Introduction
Hepatocellular carcinoma (HCC) is the most common
type of primary liver cancer and is the third leading cause of
cancer-related mortality worldwide (1). High frequency of intrahepatic and
extrahepatic metastasis in the early stage contributes to the poor
prognosis of HCC patients, which is also the vital factor involved
in the disappointing survival following curative liver resection
(2). Therefore, it is necessary to
establish the factors that act as HCC metastatic markers, while
playing an important role in the process of metastasis.
Metastasin, also known as S100A4, belongs to the
S100 family of Ca2+-binding proteins encoded by the mts1
gene (3,4). Although the relationship between
metastasin and tumor metastasis remains unclear, previous
investigations in experimental animal models indicated that
metastasin is a metastasis factor via enhancing tumor angiogenesis
(5), invasion (6) and motility (7). To our knowledge, metastasin expression
is diverse in different organs. High metastasin expression has been
found in human monocytes, macrophages and polymorphonuclear
granulocytes (8), while low level
of metastasin protein was detected in the pancreas, colon, thyroid,
lung and kidney (9). However, only
few investigations have demonstrated the expression and function of
metastasin in liver and HCC.
Epithelial mesenchymal transition (EMT) is a program
of cancer metastasis which has been characterized as loss of
epithelial cell polarity and acquisition of elongated mesenchymal
morphology, concomitant with disruption of cell adhesion, increased
cell migration, invasion and metastasis (10). In preliminary studies (11–13),
EMT was verified to be involved in the cascade of signaling events
inducing HCC metastasis (14,15).
However, the concrete mechanisms of induction of EMT in HCC remain
unclear. Metastasin was found to induce EMT in renal fibrosis and
was renamed fibroblast-specific protein (FSP1) (16). In this investigation, we found that
metastasin was significantly associated with poor prognosis of HCC
and induced EMT through upregulating SNAI1.
Materials and methods
Materials
The rabbit anti-metastasin primary antibody
(SC-292281, 1:200 dilution) for western immunoblotting was
purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The
rabbit anti-metastasin primary antibody (ZA-0257) and secondary
goat anti-rabbit antibody labeled with biotin (ZDR-5306) for
immunohistochemistry was obtained from ZSGB-BIO (Beijing, China).
The metastasin-expressing plasmid (RC201616) was from OriGene
(Rockville, MD, USA). The human siRNAs targeting both metastasin
(SC-106781) and SNAI1 (SC-38398) were purchased from Santa Cruz
Biotechnology. Other primary antibodies for western immunoblotting
included rabbit anti-SNAI1 antibody (C15D3, 1:1,000 dilution; Cell
Signaling Technology, Danvers, MA, USA), mouse anti-E-cadherin
antibody (610181, 1:1,000 dilution; BD Transduction Laboratories,
San Jose, CA, USA), rabbit anti-N-cadherin antibody (sc7939, 1:200
dilution; Santa Cruz Biotechnology), and rabbit anti-Vimentin
antibody (EPR3776, 1:1,000 dilution; Abcam, Cambridge, MA, USA).
Complete Mini Protease Inhibitor Mixture, anti-β-actin primary
antibody (A-5316) and anti-mouse secondary antibodies conjugated
with HRP (A3673) were from Sigma Chemical Co. (St. Louis, MO, USA).
The anti-rabbit secondary antibodies conjugated with HRP (ALI3403)
were from Biosource Co. (Carlsbad, CA, USA), and ECL reagents were
from Amersham/GE Healthcare (Piscataway, NJ, USA).
Cell culture and transfection
A previous study showed that there was high
metastasin expression in MHCC97H cells, while Hep3B cells expressed
metastasin slightly (17). Hence,
in the present investigation, MHCC97H cells were selected as the
cell model for metastasin knockdown and Hep3B cells were used for
metastasin-overexpression study. MHCC97H cell lines were obtained
from Fudan University of China and were maintained in our lab.
MHCC97H cells, with high metastasis potential frequently used as
cell models in the investigation of HCC metastasis, were grown here
in DMEM supplemented with 10% FBS. The Hep3B cell line with limited
metastasis capacity was obtained from the American Type Culture
Collection (ATCC, Manassas, VA, USA) and was cultured in complete
MEM medium with 10% FBS.
Both metastasin expressing plasmid and control
plasmid (pCMV6-Entry) were transfected into Hep3B cells with Roche
FuGENE® 6 Transfection reagent (Indianapolis, IN, USA)
and stably expressing clones were selected by G418 at a dose of 300
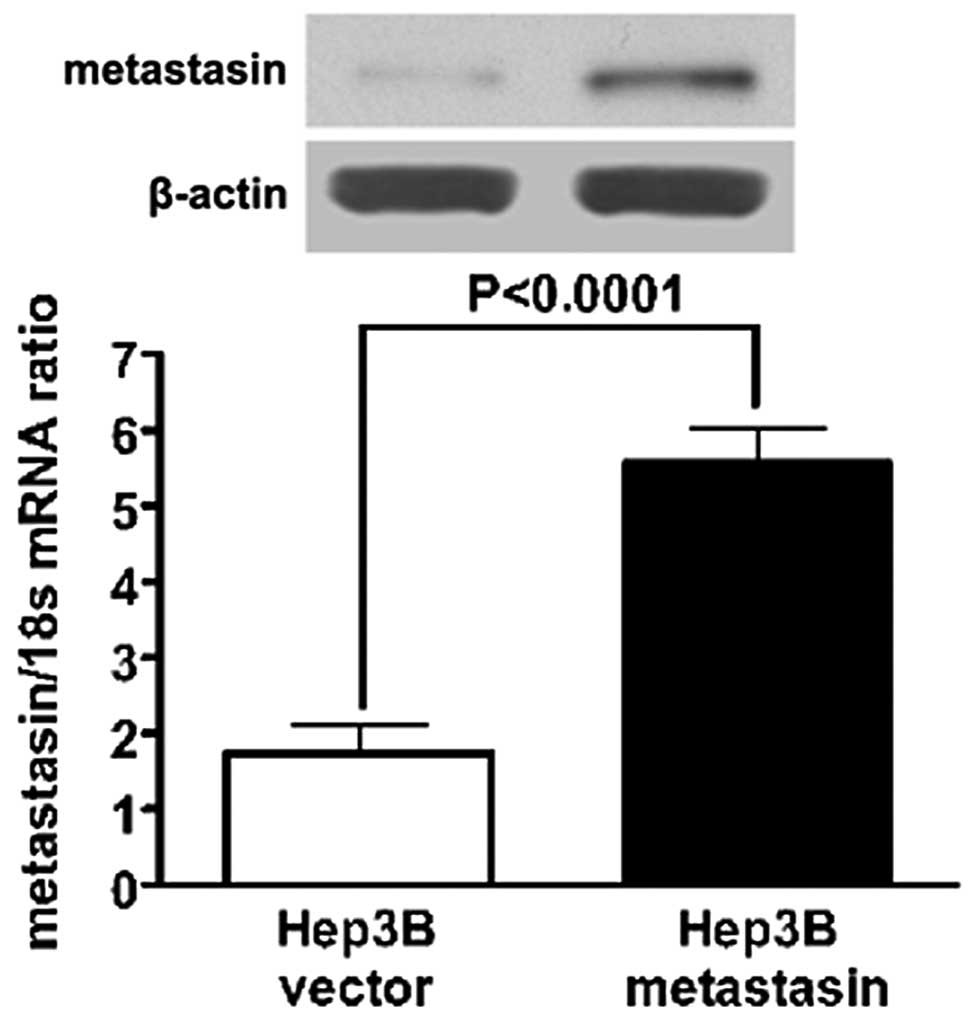
μg/ml for two weeks. Western immunoblotting and qRT-PCR assay
confirmed overexpression of metastasin in the selected stably
expressing clones (Fig. 1).
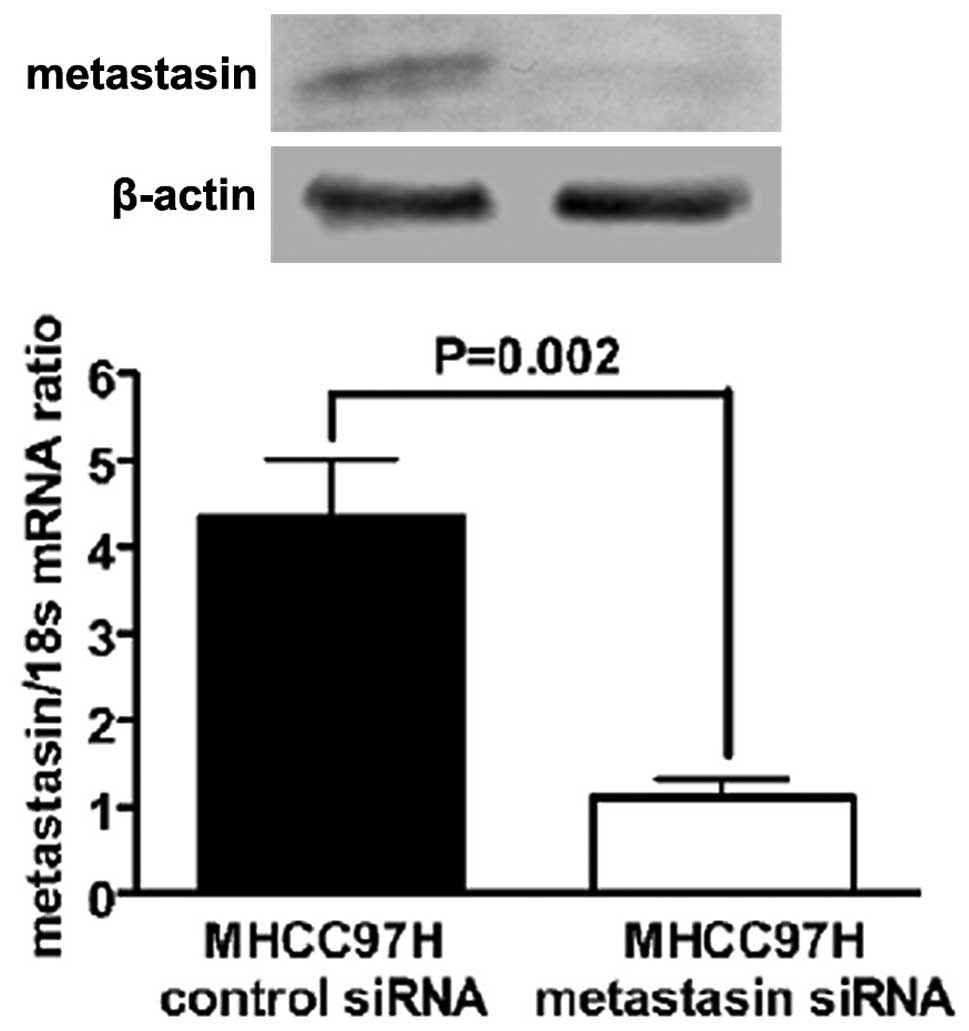
Metastasin siRNA and scramble siRNA were transfected into MHCC97H
cells using the siPORT™ NeoFX™ Transfection Agent from Applied
Biosystems (Carlsbad, CA, USA). Knockdown of metastasin was
verified by both western immunoblotting and qRT-PCR assay in
MHCC97H cells transfected with metastasin siRNA (Fig. 2). Silencing SNAI1 expression of
Hep3B cells transfected with metastasin expressing plasmid was
carried out with the siPORT™ NeoFX™ Transfection Agent.
HCC tissue specimens and the matched
HCC-adjacent normal tissue specimens
A total of 41 patients with HCC were enrolled in the
study between January 2008 and March 2009, and included 29 men and
12 women (median age, 45 years; range, 34–74 years) who had not
received preoperative chemotherapy or embolization. After receiving
the routine preoperative examination including chest X-ray,
abdominal ultrasonography and computed tomography, all patients
underwent liver resection, including radical resection for early
HCC and palliative resection for advanced HCC. Tumor tissues and
adjacent liver tissues (>2-cm distance to the resection margin)
were collected and immediately stored in paraformaldehyde for
immunohistochemistry. Clinical data were obtained from the medical
records including histopathologic Edmonson classification, clinical
tumor-node-metastasis (TNM) grading, maximum tumor diameter, and
the tumor-adjacent normal tissues, and were all confirmed by an
experienced pathologist who was blinded to clinical information. We
also obtained healthy liver tissues from 8 patients without any
liver disease. Written informed consent was obtained from all
patients. All protocols were approved by the Xi’an Jiaotong
University Ethics Committee according to the Helsinki Declaration
of 1975.
Immunohistochemistry staining
Briefly, all tissues were placed on glass slides,
rehydrated and incubated in 3% hydrogen peroxide to block the
endogenous peroxidase activity. Following trypsinization, sections
were blocked by incubation in 3% bovine serum albumin in PBS. The
primary rabbit anti-human metastasin antibody was applied to the
slides at a dilution of 1:150 and incubated at 4°C overnight. The
tissues were washed three times with PBS and treated with secondary
goat anti-rabbit antibody labeled with biotin at 37°C for 30 min.
The staining of sections was carried out using HRP-streptavidin
conjugates. All sections were visualized with diaminobenzidine and
counterstained with hematoxylin. Finally, the sections were
dehydrated in alcohol and xylene and mounted onto glass slides.
All slides were detected independently by two
experienced pathologists. The staining results were evaluated by an
immunohistochemical score combined with the percentage of tumor
cells showing specific immunoreactivity. Staining intensity was
classified into four grades: 0 (none), 1 (weak), 2 (moderate) and 3
(strong). The percentage of positive tumor cells was given with the
following grades: 0 (<5%), 1 (6–25%), 2 (26–50%), 3 (51–75%) and
4 (>75%). Staining intensity and average percentage of positive
tumor cells were assayed in ten independent high-magnification
(x400) fields. The total score was calculated by multiplying the
staining intensity and the percentage of positive tumor cells.
Total score of zero was considered as negative staining. Sections
with a total score of 1–4 were defined as the light positive
staining, while sections with a score of >4 were considered
strongly positive staining.
Quantitative real-time reverse
transcription polymerase chain reaction (qRT-PCR)
Total RNA was extracted from HCC cell lines
including Hep3B and MHCC97H using the Qiagen RNeasy kit (Valencia,
CA, USA). cDNA synthesis was carried out using the High Capacity
cDNA Reverse Transcription kit (Applied Biosystems) to transcribe 2
μg of total RNA. ABI TaqMan assays were used for qRT-PCR assay in
an ABI 7300 system. The ABI TaqMan probes used here were: 18s rRNA
(Hs99999901_s1), metastasin (Hs00243201_m1), SNAI1 (Hs
00195591_m1), E-cadherin (Hs 00170423_m1), N-cadherin (Hs
00983062_m1) and Vimentin (Hs 00185584_m1). All TaqMan probes were
obtained from Applied Biosystems. The mRNA levels of metastasin,
SNAI1, E-cadherin, N-cadherin and Vimentin were normalized to 18s
rRNA mRNA levels in the same samples. Each measurement was
performed 3 times.
Migration assay
The wound healing assay was performed as the
migration assay. Briefly, HCC cells were seeded onto 6-well plates
and cultured to confluency. Scratch wounds were made with a
1,000-μl pipette tip. The wounds were photographed with a
phase-contrast microscope at 0, 24 and 48 h. Cell migration was
quantitated by measuring the width of the wounds. The experiments
were performed with at least six replicates.
Invasion assay
A novel quantitative transwell chamber with the
Matrigel gel kit - QCM™ 96-well Cell Invasion Assay kit from
Millipore (Billerica, MA, USA) was used to detect the invasion
capacity of tumor cells, according to the manufacturer’s protocol.
Briefly, ~1×105 tumor cells were plated into the upper
chamber in serum-free medium and medium with 20% FBS was added into
the lower chamber. After 24 h, cells under the invasion chamber
were dislodged completely by the cell detachment solution and added
into the lysis buffer/dye solution mixture. The results were
obtained with a fluorescence plate reader using a 480/520-nm filter
set. The experiments were performed with at least six
replicates.
Statistical analysis
Differences between groups were compared with the
Fisher’s exact test or the Mann-Whitney test. Differences between
the Kaplan-Meier curves of HCC patients with high and low
metastasin expression in the HCC tissue in comparison with the
adjacent benign tissue were analyzed with the log-rank test.
Results
Metastasin is overexpressed in HCC
tissues and is associated with poor prognosis
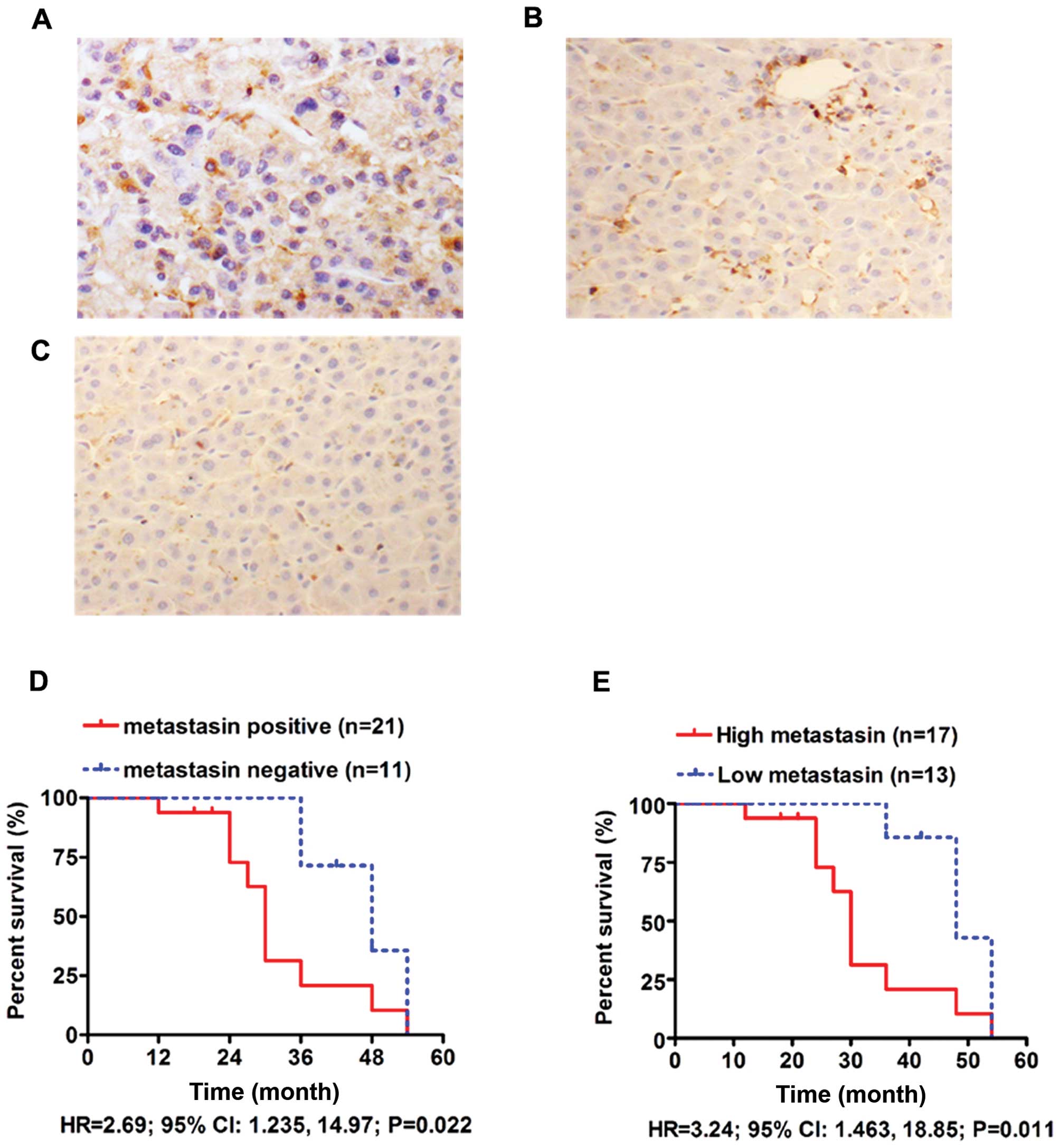
The majority of positive staining cells showed
diffuse cytoplasmic staining of metastasin, while nuclear staining
was found in a few cells (Fig. 3A).
In 41 pairs of HCC tissues and adjacent liver tissues, there were
27 (65.9%) positive metastasin staining HCC tissues compared to 8
(19.5%) positive staining adjacent liver tissues (Fig. 3A and B). The staining of metastasin
in the 8 healthy liver tissues was negative (Fig. 3C). These results clearly indicate
that metastasin was overexpressed in HCC tissues (P<0.001).
After analyzing the relationship between metastasin expression in
HCC tissues and clinicopathological characteristics (Table I), we found that positive metastasin
expression was significantly related to intrahepatic metastases
(P=0.033), portal vein invasion (P=0.001), advanced TNM staging
(P=0.037) and high Edmonson classification (P=0.04), suggesting
that metastasin expression in HCC tissues contributes to HCC
metastasis.
 | Table ICorrelation between
clinicopathological characteristics and metastasin expression in
HCC tissues. |
Table I
Correlation between
clinicopathological characteristics and metastasin expression in
HCC tissues.
| Metastasin
expression | | |
|---|
|
| | |
|---|
| Positive | Negative | Percentage | P-value |
|---|
| Gender |
| Male | 20 | 9 | 60 | 0.514 |
| Female | 7 | 5 | 58.3 | |
| Age (years) |
| ≤45 | 14 | 8 | 63.6 | 0.747 |
| >45 | 13 | 6 | 68.4 | |
| HBV infection |
| Yes | 18 | 10 | 64.3 | 0.756 |
| No | 9 | 4 | 69.2 | |
| Liver
cirrhosis |
| Yes | 23 | 11 | 67.6 | 0.594 |
| No | 4 | 3 | 57.1 | |
| AFP (ng/ml) |
| ≤400 | 12 | 8 | 60.0 | 0.440 |
| >400 | 15 | 6 | 71.4 | |
| Intrahepatic
metastasis |
| Yes | 19 | 5 | 79.2 | 0.033 |
| No | 8 | 9 | 47.1 | |
| Tumor size
(cm) |
| ≤5 | 14 | 10 | 58.3 | 0.228 |
| >5 | 13 | 4 | 76.5 | |
| Portal vein
invasion |
| Yes | 19 | 3 | 86.4 | 0.001 |
| No | 8 | 11 | 42.1 | |
| Edmonson
classification |
| I+II | 17 | 13 | 56.7 | 0.040 |
| III+IV | 10 | 1 | 90.9 | |
| TNM staging |
| I+II | 12 | 11 | 52.2 | 0.037 |
| III+IV | 15 | 3 | 83.3 | |
We obtained follow-up information from 32 of the 41
HCC cases (78%). The median duration of follow-up was 24 months.
The 3-year survival rate of the metastasin positive group was 20.8%
compared to 71.4% in the metastasin negative group. As compared by
the Kaplan-Meier survival curve, HCCs with metastasin expression
were found to have poorer survival (HR=2.69; 95% CI: 1.235, 14.97;
P=0.022) (Fig. 3D). We also
compared the survival curves between the high metastasin group, in
which HCC tissues expressed more metastasin than adjacent normal
tissues, and in the low metastasin group. The 3-year survival rate
of the high metastasin group was found to be 20.8% compared to
85.7% in the low metastasin group. The high metastasin group was
found to have clearly worse overall survival than the low
metastasin group (HR=3.24; 95% CI: 1.463, 18.85; P=0.011) (Fig. 3E). These data showed that metastasin
expression in HCC tissues was markedly associated with poor
prognosis.
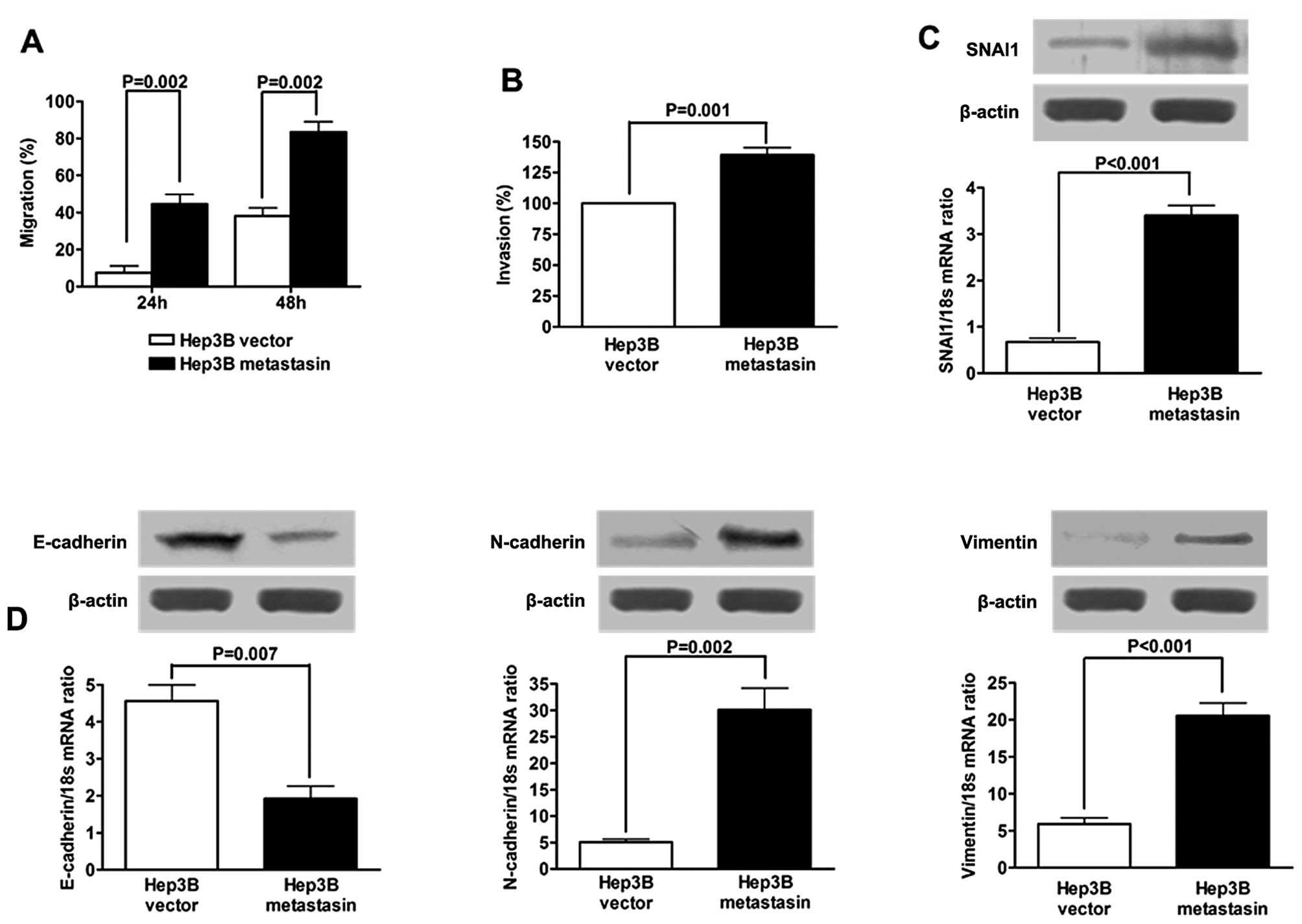
Ectopic expression of metastasin in Hep3B
cells promotes cell migration and invasion, and induces EMT
Ectopic expression of metastasin was confirmed by
both qRT-PCR and western immunoblotting (Fig. 1). To investigate the effect of
metastasin on HCC migration, wound healing assay was carried out
and showed that the migration rate of Hep3B cells stably
transfected with metastasin expressing plasmid (Hep3B metastasin
cells) was significantly faster than that of Hep3B cells
transfected with vector plasmid (Hep3B vector cells) at both 24 and
48 h after scratching (both P-values were 0.002; Fig. 4A). Similar results were obtained
from the quantitative invasion assay, which showed that the
invasion ability of Hep3B cells was greatly increased by ectopic
expression of metastasin (P=0.001; Fig.
4B).
To investigate whether metastasin promotes migration
and invasion by inducing EMT, we examined expression of EMT markers
of Hep3B cells following overexpression of metastasin. SNAI1, a
well-known EMT inducer, was found to be upregulated at both the
mRNA and the protein level. qRT-PCR assay revealed that E-cadherin
mRNA expression of Hep3B cells was decreased by ~60% by ectopic
expression of metastasin (Fig. 4C).
The protein expression of E-cadherin was verified to be suppressed
in Hep3B metastasin cells by western immunoblotting (Fig. 4D). On the other hand, as shown in
Fig. 4D, the expression of both
N-cadherin and Vimentin, which are considered mesenchymal markers,
was found enhanced at the mRNA and protein levels by metastasin
overexpression. Collectively, the data presented here indicate that
metastasin promotes migration and invasion of Hep3B cells via
induction of EMT.
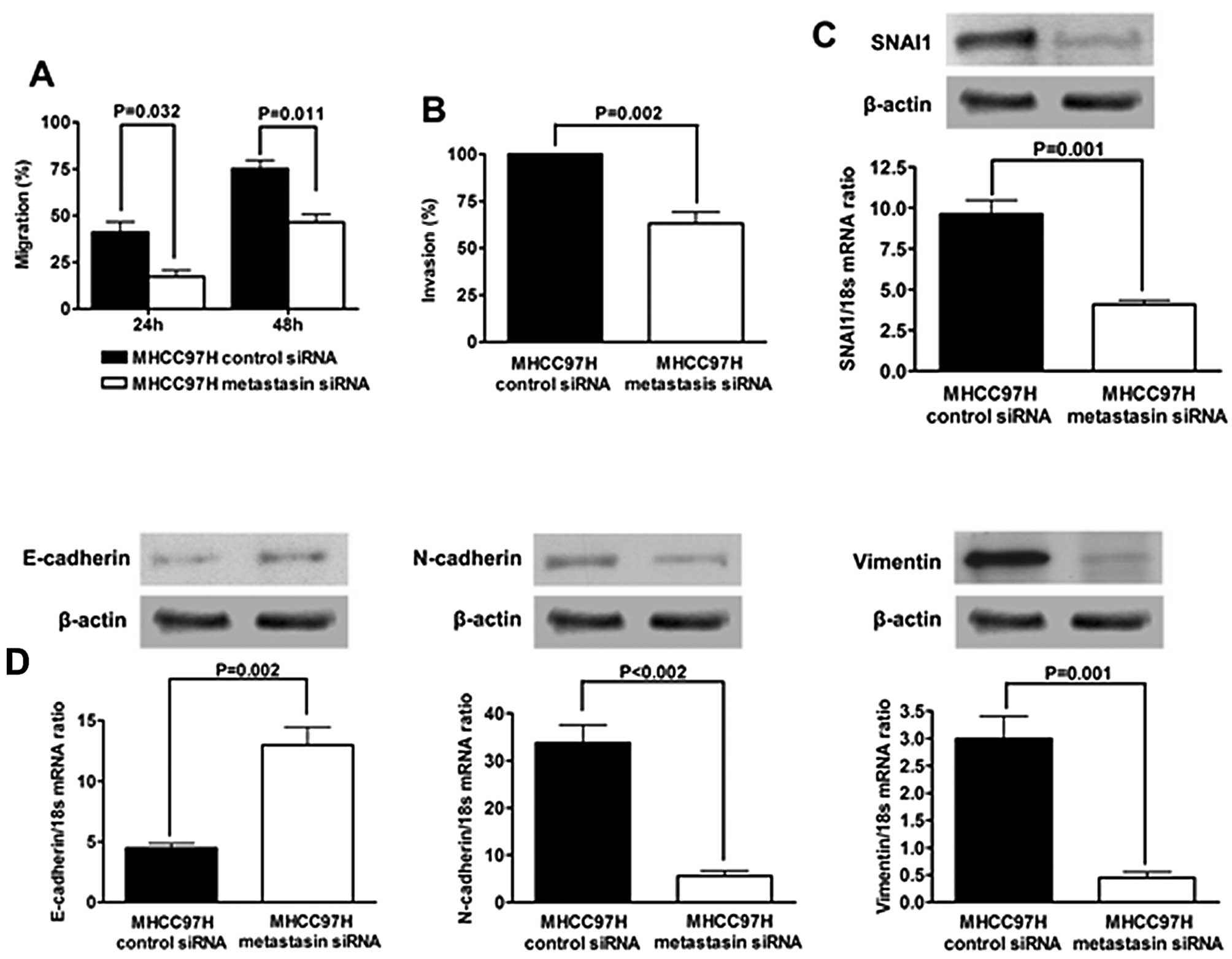
Knockdown of metastasin in MHCC97H cells
suppresses cell migration and invasion and reverses EMT
To further confirm the observation regarding
metastasin overexpression, we silenced metastasin expression of
MHCC97H cells by transfection of metastasin siRNA (Fig. 2). As shown in Fig. 5A, wound healing assay showed that
knockdown of metastasin significantly suppressed mobility of
MHCC97H cells (24 h after scratching, P=0.032; 48 h after
scratching, P=0.011). Accordingly, the invasion ability of MHCC97H
cells was markedly attenuated by silencing metastasin (P=0.002;
Fig. 5B).
Next, we tested if knockdown of metastasin
downregulated SNAI1 expression and reverted EMT in MHCC97H cells.
SNAI1 expression was significantly decreased by silencing
metastasin at both the protein and the mRNA level, as assessed by
western immunoblotting and qRT-PCR, respectively (Fig. 5C). Further examination for the
expression of EMT markers showed that knockdown of metastasin
promoted E-cadherin expression and suppressed the expression of
N-cadherin and Vimentin (Fig. 5D).
The complete opposite results obtained here further confirm the
hypothesis that metastasin induces EMT in HCC cells and then
promotes cell migration and invasion.
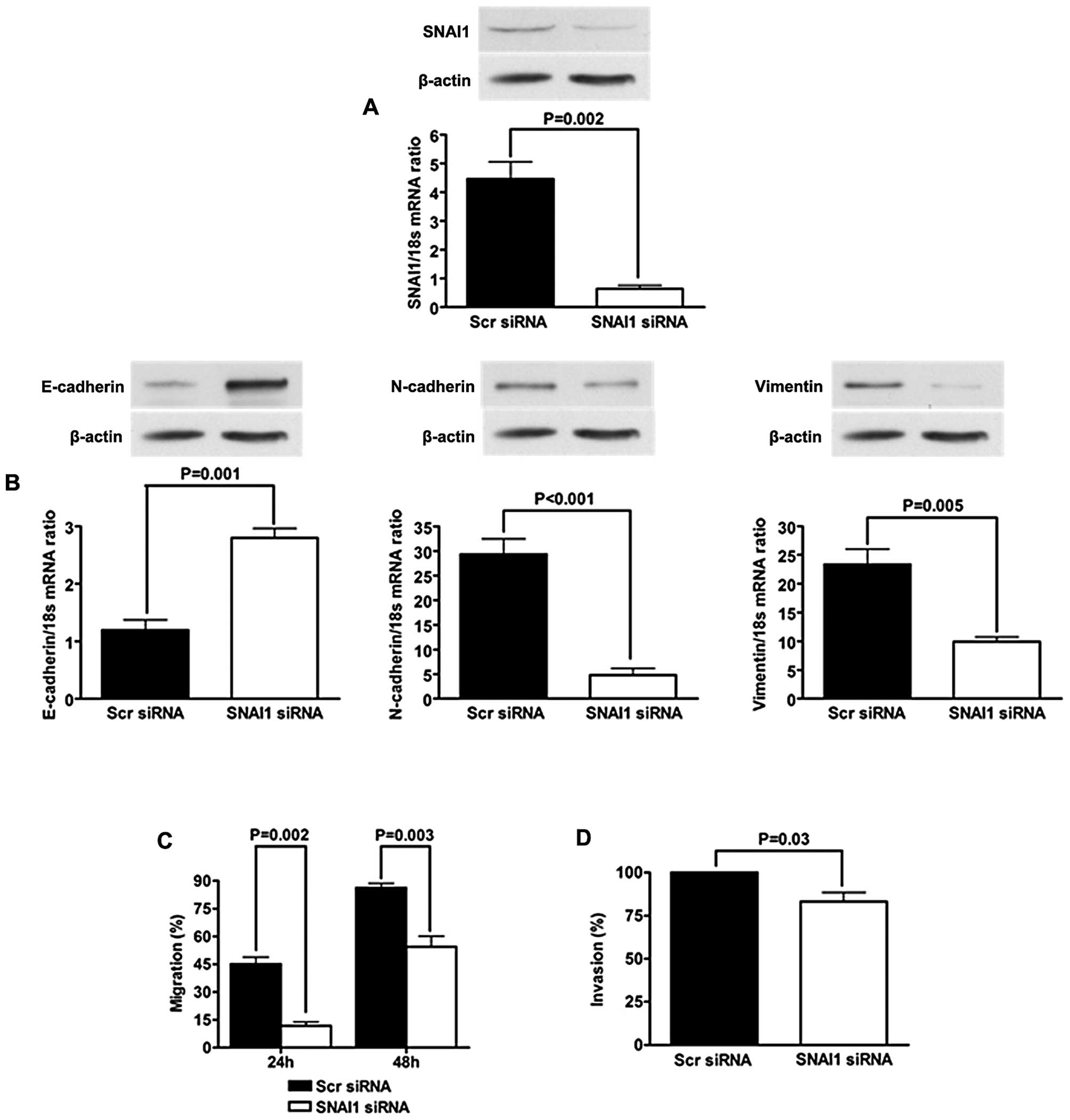
Suppression of SNAI1 blocks EMT induced
by metastasin
To investigate the role of SNAI1 in
metastasin-induced EMT, we silenced SNAI1 upregulated by metastasin
overexpression and re-evaluated EMT phenotype of Hep3B metastasin
cells. qRT-PCR and western immunoblotting confirmed that SNAI1
expression of Hep3B cells was knocked down effectively by siRNA
(Fig. 6A). E-cadherin expression
was increased significantly, while the expression of both
N-cadherin and Vimentin were decreased (Fig. 6B). The migration and invasion of
Hep3B metastasin cells were both significantly inhibited (Fig. 6C and D). These data showed that EMT
phenotype of Hep3B cells induced by metastasin was reversed
following SNAI1 knockdown.
Discussion
HCC is one of the most common malignant tumors
worldwide with increasing incidence rates. Radical hepatic
resection at the early stage is the most important and common
curative treatment for HCC. However, due to the lack of typical
symptoms at the early stage and high metastatic capacity, the
majority of HCC patients at present have no opportunity to receive
the curative resection. To date, metastasis of HCC remains largely
incurable due to its systemic characteristics and the resistance to
the existing therapeutic agents. Therefore, it is critical to
explore the mechanism underlying HCC metastasis and to develop
effective clinical prognostic biomarkers and therapeutic
agents.
The metastasin protein encoded by the human mts1
gene located at position 1q21 on chromosome 1 is a type of
polypeptide of 101 amino acids with the molecular mass of 11.5 kDa.
Previous studies revealed that the transcription of metastasin is
controlled by regulatory elements in its first intron via binding
with several transcription factors, such as I-κB (18,19).
Epigenetic investigations also found metastasin transcription was
strongly affected by the methylation of both its enhancer and
silencer elements, which could contribute to its aberrant
expression in diverse types of human cancer (20–22).
Clinical research on breast cancer patients with regional invasion
but operable stage I and II, reported by Rudland et
al(23), showed that during the
19-year follow-up period, the median survival of the metastasin
negative group was 228 months vs. 47 months in the metastasin
positive group. A similar result was reported by Lee et
al(24) showing that metastasin
was related with poor outcome of T1N0M0 breast cancer and could be
a biomarker of early metastasis of breast cancer. Aside from the
investigations on breast cancer, numerous studies of different
types of human cancer, including colon cancer (25), gastric cancer (26,27),
pancreatic ductal carcinoma (28),
renal carcinoma (29), found that
metastasin expression was significantly overexpressed in malignant
tissues and was associated with poor survival. To our knowledge,
there are few reports about the expression and function of
metastasin on HCC. In this study, we found metastasin was
aberrantly upregulated in HCC tissues and was associated with poor
outcome following liver resection, which indicates metastasin could
be a potential predictive marker and therapy target for HCC.
In the present study we initially investigated the
mechanism by which metastasin leads to poor survival of HCC. In
general, metastasin has no enzymatic activity and works via binding
and interacting with other proteins, including p53, P37, S100A1,
CCN3 (30,31). Numerous in vivo experiments
showed that metastasin promotes tumor metastasis in mouse models.
Ambartsumian et al(32)
verified that metastasin leads to higher metastatic capacity of
mammary tumor via transgenic mouse overexpressing metastasin in
mammary epithelium. The knockdown in vivo experiments on
lung carcinoma and osteosarcoma also showed silencing metastasin
suppressed the metastatic potential of tumor cells (33,34).
Although several investigations showed overexpression of metastasin
induced downregulation of E-cadherin and upregulation of MMPs
(6,35,36),
the underlying mechanism by which metastasin promotes tumor
metastasis has yet to be fully elucidated. Here, we found ectopic
expression of metastasin in Hep3B cells increased cell mobility and
invasion. SNAI1, a renowned EMT inducer, was also found upregulated
by metastasin overexpression, suggesting that metastasin induced
EMT in Hep3B cells. Further examination showing E-cadherin was
downregulated and both N-cadherin and Vimentin were upregulated
confirms the EMT phenotype of Hep3B cells induced by ectopic
expression of metastasin. Conversely, knockdown of metastasin in
MHCC97H cells led to the opposite results, which further verifies
that metastasin induces EMT phenotype of HCC cells through
upregulating SNAI1. Although metastasin was found to enhance SNAI1
expression in both Hep3B and MHCC97H cells, there is no evidence to
support that metastasin could regulate the transcription of SNAI1
directly. Our group sought to find the metastasin-binding site of
SNAI1 promoter by carrying out EMSA (unpublished data) and did not
get any positive results, which indicates that metastasin could
upregulate SNAI1 in indirect ways. To clarify the role of SNAI1
upregulated on metastasin-driven EMT, we successfully inhibited the
upregulation of SNAI1 after stably overexpressing metastasin in
Hep3B cells by SNAI1 siRNA, and found E-cadherin expression was
increased and the expression of both N-cadherin and Vimentin were
clearly decreased. Consistently, both cell mobility and invasion
were inhibited significantly. Consequently, these data indicate EMT
induced by metastasin is reversed by suppressing SNAI1 in Hep3B
cells and SNAI1 is involved in the intracellular signal cascade of
metastasin-induced EMT.
In summary, this investigation shows metastasin is
overexpressed in HCC and is markedly related with poor survival.
Metastasin induces EMT phenotype of HCC via upregulating SNAI1,
which could explain initially how metastasin leads to poor
prognosis of HCC. Therefore, metastasin is a potential clinical
biomarker and target for anti-HCC therapy, and further
investigation is required to elucidate the concrete mechanism by
which metastasin promotes HCC progression.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China to Y.Y. (81071897/H1612) and to
Q.L. (81271645/H1617 and 81072052/H1617).
References
|
1
|
Yang JD and Roberts LR: Hepatocellular
carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 7:448–458.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma W, Wong CC, Tung EK, Wong CM and Ng IO:
RhoE is frequently downregulated in HCC and suppresses HCC invasion
through antagonizing the Rho/ROCK/MYPT pathway. Hepatology.
57:152–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berge G and Maelandsmo GM: Evaluation of
potential interactions between the metastasis-associated protein
S100A4 and the tumor suppressor protein p53. Amino Acids.
41:863–873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mishra SK, Siddique HR and Saleem M:
S100A4 calcium-binding protein is key player in tumor progression
and metastasis: preclinical and clinical evidence. Cancer
Metastasis Rev. 31:163–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ambartsumian N, Klingelhofer J, Grigorian
M, et al: The metastasis-associated Mts1(S100A4) protein could act
as an angiogenic factor. Oncogene. 20:4685–4695. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saleem M, Kweon MH, Johnson JJ, et al:
S100A4 accelerates tumorigenesis and invasion of human prostate
cancer through the transcriptional regulation of matrix
metalloproteinase 9. Proc Natl Acad Sci USA. 103:14825–14830. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stein U, Arlt F, Walther W, et al: The
metastasis-associated gene S100A4 is a novel target of
beta-catenin/T-cell factor signaling in colon cancer.
Gastroenterology. 131:1486–1500. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takenaga K, Nakanishi H, Wada K, et al:
Increased expression of S100A4, a metastasis-associated gene, in
human colorectal adenocarcinomas. Clin Cancer Res. 3:2309–2316.
1997.PubMed/NCBI
|
|
9
|
Ilg EC, Schafer BW and Heizmann CW:
Expression pattern of S100 calcium-binding proteins in human
tumors. Int J Cancer. 68:325–332. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hao H, Liu J, Liu G, et al: Depletion of
GRIM-19 accelerates hepatocellular carcinoma invasion via inducing
EMT and loss of contact inhibition. J Cell Physiol. 227:1212–1219.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu K, Dai Z, Pan Q, et al: Metadherin
promotes hepatocellular carcinoma metastasis through induction of
epithelial-mesenchymal transition. Clin Cancer Res. 17:7294–7302.
2011. View Article : Google Scholar
|
|
13
|
Wang J, Chen L, Li Y and Guan XY:
Overexpression of cathepsin Z contributes to tumor metastasis by
inducing epithelial-mesenchymal transition in hepatocellular
carcinoma. PLoS One. 6:e249672011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagai T, Arao T, Furuta K, et al:
Sorafenib inhibits the hepatocyte growth factor-mediated epithelial
mesenchymal transition in hepatocellular carcinoma. Mol Cancer
Ther. 10:169–177. 2011. View Article : Google Scholar
|
|
15
|
Xia L, Huang W, Tian D, et al:
Overexpression of forkhead box C1 promotes tumor metastasis and
indicates poor prognosis in hepatocellular carcinoma. Hepatology.
57:610–624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Strutz F, Okada H, Lo CW, et al:
Identification and characterization of a fibroblast marker: FSP1. J
Cell Biol. 130:393–405. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui JF, Liu YK, Zhang LJ, et al:
Identification of metastasis candidate proteins among HCC cell
lines by comparative proteome and biological function analysis of
S100A4 in metastasis in vitro. Proteomics. 6:5953–5961. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tulchinsky EM, Georgiev GP and Lukanidin
EM: Novel AP-1 binding site created by DNA-methylation. Oncogene.
12:1737–1745. 1996.PubMed/NCBI
|
|
19
|
Cohn MA, Hjelmso I, Wu LC, Guldberg P,
Lukanidin EM and Tulchinsky EM: Characterization of Sp1, AP-1, CBF
and KRC binding sites and minisatellite DNA as functional elements
of the metastasis-associated mts1/S100A4 gene intronic enhancer.
Nucleic Acids Res. 29:3335–3346. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Liu ZL, Zhang KL, et al:
Methylation-associated silencing of S100A4 expression in human
epidermal cancers. Exp Dermatol. 18:842–848. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie R, Loose DS, Shipley GL, Xie S,
Bassett RL Jr and Broaddus RR: Hypomethylation-induced expression
of S100A4 in endometrial carcinoma. Mod Pathol. 20:1045–1054. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rehman I, Goodarzi A, Cross SS, et al: DNA
methylation and immunohistochemical analysis of the S100A4 calcium
binding protein in human prostate cancer. Prostate. 67:341–347.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rudland PS, Platt-Higgins A, Renshaw C, et
al: Prognostic significance of the metastasis-inducing protein
S100A4 (p9Ka) in human breast cancer. Cancer Res. 60:1595–1603.
2000.PubMed/NCBI
|
|
24
|
Lee WY, Su WC, Lin PW, Guo HR, Chang TW
and Chen HH: Expression of S100A4 and Met: potential predictors for
metastasis and survival in early-stage breast cancer. Oncology.
66:429–438. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang HY, Zhang JY, Cui JT, et al:
Expression status of S100A14 and S100A4 correlates with metastatic
potential and clinical outcome in colorectal cancer after surgery.
Oncol Rep. 23:45–52. 2010.PubMed/NCBI
|
|
26
|
Wang YY, Ye ZY, Zhao ZS, Tao HQ and Chu
YQ: High-level expression of S100A4 correlates with lymph node
metastasis and poor prognosis in patients with gastric cancer. Ann
Surg Oncol. 17:89–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yonemura Y, Endou Y, Kimura K, et al:
Inverse expression of S100A4 and E-cadherin is associated with
metastatic potential in gastric cancer. Clin Cancer Res.
6:4234–4242. 2000.PubMed/NCBI
|
|
28
|
Ikenaga N, Ohuchida K, Mizumoto K, et al:
S100A4 mRNA is a diagnostic and prognostic marker in pancreatic
carcinoma. J Gastrointest Surg. 13:1852–1858. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bandiera A, Melloni G, Freschi M, et al:
Prognostic factors and analysis of S100a4 protein in resected
pulmonary metastases from renal cell carcinoma. World J Surg.
33:1414–1420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tarabykina S, Griffiths TR, Tulchinsky E,
Mellon JK, Bronstein IB and Kriajevska M: Metastasis-associated
protein S100A4: spotlight on its role in cell migration. Curr
Cancer Drug Targets. 7:217–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Santamaria-Kisiel L, Rintala-Dempsey AC
and Shaw GS: Calcium-dependent and -independent interactions of the
S100 protein family. Biochem J. 396:201–214. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ambartsumian NS, Grigorian MS, Larsen IF,
et al: Metastasis of mammary carcinomas in GRS/A hybrid mice
transgenic for the mts1 gene. Oncogene. 13:1621–1630.
1996.PubMed/NCBI
|
|
33
|
Maelandsmo GM, Hovig E, Skrede M, et al:
Reversal of the in vivo metastatic phenotype of human tumor cells
by an anti-CAPL (mts1) ribozyme. Cancer Res. 56:5490–5498.
1996.PubMed/NCBI
|
|
34
|
Takenaga K, Nakamura Y and Sakiyama S:
Expression of antisense RNA to S100A4 gene encoding an S100-related
calcium-binding protein suppresses metastatic potential of
high-metastatic Lewis lung carcinoma cells. Oncogene. 14:331–337.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moriyama-Kita M, Endo Y, Yonemura Y, et
al: S100A4 regulates E-cadherin expression in oral squamous cell
carcinoma. Cancer Lett. 230:211–218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schmidt-Hansen B, Ornas D, Grigorian M, et
al: Extracellular S100A4(mts1) stimulates invasive growth of mouse
endothelial cells and modulates MMP-13 matrix metalloproteinase
activity. Oncogene. 23:5487–5495. 2004. View Article : Google Scholar : PubMed/NCBI
|