lower than in the +/+-basal diet
group, and corn oil diet caused elevation only in the +/fa
case. Serum IGF-I levels were lower in the +/+-corn oil diet than
+/+-basal diet groups, but no change was observed with the
+/fa genotype. There was no evident variation noted in serum
insulin levels among the groups.
 | Table VSerum biochemistry data at terminal
sacrifice. |
Table V
Serum biochemistry data at terminal
sacrifice.
| +/+ genotype | +/fa
genotype |
|---|
|
|
|
|---|
| Basal diet | Corn oil diet | Basal diet | Corn oil diet |
|---|
|
|
|
|
|
|---|
| No. of samples | Serum levels | No. of samples | Serum levels | No. of samples | Serum levels | No. of samples | Serum levels |
|---|
| Triglycerides
(mg/dl) | 16 | 340.8±138.7 | 15 | 311.5±221.5 | 31 | 392.3±312.6 | 31 | 279.6±146.5 |
| Total cholesterol
(mg/dl) | 16 | 119.7±22.9 | 15 | 108.3±25.4 | 31 | 125.0±38.5 | 31 | 105.7±17.5b |
| Glucose
(mg/dl) | 16 | 134.6±15.9 | 15 | 145.3±15.4 | 31 | 133.7±13.3 | 31 | 141.9±17.5b |
| Leptin (pg/ml) | 8 | 314.4±96.4 | 7 | 691.9±540.1 | 13 | 191.6±123.1 | 12 | 506.4±439.3b |
| Adiponectin
(μg/ml) | 8 | 6.7±1.4 | 7 | 7.1±3.8 | 12 | 4.0±1.0a | 12 | 6.5±1.4c |
| Insulin
(ng/ml) | 8 | 2.1±0.8 | 7 | 1.5±1.0 | 14 | 1.3±0.9 | 13 | 1.5±1.1 |
| IGF-I (ng/ml) | 8 | 612.7±85.7 | 7 | 476.4±79.3a | 12 | 609.6±178.4 | 12 | 535.3±96.6 |
Discussion
The present DMBA-induced mammary carcinogenesis
study using heterozygous (+/fa) and wild-type (+/+) lean
Zucker rats revealed higher susceptibility of +/fa rats to
DMBA induction of mammary tumors than +/+ rats, and also
differences in histopathological phenotypes of the induced
carcinomas. In particular, the latency periods of mammary carcinoma
development in +/fa rats fed basal or corn oil diet appeared
shorter than those in +/+ rats and the incidences and
multiplicities of mammary carcinomas were increased or showed a
tendency for increase in the early stages, with a greater
percentage of more advanced cancer at the termination.
Although the body weight of +/+ and +/fa rats
fed corn oil diet were higher than those of the rats fed basal
diet, the body weight differences between +/+ and +/fa rats
fed basal diet or corn oil diet were significantly smaller.
Therefore, the short latency periods and the higher incidence and
multiplicity of mammary carcinomas in the early stages in
+/fa rats were considered not to be directly due to body
weight change. On the other hand, in our preliminary study, serum
leptin concentration at 7 weeks of age was ~140 pg/ml in
+/fa, higher (P<0.01) than ~80 pg/ml (18). These results indicated that the
increased susceptibility of +/fa rats to DMBA-induced
mammary carcinogenesis might be at least partly associated with
higher leptin levels at the initiation stage. Hyperleptinemia in
juvenile stages of +/fa rats gradually normalized and no
difference in serum leptin level was found at the terminal
sacrifice between the genotypes.
Histopathologically, adenocarcinomas in +/fa
rats were more likely to present characteristic features such as
moderate/poor differentiation, nuclear atypia, prominent
interstitial cell proliferation and low ER α positivity. Expression
levels of aromatase were decreased in mammary carcinomas regardless
of the phenotype as compared to normal mammary tissue. On the other
hand, leptin receptor and leptin were expressed with various
intensities and no distinct differences were found between
carcinomas with and without atypia. Although the cause of the
lowered ER α protein expression in the moderately/poorly
differentiated carcinomas with atypia is not clear, one possibility
is that mammary epithelial cells of +/fa rats were initiated
under conditions without estrogen-dependence but with close
dependence on other growth factors, such as leptin or EGFR
(10,23). Activation of the mitogen-activated
protein kinase (MAPK) system has been demonstrated in
moderately/poorly differentiated carcinomas with atypia. Thus,
Thordarson et al(24)
reported that N-methyl-N-nitrosourea (MNU)-induced
mammary carcinomas in ovariectomized Sprague-Dawley rats showed a
more aggressive phenotype with a significant increase in MAPK
activity (phosphorylation) as compared to carcinomas in intact
rats, suggesting a relationship between loss of estrogen-dependence
and growth. Also, in an estrogen-non-responsive human breast cancer
cell line, MAPK activity was found to be increased as compared to
the original estrogen-dependent sample, suggesting that increased
activity of MAPK may contribute to the estrogen non-responsive
growth phenotype (25).
Epidemiologically, breast cancer rates among pre-and
peri-menopausal ages are reported to be higher among US-born
Chinese than those born in foreign countries, and similar findings
were found in Filipina women as well, to the extent that
contemporary rates may equal or exceed those of non-Hispanic
Whites, indicating that becoming acculturated to the western
lifestyle might be a breast cancer risk factor to some younger
Asian women (26). Plasma leptin
levels were demonstrated to be twice as high in US-born South Asian
(India, Bangladesh, Sri Lanka) women aged 18–30 years than in
European women (27), presumably
related to the increasing rate of breast cancer. In addition, in
certain Asian countries, such as India and Singapore, breast cancer
patients present at a younger age, with more advanced stage and
fewer estrogen-ER-positive tumors, as compared to western countries
(28,29). Therefore, we propose that the
present DMBA-induced mammary carcinogenesis in +/fa lean
Zucker rats may be a useful model of increasing breast cancer in
younger Asian women.
A further significant finding of the present
DMBA-induced mammary carcinogenesis study in +/fa and +/+
rats with and without 10% corn oil diet is that elevation of serum
leptin level may contribute to the growth of mammary tumors. In our
preliminary study, corn oil diet, similarly prepared as in the
present study, significantly elevated serum leptin concentrations
of 12-week-old +/+ and +/fa rats as compared to basal diet,
as also confirmed in the present study. These data are consistent
with the previous reports of overexpression of leptin and its
receptor in human breast cancer cases (30,31),
and in in vitro studies revealing that leptin can stimulate
breast cancer cell proliferation (23,32).
From epidemiological studies, it is well recognized that obesity
increases the risk of breast cancer in postmenopausal women, with a
suggested association with menstrual and reproductive factors
(33) or higher circulating levels
of leptin. However, the mechanisms have yet to be fully elucidated
(34,35).
In conclusion, +/fa rats in the present study
proved more susceptible to DMBA-induced mammary carcinogenesis than
+/+ controls, and this might be at least partly related to the
higher leptin levels in the early stages. Corn oil diet possibly
contributed to the growth of mammary tumors via elevated serum
leptin levels. In addition, an aggressive phenotype of carcinoma,
in which MAPK cascade but not estrogen signaling was activated, was
found predominantly in +/fa rats. Further studies are
required to examine the mechanisms of MAPK activation for mammary
carcinogenesis in +/fa rats.
Acknowledgements
The present study was supported in part by a
grant-in-aid for Cancer Research (19–2) and the Third-Term
Comprehensive Control Research for Cancer (H22-G-014) from the
Ministry of Health, Labour and Welfare of Japan. The authors thank
Dr Malcolm A. Moore for revision of the text and Ms. Ayako Kaneko
and Ms. Satomi Kohno for their expert technical assistance. We also
thank the National Cancer Center Research Core Facility for some of
the analyses in this study. The Core Facility was supported by the
National Cancer Center Research and Development Fund (23-A-7).
References
|
1
|
van den Brandt PA, Spiegelman D, Yaun SS,
et al: Pooled analysis of prospective cohort studies on height,
weight, and breast cancer risk. Am J Epidemiol. 152:514–527.
2000.PubMed/NCBI
|
|
2
|
Lahmann PH, Hoffmann K, Allen N, et al:
Body size and breast cancer risk: findings from the European
Prospective Investigation into Cancer and Nutrition (EPIC). Int J
Cancer. 111:762–771. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reeves GK, Pirie K, Beral V, Green J,
Spencer E and Bull D: Cancer incidence and mortality in relation to
body mass index in the Million Women Study: cohort study. BMJ.
335:11342007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iwasaki M, Otani T, Inoue M, Sasazuki S
and Tsugane S: Body size and risk for breast cancer in relation to
estrogen and progesterone receptor status in Japan. Ann Epidemiol.
17:304–312. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Key TJ, Appleby PN, Reeves GK, et al: Body
mass index, serum sex hormones, and breast cancer risk in
postmenopausal women. J Natl Cancer Inst. 95:1218–1226. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Auwerx J and Staels B: Leptin. Lancet.
351:737–742. 1998. View Article : Google Scholar
|
|
7
|
Wu MH, Chou YC, Chou WY, et al:
Circulating levels of leptin, adiposity and breast cancer risk. Br
J Cancer. 100:578–582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soma D, Kitayama J, Yamashita H, Miyato H,
Ishikawa M and Nagawa H: Leptin augments proliferation of breast
cancer cells via transactivation of HER2. J Surg Res. 149:9–14.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saxena NK, Taliaferro-Smith L, Knight BB,
et al: Bidirectional crosstalk between leptin and insulin-like
growth factor-I signaling promotes invasion and migration of breast
cancer cells via transactivation of epidermal growth factor
receptor. Cancer Res. 68:9712–9722. 2008. View Article : Google Scholar
|
|
10
|
Cirillo D, Rachiglio AM, la Montagna R,
Giordano A and Normanno N: Leptin signaling in breast cancer: an
overview. J Cell Biochem. 105:956–964. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chua SC Jr, Chung WK, Wu-Peng XS, et al:
Phenotypes of mouse diabetes and rat fatty due to mutations in the
OB (leptin) receptor. Science. 271:994–996. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bray GA: The Zucker-fatty rat: a review.
Fed Proc. 36:148–153. 1977.
|
|
13
|
Rayner DV, Dalgliesh GD, Duncan JS, Hardie
LJ, Hoggard N and Trayhurn P: Postnatal development of the ob gene
system: elevated leptin levels in suckling fa/fa rats. Am J
Physiol. 273:R446–R450. 1997.PubMed/NCBI
|
|
14
|
Lee WM, Lu S, Medline A and Archer MC:
Susceptibility of lean and obese Zucker rats to tumorigenesis
induced by N-methyl-N-nitrosourea. Cancer Lett.
162:155–160. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hakkak R, Holley AW, Macleod SL, et al:
Obesity promotes 7,12-dimethylbenz(a)anthracene-induced mammary
tumor development in female zucker rats. Breast Cancer Res.
7:R627–R633. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hakkak R, MacLeod S, Shaaf S, et al:
Obesity increases the incidence of
7,12-dimethylbenz(a)anthracene-induced mammary tumors in an
ovariectomized Zucker rat model. Int J Oncol. 30:557–563.
2007.PubMed/NCBI
|
|
17
|
de Assis S, Wang M, Goel S, Foxworth A,
Helferich W and Hilakivi-Clarke L: Excessive weight gain during
pregnancy increases carcinogen-induced mammary tumorigenesis in
Sprague-Dawley and lean and obese Zucker rats. J Nutr.
136:998–1004. 2006.PubMed/NCBI
|
|
18
|
Cho YM, Imai T, Takami S, Ogawa K and
Nishikawa A: Female heterozygous (+/fa) Zucker rats as a
novel leptin-related mammary carcinogenesis model. J Toxicol Sci.
37:1025–1034. 2012.
|
|
19
|
Phillips MS, Liu Q, Hammond HA, et al:
Leptin receptor missense mutation in the fatty Zucker rat. Nat
Genet. 13:18–19. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Imai T, Cho YM, Hasumura M and Hirose M:
Enhancement by acrylamide of
N-methyl-N-nitrosourea-induced rat mammary tumor
development-possible application for a model to detect co-modifiers
of carcinogenesis. Cancer Lett. 230:25–32. 2005.PubMed/NCBI
|
|
21
|
Cho YM, Imai T, Hasumura M and Hirose M:
Lack of enhancement of susceptibility to mammary and thyroid
carcinogenesis in rats exposed to DMBA and DHPN following
prepubertal iodine deficiency. Cancer Sci. 97:1031–1036. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ip C, Carter CA and Ip MM: Requirement of
essential fatty acid for mammary tumorigenesis in the rat. Cancer
Res. 45:1997–2001. 1985.PubMed/NCBI
|
|
23
|
Mauro L, Catalano S, Bossi G, et al:
Evidences that leptin up-regulates E-cadherin expression in breast
cancer: effects on tumor growth and progression. Cancer Res.
67:3412–3421. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thordarson G, Lee AV, McCarty M, et al:
Growth and characterization of
N-methyl-N-nitrosourea-induced mammary tumors in
intact and ovariectomized rats. Carcinogenesis. 22:2039–2047.
2001.PubMed/NCBI
|
|
25
|
Coutts AS and Murphy LC: Elevated
mitogen-activated protein kinase activity in estrogen-non
responsive human breast cancer cells. Cancer Res. 58:4071–4074.
1998.PubMed/NCBI
|
|
26
|
Gomez SL, Quach T, Horn-Ross PL, et al:
Hidden breast cancer disparities in Asian women: disaggregating
incidence rates by ethnicity and migrant status. Am J Public
Health. 100(Suppl 1): S125–S131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kalhan R, Puthawala K, Agarwal S, Amini SB
and Kalhan SC: Altered lipid profile, leptin, insulin, and
anthropometry in offspring of South Asian immigrants in the United
States. Metabolism. 50:1197–1202. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ghumare SS and Cunningham JE: Breast
cancer trends in Indian residents and emigrants portend an emerging
epidemic for India. Asian Pac J Cancer Prev. 8:507–512.
2007.PubMed/NCBI
|
|
29
|
Lim SE, Back M, Quek E, Iau P, Putti T and
Wong JE: Clinical observations from a breast cancer registry in
Asian women. World J Surg. 31:1387–1392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ishikawa M, Kitayama J and Nagawa H:
Enhanced expression of leptin and leptin receptor (OB-R) in human
breast cancer. Clin Cancer Res. 10:4325–4331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Garofalo C, Koda M, Cascio S, et al:
Increased expression of leptin and the leptin receptor as a marker
of breast cancer progression: possible role of obesity-related
stimuli. Clin Cancer Res. 12:1447–1453. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ray A, Nkhata KJ and Cleary MP: Effects of
leptin on human breast cancer cell lines in relationship to
estrogen receptor and HER2 status. Int J Oncol. 30:1499–1509.
2007.PubMed/NCBI
|
|
33
|
Iwasaki M, Otani T, Inoue M, Sasazuki S
and Tsugane S: Role and impact of menstrual and reproductive
factors on breast cancer risk in Japan. Eur J Cancer Prev.
16:116–123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grossmann ME, Ray A, Nkhata KJ, et al:
Obesity and breast cancer: status of leptin and adiponectin in
pathological processes. Cancer Metastasis Rev. 29:641–653. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hjartaker A, Langseth H and Weiderpass E:
Obesity and diabetes epidemics: cancer repercussions. Adv Exp Med
Biol. 630:72–93. 2008. View Article : Google Scholar : PubMed/NCBI
|