Introduction
The majority of cancer patients succumb to disease
as a result of invasion and metastasis, since a malignant tumor is
incurable and life-threatening (1).
Although chemotherapy is a modality in cancer treatment, drug
resistance and systemic toxicity remain major problems. Therefore,
it is important and urgent to develop safe and effective antitumor
drugs. While the mechanisms have not been fully elucidated
(1), epidemiological and laboratory
data suggest that non-steroidal anti-inflammatory agents (NSAID)
have antitumor effects. Aspirin, a safe, effective and extensively
used NSAID has antipyretic, analgesic, anti-thrombotic and
anti-inflammatory effects through the inhibition of the activity of
cyclooxygenase (COX) enzymes (2,3). In
recent years, aspirin was found to have potential in preventing and
treating tumors, which is a major area of interest in cancer
research (4). Epidemiological and
clinical studies suggest that by using aspirin as a chemopreventive
agent, it can reduce cancer risk by as much as 50% in some cancer
types (5), including colorectal,
ovarian epithelial, esophageal, bladder, lung and breast cancer as
well as glioblastoma (6–12) while showing enhancement of the
body’s antitumor immunity (13).
The molecular mechanisms of aspirin-mediated
inhibition of tumors remain unclear. Aspirin is a prototypic
inhibitor of COX. Overexpression of COX-2 induced by inflammatory
and mitogenic stimuli is commonly found in a variety of cancers
(14), which suggests that COX-2
contributes to the process of carcinogenesis. Several mechanisms by
which COX-2 contributes to the progression of cancer have been
reported, including stimulation of proliferation and inhibition of
apoptosis of cancer cells, stimulation of cancer cell invasion and
angiogenesis, and suppression of immune reponses (14,15).
Our previous study revealed that aspirin caused an inhibitory
effect on S180 sarcoma and 3AO human ovarian cancer cell growth. We
discovered that aspirin decreased the expression of COX-2 in tumor
tissues, and there was a positive correlation between the
expression of COX-2 and angiogenic factors. Considerable evidence
has shown that tumor growth and metastasis are dependent on
angiogenesis, and VEGF-A is known to be one of the most important
angiogenic factors. Antiangiogenesis may be one of the mechanisms
by which aspirin exerts its tumor chemopreventive and therapeutic
effects.
Lymph node metastasis occurs commonly in cancer with
lymphatic vessels being a main channel for the spread of cancer
cells (16,17). Recent studies suggest that
lymphangiogenesis actively contributes to metastasis based on the
observations that lymphatic vessel density is correlated with the
extent of lymph node metastasis (17,18).
Moreover, it has been revealed in some animal tumor models that the
expression of lymphangiogenic growth factors leads to the formation
of lymphatic vessels and that lymphangiogenesis is accompanied by
enhanced lymphatic metastasis (17). Prominent expression of VEGF-C has
been observed in numerous types of human cancers (17,18).
Several studies have shown that levels of VEGF-C expression in
primary tumor were correlated with lymphatic vessel invasion
(17–19). Correlations between COX-2 expression
and lymphagiogenesis have been reported in several human cancer
types (20–24). The present study focused on tumor
angiogenesis and lymphangiogenesis by evaluating the MVD, LVD and
the expression of VEGF-A and VEGF-C in tumor tissues in order to
investigate the molecular mechanisms through which aspirin inhibits
tumor growth.
Materials and methods
Tumor models
Fifty male Kunming mice aged from 6 to 8 weeks and
weighing from 20 to 24 g were obtained from the Animal Experiment
Center of Shandong University, China. The mouse model was provided
by the Institute of Medicine, Shandong Academy of Medical Science,
China. Seven days following S180 cell injection, ascites was
extracted from S180 ascites sarcoma mice under an septic condition.
Normal saline was then added to adjust the tumor cell concentration
to 1×107/ml. Tumors were generated in 40 male Kunming
mice by injection of a 0.2-ml tumor cell suspension subcutaneously
into the right flank of each mouse. Tap water and food were
provided ad libitum.
Drugs and reagents
Aspirin was obtained from Bayer S.p.A. (Milano,
Italy). DMSO was obtained from Sigma (St. Louis, MO, USA) to
dissolve the aspirin. 5-FU was obtained from Tianjin Jinyao Amino
Acids Co., Ltd., Tianjin, China. Western blotting related reagents
were purchased from the Shanghai Beyotime Institute of
Biotechnology, China.
Treatment
All tumor-bearing mice were randomly divided into 5
groups: control group, 5-FU group, high-dose aspirin group,
low-dose aspirin group and combination group with 10 mice in each
group. Treatment was initiated when the diameter of tumors was 5 mm
(referred to as day 0) at day 5 following tumor cell injection. The
high-dose and low-dose aspirin groups respectively received 250 and
50 mg/kg aspirin by oral gavage once a day for 14 days. Control
mice received an equal volume of normal saline. 5-FU (20 mg/kg) was
injected i.p. once every 3 days (day 1, 4, 7, 11 and 14).
Combination group mice received 50 mg/kg aspirin by oral gavage
once every day and 20 mg/kg 5-FU was injected i.p. once every 3
days (day 1, 4, 7, 11 and 14). The volume was measured to be 0.1
ml/10 g. This experiment was repeated three times. The two maximal
perpendicular diameters of the tumors were measured every 2 days to
document tumor growth (day 0, 2, 4, 6, 8, 10, 12 and 14). Tumor
measurements were converted to tumor volume (V) using the formula
(V = W2 × L/2); where W and L are the perpendicular
smaller and large tumor diameters, respectively, and plotted
against time. Body weight of the mice was measured twice weekly.
Mice were euthanized 24 h after the last treatment. The tumor
masses were excised from the mice and were then weighed.
Calculation of the tumor inhibitory rate was calculated using the
formula: % Inhibitory rate (IR) = [average tumor weight of the
control group (g) - average tumor weight of the treatment group
(g)]/average tumor weight of the control group (g) × 100.
Histology and immunohistochemistry
Part of the fresh tumor specimens was fixed in 10%
neutral-buffered formalin, processed and embedded into paraffin
blocks. Paraffin-embedded tumor samples were processed into tissue
array blocks, which were cut into 4-μm sections for hematoxylin and
eosin (H&E) and immunohistochemical staining. Paraffin sections
were dewaxed in five changes of xylene and rehydrated through
descending concentrations of alcohol. Endogenous peroxidase
activities were blocked using 3% hydrogen peroxide. Sections were
treated with heating in 10 mmol/l citrate buffer at pH 6.0 inside a
water bath. They were incubated overnight in a moist chamber with
the primary antibody. The secondary antibodies, biotinylated goat
anti-rabbit and anti-mouse IgG (Beijing Biosynthesis Biotechnology
Co., Ltd., Beijing, China), were applied at 1:200 in PBS for 1 h at
room temperature, stained with DAB and then counterstained with
hematoxylin. Finally, sections were dehydrated through graded
alcohols, cleared in xylene and mounted in permount. The following
antibodies were used for immunohistochemical staining: D2-40 (1:75
dilution; CWBIO, Beijing, China); CD34, VEGF-A, VEGF-C (1:100
dilution; Beijing Biosynthesis Biotechnology Co., Ltd.).
Microvessel density (MVD) was analyzed in CD34-stained vascular
endothelial cells. CD34 is expressed in the endothelial cells of
microvessels. The microvessel count was carried out in accordance
with the method of Weidner et al(25). Initially, we selected 3 dense
microvessel fields separately at the original magnification ×40 and
×100, and the numbers of CD34-stained cells were then counted at
the original magnification ×400 and averaged for statistical
analysis. The method of lymphatic vessel count was identical to
that of the microvessels.
Western blotting
Sections of fresh tumor specimens were stored at
−70°C. They were homogenized with ice-cold lysis buffer, and were
then centrifuged at 10,000 rpm for 10 min at 4°C. The protein
concentration was determined with the BCA kit. The total protein
sample was denaturated at 100°C for 10 min and separated by 10%
SDS-PAGE gels and later transferred to Protran nitrocellulose
membranes. The membranes were blocked by blocking fluid for 2 h and
incubated overnight with an anti-VEGF-A or VEGF-C antibody (1:100
dilution), after extensive washing, and a biotinylated goat
anti-rabbit IgG antibody (1:200 dilution) for 1.5 h at room
temperature, and then stained with DAB. The band intensities were
analyzed by ImageJ software (Wayne Rasband National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
All data were processed by SPSS11.5 and presented as
means ± SEM. The t-test was performed to compare means and
distributions for different treatment groups. Statistical
significance was based on two-tail P<0.05.
Results
Effects of aspirin on sarcoma 180 tumor
growth
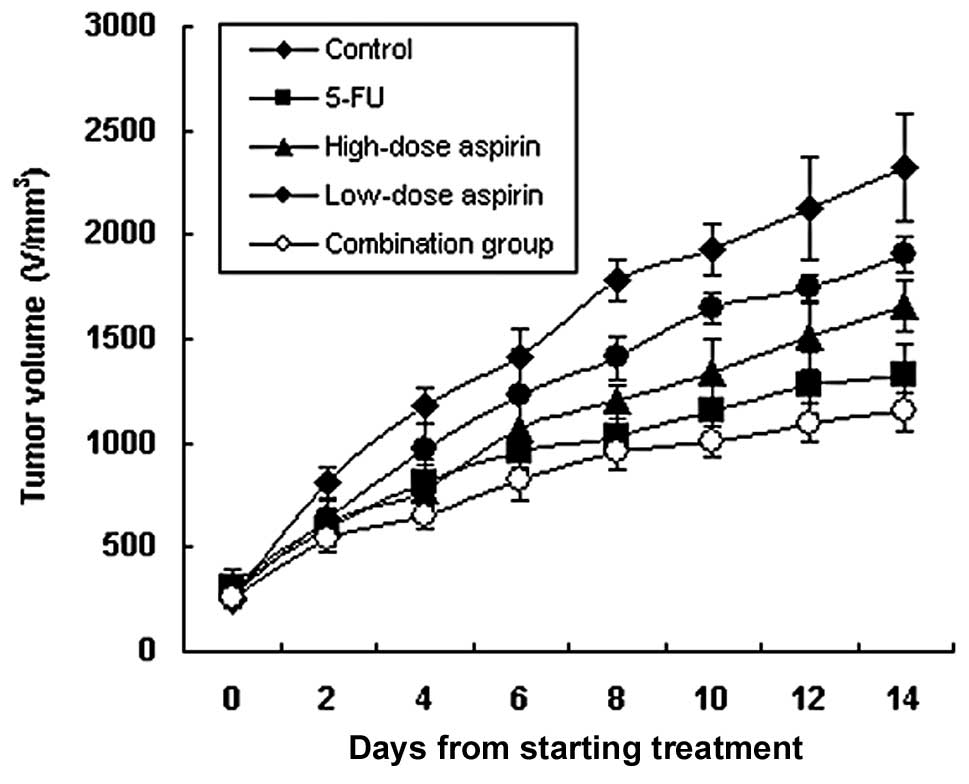
Tumors grew relatively rapidly in the mice.
Treatment with 5-FU (20 mg/kg) alone, either high-dose (250 mg/kg)
or low-dose (50 mg/kg) aspirin alone, and a combination of 5-FU (20
mg/kg) and aspirin (50 mg/kg) resulted in inhibition of tumor
growth compared to the control group. The inhibitory rate was 64.1,
33.5, 22.2 and 70.1%, respectively (Table I, Fig.
1; P<0.05 for each comparison). The inhibitory effect was
stronger in both the combination and 5-FU groups (P<0.01).
Although the inhibitory rates of the high- and low-dose aspirin
groups were lower, the mice in both groups were in a good condition
with an increased body weight after the experiment (Table I). This demonstrated that aspirin
not only inhibited the growth of S180 sarcoma, but also reduced the
body wasting effect caused by the tumor.
 | Table IInhibitory effect of aspirin on S180
sarcoma (mean ± SEM, n=10). |
Table I
Inhibitory effect of aspirin on S180
sarcoma (mean ± SEM, n=10).
| Body weight (g) | | |
|---|
|
| | |
|---|
| Group | Before
experiment | After experiment | Tumor weight (g) | IR (%) |
|---|
| Control | 24.9±2.5 | 27.9±4.6 | 2.84±0.71 | - |
| 5-FU | 23.0±2.6 | 26.3±3.0 | 1.02±0.53b | 64.1 |
| High-dose
aspirin | 23.7±3.1 | 28.3±2.3 | 1.89±0.58a | 33.5 |
| Low-dose aspirin | 24.3±4.0 | 29.2±3.9 | 2.21±0.89a | 22.2 |
| Combination group
(5-FU and aspirin) | 23.8±3.0 | 28.5±1.6 | 0.85±0.27b | 70.1 |
Pathological and morphometric analysis of
sarcoma 180 tumors after treatment
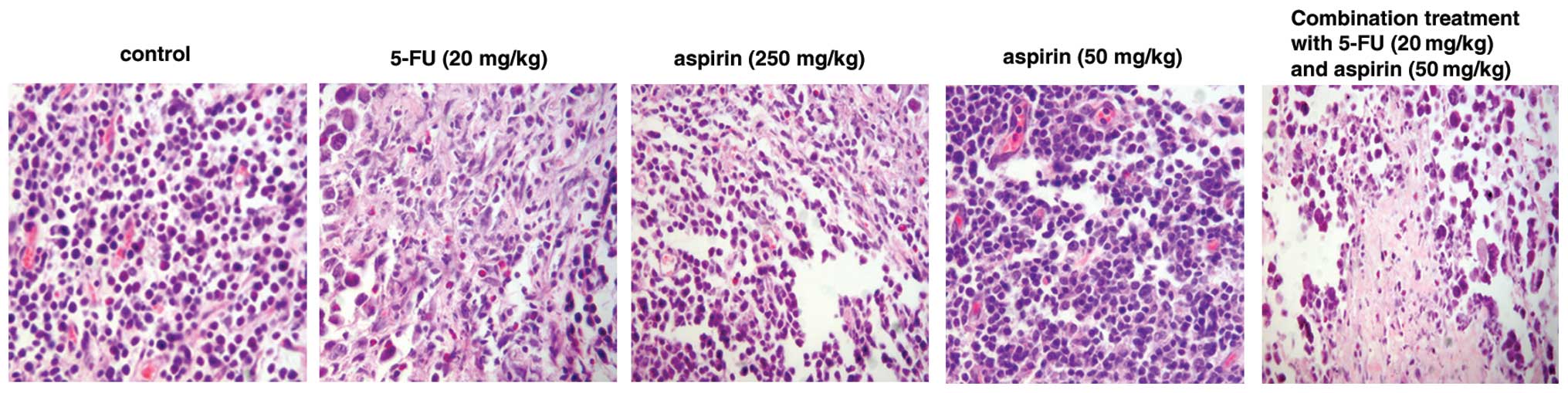
H&E staining showed that S180 sarcoma cells were
distributed as sheets and nests. Tumor cells varied in size and
shape. High- and low-dose aspirin, and the 5-FU and combination
groups exhibited morphological changes characteristic of apoptotic
tumor cells such as nuclear pyknosis and karyorrhexis. Microvessel
density was significantly lower than that in the control group
(Fig. 2). In the high-dose aspirin,
5-FU and combination groups the tumors exhibited large patchy
necrosis along with the frequent observation of monocyte
infiltration.
Expression of VEGF-A and VEGF-C in
sarcoma 180 tumors
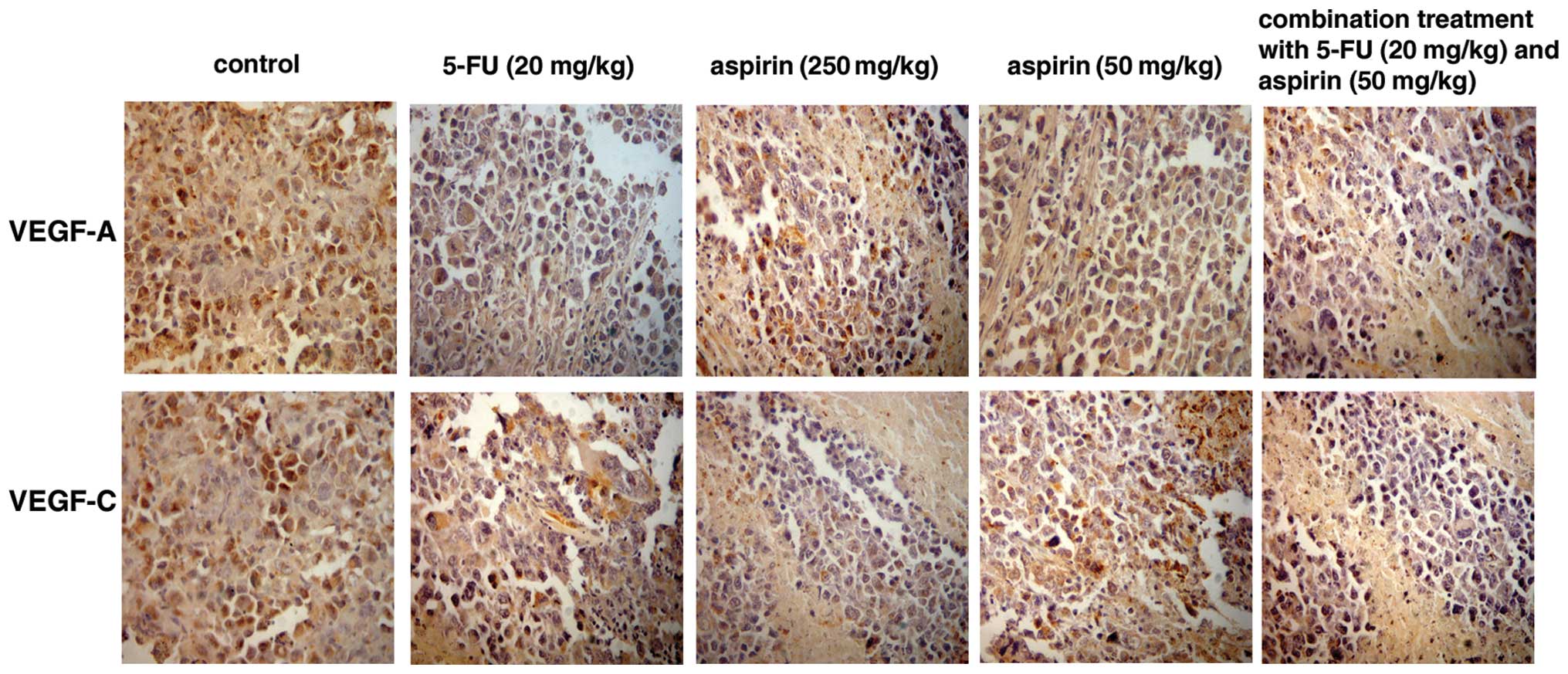
Based on immunohistochemical staining, VEGF-A and
VEGF-C were expressed in the cytoplasm of tumor cells. Cells
positive for VEGF-A and VEGF-C were stained brown (Fig. 3). The expression of VEGF-A and
VEGF-C in the control group was markedly higher than that in the
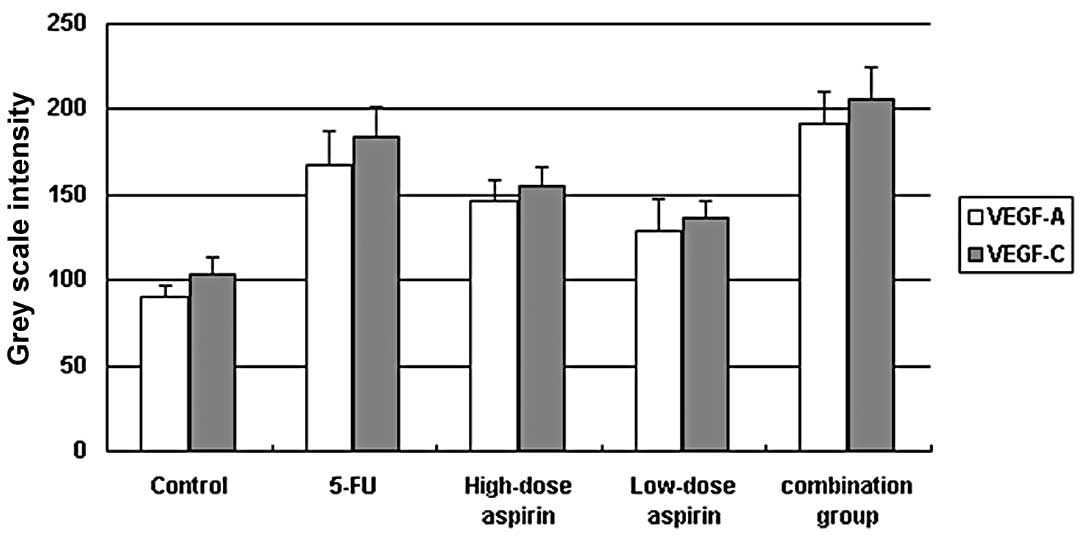
other treatment groups. Grey scale intensity variants of VEGF-A and
VEGF-C immunoreactivity were evaluated by Leica Qwin V3 software.
Sections were evaluated in each of 5 randomly selected positive
regions at the original magnification ×200. Fig. 4 indicates an inverse relationship
between the grey scale intensity and the protein expression. Higher
grey scale intensity indicates weaker protein expression, and lower
intensity indicates stronger protein expression. Treatment with
5-FU and aspirin resulted in a reduction in VEGF-A and VEGF-C
expression. VEGF-A and VEGF-C expression decreased significantly in
both the 5-FU alone and combination groups when compared with the
other treatment groups showing a dose-dependency on high- and
low-dose aspirin. In each comparison, there was a significant
difference (P<0.05).
Effect of aspirin treatment on VEGF-A and
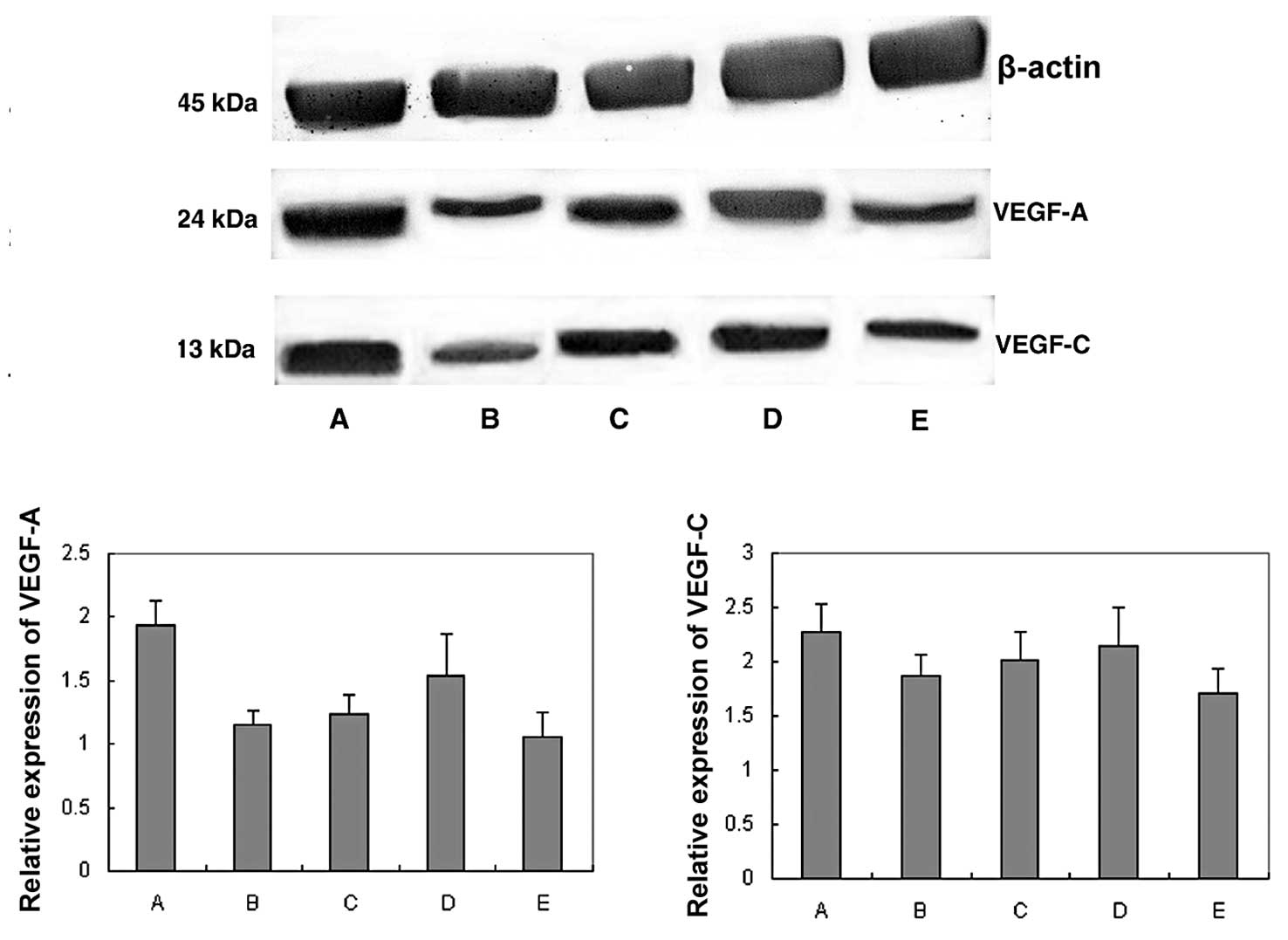
VEGF-C protein expression as assessed by western blot analysis
VEGF-A and VEGF-C expression was normalized to
β-actin expression by band intensity. As known in Fig. 5, VEGF-A and VEGF-C expression was
reduced in the high- and low-dose aspirin, 5-FU and combination
groups. Band intensities were analyzed by ImageJ software. VEGF-A
and VEGF-C expression decreased significantly in the S180 sarcoma
tumors treated with 5-FU alone or with the combination with
aspirin. The decreased expression also showed a dose-dependent
trend in the high- and low-dose aspirin groups.
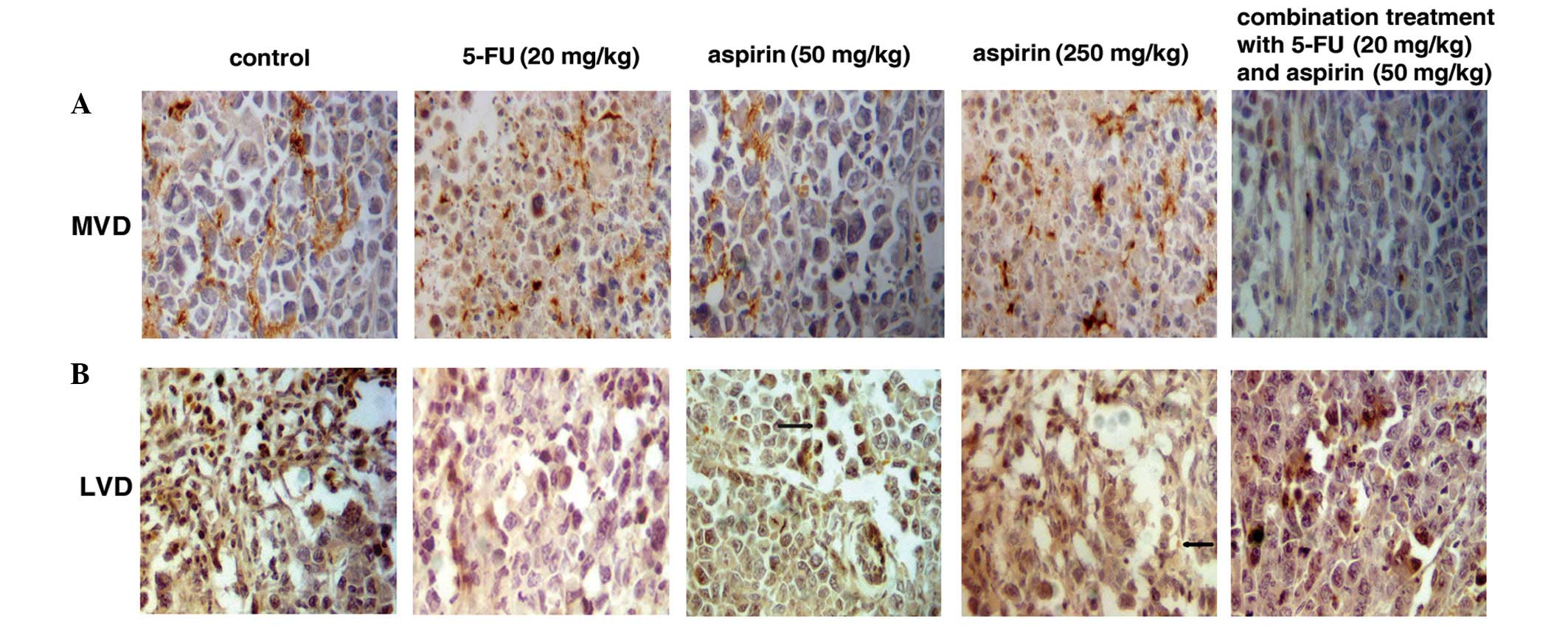
MVD and LVD in sarcoma 180 tumors
Cells positive for CD34 were stained brown.
Microvessel distribution is shown in Fig. 6A. Necrotic tumor cells were
frequently present in the 5-FU-treated group and in the high-dose
aspirin group. The 5-FU alone, high- and low-dose aspirin and
combination groups all demonstrated inhibition of MVD in comparison
to the control group (P<0.05 for each comparison), which
suggests that aspirin inhibits angiogenesis (Table II).
 | Table IICounts of MVD and LVD in S180 sarcoma
tissue (mean ± SEM, n=10). |
Table II
Counts of MVD and LVD in S180 sarcoma
tissue (mean ± SEM, n=10).
| Group | MVD | LVD |
|---|
| Control | 11.65±4.34 | 6.45±2.03 |
| 5-FU | 7.13±2.16a | 5.20±1.61 |
| High-dose
aspirin | 7.55±2.73a | 4.63±1.84a |
| Low-dose aspirin | 9.87±3.89a | 5.47±1.34 |
| Combination
group | 6.82±1.30a | 4.95±1.03a |
D2-40-positive tubular structures
D2-40-positive tubular structures were stained
brown. The vessel walls were thin and irregular in structure
(Fig. 6B). The 5-FU alone, aspirin
and the combination groups had an inhibitory effect on LVD. The
high-dose aspirin (250 mg/kg) group showed a noticeable inhibition
of LVD in comparison to the control group (P<0.05) (Table II). The high-dose aspirin group
exhibited positive tumor cells generating non-endothelial
cell-lined channels (Fig. 6B, arrow
fourth panel). The low-dose aspirin group displayed a tumor cell
around a lymphatic vessel (Fig. 6B,
arrow, middle panel). The result revealed that LVD of the control
group was the highest in the 5 groups and the lumen showed obvious
expansion. The 5-FU, high- and low-dose aspirin groups had an
inhibitory effect on LVD and VEGF-C. High-dose aspirin (250 mg/kg)
markedly inhibited lymphatic vessel density when compared with the
control group.
Discussion
This research demonstrated the inhibitory effect of
aspirin on murine S180 sarcoma growth in vivo and its
possible mechanisms. The inhibitory rates of the 5-FU, high-dose
(250 mg/kg) and low-dose (50 mg/kg) aspirin, and combination of
5-FU with aspirin groups were 64.1, 33.5, 22.2 and 70.1%,
respectively. Unexpectedly, aspirin enhanced the antitumor effect
of 5-FU, and the mice treated with aspirin were in good condition.
In conclusion, aspirin is capable of reducing the body wasting
caused by tumors.
Aspirin demonstrated a potential in decreasing the
expression of VEGF-A and MVD. Tumor tissue in the high-dose aspirin
group showed patchy necrosis and decreased MVD. These results imply
that antiangiogenesis plays a critical role in aspirin-induced
antitumor activity. This is possibly due to the attributes of
aspirin that directly affect vascular endothelial cells and
decrease VEGF-A production along with MVD, thereby causing tumor
tissue ischemia and even necrosis. Cox-2 is important in regulating
angiogenesis, and its reported mode of action on endothelial cells
is indirect (26). Our previous
study showed that aspirin decreases the expression of Cox-2 in
tumor tissue. Therefore, we concluded that aspirin inhibits
angiogenesis by directly targeting endothelial cells through an
independent Cox pathway. There are other possible pathways that
could be responsible including pathways regulating ERK kinase,
cyclin E and CDC2 (27). However,
further research is needed to ascertain the pathway responsible for
the inhibitory effect of aspirin on angiogenesis.
It is difficult to distinguish blood vessels from
lymphatic vessels using regular H&E staining. D2-40 was
recently discovered to be a tumor-associated lymphatic vessel
endothelial cell marker with high specificity (28). D2-40 was used to identify lymphatic
endothelial cells in this research. The treatment groups had an
inhibitory effect on LVD and VEGF-C particularly in the high-dose
aspirin group (250 mg/kg). We concluded that aspirin inhibits tumor
lymphangiogenesis by reducing VEGF-C consequently inhibiting the
growth of murine S180 sarcoma. Moreover, we found that positive
tumor cells generated non-endothelial cell-lined channels (arrow in
Fig. 6B), which indicated that fast
growing tumors require various nutrients and need additional
channels in order to transport nutrients. A tumor cell around a
lymphatic vessel (arrow in Fig. 6B)
was noted, which indicated that the tumor cell might spread through
the lymphatic vessel. Several studies had suggested that the level
of Cox-2 expression in tumor tissue is correlated with lymph node
metastasis (21–23), and Cox-2 promotes tumor
lymphangiogenesis and consequent lymph node metastasis (29). We indicated here, that aspirin
inhibits tumor lymphangiogenesis through VEGF-C dependent on the
reduced activity of Cox-2.
A recent study showed that daily usage of low-dose
aspirin (75–300 mg) can reduce the incidence of cancer by ~25%
after a delay of ~3 years. This provides strong evidence supporting
our conclusion, but it also suggests that the side effects of
aspirin such as anemia and bleeding might account for the
subsequent reduction in cancer death (4). In our study, tumor-bearing mice
treated with aspirin had slight to mild side effects. This was
possibly due to the short treatment duration. Aspirin is indeed
safer than most chemotherapeutic agents; however, we still need to
optimize the dose for cancer treatment.
In summary, our findings demonstrated that aspirin
can inhibit tumor angiogenesis by reducing the production of
VEGF-A, and inhibiting tumor lymphangiogenesis through the
reduction of VEGF-C in a dose-dependent manner, which explains why
aspirin inhibits the tumor growth of murine S180 sarcoma in
vivo. We also found that aspirin synergistically enhanced the
antitumor effect of 5-FU. In addition, the significance of these
finding needs to be established in a broader context by conducting
further studies in a variety of tumor models. To the best of our
knowledge, our findings indicate that aspirin and other NSAIDs
inhibit lymphangiogenesis, possibly through COX-2-dependent
regulation of VEGF-C expression. Although further validation is
required, the proposed effect of aspirin specifically on tumor
angiogenesis and lymphangiogenesis may have therapeutic
implications in chemoprevention, adjuvant chemotherapy and
treatment of metastatic disease.
Acknowledgements
This study was supported by funding from the
National Nature Science Foundation of China (nos. 81073102 and
30873408) and Development Project of Science and Technology in
Jinan (no. 201004012).
References
|
1
|
Vinogradova Y, Hippisley-Cox J, Coupland
C, et al: Risk of colorectal cancer in patients prescribed statins,
nonsteroidal anti-inflammatory drugs, and cyclooxygenase-2
inhibitors: nested case-control study. Gastroenterology.
133:393–402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Doherty GA and Murray FE: Cyclooxygenase
as a target for chemoprevention in colorectal cancer: lost cause or
a concept coming of age? Expert Opin Ther Targets. 13:209–218.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rostom A, Dube C, Lewin G, et al:
Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2
inhibitors for primary prevention of colorectal cancer: a
systematic review prepared for the U.S. Preventive Services Task
Force. Ann Intern Med. 146:376–389. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rothwell PM, Price JF, Fowkes FG, et al:
Short-term effects of daily aspirin on cancer incidence, mortality,
and non-vascular death: analysis of the time course of risk sand
benefits in 51 randomised controlled trials. Lancet. 379:1602–1612.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li S, Miner K, Fannin R, et al:
Cyclooxygenase-1 and 2 in normal and malignant human ovarian
epithelium. Gynecol Oncol. 92:622–627. 2004. View Article : Google Scholar
|
|
6
|
Thun MJ, Henley SJ and Patrono C:
Nonsteroidal anti-inflammatory drugs as anticancer agents:
mechanistic, pharmacologic, and clinical issues. J Natl Cancer
Inst. 94:252–266. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dube C, Rostom A, Lewin G, et al: The use
of aspirin for primary prevention of colorectal cancer: a
systematic review prepared for the U.S. Preventive Services Task
Force. Ann Intern Med. 146:365–375. 2007. View Article : Google Scholar
|
|
8
|
Sadeghi S, Bain CJ, Pandeya N, et al:
Aspirin, nonsteroidal anti-inflammatory drugs, and the risks of
cancers of the esophagus. Cancer Epidemiol Biomarkers Prev.
17:1169–1178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gee JR, Jarrard DF, Bruskewitz RC, et al:
Reduced bladder cancer recurrence rate with cardioprotective
aspirin after intravesical bacille Calmette-Guerin. BJU Int.
103:736–739. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kelly JP, Coogan P, Strom BL, et al: Lung
cancer and regular use of aspirin and nonaspirin nonsteroidal
anti-inflammatory drugs. Pharmacoepidemiol Drug Saf. 17:322–327.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alfonso LF, Srivenugopal KS, Arumugam TV,
et al: Aspirin inhibits camptothecin-induced p21CIP1
levels and potentiates apoptosis in human breast cancer cells. Int
J Oncol. 34:597–608. 2009.PubMed/NCBI
|
|
12
|
Kim SR, Bae MK, Kim JY, et al: Aspirin
induces apoptosis through the blockade of IL-6-STAT3 signaling
pathway in human glioblastoma A172 cells. Biochem Biophys Res
Communm. 387:342–347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iwama T: NSAIDs and colorectal cancer
prevention. J Gastroenterol. 44:72–76. 2009. View Article : Google Scholar
|
|
14
|
Dannenberg AJ, Altorki NK, Boyle JO, et
al: Cyclo-oxygenase 2: a pharmacological target for the prevention
of cancer. Lancet Oncol. 2:544–551. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang D, Mann JR and DuBois RN: The role of
prostaglandins and other eicosanoids in the gastrointestinal tract.
Gastroenterology. 128:1445–1461. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alitalo K, Tammela T and Petrova TV:
Lymphangiogenesis in development and human disease. Nature.
438:946–953. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Achen MG and Stacker SA: Tumor
lymphagiogenesis and metastatic spread-new players begin to emerge.
Int J Cancer. 119:1755–1760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pepper MS, Tille JC, Nisato R, et al:
Lymphangiogenesis and tumor metastasis. Cell Tissue Res.
314:167–177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yonemura Y, Endo Y, Fujita H, et al: Role
of vascular endothelial growth factor C expression in the
development of lymph node metastasis in gastric cancer. Clin Cancer
Res. 5:1823–1829. 1999.PubMed/NCBI
|
|
20
|
Su JL, Shih JY, Yen ML, et al:
Cyclooxygenase-2 induces EP1- and HER-2/Neu-dependent vascular
endothelial growth factor-C up-regulation: a novel mechanism of
lymphangiogenesis in lung adenocarcinoma. Cancer Res. 64:554–564.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Timoshenko AV, Chakraborty C, Wagner GF,
et al: COX-2-mediated stimulation of the lymphangiogenic factor
VEGF-C in human breast cancer. Br J Cancer. 94:1154–1163. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Siironen P, Ristimaki A, Narko K, et al:
VEGF-C and COX-2 expression in papillary thyroid cancer. Endocr
Relat Cancer. 13:465–473. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Soumaoro LT, Uetake H, Takagi Y, et al:
Coexpression of VEGF-C and Cox-2 in human colorectal cancer and its
association with lymph node metastasis. Dis Colon Rectum.
49:392–398. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang J, Ji J, Yuan F, et al:
Cyclooxygenase-2 expression is associated with VEGF-C and lymph
node metastases in gastric cancer patients. Biomed Pharmacother.
59:S285–S288. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weider N, Carroll PR, Flax J, et al: Tumor
angiogenesis correlation with metastasis in invasive prostate
carcinoma. Am J Pathol. 143:755–761. 1993.
|
|
26
|
Borthwick GM, Johnson AS, Partington M, et
al: Therapeutic levels of aspirin and salicylate directly inhibit a
model of angiogenesis through a Cox-independent mechanism. FASEB J.
20:2009–2016. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pillinger MH, Capodici C, Rosenthal P, et
al: Modes of action of aspirin-like drugs: salicylates inhibit erk
activation and integrin-dependent neutrophil adhesion. Proc Natl
Acad Sci USA. 95:14540–14545. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saad RS, Kordunsky L, Liu YL, et al:
Lymphatic microvessel density as prognostic marker in colorectal
cancer. Mod Pathol. 19:1317–1323. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
lwata C, Kano MR, Komuro A, et al:
Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via
reduction of lymphangiogenesis. Cancer Res. 67:10181–10189. 2007.
View Article : Google Scholar : PubMed/NCBI
|