Introduction
Neuroblastoma (NB) is the most common malignant
extra cranial solid tumor in children. NB is an extremely
heterogeneous disease, both clinically and biologically, resulting
from the plasticity of the embryonic neural crest, in which this
tumor originates (1,2). Clinically, it has been shown that
cellular heterogeneity and extent of maturation (such as,
stroma-rich and -poor tumors or high-and low-risk tumors, based on
histological grade) correlate with clinical manifestation, and
these properties have been used for the classification and
prognosis of the disease (3). Cell
lines established from human NB also show the same cellular
heterogeneity. Based on morphological appearance, biochemical
properties, and growth patterns, three major cell types have been
identified in NB cell lines: N (neuroblastic)-, S (substrate
adherent)- and I (intermediate)-type NB cells. Significant
similarities between normal neural crest stem cells and I-type NB
cells in self-renewal, multipotent and differentiation ability may
indicate that I-type cells represent a population of NB stem cells
or malignant neural crest stem cells (4–6).
The octamer-binding protein 4 (OCT4), a member of
the POU family of transcription factors, together with SOX-2 and
NANOG, regulates self-renewal and differentiation in embryonic stem
cells (ESCs) (7). Recent findings
demonstrated that OCT4 also plays an oncogenic role and is
expressed in some cancer stem cells (8–11).
Furthermore, previous studies detected the expression of OCT4 in NB
primary samples, metastatic bone marrow aspirates, cell lines, and
OCT4 is facilitated to identify NB cancer stem cells as the
stemness gene (12–17). OCT4 is proved to have a potential
role in maintaining NB cancer stem cell niche localized in the
hypoxic zones of the solid tumors in vivo(12) and is used for identification of
tumor-derived endothelial cells as a putative marker (17).
In this study, we investigated OCT4 expression in
the BE (2)-C human NB I-type cell line, typical NB cell line, and
analyzed the possible relationship between the expression levels of
OCT4 and tumor genesis. Our study demonstrates a role of OCT4
expression levels in the regulation of BE (2)-C self-renewal
capacity and differentiation.
Materials and methods
Cell culture and differentiation
assays
The BE (2)-C human NB I-type cell lines (ATCC
CRL-2268) were cultured in DMEM with F12 (Sigma) containing 10%
fetal bovine serum (FBS; Invitrogen). In differentiation assays,
all-trans retinoic acid (RA) and 5-bromo-2′-deoxyuridine (BrdUrd)
were dissolved in DMSO and 10 mM solutions were stocked. BE (2)-C
cells were grown for two weeks in the presence of 10 μM of RA or 10
μM BrdUrd.
Lentiviral constructs and
transfection
FUGW-OCT4 was constructed by replacing GFP with OCT4
ORF (NM_002701) of lentiviral vector FUGW (18). The sequence corresponding to OCT4
full length ORF was amplified from hESC cDNA library (Invitrogen,
A10303-01) with AgeI and EcoRI restriction site added
to 5′ and 3′ of the PCR fragment, respectively. The fragment was
digested and ligated to FUGW vector that has a ubiquitin-C promoter
used to overexpress human OCT4 in BE (2)-C cells.
To downregulate OCT4 expression, we constructed
siRNA clones as following: oligonucleotides encoding shRNA were
inserted downstream of and driven to express by a human U6
promoter. Each oligonucleotide pair contained a 5′ AgeI and
3′ EcoRI overhang; an RNA polymerase III termination
sequence, the OCT4 target sequence and its antisense were separated
by a piece of short loop sequence. For sequence OCT4 si_c
CCCTCACTTCACTGCACTGTA, which was reported to be able to efficiently
knock down OCT4, the loop is CTCGAG (19). Another target sequence was selected
using BLOCK-iTTM RNAI Designer (Invitrogen). OCT4 si:
CCGTGAAGCTGGAGAAGGA, while in this case TTCAAGAGA is the loop. A
non-functional sequence TTCTCCGAACGTGTCACGT was included as
control. Oligonucleotides were synthesized (Invitrogen), annealed,
and ligated into the vector PSCN according to a previously
described manipulation procedure (20).
Cell proliferation analysis
Cells were seeded onto 96-well plates at 2,500
cells/well and transfected with lentiviral vector after 12 h. After
culturing for various durations, cell growth rates were measured by
using Cell Counting Kit-8 (CCK-8; Dojindo Laboratories), according
the manufacturer’s instructions.
Soft agar colony formation assay
After 24 h of transfection, cells were dissociated
into single cell suspensions and mixed in 0.3% Noble agar (in DMEM
containing 10% FBS) and seeded onto 6-well plates containing 0.6%
Noble agar in the same growth medium at 1,000 cells/well. After 14
days of incubation, colonies were stained with 5 mg/ml MTT and
photographed. All experiments were carried out in triplicate.
Western blot analysis
Cells were lysed in 1% Triton X-100 buffer. Total
cell protein homogenates (20 μg) were separated on
SDS-polyacrylamide gels, transferred to a polyvinylidene fluoride
membrane and probed with 1:1000 rabbit polyclonal Oct4 (Abcam),
1:5000 mouse monoclonal to 68 kDa Neurofilament (Abcam), 1:1000
monoclonal anti-peripherin (mouse IgG1 isotype, Sigma), 1:1000
vimentin (R28) (Cell Signaling Technology), 1:1000 S100α chain
(Santa Cruz Biotechnology, Inc.). Horseradish peroxidase-conjugated
goat anti-rabbit and goat anti-mouse IgG were used as secondary
antibodies. Mouse monoclonal anti-human GAPDH was used as internal
control. The membranes were developed with a SuperSignal West Pico
chemiluminescence kit (Pierce).
RNA extraction and real-time quantitative
reverse-transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from cultured cells using
TRIzol reagent (Invitrogen) and reverse transcribed into cDNA with
the MMLV Reverse Transcriptase kit (Promega) according to the
manufacturer’s instructions. Quantitative RT-PCR analysis was
carried out with the SYBR Premix Ex Taq (Takara, Dalian) using the
Bio-Rad iQ5 real-time PCR system according to the manufacturer’s
instructions. Sequences for mRNAs from the nucleotide data bank
(National Center for Biotechnology Information, USA) were used to
design primer pairs with the PrimerBank website. Sequences were:
human Oct4 (sense: GGGAGATTGATAACTGGTGTGTT, antisense:
GTGTATATCCCAGGGTGATCCTC); human peripherin (sense:
CCAAGTACGCGGACCTGTC, antisense: CTCGCACGTTAGACTCTGGA); human
vimentin (sense: GAACGCCAGATGCGTGAAATG, antisense:
CCAGAGGGAGTGAATCCAGATTA); human neurofilament 68 (NF68) (sense:
ATGAGTTCCTTCAGCTACGAGC, antisense: GGGCATCAACGATCCAGAGC); human
S100 (sense: GACCCTCATCAACGTGTTCCA, antisense:
CCACAAGCACCACATACTCCT). Appropriate regions of human β-actin were
used as controls.
Results
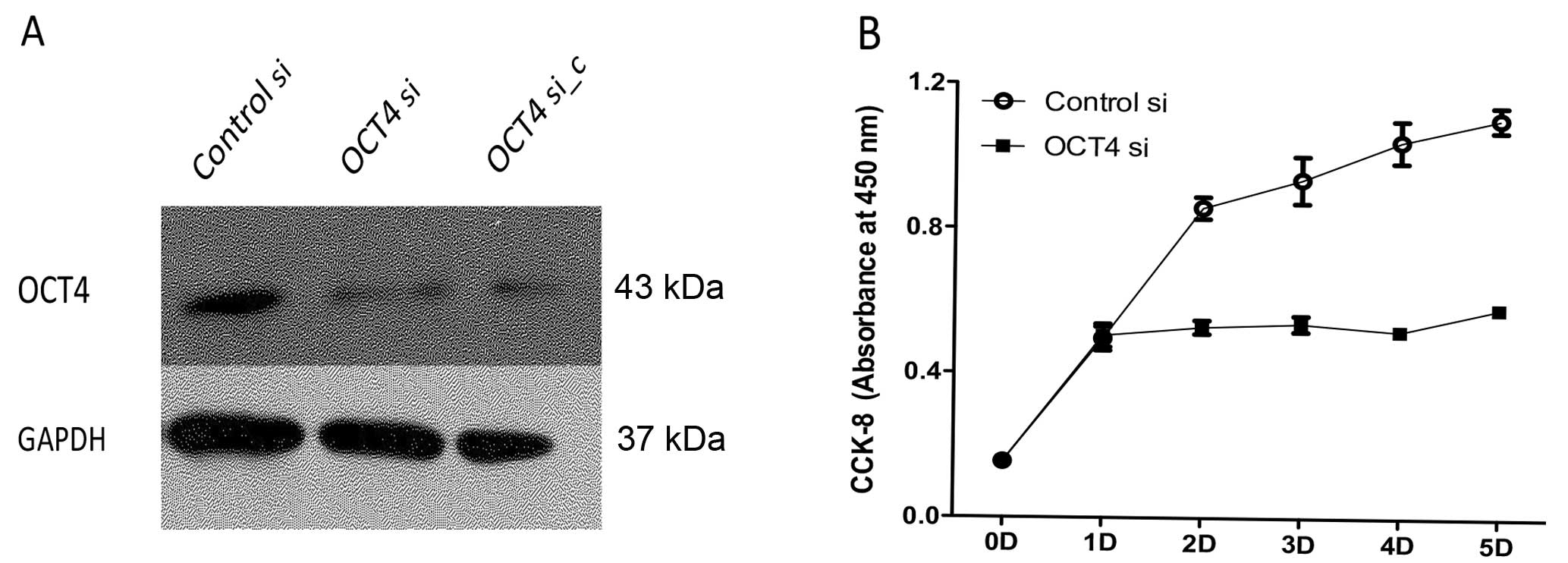
Downregulation of OCT4 in BE (2)-C cells
inhibits proliferation of BE (2)-C cells and promotes cell
differentiation into S-type
Lentiviral vector produced from either constructs
was effective in downregulating OCT4 expression in BE (2)-C cells,
and >90% of OCT4 protein was suppressed by RNA interference,
insistent with si_c from other researcher’s interference sequence
(Fig. 1A). This effect was not
observed in BE (2)-C cells infected with the control vector. A
reduction in cell growth rate was observed in cells with
downregulated OCT4 than the controls measured by CCK-8 absorption
(Fig. 1B).
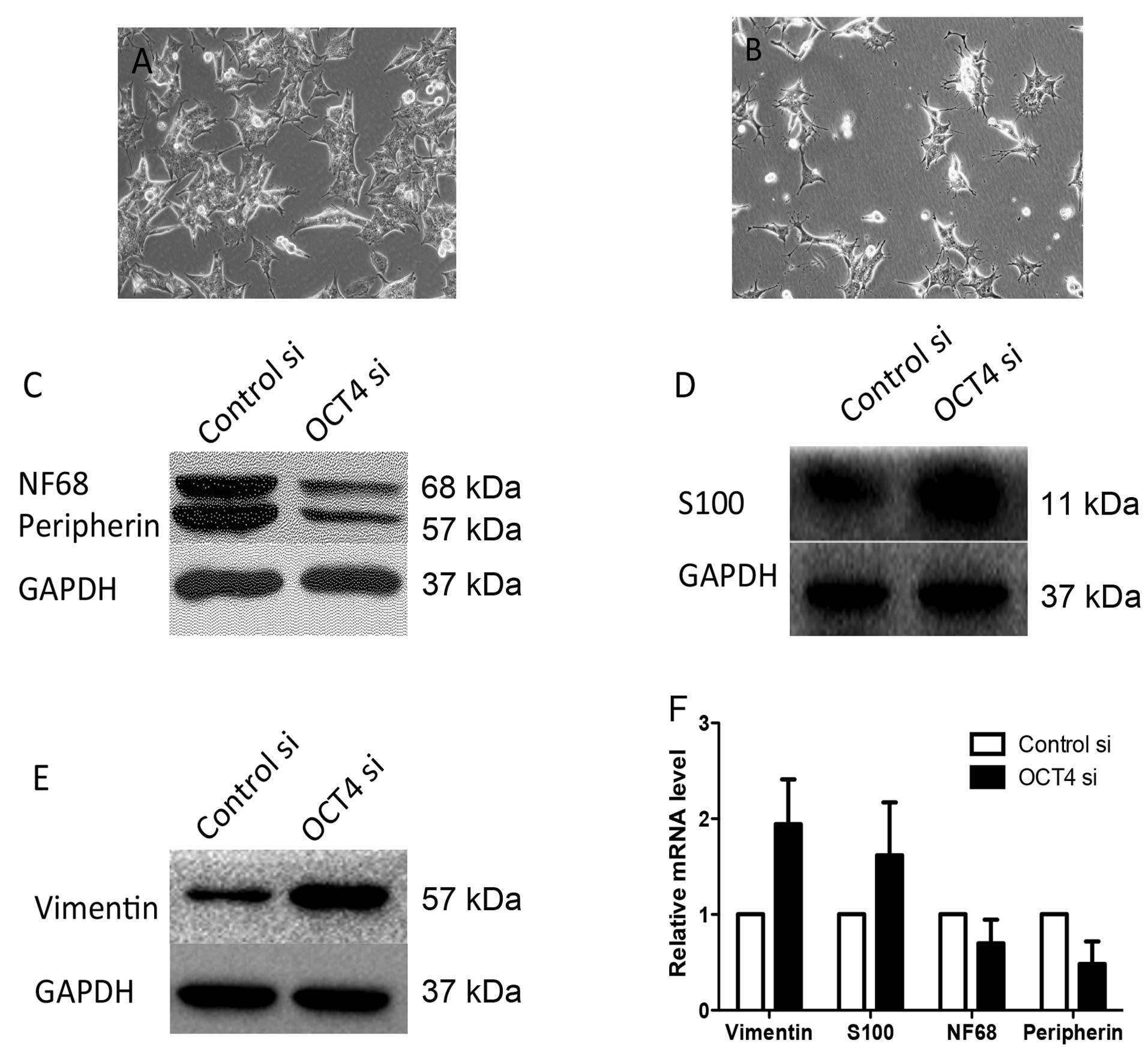
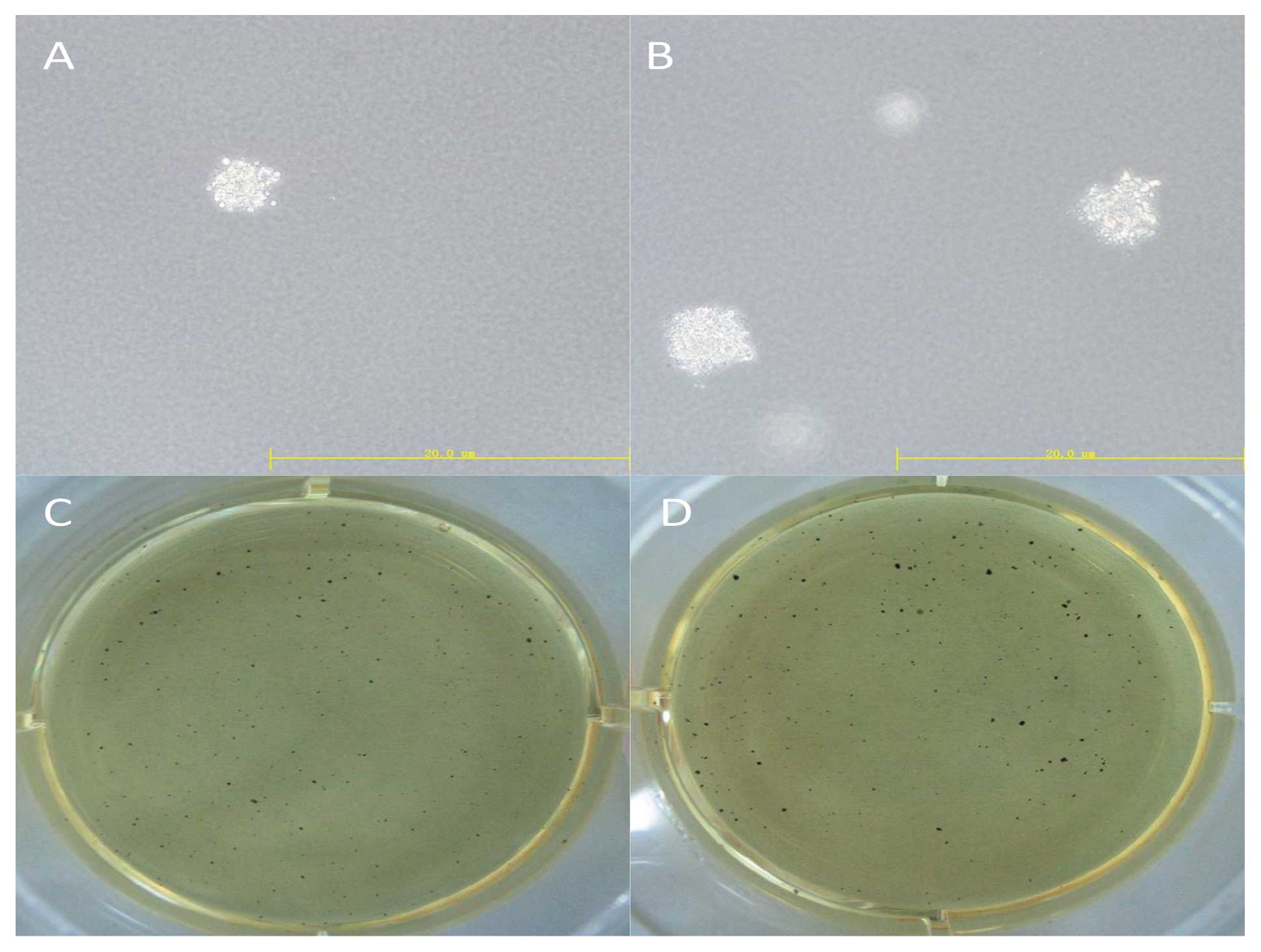
OCT4 si cells showed morphological alteration of S
type cells (Fig. 3B) and the soft
agar assay indicated colony formation of cells with downregulated
OCT4 was unable to form colonies on soft agar (Fig. 2). Cell lineage markers were measured
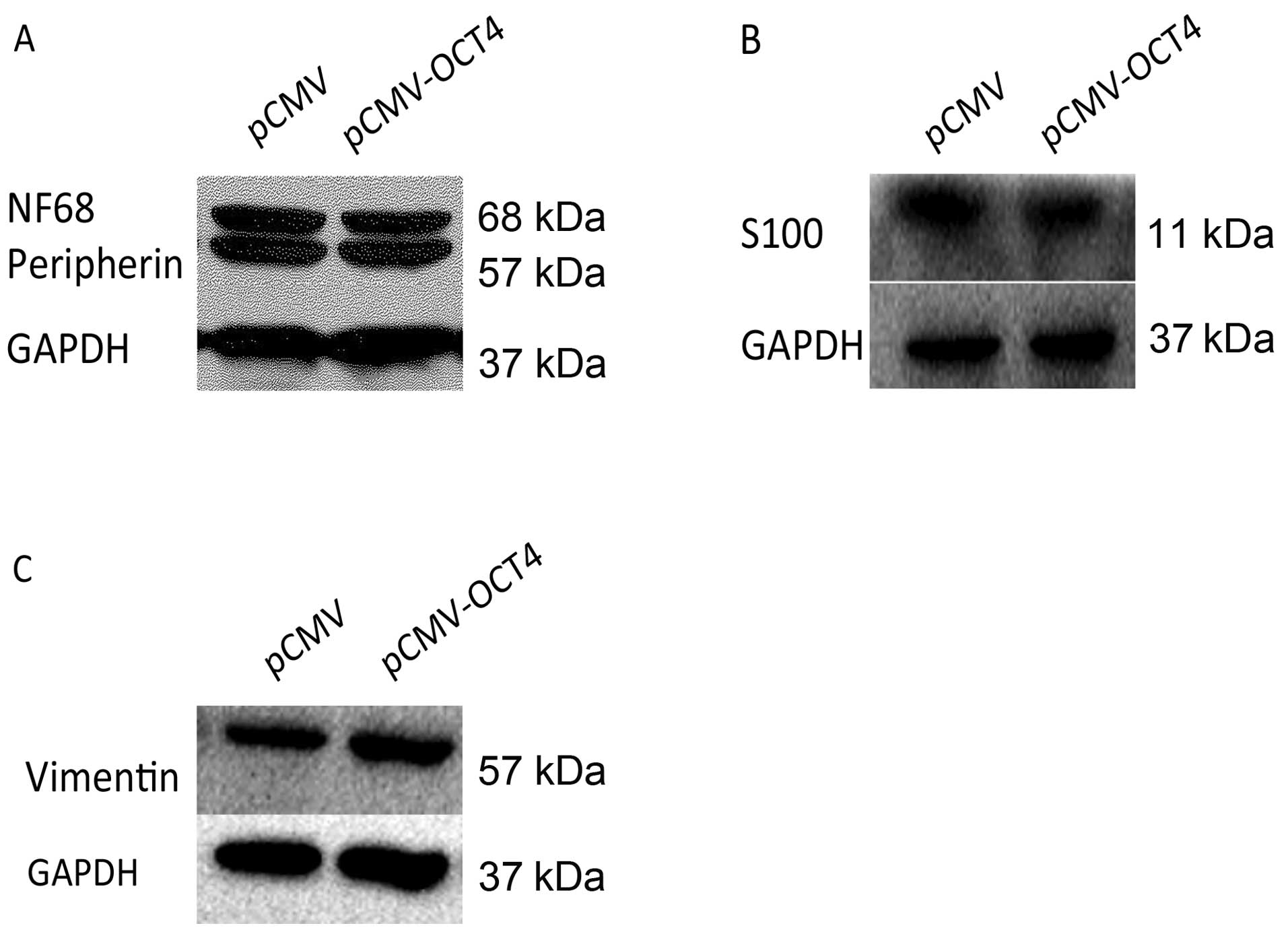
to assess the effect of OCT4 RNAi on BE (2)-C differentiation.
Compared to cells infected with normal control vector by western
blot analysis, BE (2)-C/OCT4 si cells showed an apparent decrease
in the expression of N-type cell markers, peripherin and NF68,
which was accompanied by an increase in the expression of the
S-type cell markers, vimentin and S100 (Fig. 3C–E). The corresponding N- or S-type
marker changes were also confirmed by quantitative RT-PCR analysis,
which showed similar upregulation of vimentin and S100 and
downregulation of peripherin and NF68 (Fig. 3F). Therefore, these results suggest
that downregulation of OCT4 in BE (2)-C cells promotes cell
differentiation into S-type cells.
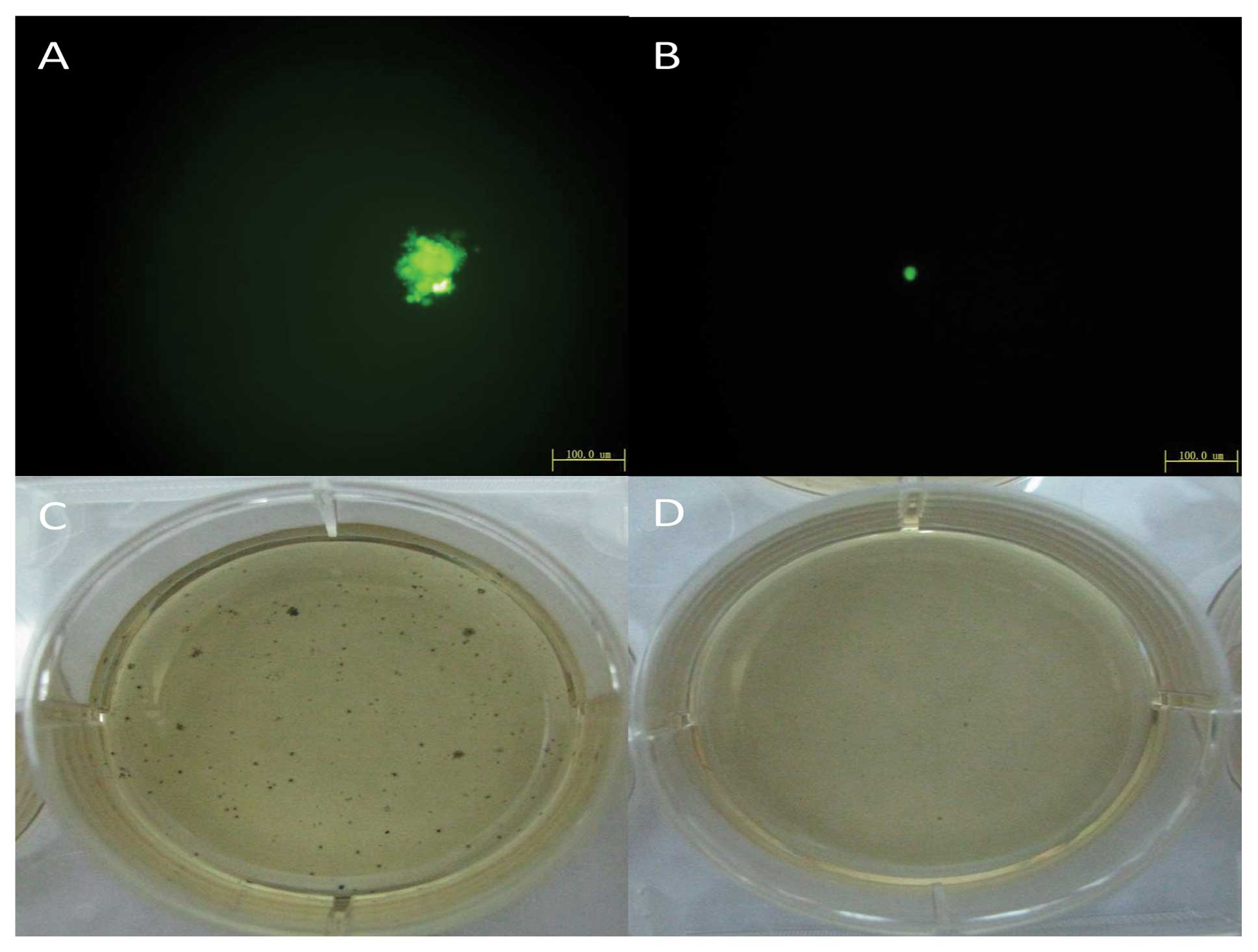
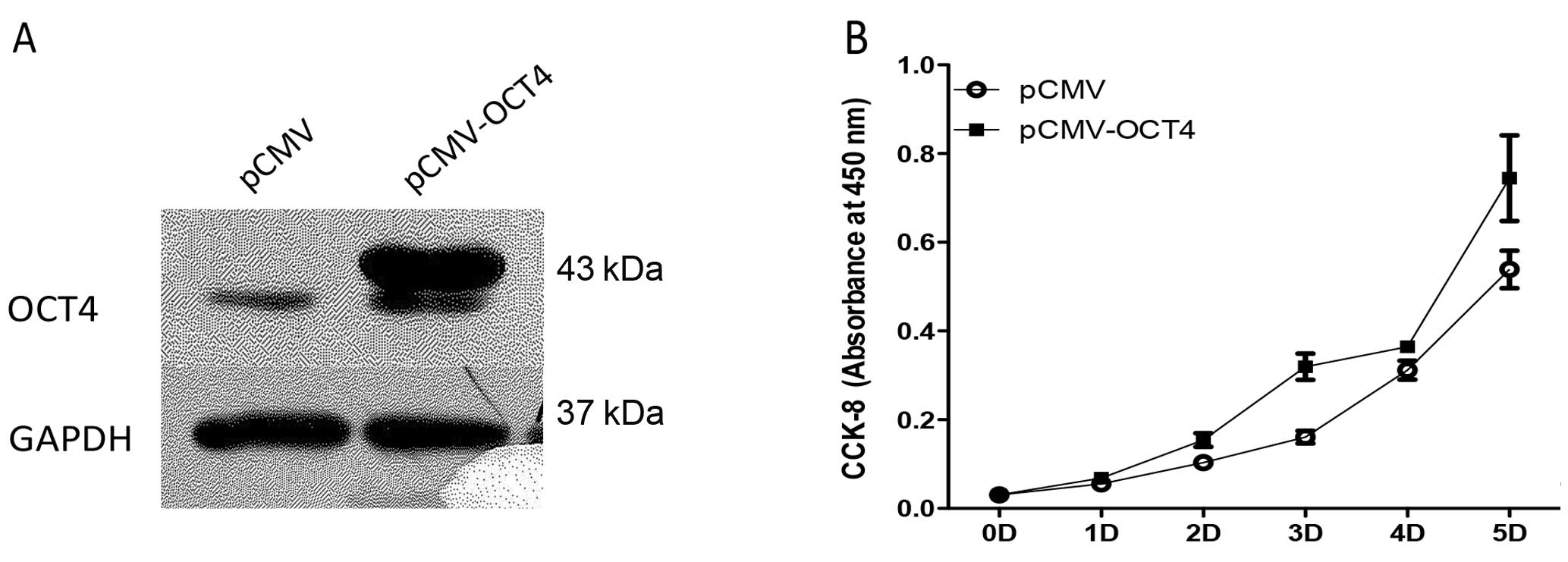
Upregulation of OCT4 in BE (2)-C cells
promotes proliferation without apparent differentiation
The lentiviral mediated OCT4 overexpression in BE
(2)-C cells is shown by western blotting in Fig. 4A. OCT4-overexpressing BE (2)-C cells
grew faster (Figure 4B) and colony
formation of cells with upregulated OCT4 increased 6.5±2.2% using
the soft agar assay (Fig. 5).
However, protein levels of lineage differentiation marker of N-type
(peripherin, NF68) and S-type (vimentin, S100) remained relatively
unaltered in the OCT4 upregulated cells as in the control cells
(Fig. 6).
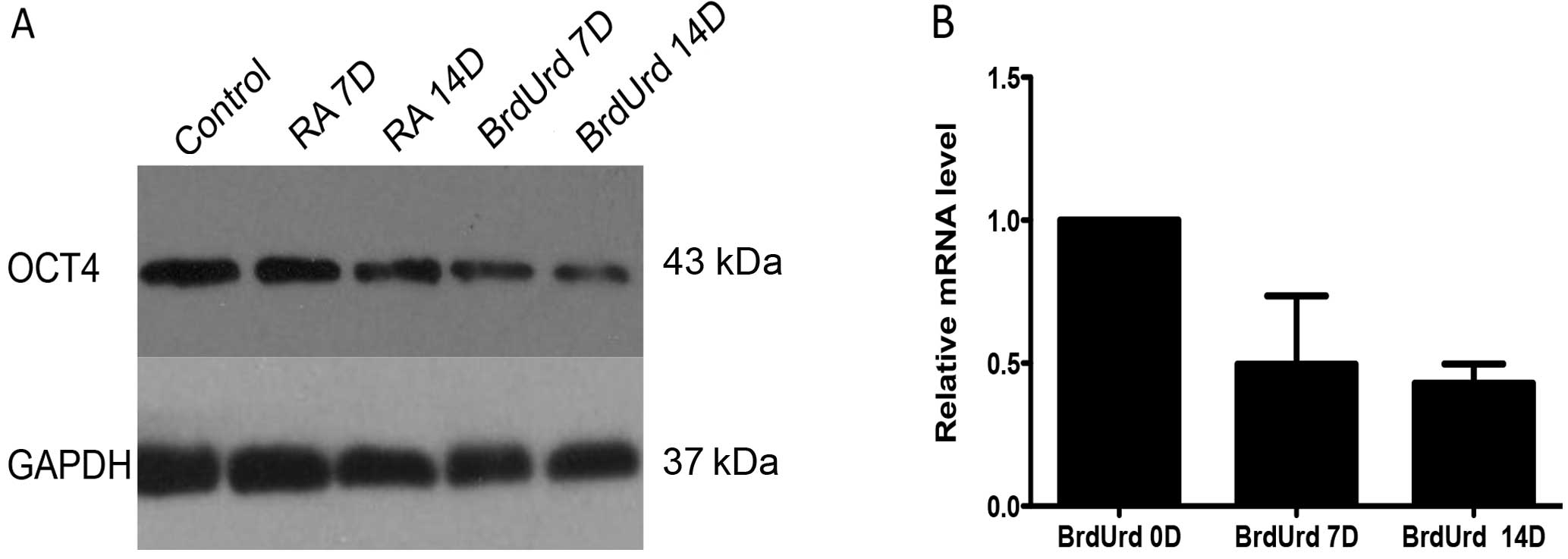
Reduced OCT4 expression accompanied by
BrdUrd induces differentiation
RA and BrdUrd have been proved to induce
differentiation of BE (2)-C into N-type and S-type cells,
respectively, in vitro(5,21).
Following treatment of BrdUrd, in addition to the expected S-type
morphological alteration, cells showed a progressive reduction of
OCT4 expression levels in both protein and RNA levels, and there
was no related change in induced N-type cells (Fig. 7).
Discussion
Cancer stem cells possess the ability of high
proliferation, self-renewal and colony formation, and they finally
cause cancer; they are similar to normal stem cells. Previous
studies have isolated tumor-initiating cells with stem cell
biological characteristics using various technologies in
neuroblastoma (NB) (4,21–24).
Among them, the method of continuous NB cell culture in
vitro identified three morphologically and biochemically
phenotypic variants, a neuroblastic subunit (N-type), a flattened,
substrate adherent subunit (S-type) representing Schwann/glial
cells, and a third type characterized between N- and S-type, which
is recognized as Intermediate type (I-type). Based on the following
findings, I-type cells most closely resemble a population of
malignant neural crest stem cells; first, I-type cells express
markers of both N- and S-type cells and the specific stem cell
proteins CD133 and c-kit; second, I-type cells differentiate to N-
or S-type derivatives spontaneously or by specific agents; and
third, I-type cells exhibit the greatest malignant potential in
immune-deficient mice and higher clonogenic activity in soft agar
than the other two types. Finally, presence of a larger number of
I-type cells in the tumor has been reported to correlate with a
poorer prognosis (6).
OCT4 has been used as a cell marker in NB to
identify progenitor cells (12–17).
Our study showed that the expression levels of OCT4 strongly
correlated with the stemness of NB I-type cells BE (2)-C. High
expression of OCT4 increases grades and correlates with tumor
progression and malignancy in glioma, a nervous system malignant
tumor (8). With the overexpression
of OCT4, BE (2)-C cells acquired increased proliferation rate and
formed relatively more and larger sphere colonies on soft agar,
indicating that OCT4 also played a role in increasing malignancy in
BE (2)-C. This is consistent with a previous study that indicates
BE (2)-C spheres grown in serum-free non-adherent culture show
increased expression of OCT4 (16).
In addition, we showed that with the downregulation of OCT4, BE
(2)-C grew slowly compared with control, and was unable to form
colonies on soft agar. Soft agar is used as semisolid support media
to prevent the migration of cells, which also leads to the
formation of spatially distinct colonies. Anchorage-independent
growth ability is a property of stem cells and formation of new
colonies suggest self-renewal (21). This proved that OCT4 was essential
in maintaining the clonogenic self-renewal capacity of BE (2)-C.
Inability of growth in soft agar may result from defects in the
regulation of proliferation or differentiation.
Since I-type NB cells can spontaneously
differentiate into N- or S-type cell in vitro, we
investigated the role of OCT4 in the regulation of BE (2)-C cell
differentiation. Peripherin is expressed by neural crest-derived
peripheral neurons and neurofilament 68 (NF68) is an N-type
specific marker protein (5), while
vimentin is an intermediate filament highly expressed in S-type
cells and S100 is a common marker for Schwann/glial cells (21). BE (2)-C cells with OCT4
downregulation became more flattened, substrate-adherent, which is
a morphological characteristic of Schwann cells. In reduced
expression of OCT4 cells, vimentin and S100 had a higher expression
in both RNA and protein levels, while peripherin and NF68 expressed
lower levels. Collectively, these results demonstrated that
downregulation of OCT4 prompt BE (2)-C cells to exit from the
I-type cell state and to differentiate into the Schwann/glial type.
S-type non-neuronal cells are non-malignant and do not form tumors
in athymic mice compared to I-type or N-type cells (5). OCT4 has been implicated in a variety
of cellular functions including maintaining the pluripotent
phenotype of embryonic stem cells (ESCs) and directing the
differentiation of ESCs to particular cell lineages (25). It has also been used as a cancer
stem cell marker and has the function to maintain cancer stem cell
undifferentiation and malignancy in some types of cancer (19,26,27).
Hence, OCT4 is required to maintain the undifferentiating I-type
state and a loss of OCT4 in NB cells might cause a differentiation
into more benign S-types. This may also partially explain the
inability of soft agar colony formation and further prove that OCT4
may enhance the malignancy and stem cell characteristics of NB
cells.
In vivo, I-type cells originate from neural
crest stem cells and represent a progenitor of N- and S-type cells
(5,6). In vitro, I-type NB cells were
induced into N-type cells by treating with retinoic acid (RA),
whereas they were induced into S-type cells using BrdUrd (4,21). Our
study revealed that BrdUrd induced differentiation of BE (2)-C into
S-type accompanied with decreased expression of OCT4, whereas the
RA-induced N-type cells did not show apparent changes of OCT4
expression. Together with the observation of the BE (2)-C S-type
differentiation with OCT4 downregulation, it is further suggested
that OCT4 plays a role in regulating the BE (2)-C differentiation
via the Schwann/glial pathway. Downregulation of OCT4 may cause BE
(2)-C differentiation into S-type cells and vice versa.
Our study has some limitations. The mechanism of how
OCT4 regulates the differentiation of BE (2)-C has not been
investigated in this study and this experiment was only performed
in vitro, thus it remains to be confirmed in
vivo.
In summary, our study is consistent with the
hypothesis that I-type NB cells are malignant neural crest stem
cells. OCT4 may enhance the stem characteristics and be required to
maintain the undifferentiated state of NB I-type cells by
regulating the Schwann/glial pathway of BE (2)-C cells. Our study
may provide a target for the treatment of NB.
Acknowledgements
This research was supported by the National Natural
Science Foundation of China, grant no. 30801198.
References
|
1
|
Brodeur GM: Neuroblastoma: biological
insights into a clinical enigma. Nat Rev Cancer. 3:203–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maris JM, Hogarty MD, Bagatell R and Cohn
SL: Neuroblastoma. Lancet. 369:2106–2120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shimada H, Umehara S, Monobe Y, et al:
International neuroblastoma pathology classification for prognostic
evaluation of patients with peripheral neuroblastic tumors: a
report from the Children’s Cancer Group. Cancer. 92:2451–2461.
2001.
|
|
4
|
Ross RA, Spengler BA, Domenech C, Porubcin
M, Rettig WJ and Biedler JL: Human neuroblastoma I-type cells are
malignant neural crest stem cells. Cell Growth Differ. 6:449–456.
1995.PubMed/NCBI
|
|
5
|
Walton JD, Kattan DR, Thomas SK, et al:
Characteristics of stem cells from human neuroblastoma cell lines
and in tumors. Neoplasia. 6:838–845. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ross RA and Spengler BA: Human
neuroblastoma stem cells. Semin Cancer Biol. 17:241–247. 2007.
View Article : Google Scholar
|
|
7
|
Babaie Y, Herwig R, Greber B, et al:
Analysis of Oct4-dependent transcriptional networks regulating
self-renewal and pluripotency in human embryonic stem cells. Stem
Cells. 25:500–510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du Z, Jia D, Liu S, et al: Oct4 is
expressed in human gliomas and promotes colony formation in glioma
cells. Glia. 57:724–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chiou SH, Wang ML, Chou YT, et al:
Coexpression of Oct4 and Nanog enhances malignancy in lung
adenocarcinoma by inducing cancer stem cell-like properties and
epithelial-mesenchymal transdifferentiation. Cancer Res.
70:10433–10444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ikushima H, Todo T, Ino Y, et al:
Glioma-initiating cells retain their tumorigenicity through
integration of the Sox axis and Oct4 protein. J Biol Chem.
286:41434–41441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu CG, Lu Y, Wang BB, et al: Clinical
implications of stem cell gene Oct-4 expression in breast cancer.
Ann Surg. 253:1165–1171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Das B, Tsuchida R, Malkin D, Koren G,
Baruchel S and Yeger H: Hypoxia enhances tumor stemness by
increasing the invasive and tumorigenic side population fraction.
Stem Cells. 26:1818–1830. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Melone MA, Giuliano M, Squillaro T, et al:
Genes involved in regulation of stem cell properties: studies on
their expression in a small cohort of neuroblastoma patients.
Cancer Biol Ther. 8:1300–1306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pietras A, Hansford LM, Johnsson AS, et
al: HIF-2alpha maintains an undifferentiated state in neural
crest-like human neuroblastoma tumor-initiating cells. Proc Natl
Acad Sci USA. 106:16805–16810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Newton TC, Wolcott K and Roberts SS:
Comparison of the side populations in pretreatment and postrelapse
neuroblastoma cell lines. Transl Oncol. 3:246–251. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nishimura N, Hartomo TB, Pham TV, et al:
Epigallocatechin gallate inhibits sphere formation of neuroblastoma
BE(2)-C cells. Environ Health Prev Med. 17:246–251. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pezzolo A, Parodi F, Marimpietri D, et al:
Oct-4(+)/Tenascin C(+) neuroblastoma cells serve as progenitors of
tumor-derived endothelial cells. Cell Res. 21:1470–1486. 2011.
|
|
18
|
Lois C, Hong EJ, Pease S, Brown EJ and
Baltimore D: Germline transmission and tissue-specific expression
of transgenes delivered by lentiviral vectors. Science.
295:868–872. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen YC, Hsu HS, Chen YW, et al: Oct-4
expression maintained cancer stem-like properties in lung
cancer-derived CD133-positive cells. PLoS One. 3:e26372008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kutner RH, Zhang XY and Reiser J:
Production, concentration and titration of pseudotyped HIV-1-based
lentiviral vectors. Nat Protoc. 4:495–505. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cui H, Ma J, Ding J, Li T, Alam G and Ding
HF: Bmi-1 regulates the differentiation and clonogenic self-renewal
of I-type neuroblastoma cells in a concentration-dependent manner.
J Biol Chem. 281:34696–34704. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marzi I, D’Amico M, Biagiotti T, et al:
Purging of the neuroblastoma stem cell compartment and tumor
regression on exposure to hypoxia or cytotoxic treatment. Cancer
Res. 67:2402–2407. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mahller YY, Williams JP, Baird WH, et al:
Neuroblastoma cell lines contain pluripotent tumor initiating cells
that are susceptible to a targeted oncolytic virus. PLoS One.
4:e42352009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Coulon A, Flahaut M, Muhlethaler-Mottet A,
et al: Functional sphere profiling reveals the complexity of
neuroblastoma tumor-initiating cell model. Neoplasia. 13:991–1004.
2011.PubMed/NCBI
|
|
25
|
Niwa H: How is pluripotency determined and
maintained? Development. 134:635–646. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chiou SH, Yu CC, Huang CY, et al: Positive
correlations of Oct-4 and Nanog in oral cancer stem-like cells and
high-grade oral squamous cell carcinoma. Clin Cancer Res.
14:4085–4095. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim R-J and Nam J-S: OCT4 Expression
enhances features of cancer stem cells in a mouse model of breast
cancer. Lab Anim Res. 27:147–152. 2011. View Article : Google Scholar : PubMed/NCBI
|