Introduction
Neuroblastoma is the most common extracranial solid
tumor in infancy and childhood (1).
Currently, chemotherapy remains the key therapy for neuroblastoma
in the clinic. However, inevitable severe side-effects and multiple
drug resistance (MDR) limit its application. The mechanism of
chemoresistance is complicated and current research involving
reversal of MDR is still in the experimental stage (2–4). It is
necessary to develop novel methods to enhance chemosensitivity and
to reverse the MDR. Using high-intensity focused ultrasound (HIFU)
treatment, the fringe area of tumor tissue treated by low intensity
ultrasound is usually damaged, but is not necrotic (5–7). In
addition, the tumor tissue is sensitive to chemotherapeutic drugs,
even tissue that was insensitive to chemotherapy prior to HIFU
therapy (8). The ability of LIPUS
to reverse tumor MDR and enhance the sensitivity to chemotherapy
drugs has, therefore, been confirmed (9–12).
The aim of the present study was to investigate the
mechanism underlying low-intensity pulsed ultrasound (LIPUS) in
reversing MDR of the human neuroblastoma multidrug resistant cell
line SK-N-SH/MDR1. We applied various ultrasound intensities to
determine the optimum ultrasonic to increase the sensitivity of
SK-N-SH/MDR1 to chemotherapeutic drugs. We then analyzed the
membrane alterations and MDR-related proteins [P-glycoprotein
(P-gp), multidrug resistance protein 1 (MRP1), and
glutathione-S-transferase-π (GST-π)] of SK-N-SH/MDR1 following
optimum ultrasonic to elucidate the possible mechanism involved in
reversing MDR.
Materials and methods
Cell culture
The HEK 293 cell line was a gift from professor
Tong-Chuan He (Laboratory of Molecular Oncology, University of
Chicago, USA). The human neuroblastoma cell line SK-N-SH was
purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA) and was preserved in our laboratory. The cells
were maintained in Dulbecco’s modified Eagle’s medium/Nutrient
Mixture F-12 (DMEM/F12) supplemented with 10% fetal bovine serum
(PAA Laboratories GmbH, Cölbe, Germany) at 37°C with 5%
CO2.
Preparation of high titer adenovirus
vector supernatant
Recombinant adenoviral vectors expressing green
fluorescence protein gene (GFP) and multidrug resistance gene 1
(MDR1) (Ad-GFP-MDR1) and only GFP (Ad-GFP) were previously prepared
in our laboratory (13). HEK 293
cells transducted with an appropriate multiplicity of infection
(MOI) of Ad-GFP-MDR1 and Ad-GFP were harvested and frozen using a
dry ice/methanol bath, immediately thawed in a 37°C water bath and
vortexed. After expanding and purifying the cells using density
gradient centrifugation (14), the
high titer recombinant adenoviruses Ad-GFP-MDR1 and Ad-GFP were
harvested.
Transduction of SK-N-SH cells with
adenoviral vector supernatant
Logarithmic phase SK-N-SH cells were divided into 3
groups. Cells in group 1 transducted with Ad-GFP-MDR1, which served
as the experimental group, were referred to as SK-N-SH/MDR1. Cells
in group 2 transducted with Ad-GFP served as the control group and
were referred to as SK-N-SH/GFP. A third group of untransducted
cells served as a blank control, referred to as SK-N-SH.
The cells were plated on 96-well plates at a density
of 2.0×105 cells/well. After culturing for 16 h, the
cells of each group were divided into 6 subgroups and transducted
with adenoviral vector according to increasing MOI: MOI = 5, 10,
50, 100, 200 or 400. Each subgroup contained 6 repeated pores. The
efficiency of transduction was quantified using fluorescence
microscopy and flow cytometry was performed 48 h after
transduction. The experiments were repeated in triplicate.
Ultrasound equipment and irradiation
A low-intensity ultrasonic irradiation system
provided by Chongqing Medical University Ultrasonic Engineering
Institute (transducer diameter, 1.8 cm; frequency 0.3 MHz; China)
was used. SK-N-SH/MDR1 cells were suspended in culture serum at
1×105 cells/well and prepared for irradiation. The
SK-N-SH/MDR1 cell suspension was randomly divided into three
groups: the 0.5 W/cm2 group, the 1.0 W/cm2
group and the 1.5 W/cm2 group. The exposure times for
the experimental group were 10, 20, 30, 40, 60, 90, 120, 160 and
200 sec with sham irradiation for the control subgroup. Each
subgroup contained 1 ml cell suspension.
Analysis of the optimum irradiation of
reversing MDR of SK-N-SH/MDR1 using methyl-thiazolyl-tetrazolium
(MTT) assay
Following irradiation, 150 μl of each subgroup of
cell suspensions were plated on 96-well plates. The cell
suspensions in the experimental and the control subgroup were
divided into 3 repeated pores. Subsequently, the cells were stained
with 20 μl of 5.0 mg/ml sterile MTT solution
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide;
Sigma, St. Louis, MO, USA] for 4 h at 37°C, after which the medium
was removed and thoroughly mixed with 100 μl dimethyl sulfoxide
(DMSO; Merck Inc., Whitehouse Station, NJ, USA) to dissolve
formazan crystals. The cells were then agitated for 10 min, and
their absorbance was measured at 490 nm using a spectrophotometric
microplate reader (Bio-Rad Laboratories In., Hercules, CA, USA).
Each treatment group was analyzed in triplicate, and the experiment
was repeated 3 times. The optimum ultrasonic irradiation conditions
were selected by the value of M calculated using the following
formula: M = average OD value of the experimental cells/average OD
value of the control cells.
Analysis of chemosensitivity using MTT
assay
To assess multidrug resistance of SK-N-SH,
SK-N-SH/GFP, SK-N-SH/MDR1, and SK-N-SH/MDR1 with optimum
irradiation, cells were plated on 96-well plates at a density of
3.0×105 cells/well and incubated for 24 h. Then, the
medium was removed, replaced with fresh medium containing different
concentrations of daunorubicin (DNR; Pharmacia Italia S.p.A, Milan,
Italy), adriamycin (ADM; Pharmacia Italia S.p.A), vincristine (VCR;
Wanle Pharmaceutical Factory, Shenzhen, China), and
cyclophosphamide (CTX; Jiangsu Hengrui Pharmaceutical Co., Ltd.,
Jiangsu, China) and incubated for another 48 h. The cells were then
stained with 20 μl of MTT solution (5.0 mg/ml) for 4 h at 37°C, and
the medium was removed and thoroughly mixed with 100 μl dimethyl
sulfoxide (DMSO) to dissolve formazan crystals. Absorbance was read
at 490 nm using a spectrophotometric microplate reader (Bio-Rad
Laboratories). The inhibition ratio of tumor cells at each drug
concentration was calculated using the following formula:
Inhibition ratio (%) = (1 - average OD value of the experimental
cells/average OD value of the control cells) × 100. The half
inhibiting concentration (IC50) of each chemotherapeutic
drug was determined from the inhibition ratio for each
concentration (15).
Scanning electron microscopy (SEM)
The structural changes of SK-N-SH/MDR1 cells exposed
to optimum ultrasonic or sham irradiation were observed by SEM. The
cells were washed with PBS twice and then suspended by PBS. The
cell suspensions were dropped on a cover glass with a gold-plated
membrane for 30 min. The cells were fixed with 4% formalin for 2–3
min and then washed with triple PBS for 10 min. The cells were then
fixed with 1% osmic acid for 20–30 min and then washed with iced
distilled water three times. The cells were soaked in the 2% tannin
at 4°C overnight, dehydrated using graded ethanol, and lyophilized
by tertiary butyl alcohol overnight. Finally, the cells were coated
using a vacuum spray plating instrument and images were
captured.
Immunofluorescence
Immunofluorescent staining was used to compare the
localization and expression of P-gp, MRP1 and GST-π in SK-N-SH/MDR1
exposed to optimum ultrasonic vs. sham irradiation. The cell slides
were fixed with 2% paraformaldehyde and washed with PBS three
times. The cell slides were blocked with rabbit serum for 10 min at
room temperature and then incubated with rabbit polyclonal
anti-P-gp antibody (diluted 1:200, Santa Cruz Biotechnology, Santa
Cruz, CA, USA), rabbit polyclonal anti-MRP1 antibody (diluted
1:200, Santa Cruz Biotechnology) and rabbit polyclonal anti-GST-π
antibody (diluted 1:200, Santa Cruz Biotechnology), respectively,
at 4°C overnight. Negative controls were conducted by exchange of
primary antibody for PBS. After rinsing with PBS, the cell slides
were incubated with anti-rabbit IgG conjugated with TRITC (diluted
1:100, Sigma) for 1 h at 37°C in darkness. The protein expression
was observed by fluorescent microscopy after being counterstained
with Hoechst and mounted with water-solubility mounting agents.
Western blotting
Western blot analysis was performed to investigate
the expression of P-gp, MRP1 and GST-π. The cells were harvested
and lysed in lysis buffer (0.5% Nonidet P-40, 10 mM Tris-HCl pH
7.4, 150 mM NaCl, 1 mM EDTA and 1 mM Na3VO4)
supplemented with protease inhibitors and 1 mM phenylmethylsulfonyl
fluoride (PMSF). Equal amounts of proteins were separated by
SDS-PAGE for western blot analysis, and the proteins were
transferred to polyvinylidene fluoride (PVDF) membranes (Millipore
Corp., Billerica, MA, USA), which were then blocked for 1 h with 5%
non-fat milk in 10 mM Tris-HCl pH 7.5, 100 mM NaCl and 0.1% (w/v)
Tween-20. The membranes were first incubated with antibodies
against β-actin, P-gp, MRP1 or GST-π (all from Santa Cruz
Biotechnology) overnight at 4°C, followed by a 1.5-h incubation
with horseradish peroxidase-conjugated secondary antibody. The
protein signals were detected using an enhanced chemiluminescence
kit (Biyuntian Biotech, Beijing, China) and analyzed using the
Bio-Rad (USA) imaging system and the associated software according
to the manufacturer’s instructions.
Statistical analysis
Results are presented as the means ± standard
deviation (SD). ANOVA and paired-samples t-test were performed to
compare mean values between groups. The significance level was set
at 5% for each analysis.
Results
Production of recombinant adenoviruses in
HEK 293 cells
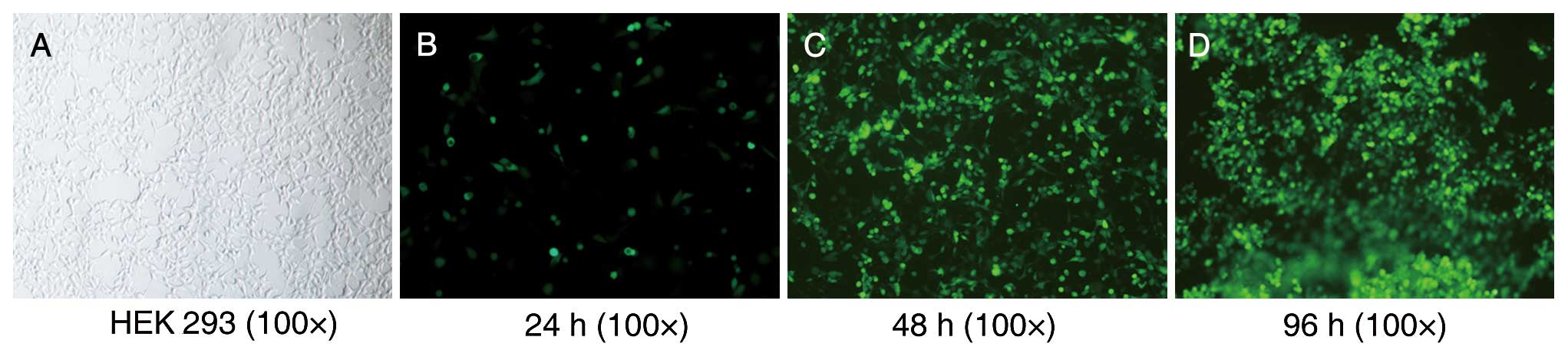
After culturing for 4–5 days, the HEK 293 cells
transducted with the recombinant adenoviruses Ad-GFP-MDR1 and
Ad-GFP transducted cells were observed floating in the media under
a fluorescence microscope (Fig. 1).
The viral titers of Ad-GFP-MDR1 and Ad-GFP ranged between 2.0 and
3.0×109 plaque forming units (PFU)/ml.
Fluorescence and adenovirus
quantification in SK-N-SH cells
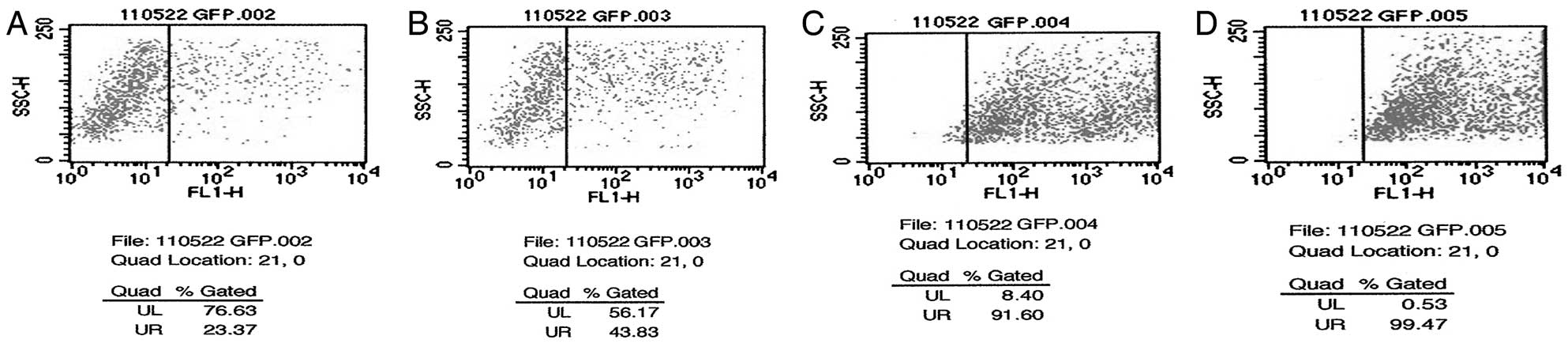
The expression of GFP in SK-N-SH cells was observed
48 h after transduction using flow cytometry. As shown in Fig. 2, the transduction efficiency
increased with increasing concentrations of adenovirus. Both the
survival rate (over 80%) and the transduction efficiency (~90%) of
SK-N-SH cells were relatively high when the adenovirus MOI = 100.
Thus, an MOI = 100 was used in further experiments. A
multidrug-resistant cell line SK-N-SH/MDR1 was transduced from an
SK-N-SH cell line of neuroblastoma with adenoviral vectors encoding
the MDR1 and the GFP (Ad-GFP-MDR1).
Optimum irradiation of reversing MDR of
SK-N-SH/MDR1
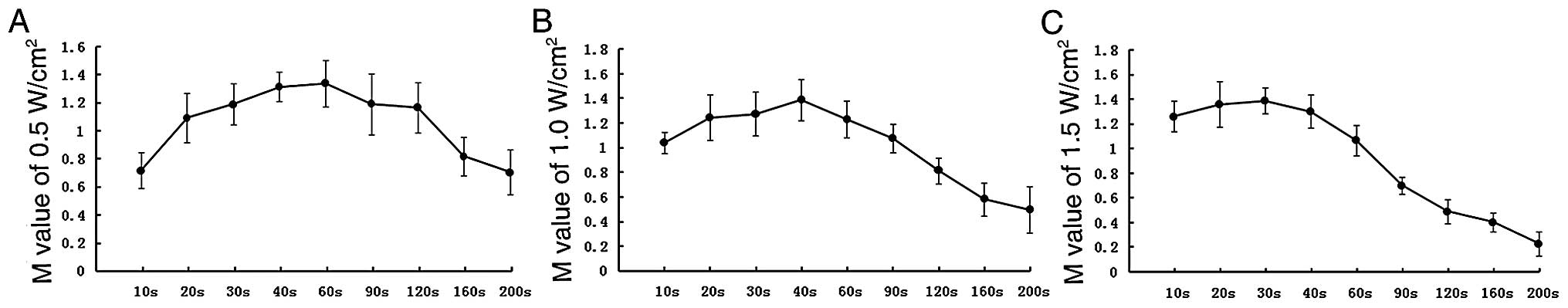
LIPUS may damage the structural integrity of the
membrane of tumor cells mechanically via sonoporation (16–19).
In our experiment, the effect of proliferation could be excluded by
reason of detecting M value immediately after irradiation. As shown
in Fig. 3, with the irradiation
dose increased, sonoporation enhanced, more MTT entered tumor cells
as a result of cell membrane permeability increasing, M value
increased. When the irradiation dose was beyond the peak, tumor
cells were killed and M value decreased. It could be concluded that
the largest number of anticancer drugs enter the living cells for
the strongest permeability of cell membrane at the peak M value,
and the peak M value of SK-N-SH/MDR1 for different doses of
irradiation was the optimum ultrasonic. Thus, 0.3 MHz, 1.0
W/cm2 and 40 sec was chosen as the optimum ultrasonic
condition, so that the experimental conditions would be consistent
for the next experiment.
Sensitivity to anticancer drugs
The MTT assay allowed us to verify the drug
sensitivity of SK-N-SH, SK-N-SH/MDR1, and SK-N-SH/MDR1 with optimum
ultrasound (SK-N-SH/ MDR1+U) to anticancer drugs DNR, ADM, VCR, CTX
and 5-FU, which are commonly used drugs in cancer therapy. As shown
in Table I, the MDR1 transductants
exhibited decreased sensitivity to the anticancer drugs (P<0.05)
which confirmed the multidrug-resistant cell line SK-N-SH/MDR1 was
constructed successfully. Notably, the IC50 of
SK-N-SH/MDR1 with the optimum irradiation was significantly lower
than that of SK-N-SH and SK-N-SH/MDR1 cells, and the difference was
statistically significant (P<0.05). This finding shows that the
optimum irradiation could reverse the MDR of SK-N-SH/MDR1.
 | Table IIC50 (μg/ml) for DNR, ADM,
VCR and CTX in SK-N-SH, SK-N-SH/GFP, SK-N-SH/MDR1 and
SK-N-SH/MDR1+U. |
Table I
IC50 (μg/ml) for DNR, ADM,
VCR and CTX in SK-N-SH, SK-N-SH/GFP, SK-N-SH/MDR1 and
SK-N-SH/MDR1+U.
| IC50
(μg/ml) |
|---|
|
|
|---|
| Cell line | DNR | ADM | VCR | CTX |
|---|
| SK-N-SH | 0.066±0.011 | 0.065±0.017 | 0.366±0.043 | 2.039±0.213 |
| SK-N-SH/GFP | 0.070±0.020 | 0.047±0.028 | 0.314±0.102 | 1.930±0.572 |
| SK-N-SH/MDR1 | 0.629±0.092a | 0.484±0.118a | 1.526±0.184a | 8.022±1.044a |
| SK-N-SH/MDR1+U | 0.026±0.014b | 0.041±0.011b | 0.191±0.017b | 1.364±0.232b |
Effect of LIPUS on the morphology and
microstructure of SK-N-SH/MDR1
To verify the effects of LIPUS on morphology and
microstructure of SK-N-SH/MDR1, we observed the cells by microscopy
following different doses of irradiation. We found that with an
increase in irradiation intensity, the SK-N-SH/MDR1 became swollen
and round. The clearest difference occurred at the optimum
irradiation (Fig. 4B). As the
intensity of irradiation increased continuously, the cells were
injured, they became necrotic and condensed (Fig. 4C).
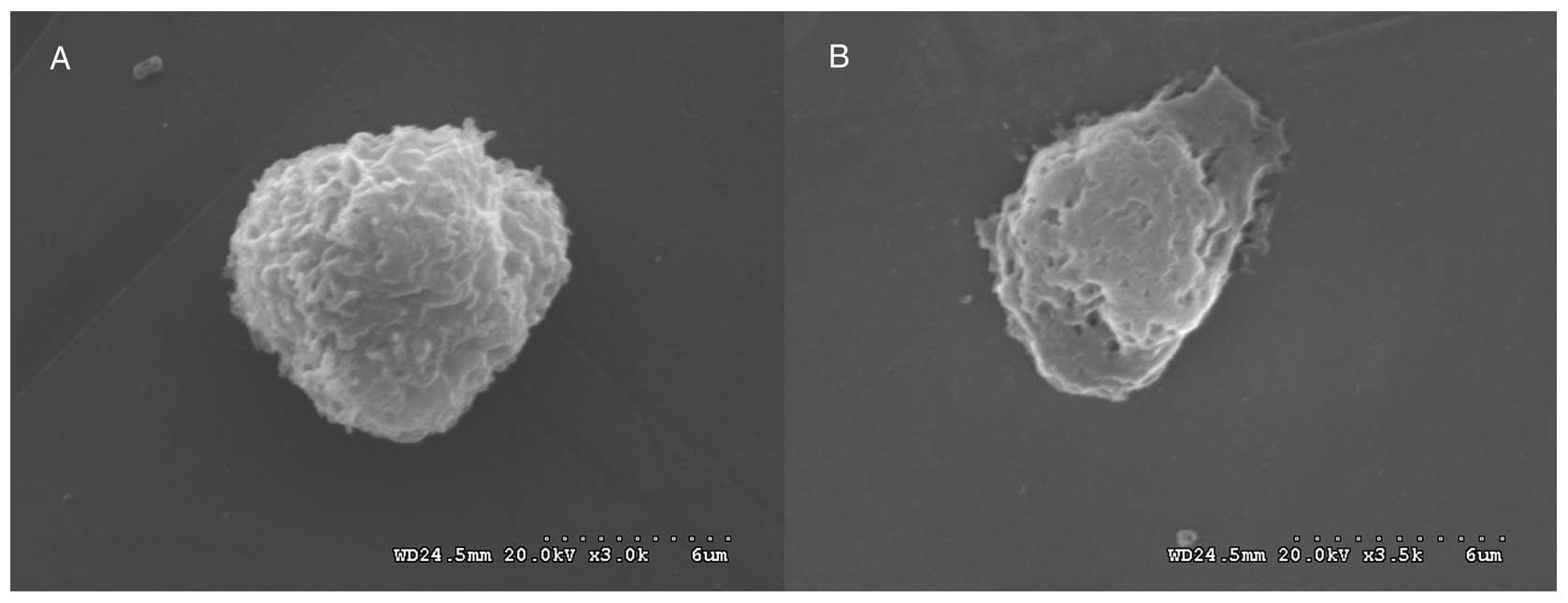
To compare the changes prior to and following the
optimum irradiation on a microstructural level, SEM was performed.
In Fig. 5, SEM shows that
SK-N-SH/MDR1, covered by microvilli on their cell surfaces,
contained holes of various diameters in their cell membranes, and
the number of microvilli was reduced (and even disappeared)
following optimum irradiation.
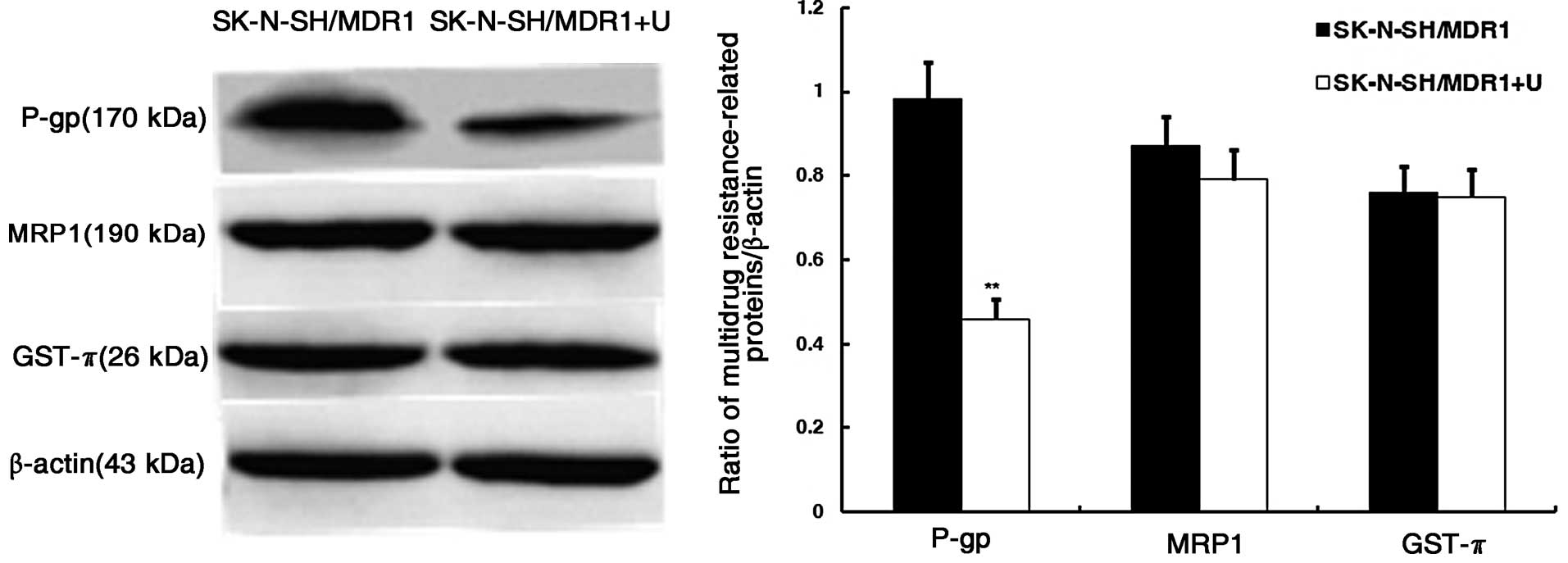
Distribution and expression changes of
P-gp, MRP and GST-π in SK-N-SH/MDR1 in optimum irradiation
Immunofluorescent staining revealed P-gp expression
was significantly decreased in the membranes under the optimum
irradiation, whereas MRP and GST-π in the cytoplasm and nucleus,
respectively, did not change significantly, as indicated by
fluorescent-red stain (Fig. 6). The
expression of P-gp, MRP, and GST-π in SK-N-SH by western blot
analysis also revealed that P-gp was downregulated, but MRP and
GST-π were not differently expressed under the optimum irradiation
(Fig. 7).
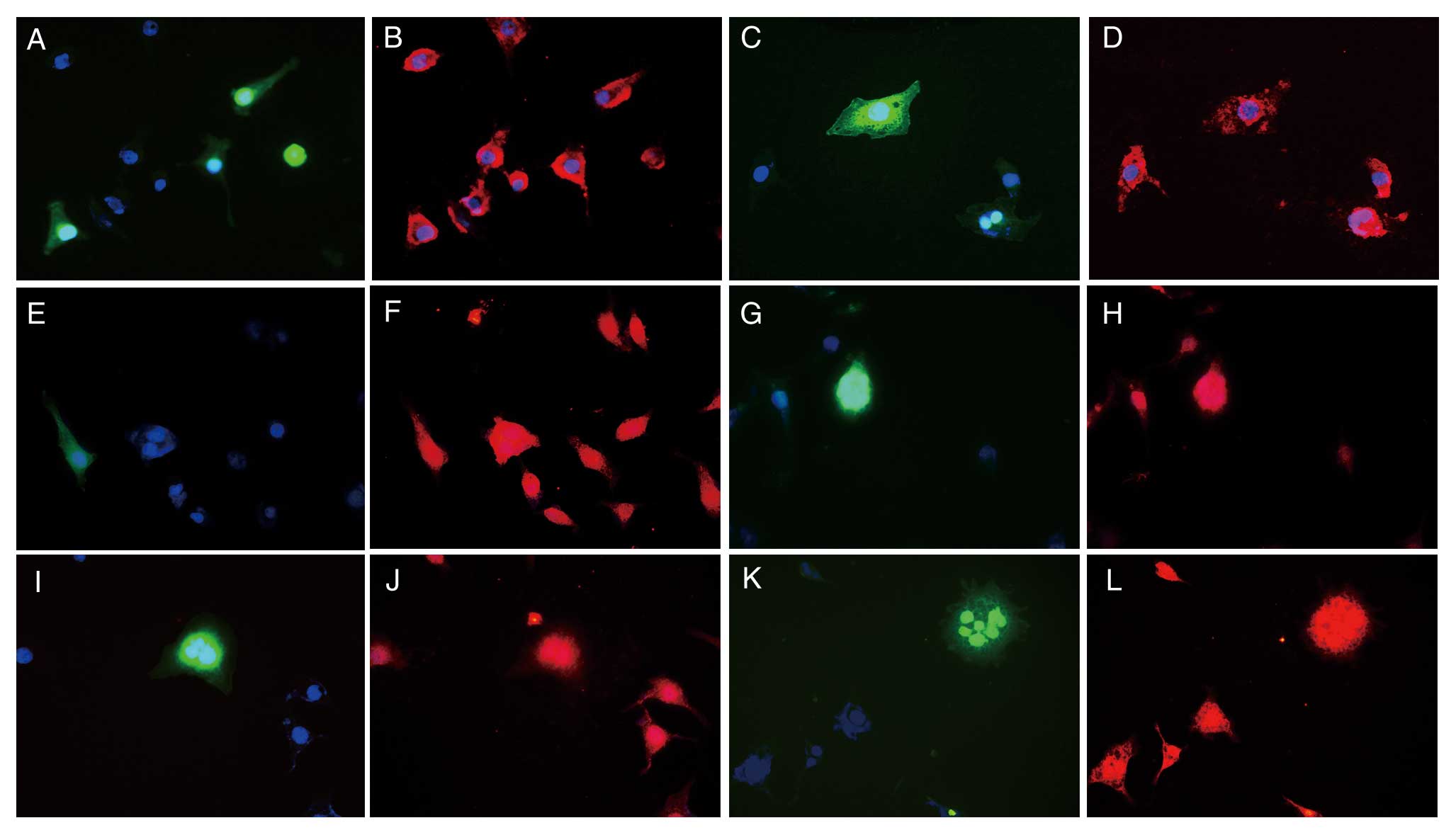 | Figure 6Distribution and expression changes of
the P-gp, MRP1 and GST-π in SK-N-SH/MDR1 in optimum irradiation as
determined by immunofluorescent staining analysis (magnification,
×400). (A–D) P-gp; (E–H) MRP1; (I–L) GST-π; (A, B, E, F, I and J)
SK-N-SH/MDR1 with sham ultrasonic; (C, D, G, H, K and L)
SK-N-SH/MDR1 with optimum ultrasonic; blue staining shows nucleus;
green staining shows the GFP expression of Ad-GFP-MDR1; red
staining shows multidrug resistance-related proteins. |
Discussion
According to Jemal et al(20), malignant cancer is a leading cause
of mortality (24.45%). Chemotherapy is often ineffectual due to the
MDR of some types of cancer. Tumor cell membrane barriers and the
high expression of MDR-related proteins such as P-gp, MRP1 and
GST-π are the critical factors blocking anticancer drugs from
playing their role (21–23). HIFU demonstrates advantages in tumor
treatment (24–26). However, HIFU has significant
side-effects such as long treatment times that result in dermal
burns and lack of absorption by necrotic tumor tissue (27,28).
During the clinical application of HIFU, it was discovered that the
fringe area of tumor tissue treated by LIPUS was more sensitive to
anticancer drugs. In addition, even tissue that was insensitive to
chemotherapy prior to HIFU therapy become sensitive, which
confirmed that LIPUS could reverse the MDR of cancer cells.
In our study, resistant cell line SK-N-SH/MDR1 was
constructed, and it was confirmed that 0.3 MHz, 1.0
W/cm2, a duration of 40 sec was the optimum ultrasonic
of reversing MDR of SK-N-SH/MDR1, through the experiments of
sensitivity to anticancer drugs. The tumor cell membrane barrier is
one of the critical factors that block anticancer drugs. Under the
optimum ultrasonic, we observed by microscopy that the SK-N-SH/MDR1
cells were plump but not killed, and we also found, using SEM, that
the cell membranes were perforated and their permeability was
increased. Thus, LIPUS enhances the permeability of tumor cell
membranes, allowing a larger amount of anticancer drugs into the
cells. Currently, the majority of reversal agents inhibit the
function of P-gp by competitive inhibition; however, the dose
required for a visible effect is so toxic that it prohibits their
use in the clinical setting (29,30).
According to our immunofluorescence and western blot results, the
P-gp was sparsely distributed in the cell membrane and
significantly reduced under the optimum ultrasonic P<0.01.
However, MRP1 and GST-π in the cytoplasm and nucleus, respectively,
were not significantly differently expressed P<0.05. We inferred
that due to cell membrane damaged by sonoporation of LIPUS, P-gp
was significantly reduced, and due to the structure and molecular
environment of P-gp were destroyed, the discharge of anticancer
drugs was reduced. The effect of optimum ultrasonic, however, was
too low to damage the inner of cells and to kill cells, thus, MRP
and GST-π expressed in the cytoplasm and nucleus were not
affected.
According to previous reports (31–34),
LIPUS may also strengthen the transport of genes, proteins, and
drugs to target organ tissues and cells, consequently improving
their therapeutic effects. LIPUS may therefore become an important
therapeutic modality in the future.
In conclusion, LIPUS can effectively reverse the MDR
of SK-N-SH/MDR1. The underlying mechanism may involve damage to the
cell structure and increased permeability of the cell membrane
allowing more chemotherapeutic drugs to pass into the tumor cells.
In addition, a reduction in the amount of P-gp due to LIPUS may
prevent chemotherapeutic drugs from leaving the tumor cells. The
potential of LIPUS in reversing MDR and improving the sensitivity
to chemotherapeutic drugs provides a more targeted, safe, and
stable method to treat tumors with fewer side-effects.
Acknowledgements
The authors thank Professor Tong-Chuan He
(Laboratory of Molecular Oncology, University of Chicago, USA) for
providing the HEK 293 cell line. The present study was supported by
the National Natural Science Foundation of China (no.
39970768).
References
|
1
|
Liao XM and Leung KN: Indirubin-3′-oxime
induces mitochondrial dysfunction and triggers growth inhibition
and cell cycle arrest in human neuroblastoma cells. Oncol Rep.
29:371–379. 2013.
|
|
2
|
Shen H, Xu W, Chen Q, Wu Z, Tang H and
Wang F: Tetrandrine prevents acquired drug resistance of K562 cells
through inhibition of mdr1 gene transcription. J Cancer Res Clin
Oncol. 136:659–665. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yuan Y, Shen H, Hu XY, Gu FY, Li MD and
Zhong X: Multidisciplinary treatment with chemotherapy, targeted
drug, and high-intensity focused ultrasound in advanced pancreatic
carcinoma. Med Oncol. 29:957–961. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma B, Chai S, Li N, To KK, Kan WL, Yang D
and Lin G: Reversal of P-glycoprotein-mediated multidrug resistance
by a synthetic α-aminoxy peptidomimetic. Int J Pharm. 424:33–39.
2012.
|
|
5
|
Marin A, Sun H, Husseini GA, Pitt WG,
Christensen DA and Rapoport NY: Drug delivery in pluronic micelles:
effect of high-frequency ultrasound on drug release from micelles
and intracellular uptake. J Control Release. 84:39–47. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng Y, Tian ZM, Wan MX and Zheng ZB: Low
intensity ultrasound-induced apoptosis in human gastric carcinoma
cells. World J Gastroenterol. 14:4873–4879. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Man J, Shelton RM, Cooper PR and Scheven
BA: Low-intensity low-frequency ultrasound promotes proliferation
and differentiation of odontoblast-like cells. J Endod. 38:608–613.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rapoport N: Combined cancer therapy by
micellar-encapsulated drug and ultrasound. Int J Pharm.
277:155–162. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y, Cho CW, Yan X, Henthorn TK,
Lillehei KO, Cobb WN and Ng KY: Ultrasound-induced hyperthermia
increases cellular uptake and cytotoxicity of P-glycoprotein
substrates in multi-drug resistant cells. Pharm Res. 18:1255–1261.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cho CW, Liu Y, Cobb WN, Henthorn TK,
Lillehei K, Christians U and Ng KY: Ultrasound-induced mild
hyperthermia as a novel approach to increase drug uptake in brain
microvessel endothelial cells. Pharm Res. 19:1123–1129. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu T, Hu K, Bai J and Wang Z: Reversal of
adriamycin resistance in ovarian carcinoma cell line by combination
of verapamil and low-level ultrasound. Ultrason Sonochem. 10:37–40.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu T, Huang X, Hu K, Bai J and Wang Z:
Mechanisms of reversal of adriamycin resistance in human ovarian
carcinoma cell line by ultrasound. Int J Gynecol Cancer. 14:76–81.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao L, Jin X, Xu Y, Guo Y, Liang R, Guo
Z, Chen T, Sun Y and Ding X: Functional study of the novel
multidrug resistance gene HA117 and its comparison to multidrug
resistance gene 1. J Exp Clin Cancer Res. 29:98–105. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo J, Deng ZL, Luo X, Tang N, Song WX,
Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, Vogelstein B and
He TC: A protocol for rapid generation of recombinant adenoviruses
using the AdEasy system. Nat Protoc. 2:1236–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao L, Sun Y, Li X, Jin X, Xu Y, Guo Z,
Liang R, Ding X, Chen T and Wang S: Multidrug resistance strength
of the novel multidrug resistance gene HA117: compared with MRP1.
Med Oncol. 28:1188–1195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xia CY, Liu YH, Wang P and Xue YX:
Low-frequency ultrasound irradiation increases blood-tumor barrier
permeability by transcellular pathway in a rat glioma model. J Mol
Neurosci. 48:281–290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O’Reilly MA, Waspe AC, Ganguly M and
Hynynen K: Focused-ultrasound disruption of the blood-brain barrier
using closely-timed short pulses: influence of sonication
parameters and injection rate. Ultrasound Med Biol. 37:587–594.
2011.
|
|
18
|
McDannold N, Vykhodtseva N and Hynynen K:
Effects of acoustic parameters and ultrasound contrast agent dose
on focused-ultrasound induced blood-brain barrier disruption.
Ultrasound Med Biol. 34:930–937. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hayashi S, Yamamoto M, Tachibana K, Ueno
Y, Bu G and Fukushima T: Mechanism of photofrin-enhanced
ultrasound-induced human glioma cell death. Anticancer Res.
29:897–905. 2009.PubMed/NCBI
|
|
20
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
21
|
Lazaris AC, Kavantzas NG, Zorzos HS,
Tsavaris NV and Davaris PS: Markers of drug resistance in relapsing
colon cancer. J Cancer Res Clin Oncol. 128:114–118. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berger W, Setinek U, Hollaus P, Zidek T,
Steiner E, Elbling L, Cantonati H, Attems J, Gsur A and Micksche M:
Multidrug resistance markers P-glycoprotein, multidrug resistance
protein 1, and lung resistance protein in non-small cell lung
cancer: prognostic implications. J Cancer Res Clin Oncol.
131:355–363. 2005. View Article : Google Scholar
|
|
23
|
Sui H, Fan ZZ and Li Q: Signal
transduction pathways and transcriptional mechanisms of
ABCB1/Pgp-mediated multiple drug resistance in human cancer cells.
J Int Med Res. 40:426–435. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Satava RM: Advanced technologies and the
future of medicine and surgery. Yonsei Med J. 49:873–878. 2008.
View Article : Google Scholar
|
|
25
|
Orsi F, Arnone P, Chen W and Zhang L: High
intensity focused ultrasound ablation: a new therapeutic option for
solid tumors. J Can Res Ther. 6:414–420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zini C, Hipp E, Thomas S, Napoli A,
Catalano C and Oto A: Ultrasound- and MR-guided focused ultrasound
surgery for prostate cancer. World J Radiol. 4:247–252. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu G, Luo G, He L, Li J, Shan H, Zhang R,
Li Y, Gao X, Lin S and Wang G: Follow-up of high-intensity focused
ultrasound treatment for patients with hepatocellular carcinoma.
Ultrasound Med Biol. 37:1993–1999. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mougenot C, Köhler MO, Enholm J, Quesson B
and Moonen C: Quantification of near-field heating during
volumetric MR-HIFU ablation. Med Phys. 38:272–282. 2011.PubMed/NCBI
|
|
29
|
Hung HY, Ohkoshi E, Goto M, Bastow KF,
Nakagawa-Goto K and Lee KH: Antitumor agents. 293 Nontoxic
dimethyl-4,4′-dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-dicarboxylate
(DDB) analogues chemosensitize multidrug-resistant cancer cells to
clinical anticancer drugs. J Med Chem. 55:5413–5424.
2012.PubMed/NCBI
|
|
30
|
Tang X, Gu X, Ren Z, Ma Y, Lai Y, Peng H,
Peng S and Zhang Y: Synthesis and evaluation of substituted
dibenzo[c,e]azepine-5-ones as P-glycoprotein-mediated multidrug
resistance reversal agents. Bioorg Med Chem Lett. 22:2675–2680.
2012.
|
|
31
|
Scheven BA, Millard JL, Cooper PR, Lea SC,
Walmsley AD and Smith AJ: Short-term in vitro effects of low
frequency ultrasound on odontoblast-like cells. Ultrasound Med
Biol. 33:1475–1482. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hassan MA, Buldakov MA, Ogawa R, Zhao QL,
Furusawa Y, Kudo N, Kondo T and Riesz P: Modulation control over
ultrasound-mediated gene delivery: evaluating the importance of
standing waves. J Control Release. 141:70–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang H, Jiang H, Wang H, Zhao J, Chen B
and Wang X: Ultrasound mediated drug-loaded nanoparticles crossing
cell membranes as a new strategy to reverse cancer multidrug
resistance. J Nanosci Nanotechnol. 11:1834–1840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang Q, Deng J, Xie Z, Wang F, Chen S,
Lei B, Liao P, Huang N, Wang Z, Wang Z and Cheng Y: Effective gene
transfer into central nervous system following
ultrasound-microbubbles- induced opening of the blood-brain
barrier. Ultrasound Med Biol. 38:1234–1243. 2012. View Article : Google Scholar : PubMed/NCBI
|