Introduction
Hepatocellular carcinoma (HCC) is the most common
form of liver cancer and is one of the leading causes of
cancer-related mortality worldwide (1). Invasion and metastasis of liver cancer
contribute to treatment failure in the majority of cancer patients.
Liver cancer is generally diagnosed at a later stage of cancer
development. Liver metastasis and treatment are related to the
characteristics of the tumor and the immune system of the host.
Understanding the apoptotic pathways and their corresponding
inhibitors enables us to formulate strategies for cancer therapy.
The overexpression of genes that are associated with cell growth
and programed cell death depend on the activity of the
p53-associated pathways. During malignant progression, the human
papillomaviruses integrate into the liver cell genome resulting in
a loss of expression of oncogenes. The gene proteins may lead to
interference with the tumor suppression protein p53, which is
activated in response to stress and plays an important role in the
regulation of cell cycle, DNA repair and apoptosis (2). Genomic alterations of p53 can be found
in cancer and deletion of p53 in cancer cells could lead to their
resistance to apoptosis (2). Tumor
suppressor p53 is involved in transcriptional activation of the
human Bax gene (3,4). Apoptosis-inducing factor plays a role
in the regulation of caspase-independent cell death (5,6). The
most common risk factor for HCC is the hepatitis B (HBV) or
hepatitis C viral (HCV) infection, of which there is a high
incidence rate in China (1).
Approximately one fourth of the carriers could develop liver
cancer. An antiviral approach against human transcriptional
inactivation of viral infection is used for HCV infection.
Complementary medicine using herbal ingredients against
transcriptional inactivation of cancer cells showed minimal system
toxicity and could be a promising agent for liver cancer therapy
particularly at an early stage of liver carcinogenesis. Cancer
patients can have a higher survival rate with the complementary
treatment. Tetrandrine is a bisbenzylisoquinoline alkaloid, a
naturally occurring compound isolated from the root of Stephania
tetrandra, which was reported to exhibit a variety of
pharmacological properties including anti-inflammatory,
anti-rheumatic and anti-hypertensive effects (7). It can inhibit the proliferation of
HeLa and HepG2 cells in vitro and suppress ascites tumors in
mice (8). Tetrandrine was reported
to suppress Wnt/β-catenin signaling and tumor growth of human
colorectal cancer (9). A previous
study showed that tetrandrine induced apoptosis by activating
reactive oxygen species and repressing Akt activity in human liver
cancer cells (10). The results
suggest that mediation of the ROS/Akt pathway by tetrandrine can
enhance the beneficial effects of tetrandrine in cells. Zhang et
al showed that tetrandrine was used together with cisplatin to
enhance growth suppression of ovarian cells and apoptosis (11). In vivo study of the combined
effects of tetrandrine and cisplatin exhibited anticancer effects
in the rat (11). The effect of
tetrandrine with radiation on human esophageal cancer cell line TE1
showed that the expression of cyclin B1 protein increased while
radiation-induced G2 arrest was abrogated (12). The study suggests that the enhanced
cytotoxicity and activation of ROS-dependent caspase-3 activity
could induce programmed cell death in cancer (13). Another study indicated that two
distinct pathways could lead to the apoptosis of cancer cells
(14). Apoptosis of cancer cells
was considered to be associated with mitochondrial release of
inducing factors that occurs downstream of cytochrome c
release in response to oxidative stress (15). A recent study showed that
tetrandrine induced apoptosis via caspase cascade in human bladder
cancer cells (16). Although
tetrandrine was reported to have multiple biologic activities, the
details of its anticancer properties are lacking. Therefore, we
investigated the effect of tetrandrine on Huh-7 cancer cells.
Materials and methods
Cell culture and reagents
The human liver cell line Huh-7 was obtained from
the American Type Culture Collection (USA) and cultured in DMEM
(Gibco, USA) supplemented with RPMI and 0.25% trypsin-EDTA at 37°C
in an atmosphere of 5% CO2–95% air. Tetrandrine was
obtained from Sigma Chemicals (St. Louis, MO, USA).
In vitro cell viability assay
Cell proliferation was measured by a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide assay
(MTT) according to the manufacturer’s protocols. Huh-7 cells
(1×105 cells/well) were placed in 96-well microtiter
plates (Corning) and incubated overnight. Cells were treated with
either 1% serum DMEM as a control or with various concentrations of
tetrandrine in 1% DMEM for 24, 48 or 72 h, respectively. At the end
of the incubation period, 20 μl of a 5-mg/ml solution of MTT
prepared in PBS was placed into each well and the cells were
incubated for an additional 4 h. Cells were lysed in 200 μl DMSO
and absorbance was measured at 570 nm. Six replicate wells were
used for each analysis. The data represent the means ± SD of three
independent experiments with 95% confidence intervals.
Western blot analysis of gene
expression
Western blotting assay was used to analyze the
expressions of apoptotic proteins in Huh-7 cells. Cells
(1×106) seeded in 6-well plates were exposed to various
concentrations of tetrandrine for 24, 48 and 72 h. The cells were
harvested and lysated (40 μg of protein per lane) and fractionated
by 10% SDS-PAGE as described below. The protein content was
determined according to the Bradford method (17). Cells were collected in the 15-ml
falcon and were re-suspended in 400 μl of cell DNA lysis buffer in
a 1.5-ml microtube with vigorous mixing. Twenty microliters of 10
mg/ml proteinase K were added to each microtube. The mixtures were
incubated at 37°C for 3 h. Saturated NaCl solution (50 μl) was
added to each sample tube prior to centrifugation at 7,000 × g for
15 min at room temperature. The supernatant containing DNA was
collected. Ice cold absolute ethanol (1 ml) was added to the tubes
with mixing followed by centrifugation at 14,000 × g at 4°C for 20
min. The pellets were washed with 70% ethanol once. They were spun
again at 14,000 × g at 4°C for 20 min. The DNA pellet was allowed
to air dry. TE buffer (50 μl) containing 0.2 mg/ml of RNase A was
added to the DNA pellets for RNA digestion prior to incubation at
37°C for 90 min. DNA solution (2 μl) from each sample was added to
998 μl TE buffer. The concentrations of the DNA in the diluted
solutions were measured by UV spectrophotometry (Beckman, DU 650)
at 260 nm.
Gel electrophoresis of DNA
Agarose (0.3 g) was added to 20 ml TBE buffer.
Ethidium bromide (2 μl) was added to the agarose solution followed
by the addition of 10 μl of the dissolved DNA samples and 2 μl 6X
DNA loading dye. The mixtures were loaded on the 1.5% agarose gel
and it was run at 75 V for 1 h. The DNA bands were visualized under
UV illuminator. The gel was photographed for documentation.
Measurement of gene expression in
tetrandrine-induced cancer cells
Huh-7 cells (1×106) were seeded on 6-well
plates with different concentrations of tetrandrine (0, 7.5, 15 and
20 μM). After 24 h of incubation, cells were harvested by
trypsinization and washed with 1X PBS. The cell platelets were
collected by centrifugation at 600 g for 2 min followed by the
addition of 1 ml TRIzol reagent. The mixtures were then incubated
at room temperature for 5 min prior to the addition of 266 μl
chloroform. Subsequently, the samples were centrifuged at 14,000
rpm for 15 min. The supernatant was mixed with 70% ethanol in 1:1
volume ratio. RNeasy Mini kit™ from Qiagen was used for the total
RNA extraction. The mRNA extraction was conducted according to the
manufacturer’s method. The total RNA concentration of each sample
assay was measured. The same amount of total RNA was used in each
sample for cDNA synthesis. Transcriptor First Strand cDNA Synthesis
kit from Roche was used for cDNA synthesis.
Reverse-transcription-PCR (RT-PCR) and
detection of gene products
Each RT-PCR reaction mixture contained 11.2 μl
water, 4 μl 5X Green GoTaq Flexi Buffer, 1.2 μl MgCl2
(25 mM), 1 μl primer, 0.4 μl dNTP, 0.2 μl GoTaq® Hot
Start Polymerase 5 U/μl, 2 μl cDNA template. The reaction was
conducted at 95°C for 5 min and the denaturation was at 95°C for 30
sec. Annealing and extension were performed in one step over 1 min
(Table I). The PCR products were
detected by running a 2% agarose gel. Agarose (0.4 g) was dissolved
in 20 ml 1X TBE buffer. The 2% agarose gel was run at 100 V for 30
min to separate the PCR products.
 | Table IThe annealing and extension
temperature used for different target genes. |
Table I
The annealing and extension
temperature used for different target genes.
| Target gene | Annealing and
extension temperature (°C) |
|---|
| Bax | 60 |
| Bid | 60 |
| Bcl-2 | 55 |
| GAPDH | 60 |
| p21 | 65 |
| Survivin | 60 |
Measurement of protein expression in
tetrandrine-induced apoptotic cells
Huh-7 cells (1×106) were seeded into a
100-mm dish with different concentrations of tetrandrine for 24 h
before being trypsinized and collected by centrifugation. The
number of cells collected in each sample was counted using a
hemocytometer. Whole cells lysis buffer (200 μl) was added per
1×106 cells and incubated at 37°C for 30 min. The
samples were then placed in boiling water for 10 min prior to
centrifugation at 14,000 × g for 15 min. The supernatant of the
samples was collected in a new micro-centrifuge tube for the
protein concentration determination (17).
Protein gel electrophoresis by
SDS-PAGE
Mini-PROTEAN® III cell with a 10-tooth
comb from Bio-Rad was used for SDS-PAGE according to a previous
method with modifications (17). A
small quantity of isopropanol was layered on the top of the running
gel solution before the excess isopropanol was discarded and
dH2O was used to wash the gel. Stacking gel solution
(4%) was added onto the top of the running gel. The gel was allowed
to stand until it polymerized. Sample loading dye (2X) was added to
the protein samples in a 1:1 ratio prior to boiling at 100°C for 10
min. Samples were loaded onto the wells and 1.5 μl of PageRuler™
Prestained Protein Ladder was added as a protein marker. The gel
was allowed to run at constant voltage. Fifty V were used for
running the stacking gel whereas 80, 100 and 120 V were used for
running 10, 12 and 15% running gel, respectively. The SDS-PAGE was
stopped after the dye front containing bromophenol blue reached the
bottom of the gel.
Western blot analysis
Bio-Rad Semi-dry Trans-Blot electroblotter was used
to transfer the protein onto the membrane according to the
manufacturer’s protocols. The gel was immersed into transfer buffer
for 15 min for equilibration. The PVDF membrane was activated using
100% methanol and was washed twice using dH2O to remove
methanol prior to equilibration in the transfer buffer for 15 min.
The running gel was placed above the membrane with another 3 pieces
of paper. Constant current (0.1A) was used to transfer proteins for
2 h. The PVDF membrane was rinsed with TBST buffer twice. The
desired dilution of antibody was added to the suitable percentage
of non-fat milk solution which was the same as that used in
blocking. The membrane was finally blotted with different
antibodies overnight at 4°C. The membrane was washed with 1X TBST
containing 0.2% Tween-20 three times, 15 min each. After washing,
the membrane was blotted with the corresponding secondary antibody.
The dilution for anti-mouse and anti-rabbit were 1:10,000 and
1:6,667, respectively. The secondary antibodies were diluted in
non-fat milk solution. The blocking solution contained the same
concentration of non-fat milk as that for the primary antibody. The
membrane was agitated for 1 h at room temperature. After probing
with secondary antibody, the membrane was washed with 1X TBST
containing 0.1% Tween-20 solution three times, each with 10
min.
Rodeo™ ECL Western Blotting Reagents from USB
Biochemicals was used for the signal development. The two reagents
in the kit were mixed in a 1:1 ratio. The mixture was equilibrated
at room temperature for 5 min prior to use. Excess TBST solution on
the membrane was removed by blotting it onto the M-fold tissue
paper. The western blotting reagent mixture was added slowly onto
the membrane with the size mobilized with proteins faced upward.
Protein bands on the membrane were visualized after exposing to
Fuji Super RX film. The intensities of the bands were analyzed
using ImageJ program.
Cell cycle analysis of
tetrandrine-treated cells
Huh-7 cells (1×106) were seed into a
100-mm dish with different concentrations of tetrandrine for 24, 24
and 72 h. The tetrandrine-treated Huh-7 cells were trypsinized and
were collected in a 15-ml falcon prior to centrifugation at 3,000 ×
g for 3 min. The cells were re-suspended in 1 ml 1X PBS prior to
centrifugation at 3,000 × g for 3 min. The cells were collected by
centrifugation in sequence after washing in 2 ml of 70% ethanol,
RNase containing PBS solution, 1 ml propidium iodide solution. The
cells in 1 ml propidium iodide solution were centrifuged at 3,000 ×
g for 3 min prior to flow cytometry analysis. The DNA content of
the treated cells was recorded and analyzed by FACSCanto flow
cytometer.
Statistical analysis
Statistical analysis was conducted using ANOVA. All
experiments were performed three times independently. Data are
expressed as the means ± SD.
Results
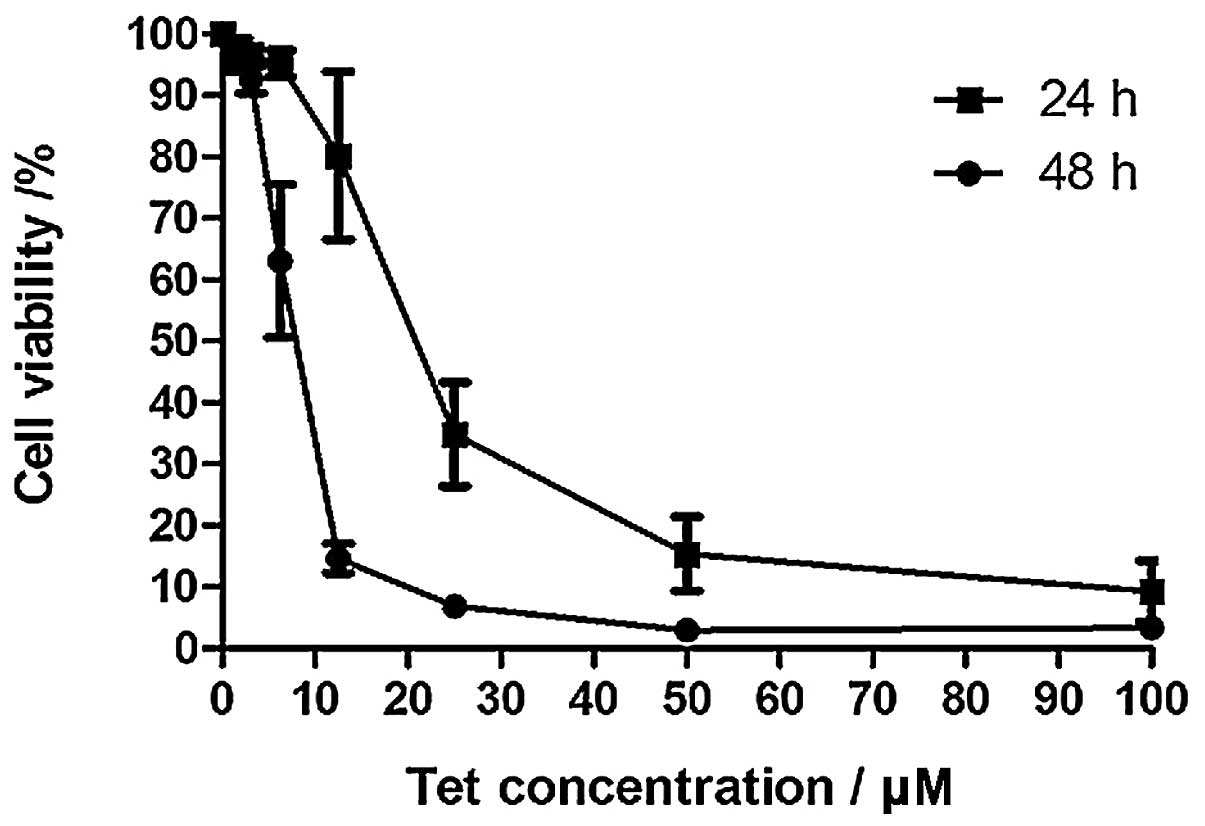
Fig. 1 shows the
cell viability of Huh-7 cells following treatment with different
concentrations of tetrandrine for 24 and 48 h. The viability of
cells significantly decreased when tetrandrine concentration was
increased. The IC50 of cells after 24 and 48 h of
incubation were found to be 20.8 and 8.0 μM, respectively. After 48
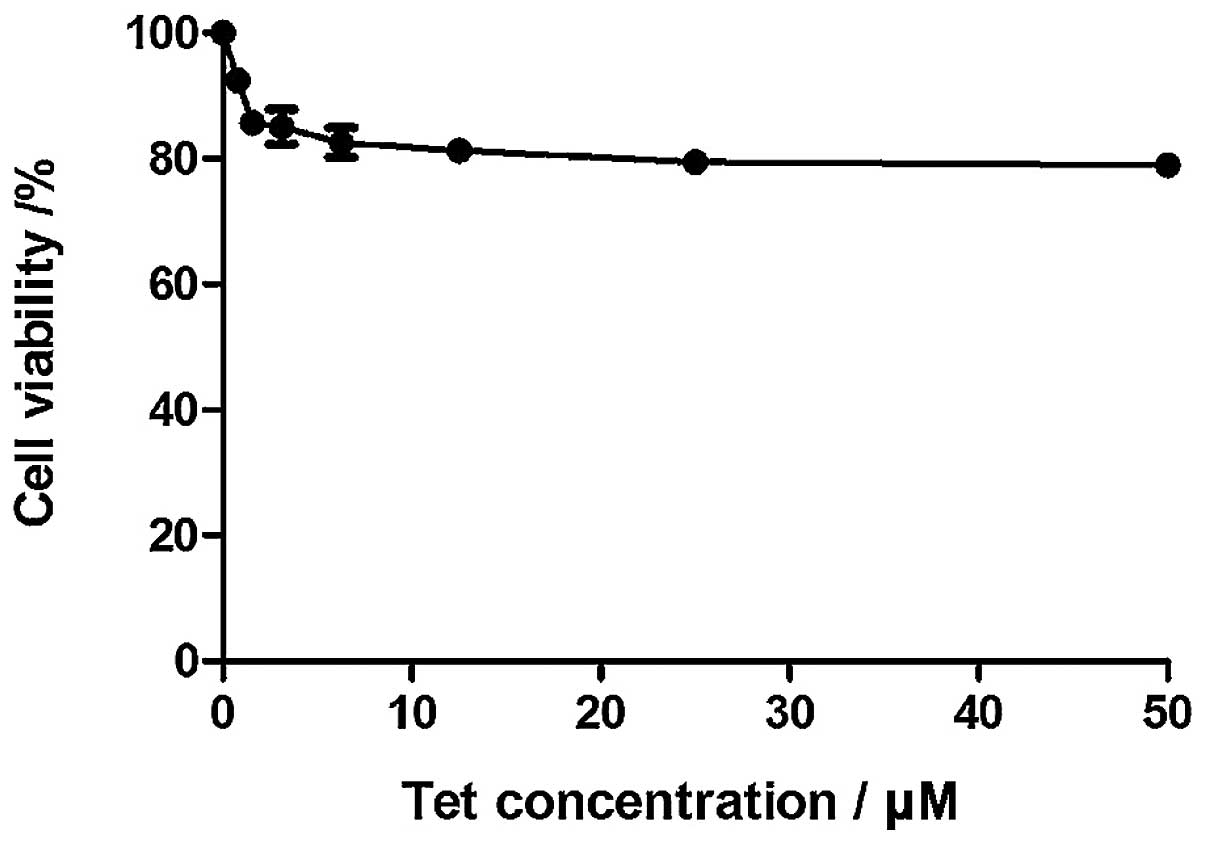
h of incubation, the cell viability was reduced. Fig. 2 shows the effect the tetrandrine on
WRL68 cells. The cell viability of WRL68 cells decreased when
tetrandrine concentration increased, but leveled off when its
concentration reached 3.125 μM. No IC50 for WRL68 could
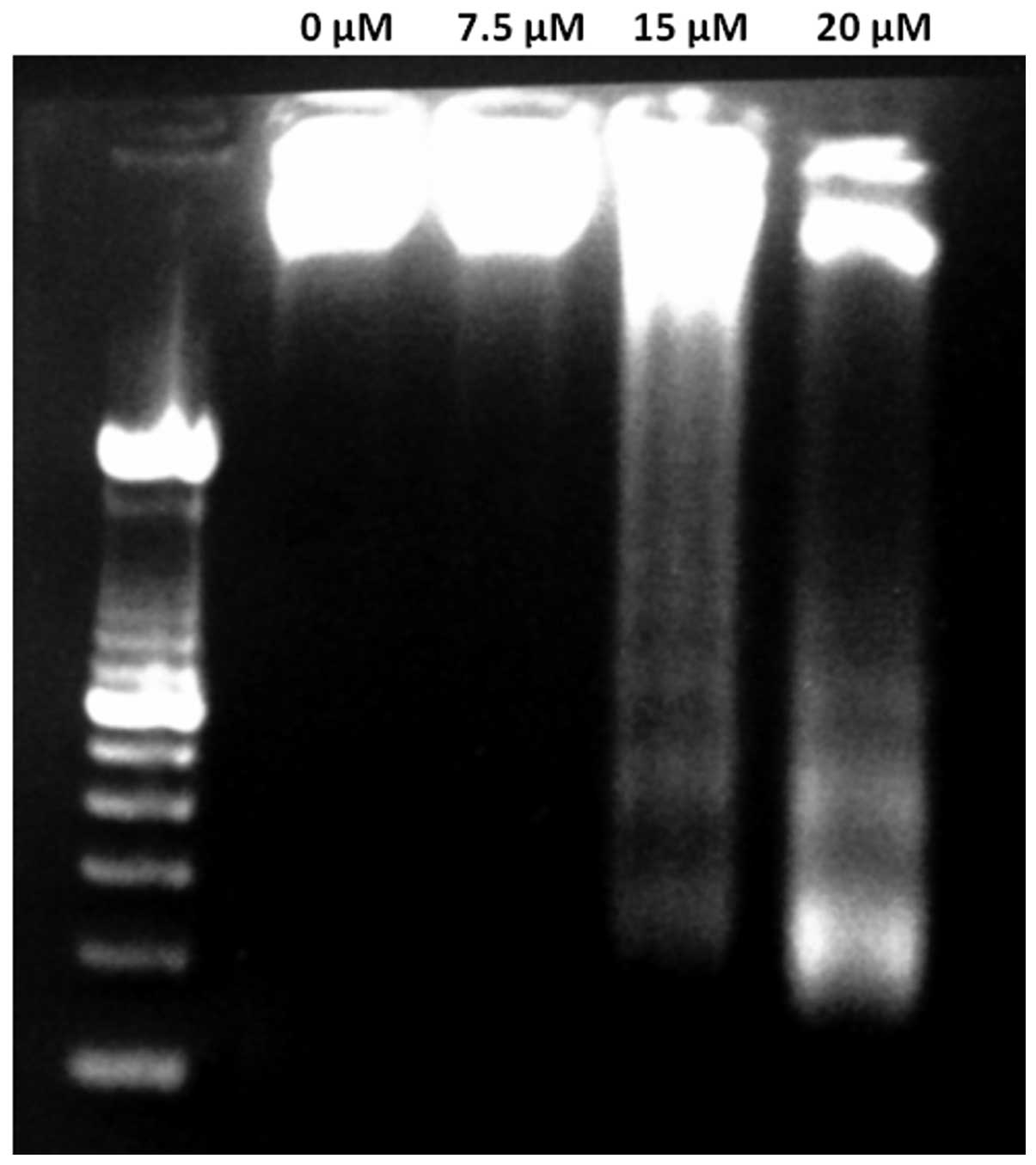
be determined. Fig. 3 shows the DNA
fragmentation of tetrandrine-treated Huh-7 cells. DNA ladders were
observed in the tetrandrine-treated samples but not in the control.
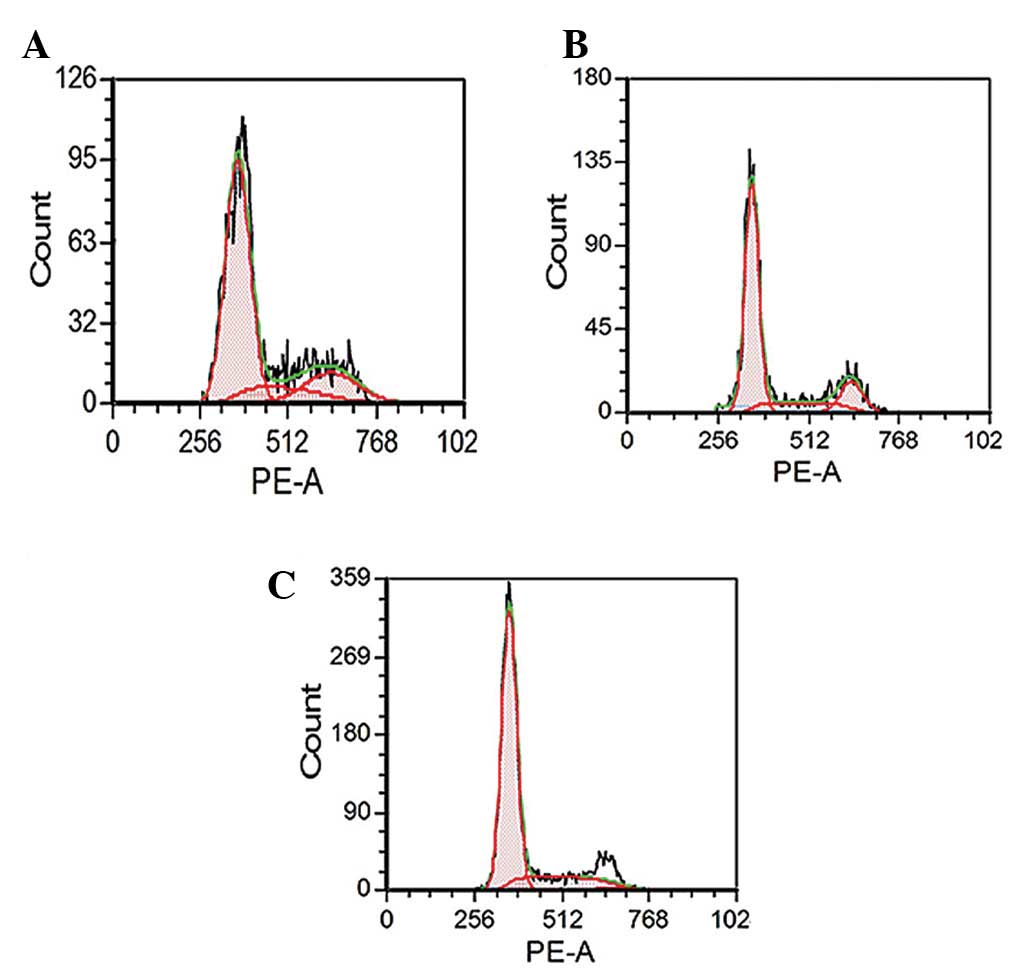
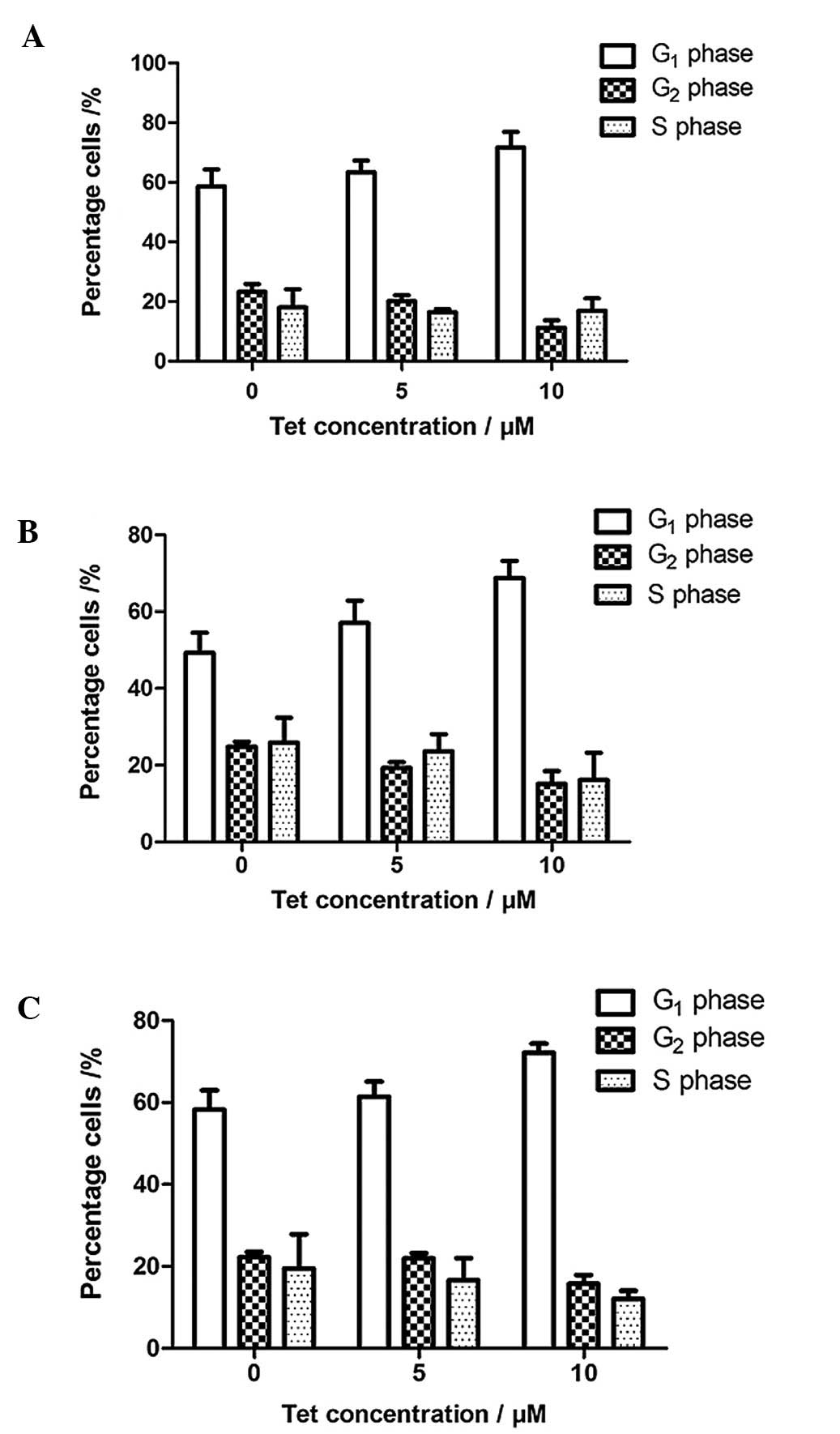
Fig. 4 shows the flow cytometry
analysis of Huh-7 cells after treatment with tetrandrine for 24 h.
The similar pattern of results of flow cytometry after 48 and 72 h
of incubation of cells was recorded. The results are summarized in
Fig. 5 and indicate the
distribution of the cell populations in G1, S and
G2 phase following treatment with tetrandrine. The cell
percentage in each phase of the cell cycle was analyzed by FACS
Express. The cell population in the G1 phase increased
from 58.63 to 71.72% after 24 h, from 49.30 to 68.74% after 48 h
and from 58.30 to 72.15% after 72 h of treatment. For the cell
population within the S phase, the percentages decreased slightly
from 18.07 to 16.95% for 24 h, from 25.88 to 16.14% for 48 h and
from 19.47 to 12.09% for 72 h of incubation. The cell population
within the G2 phase decreased at all the time points. It
decreased from 2.30 to 11.33%, from 24.83 to 15.14% and from 22.24
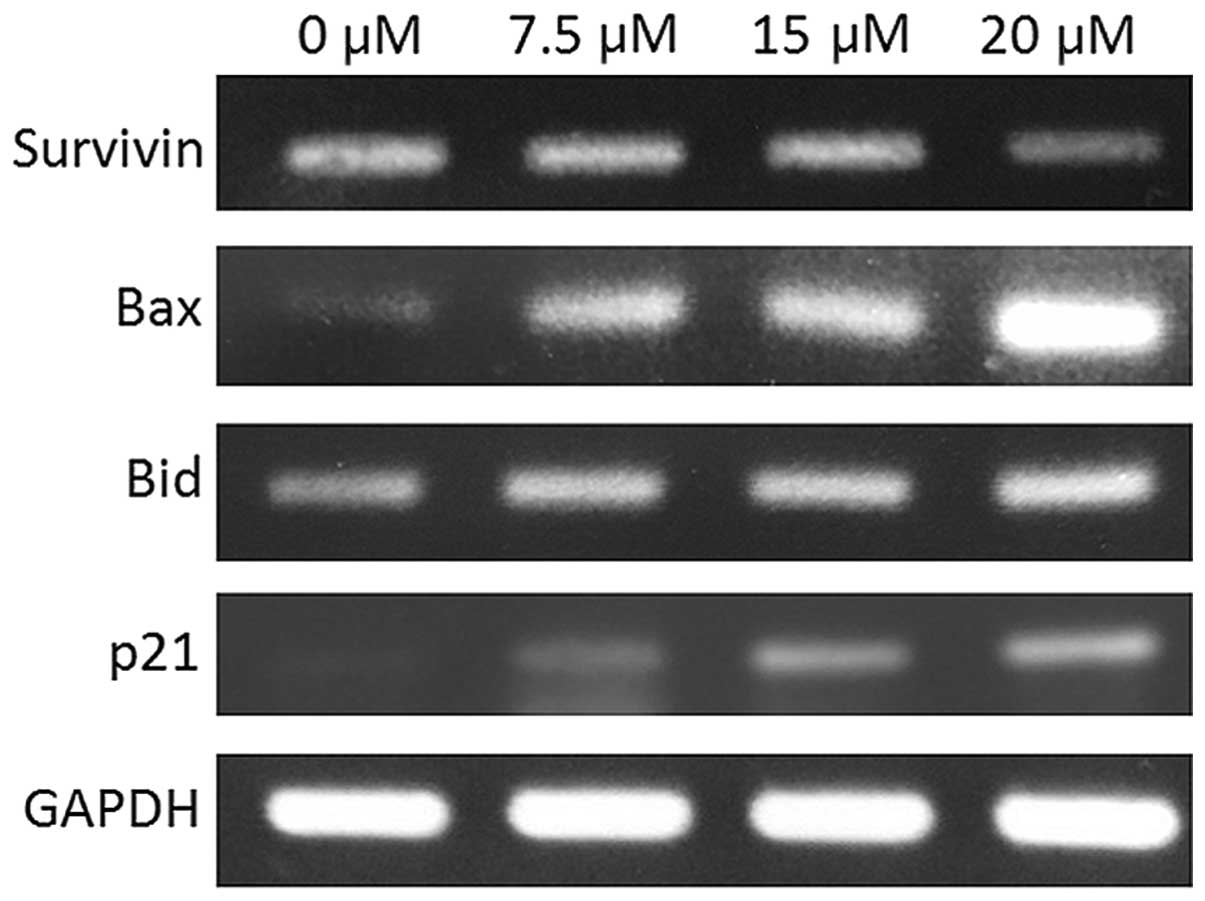
to 15.76% for 24, 48 and 72 h of incubation, respectively. Fig. 6 shows the mRNA expression of
survivin, Bax, Bid and p21 in tetrandrine-treated cancer cells. The
expression levels of these genes were evaluated by the RT-PCR.
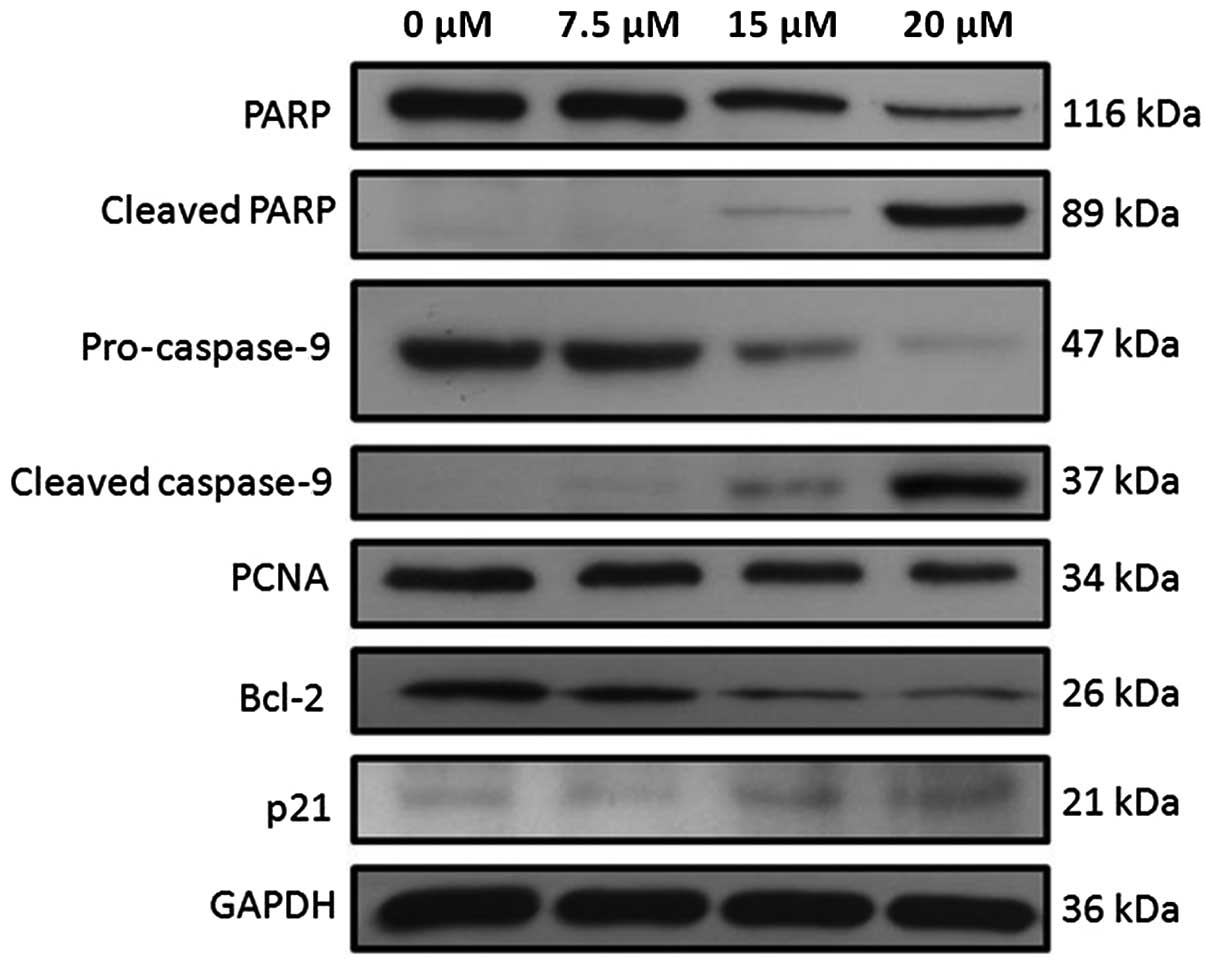
Fig. 7 shows the western blots of
different apoptotic proteins after Huh-7 cells were treated with
tetrandrine. When the tetrandrine concentration was increased from
0 to 20 μM, the band intensities for the full-length PARP,
pro-caspase-9, PCNA and Bcl-2 were found to be decreased while the
intensities for bands of cleaved PARP, cleaved caspase-9 and p21
were found to be increased with the increased tetrandrine
concentration. To ensure even loading of the total proteins, the
blots were stripped and re-probed with anti-GAPDH. GAPDH was used
as the control.
Discussion
The cell viability of Huh-7 decreased in a
dose-dependent manner but remained almost unchanged in WRL68, the
normal liver cells. The IC50 decreased from 20.8 to 8.0
μM for Huh-7 cells with time. The low value of IC50
suggests that tetrandrine is a considerable candidate as an
anticancer agent. The viability of WRL68 cells dropped and leveled
off approximately 80.0% at 50 μM. No IC50 for WRL68
could be determined from the MTT assay. The results suggest
tetrandrine is one of the few phytochemicals that can
differentially act against cancer cell viability.
Cell death of Huh-7 cells induced by
tetrandrine is mediated through apoptosis
The results from the DNA fragmentation assay clearly
indicated that DNA ladders were detected in the tetrandrine-treated
Huh-7 cells. The results provide supporting evidence that
tetrandrine induces apoptosis of Huh-7 cells with increased
concentration of tetrandrine. It suggests that more genomic DNA
molecules were cleaved to form smaller DNA fragments at a higher
tetrandrine concentration. The Huh-7 cell line is a p53 gene
mutated cell line. In the absence of the functional p53 protein,
apoptosis can still occur in Huh-7 cells suggesting that apoptosis
does not require the activation of the p53 gene. The results
suggest that tetrandrine could induce apoptosis through the
p53-independent pathway.
Tetrandrine induces G1 phase
cell cycle arrest
Studies show that there is a link between cell
proliferation and apoptosis of Huh-7. The results of the cell cycle
analysis by flow cytometry showed that the percentage of cells in
the S and G2 phases decreased whereas G1
phase increased with increasing concentrations of tetrandrine at
all the time points. It suggests that tetrandrine inhibits cell
proliferation at a very early stage within the cell cycle.
Tetrandrine induced programmed cell death via mediation of p21 and
PCNA gene expression possibly through binding of p21 to cyclin-CDK
2 or -CDK 4 complexes and subsequently inhibits their activities.
p21 plays an important role in cell cycle regulation by controlling
the cell cycle progression from G1 to S phase. PCNA is
synthesized in the early G1 and S phases during the cell
cycle. It acts as an auxiliary factor for DNA polymerase δ in DNA
synthesis during the S phase of the cell cycle. It is an important
protein responsible for the regulation of DNA synthesis. The
binding of p21 to PCNA inhibits the role of PCNA during DNA
replication. Therefore, the decreased protein expression for PCNA
indicates that there were fewer cells entering S phase after
treatment with tetrandrine. The results showed that a higher
inhibitory effect on Huh-7 cells was observed in proceeding from
G1 to S phase with higher concentrations of tetrandrine.
Therefore, there were fewer proliferating cells and a larger
population of cells was retained in G1 phase after
treatment. The data from RT-PCR and western blot analysis also
support the notion that tetrandrine induces G1 phase
cell cycle arrest.
Tetrandrine-induced apoptosis involves
the intrinsic, caspase-dependent pathway
Caspases are involved in both the initiation and
execution of the programmed cell death. Western blot analysis
showed that tetrandrine induced apoptosis of Huh-7 through caspase
activity. The result showed that the protein expression for
pro-caspase-9 decreased in a concentration-dependent manner.
Furthermore, the expression for the cleaved, active caspase-9 was
found to be similarly increased. As caspase-9 is the initiator for
the intrinsic apoptotic pathway, the cleavage of pro-caspase-9 to
form the active caspase-9 is essential for inducing cell death in
tetrandrine-treated Huh-7 cells and the intrinsic apoptotic pathway
is involved.
In the intrinsic apoptotic pathway, the caspase
cascade involves active caspase-9 and pro-caspase-3. Following
treatment of Huh-7 cells with tetrandrine, the protein expression
for full-length PARP decreased in Huh-7 cells. The amount of
cleaved PARP increased. PARP, an important protein for DNA repair,
is the molecular substrate of active caspase-3. The occurrence of
PARP cleavage is associated with DNA fragmentation in cells
resulting in cell death. The result is concordant with the DNA
integrity analysis for Huh-7 cells shown in Fig. 3. In the gene expression analysis in
Huh-7 cells (Fig. 6), survivin
expression was found to be decreased in a concentration-dependent
manner. It is reported that survivin is an anti-apoptotic protein
that exerts its function by binding to caspase-3 and hence the
caspase-3 activity is suppressed. The decreased gene expression
suggests that tetrandrine could promote apoptosis by suppressing
the expression of survivin.
Tetrandrine induces expression of
proteins in Bcl-2
The proteins in the Bcl-2 family can be classified
into two categories, the pro-apoptotic and anti-apoptotic proteins.
These proteins are involved in the apoptotic pathway associated
with mitochondrial control. The gene expressions of Bax and Bid
increased. Both the Bax and Bid are pro-apoptotic proteins whereas
Bcl-2 is an anti-apoptotic protein. The pro- and anti-apoptotic
proteins exert their function in opposite ways. It is reported that
Bcl-2 was overexpressed whereas the expression of Bax was
downregulated in HCC (4). The
elevated gene expressions of Bax and Bid, and the suppressed gene
expression of Bcl-2, suggest that these proteins were involved in
the apoptosis of Huh-7 cells.
Acknowledgements
This study was supported by grants from the Luck
Tissue Mfy Ltd. We thank Matt Cheung for his technical
assistance.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Schuler M, Bossy-Wetzel E, Goldstein JC,
Fitzgerald P and Green DR: p53 induces apoptosis by caspase
activation through mitochondrial cytochrome c release. J Biol Chem.
275:7337–7342. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chipuk JE, Maurer U, Green DR and Schuler
M: Pharmacologic activation of p53 elicits Bax-dependent apoptosis
in the absence of transcription. Cancer Cell. 4:371–381. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oda E, Ohki R, Murasawa H, Nemoto J,
Shibue T, Yamashita T, Tokino T, Taniguchi T and Tanaka N: Noxa, a
BH 3-only member of the Bcl-2 family and candidate mediator of
p53-induced apoptosis. Science. 288:1053–1058. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cregan SP, Fortin A, MacLaurin JG,
Callaghan SM, Cecconi F, Yu SW, Dawson TM, Dawson VL, Park DS and
Kroemer G: Apoptosis-inducing factor is involved in the regulation
of caspase-independent cell death. J Cell Biol. 158:507–517. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyashita T and Reed JC: Tumor suppressor
p53 is a direct transcriptional activator of the human bax gene.
Cell. 80:293–299. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ng LT, Chiang LC, Lin YT and Lin CC:
Antiproliferative and apoptotic effects of tetrandrine on different
human hepatoma cell lines. Am J Chin Med. 34:125–135. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoo SM, Oh SH, Lee SJ, Lee BW, Ko WG, Moon
CK and Lee BH: Inhibition of proliferation and induction of
apoptosis by tetrandrine in HepG2 cells. J Ethnopharmacol.
81:225–229. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He BC, Gao JL, Zhang BQ, Luo Q, Shi Q, Kim
SH, Huang E, Gao Y, Yang K, Wagner ER, Wang L, Tang N, Luo J, Liu
X, Li M, Bi Y, Shen J, Luther G, Hu N, Zhou Q, Luu HH, Haydon RC,
Zhao Y and He TC: Tetrandrine inhibits Wnt/β-catenin signaling and
suppresses tumor growth of human colorectal cancer. Mol Pharmacol.
79:211–219. 2011.
|
|
10
|
Liu C, Gong K, Mao X and Li W: Tetrandrine
induces apoptosis by activating reactive oxygen species and
repressing Akt activity in human hepatocellular carcinoma. Int J
Cancer. 129:1519–1531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Wang C, Wang H, Wang K, Du Y and
Zhang J: Combination of Tetrandrine with cisplatin enhances
cytotoxicity through growth suppression and apoptosis in ovarian
cancer in vitro and in vivo. Cancer Lett. 304:21–32. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu J, Liu F, Sun M, Sun Z and Sun S:
Enhancement of radiosensitivity and the potential mechanism on
human esophageal carcinoma cells by tetrandrine. Cancer Biother
Radiopharm. 26:437–442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li X, Zhen D, Lu X, Xu H, Shao Y, Xue Q,
Hu Y, Liu B and Sun W: Enhanced cytotoxicity and activation of
ROS-dependent c-Jun NH2-terminal kinase and caspase-3 by
low doses of tetrandrine-loaded nanoparticles in Lovo cells: a
possible Trojan strategy against cancer. Eur J Pharm Biopharm.
75:334–340. 2010.PubMed/NCBI
|
|
14
|
Arnoult D, Parone P, Mattinou JC,
Antonsson B, Estaquier J and Ameisen JC: Mitochondrial release of
apoptosis-inducing factor occurs downstream of cytochrome c release
in reponse to several proapoptotic stimuli. J Cell Biol.
159:923–929. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Susin SA, Daugas E and Ravagnan L: Two
distinct pathways leading to nuclear apoptosis. J Exp Med.
192:571–580. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Su B, Liu R, Wu D and He D:
Tetrandrine induces apoptosis and triggers caspase cascade in human
bladder cancer cells. J Sur Res. 166:e45–51. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|