Introduction
Oral submucous fibrosis (OSF) is a chronic insidious
disease predisposing to cancer. The rate of carcinoma incidence in
OSF cases reaches up to 7.6%. Areca nut (betel) chewing is one of
the primary etiologic factors for OSF and carcinogenesis. The areca
nut is indentified as a first class carcinogen by IARC (1,2).
Histologically, most OSF is characterized by epithelial atrophy and
progressive accumulation of collagen fibers in the lamina propria
while a minority is characterized by epithelial atypical
hyperplasia. In tissues with OSF-associated carcinogenesis, both
epithelial atrophy and atypical epithelia can exist or coexist.
There is abnormal expression of proteins related to cell cycle
arrest and apoptosis in OSF and in tissues showing carcinogenesis.
The role and mechanism of arecoline in epithelial atrophy and
epithelial hyperplasia require further investigation (3–10).
Eukaryotic cell division is a precise and orderly
process with multi-factorial controls. The cell cycle can be
divided into the four phases known as G1, S,
G2 and M, with two important restriction points. The
restriction points are G1/S and G2/M phase,
and only across these two limiting points can there be final
completion of cell replication and division. Normal cells with DNA
damage and cells affected by other external stimuli can undergo
G1/S and/or G2/M phase blockage, delaying
progression of the cell cycle. Yet, these normal cells win enough
time to repair DNA damage before DNA replication and cell division
(11). Cyclins and expression of
cyclin-dependent-kinase (CDKs) form an active complex which
positively regulates the cell cycle. CDK inhibitors (CKIs) combine
with CDKs, and inhibit the activity of CDKs, thus playing a role as
a negative regulator of the cell cycle. Cyclin D/CDK4/6 complexes
are necessary to promote cell cycle through the G1/S
phase restriction point. Cyclin E and cyclin A further promote the
phosphorylation of Rb, induction of E2F1 in the intracellular
accumulation, and promote a series of cyclins and cyclin-dependent
protein kinases in the cell across the late G1 phase to
S phase. The ultimate target of the G2 checkpoint
signaling pathway is the cyclin-dependent kinase (CDK) complex,
CDK1-cyclin B1. Deregulation of cell cycle machinery is an
important step in malignant transformation. Cyclin D1 is one of the
most important proto-oncogenes and cell cycle regulators. Its
protein is known as cyclin D1 and is expressed in the G1
phase of the cell cycle (12,13).
Similar types of stimuli elicit different responses
in different cells. In the present study, we investigated the
effects of arecoline on the HaCaT epithelial and Hel fibroblast
cell lines. First, human keratinocyte cells of the HaCaT cell line
and human embryo lung fibroblasts of the Hel cell line were treated
with high doses of arecoline for a short time to observe the
survival rate, cell cycle distribution and apotosis. Secondly, the
molecular mechanism of epithelium atrophy induced by arecoline was
studied.
Materials and methods
Cell lines
Keratinocytes of the naturally immortalized normal
cell line, HaCaT, were obtained from the Laboratory of Apoptosis,
College of Life Science, Hunan Normal University, China. The human
embryonic lung fibroblast cell line (human normal fibroblast cell
line), Hel, was established by the Laboratory of Molecular
Virology, College of Life Science, Hunan Normal University, China
(14).
Cell proliferation assay
The impact of arecoline treatment on cell
proliferation was measured by the MTT assay as described previously
(15). Briefly, keratinocytes from
the natural immortalized normal cells, HaCaT, and human embryonic
lung fibroblast cells, Hel, (104 cells/well) were
cultured in triplicate with 10% FCS RPMI-1640 in 96-well plates in
the presence or absence of 25, 50, 75, 100 and 125 μg/ml arecoline
for 0, 24, 48 and 72 h, respectively. The cells were then exposed
to 5 mg/ml MTT for 4 h. The generated formazan was dissolved with
dimethyl sulfoxide, and the absorbance was measured at 570 nm using
an ELX-800 microplate reader (Bio Tek Instruments, Inc., Winooski,
VT, USA).
Flow cytometric analysis of the cell
cycle
The previously described keratinocytes and lung
fibroblast cells were cultured in 10% FCS RPMI-1640 to ~70%
confluence and then treated with, or without, 25, 50, 75, 100 and
125 μg/ml arecoline for 12 h, respectively. The adherent cells were
trypsinized, harvested and fixed in 70% ethanol at 4°C.
Subsequently, the cells were washed with cold PBS and stained with
propidium iodide (PI) in working solution (0.5 mg/ml RNase, and 0.1
mg/ml PI in PBS). The cell cycle was characterized by flow
cytometric analysis using a FACScan cytofluorimeter, and the data
were analyzed by the CellQuest Pro Software (Becton-Dickinson, San
Jose, CA. USA).
Reverse transcription-PCR analysis
Both cell lines were cultured in RPMI-1640 to 80%
confluence and then treated with, or without, 25, 50, 75, 100 and
125 μg/ml arecoline for 12 h, respectively. The cells were
harvested, and their total RNA was extracted. One microgram total
RNA was reversely transcripted into cDNA, as described previously
(15,16) and was subsequently used as the
template for PCR reaction. The first step in the PCR reaction
involved denaturing at 94°C for 5 min, followed by 30 cycles of
94°C for 45 sec, 55°C for 45 sec, and 72°C for 1 min, followed by
72°C for 7 min for CDK2, CDK4, cyclin A, cyclin B, cyclin D1,
cyclin E, E2F1 and CDC2. The primers CDK2, CDK4, cyclin A, cyclin
B, cyclin D1, cyclin E, E2F1, CDC2 and β-actin were synthesized as
shown in Table I. All RT-PCR
reactions were repeated at least three times with varying cycle
numbers to avoid potentially false results. β-actin was used as an
endogenous control for normalization. The PCR products were
analyzed by agarose-gel electrophoresis and ethidium bromide
staining.
 | Table ISequences of the primers for
RT-PCR. |
Table I
Sequences of the primers for
RT-PCR.
| Gene | Forward primer | Reverse primer | Product (bp) |
|---|
| CDK2 |
CCTGGAGATTCTGAGATTGA |
GGGAAACTTGGCTTGTAAT | 113 |
| CDK4 |
GCATCCCAATGTTGTCCG |
AGGCAGCCCAATCAGGTC | 499 |
| Cyclin A |
CCTGCGTTCACCATTCAT |
TCTTCTCCTACTTCAACTAACC | 383 |
| Cyclin B |
TTGGTTGATACTGCCTCTC |
TCTGACTGCTTGCTCTTC | 204 |
| Cyclin D1 |
GAACAGAAGTGCGAGGAG |
GCGGTAGTAGGACAGGAA | 477 |
| Cyclin E |
TGGATGTTGACTGCCTTG |
TCTATGTCGCACCACTGA | 115 |
| E2F1 |
CACTGAATCTGACCACCAA |
ACCATAACCATCTGCTCTG | 400 |
| CDC2 |
AGAGTTCTTCACAGAGACT |
GGATGATTCAGTGCCATT | 488 |
| β-actin |
CCGTGACCTGACTGACTACCTC |
ATACCGCAAGATTCCATACCC | 276 |
Western blotting
The ketatinocytes and the epithelial cells were
cultured in RPMI-1640 up to 80% confluence and then treated with,
or without, 25, 50, 75, 100 and 125 μg/ml arecoline for 12 h,
respectively. The cells were harvested and lysed in the lysis
buffer [1% Nonidet P-40, 50 mM Tris-HCl, pH 7.5, 50 mM NaF, 2 mM
EDTA, 400 mM NaCl, 10% glycerol plus complete protease inhibitor
mixture (Roche Diagnostics)]. The protein concentrations were
determined using the bicinchoninic acid protein assay kit (Pierce
Chemical, Rockford, IL, USA). The cell lysates (50 μg) from
individual samples were separated by SDS-PAGE and transferred onto
nitrocellulose membranes (HyClone Laboratories, Logan, UT, USA).
The membranes were blocked with 5% nonfat milk in Tris-buffered
saline/Tween-20 (25 mM Tris-HCl, 150 mM NaCl, pH 7.5, and 0.05%
Tween-20) and probed with the primary antibody overnight at 4°C.
After washing with Tris-buffered saline/Tween-20 three times, the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA), and the specific signals were visualized using ECL
detection system. Antibodies against cyclin D1, CDK4, CDK2, E2F1,
cyclin B, CDC2, cyclin A and cyclin E were purchased from Cell
Signaling Technology Inc. (Beverly, MA, USA).
Statistical analysis
Differences in nonparametric variables were analyzed
by the Fisher's exact test using the EPI software (EPI Info,
version 3.2.2, www.CDC.gov/epiinfo/). Differences in
the quantitative variables between groups were analyzed by the
Student's t-test using SPSS 11.0 program (SPSS, Chicago, IL, USA).
A P-value <0.05 was considered to indicate a statistically
significant result.
Results
Effect of arecoline on HaCaT cell
morphology
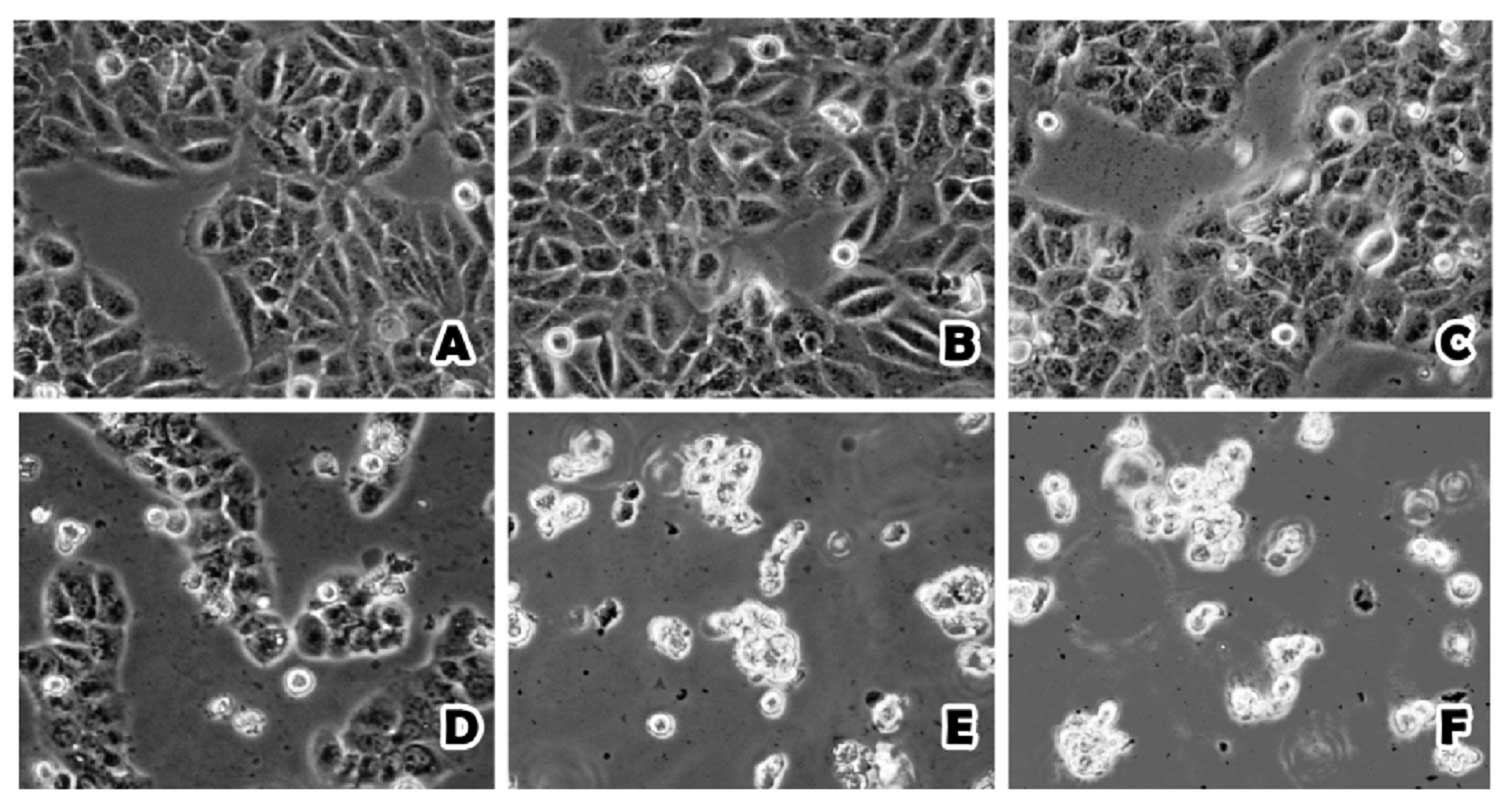
The HaCaT cell morphology was significantly altered
following treatment of arecoline at different concentrations after
48 h. The outlines of the HaCaT cells were clear when treated with
0 and 25 μg/ml, but when the concentration of arecoline increased
to 50 μg/ml, the boundaries of the HaCaT cells became indistinct
and some of the cells were dead. There was massive cell death and a
lack of boundaries between the cells when the concentration of
arecoline was increased to 75, 100 and 125 μg/ml (Fig. 1). In contrast, the Hel cells were
less affected by arecoline treatment. Only when the concentration
of arecoline was increased to 100 and 125 μg/ml, did the Hel cell
morphology begin to change slightly (Fig. 2).
Arecoline suppresses HaCaT cell
proliferation
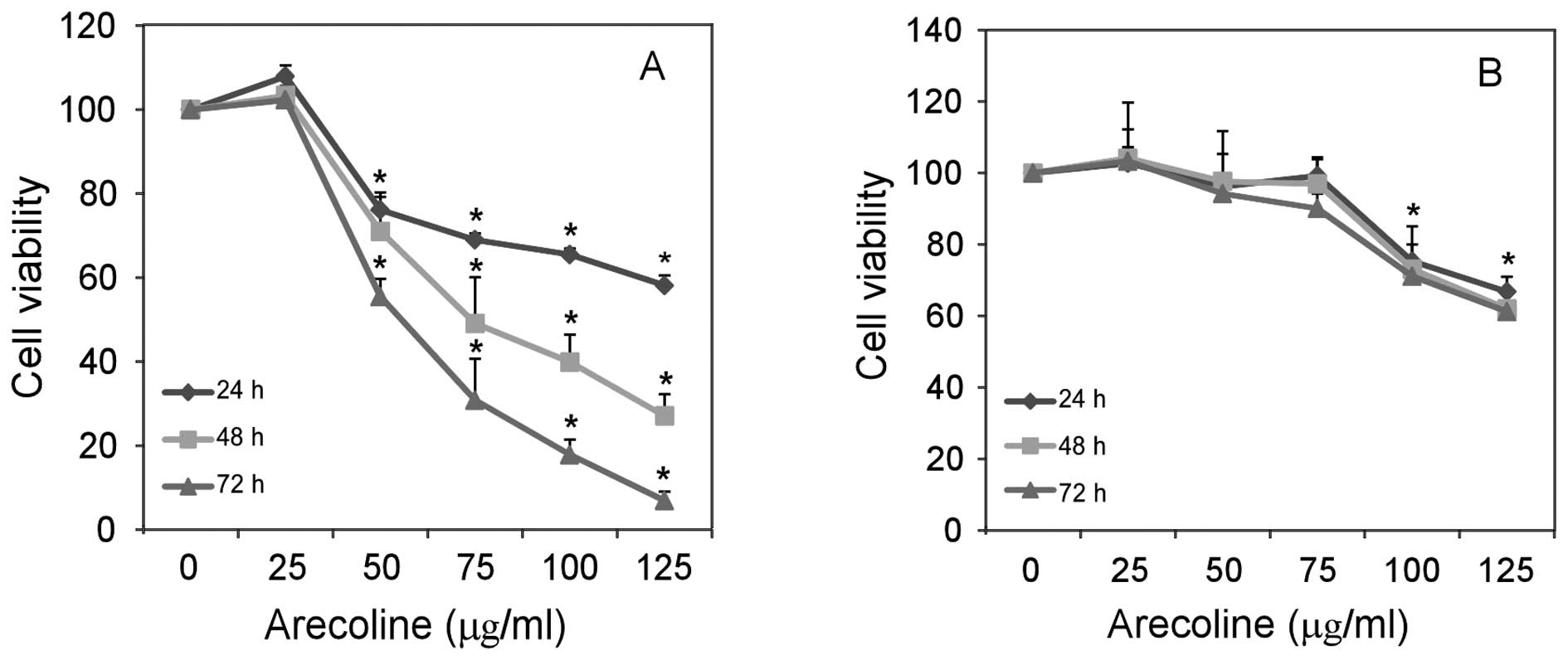
To further test whether arecoline modulates HaCaT
cell growth, HaCaT and Hel cells were treated with 0, 25, 50, 75,
100 and 125 μg/ml arecoline, and their spontaneous proliferation
was determined by MTT assays. Treatment with arecoline
significantly inhibited the proliferation of HaCaT cells (Fig. 3A), but only slightly reduced Hel
cell growth in vitro (Fig.
3B). Therefore, treatment with arecoline inhibited the
proliferation of epithelial cells, but not fibroblasts in
vitro.
Arecoline induces the cell cycle arrest
of HaCaT cells
Inhibition of cell proliferation usually is mediated
by inducing cell cycle arrest. To test this possibility, HaCaT and
Hel cells were treated with 0, 25, 50, 75, 100 and 125 μg/ml
arecoline and the cell cycle distribution was determined by FACS
analysis (Tables II and III). In comparison to the control cells
without arecoline treatment, following treatment with ≥75 μg/ml
arecoline a higher percentage of HaCaT cells remained at the
G0/G1 phase of the cell cycle (from 49.02 to
73.00%) at 12 h, accompanied by a reduced percentage of cells in
the S phase (from 32.05 to 20.50%). However, arecoline treatment
did not significantly alter Hel cell cycling even after treatment
with arecoline for 12 h. Hence, these data demonstrate that
arecoline treatment induces cell cycle arrest by inhibiting the
G1 to S phase transition of the cell cycle in epithelial
cells in vitro.
 | Table IIFlow cytometric analysis of HaCaT
cells following treatment with different concentrations of
arecoline. |
Table II
Flow cytometric analysis of HaCaT
cells following treatment with different concentrations of
arecoline.
| Phase of the cell
cyclea |
|---|
|
|
|---|
| Treatment |
G0-G1 (%) | S (%) | G2-M
(%) |
|---|
| Arecoline
(μg/ml) |
| 0 | 49.02 | 32.05 | 18.93 |
| 25 | 51.06 | 31.94 | 17.00 |
| 50 | 53.62 | 30.69 | 15.69 |
| 75 | 73.00 | 20.50 | 6.50 |
| 100 | 91.23 | 5.61 | 3.16 |
| 125 | 89.67 | 5.13 | 5.20 |
 | Table IIIFlow cytometric analysis of Hel cells
following treatment with different concentrations of arecoline. |
Table III
Flow cytometric analysis of Hel cells
following treatment with different concentrations of arecoline.
| Phase of the cell
cyclea |
|---|
|
|
|---|
| Treatment |
G0-G1 (%) | S (%) | G2-M
(%) |
|---|
| Arecoline
(μg/ml) |
| 0 | 52.52 | 33.35 | 14.13 |
| 25 | 49.09 | 34.02 | 16.89 |
| 50 | 50.04 | 30.17 | 19.79 |
| 75 | 51.75 | 30.21 | 18.04 |
| 100 | 54.02 | 31.64 | 14.34 |
| 125 | 54.86 | 30.92 | 14.22 |
Arecoline regulates the transcription
level of cell cycle regulatory molecules in the HaCaT epithelial
cells
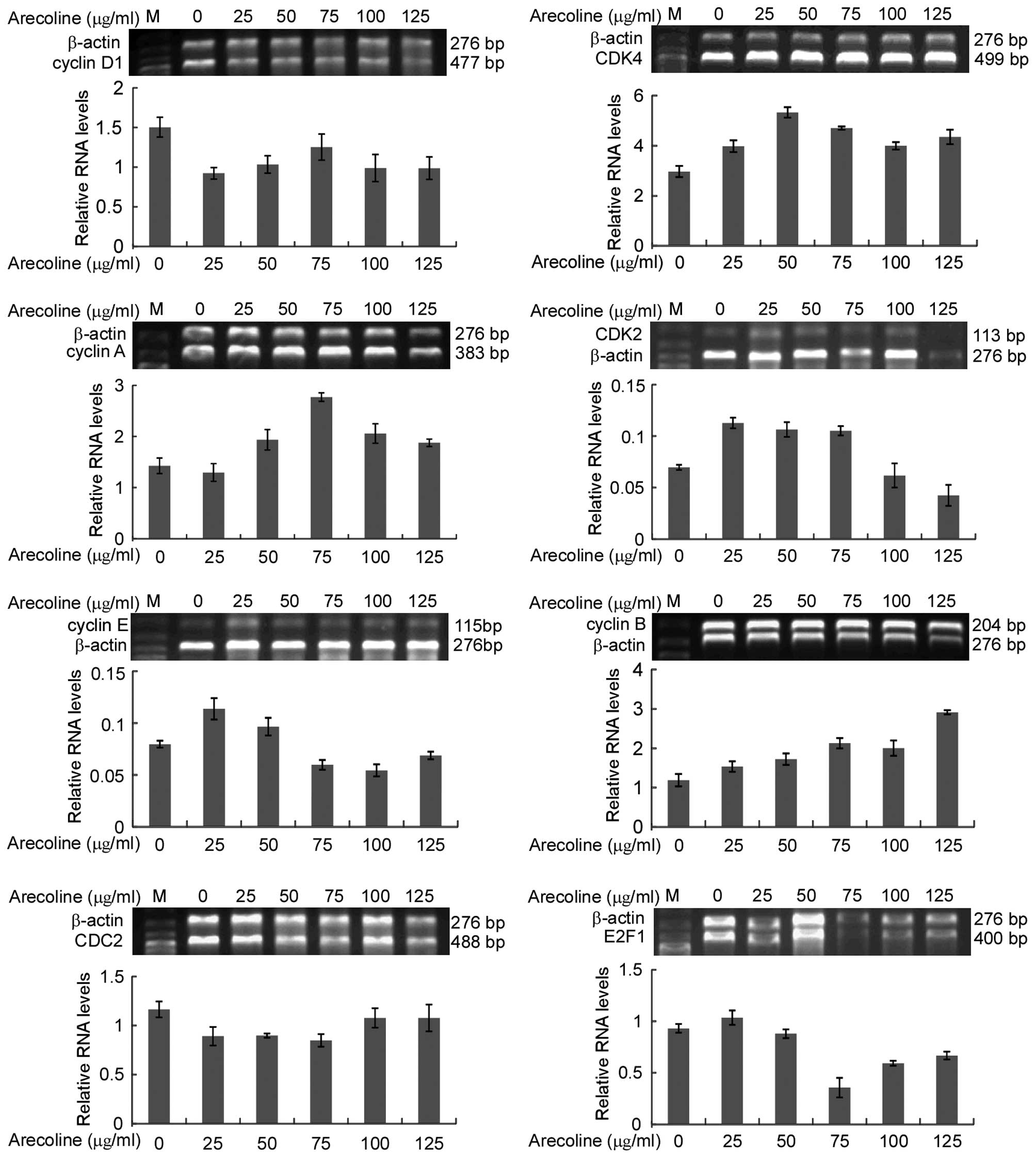
To reveal the possible mechanism of the effect of
arecoline on epithelial cells in vitro, the levels of mRNA
transcripts of several cell cycle regulatory molecules (CDK2, CDK4,
cyclin A, cyclin B, cyclin D1, cyclin E, E2F1, CDC2) were
determined by semi-quantitative RT-PCR. Cyclin D1 mRNA expression
was downregulated following arecoline stimulation, while there was
no significant concentration gradient dependence. The mRNA
expression of cyclin B was upregulated. Low concentrations of
arecoline stimulated CDK4 expression. However, its expression was
suppressed following treatment with high concentrations of
arecoline. Cyclin A expression level was increased in the HaCaT
cells following treatment with arecoline. Cyclin E expression level
was decreased with an increase in arecoline concentrations. CDK2
had the same tendency as cyclin E. There was no significant
difference in CDC2 levels (Fig.
4).
Arecoline regulates the protein levels of
cell cycle regulatory molecules in the HaCaT epithelial cells
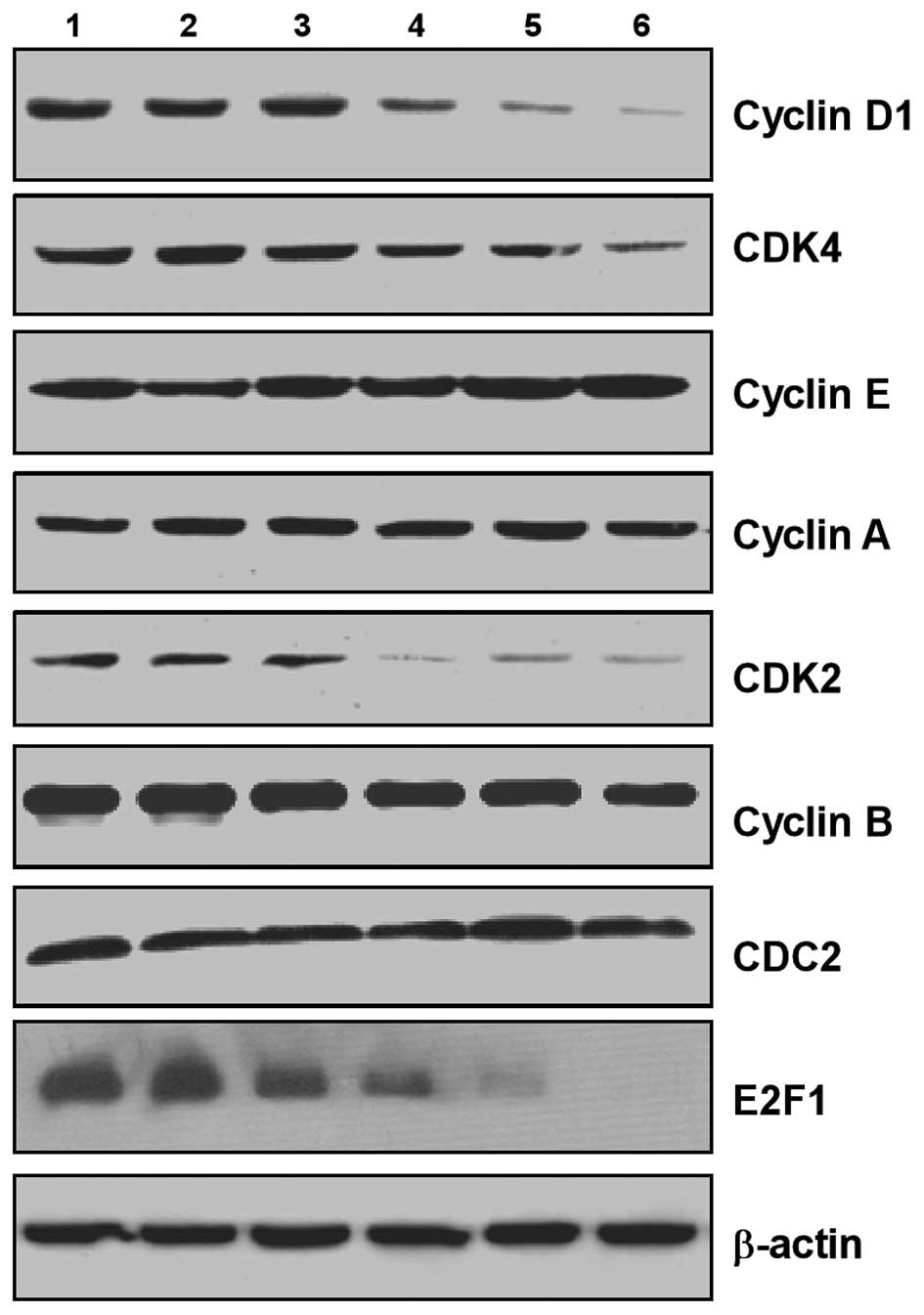
To confirm the change mechanism of cell cycle
regulatory molecules, we tested the protein levels of CDK2, CDK4,
cyclin A, cyclin B, cyclin D1, cyclin E, E2F1 and CDC2 by western
blotting. The expression levels of cyclin D1, CDK4, CDK2 and E2F1
were significantly downregulated. Expression of cyclin A and cyclin
E was slightly upregulated in HaCaT cells following arecoline
stimulation. There was no significant difference in cyclin B and
CDC2 expression (Fig. 5).
Discussion
Betel nut chewing is the most common cause of OSF.
Arecoline is the main component of the betel nut, and it is
associated with the occurrence and development of OSF through
cytotoxicity, genotoxicity and DNA damage. Arecoline inhibits oral
keratinocytes and vascular endothelial cell growth (17,18).
However, using identical arecoline stimulation, OSF epithelial
tissues became atrophic and presented vascular occlusion, and
showed the beginning of the accumulation of fibroblasts and
collagen fibers. Similar types of stimuli elicit differential
responses in different cell types. In the present study, we studied
the effects of arecoline on HaCaT epithelial cells and Hel
fibroblasts.
Our data demonstrated the effects of arecoline on
HaCaT cell morphology. The cell morphologies were significantly
different dependent on the arecoline concentration after 48 h of
treatment. In contrast, the Hel cells showed no effects following
arecoline treatment. MTT assay revealed that arecoline suppressed
HaCaT cell proliferation. Treatment with arecoline significantly
inhibited the proliferation of HaCaT cells, but only slightly
reduced Hel cell growth in vitro. Therefore, treatment with
arecoline inhibited the proliferation of epithelial cells, but not
fibroblasts, in vitro. Our results confirmed our hypothesis:
different cells have differential responses to similar types of
stimuli.
Cell cycle checkpoints are biochemical pathways that
ensure the orderly and timely progression and completion of
critical events, such as DNA replication and chromosome segregation
(9,19). In the present study, we found that
arecoline induced HaCaT cell cycle arrest. In comparison with
control cells without arecoline treatment, following treatment with
≥75 μg/ml arecoline a higher percentage of HaCaT cells remained at
the G0/G1 phase of the cell cycle,
accompanied by a reduced percentage of cells in the S phase.
However, arecoline treatment did not significantly alter Hel cell
cycling. Huang et al(13)
found that arecoline decreased interleukin-6 production and induced
apoptosis and cell cycle arrest in human basal cell carcinoma
cells. Arecoline, a major alkaloid of the areca nut, was found to
inhibit p53, repress DNA repair, and trigger DNA damage response in
human epithelial cells (21).
Cyclin D1 expression and its possible regulation in chewing tobacco
have been shown to mediate oral squamous cell carcinoma progression
(22).
To reveal the possible mechanism of arecoline effect
on epithelial cells in vitro, the levels of mRNA transcripts
of cell cycle regulatory molecules were determined by
semi-quantitative RT-PCR and western blotting. Short-term high-dose
arecoline exerted little effect on Hel fibroblast cell growth, but
significantly inhibited HaCaT epithelial cell growth and arrested
HaCaT cells at the G1/S phase, which was mainly through
decreased protein expression and gene transcription of cyclin D1,
CDK4, CDK2, E2F1, but had no obvious effects on cyclin B/CDC2 which
is related to the G2/M phase. Thus, epithelial atrophy
in OSF is mainly attibuted to cell cycle arrest and imbalance of
proliferation and apoptosis induced by arecoline. Studies have
shown that expression of the transcription factor E2F1 was
increased in tumors and proliferative diseases (11,17,18,23–25).
When cells cross the G1/S restriction point, the cell
cycle path becomes irreversible with autonomy until the end of the
cell cycle (26–29). Arecoline had no significant effect
on fibroblasts in the G1/S phase, which may explain the
reasons for subcutaneous fibroblast proliferation-induced submucosa
collagen accumulation (28,29).
In summary, arecoline inhibits HaCaT epithelial cell
proliferation and survival, in a dose-dependent manner with cell
cycle arrest in the G1/S phase, while this observation
is not obvious in Hel fibroblast cells. Arecoline downregulates the
expression of G1/S phase regulatory proteins cyclin D1,
CDK4, CDK2 and E2F1 in HaCaT epithelial cells. Potentially, our
findings may aid in the prevention of arecoline-associated human
OSF.
 | Table ISequences of the primers for
RT-PCR. |
Table I
Sequences of the primers for
RT-PCR.
| Gene | Forward primer | Reverse primer | Product (bp) |
|---|
| CDK2 |
CCTGGAGATTCTGAGATTGA |
GGGAAACTTGGCTTGTAAT | 113 |
| CDK4 |
GCATCCCAATGTTGTCCG |
AGGCAGCCCAATCAGGTC | 499 |
| Cyclin A |
CCTGCGTTCACCATTCAT |
TCTTCTCCTACTTCAACTAACC | 383 |
| Cyclin B |
TTGGTTGATACTGCCTCTC |
TCTGACTGCTTGCTCTTC | 204 |
| Cyclin D1 |
GAACAGAAGTGCGAGGAG |
GCGGTAGTAGGACAGGAA | 477 |
| Cyclin E |
TGGATGTTGACTGCCTTG |
TCTATGTCGCACCACTGA | 115 |
| E2F1 |
CACTGAATCTGACCACCAA |
ACCATAACCATCTGCTCTG | 400 |
| CDC2 |
AGAGTTCTTCACAGAGACT |
GGATGATTCAGTGCCATT | 488 |
| β-actin |
CCGTGACCTGACTGACTACCTC |
ATACCGCAAGATTCCATACCC | 276 |
 | Table IIFlow cytometric analysis of HaCaT
cells following treatment with different concentrations of
arecoline. |
Table II
Flow cytometric analysis of HaCaT
cells following treatment with different concentrations of
arecoline.
| Phase of the cell
cyclea |
|---|
|
|
|---|
| Treatment |
G0-G1 (%) | S (%) | G2-M
(%) |
|---|
| Arecoline
(μg/ml) |
| 0 | 49.02 | 32.05 | 18.93 |
| 25 | 51.06 | 31.94 | 17.00 |
| 50 | 53.62 | 30.69 | 15.69 |
| 75 | 73.00 | 20.50 | 6.50 |
| 100 | 91.23 | 5.61 | 3.16 |
| 125 | 89.67 | 5.13 | 5.20 |
 | Table IIIFlow cytometric analysis of Hel cells
following treatment with different concentrations of arecoline. |
Table III
Flow cytometric analysis of Hel cells
following treatment with different concentrations of arecoline.
| Phase of the cell
cyclea |
|---|
|
|
|---|
| Treatment |
G0-G1 (%) | S (%) | G2-M
(%) |
|---|
| Arecoline
(μg/ml) |
| 0 | 52.52 | 33.35 | 14.13 |
| 25 | 49.09 | 34.02 | 16.89 |
| 50 | 50.04 | 30.17 | 19.79 |
| 75 | 51.75 | 30.21 | 18.04 |
| 100 | 54.02 | 31.64 | 14.34 |
| 125 | 54.86 | 30.92 | 14.22 |
Acknowledgements
The study was supported by the International
Cooperation Program Funds of the China Hunan Provincial Science and
Technology Department (project no. 2012WK4005) and the Research
Programe from the Science and Technology Department of Hunan
Province, China (project no. 2012FJ4076). We would like to thank Dr
Fred Bogott for his excellent English editing of the
manuscript.
Abbreviations:
|
OSF
|
oral submucous fibrosis
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
E2F1
|
E2F transcription factor 1
|
|
CDK4
|
cyclin-dependent kinase 4
|
|
CDK2
|
cyclin-dependent kinase 2
|
References
|
1
|
Chaturvedi P, Vaishampayan SS, Nair S, et
al: Oral squamous cell carcinoma arising in background of oral
submucous fibrosis: A clinicopathologically distinct disease. Head
Neck. Sep 13–2012.(Epub ahead of print). View Article : Google Scholar
|
|
2
|
Mehrotra D and Kumar S, Agarwal GG,
Asthana A and Kumar S: Odds ratio of risk factors for oral
submucous fibrosis in a case control model. Br J Oral Maxillofac
Surg. Aug 27–2012.(Epub ahead of print).
|
|
3
|
Khan S, Chatra L, Prashanth SK, Veena KM
and Rao PK: Pathogenesis of oral submucous fibrosis. J Cancer Res
Ther. 8:199–203. 2012. View Article : Google Scholar
|
|
4
|
Kothari MC, Hallur N, Sikkerimath B, Gudi
S and Kothari CR: Coronoidectomy, masticatory myotomy and buccal
fat pad graft in management of advanced oral submucous fibrosis.
Int J Oral Maxillofac Surg. 41:1416–1421. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ranganathan K and Kavitha R: Proliferation
and apoptosis markers in oral submucous fibrosis. J Oral Maxillofac
Pathol. 15:148–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khan I, Agarwal P, Thangjam GS, Radhesh R,
Rao SG and Kondaiah P: Role of TGF-β and BMP7 in the pathogenesis
of oral submucous fibrosis. Growth Factors. 29:119–127. 2011.
|
|
7
|
Tadakamadla J, Kumar S and Mamatha GP:
Evaluation of serum copper and iron levels among oral submucous
fibrosis patients. Med Oral Patol Oral Cir Bucal. 16:e870–e873.
2011. View Article : Google Scholar
|
|
8
|
Wang YC, Tsai YS, Huang JL, et al:
Arecoline arrests cells at prometaphase by deregulating mitotic
spindle assembly and spindle assembly checkpoint: implication for
carcinogenesis. Oral Oncol. 46:255–262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee PH, Chang MC, Chang WH, et al:
Prolonged exposure to arecoline arrested human KB epithelial cell
growth: regulatory mechanisms of cell cycle and apoptosis.
Toxicology. 220:81–89. 2006.PubMed/NCBI
|
|
10
|
Lu SY, Chang KW, Liu CJ, Tseng YH, Lu HH,
Lee SY and Lin SC: Ripe areca nut extract induces G1
phase arrests and senescence-associated phenotypes in normal human
oral keratinocyte. Carcinogenesis. 27:1273–1284. 2006.PubMed/NCBI
|
|
11
|
Tu HF, Liu CJ, Chang CS, Lui MT, Kao SY,
Chang CP and Liu TY: The functional (−1171 5A→6A) polymorphisms of
matrix metalloproteinase 3 gene as a risk factor for oral submucous
fibrosis among male areca users. J Oral Pathol Med. 35:99–103.
2006.
|
|
12
|
Chiu CJ, Chiang CP, Chang ML, et al:
Association between genetic polymorphism of tumor necrosis
factor-alpha and risk of oral submucous fibrosis, a pre-cancerous
condition of oral cancer. J Dent Res. 80:2055–2059. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang LW, Hsieh BS, Cheng HL, Hu YC, Chang
WT and Chang KL: Arecoline decreases interleukin-6 production and
induces apoptosis and cell cycle arrest in human basal cell
carcinoma cells. Toxicol Appl Pharmacol. 258:199–207. 2012.
View Article : Google Scholar
|
|
14
|
Tsai YS, Lee KW, Huang JL, et al:
Arecoline, a major alkaloid of areca nut, inhibits p53, represses
DNA repair, and triggers DNA damage response in human epithelial
cells. Toxicology. 249:230–237. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mishra R and Das BR: Cyclin D1 expression
and its possible regulation in chewing tobacco mediated oral
squamous cell carcinoma progression. Arch Oral Biol. 54:917–923.
2009. View Article : Google Scholar
|
|
16
|
Liu L, Xie H, Chen X, Shi W, Xiao X, Lei D
and Li J: Differential response of normal human epidermal
keratinocytes and HaCaT cells to hydrogen peroxide-induced
oxidative stress. Clin Exp Dermatol. 37:772–780. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou Y, Zeng Z, Zhang W, et al:
Lactotransferrin: a candidate tumor suppressor-Deficient expression
in human nasopharyngeal carcinoma and inhibition of NPC cell
proliferation by modulating the mitogen-activated protein kinase
pathway. Int J Cancer. 123:2065–2072. 2008. View Article : Google Scholar
|
|
18
|
Zhou Y, Zeng Z, Zhang W, et al:
Identification of candidate molecular markers of nasopharyngeal
carcinoma by microarray analysis of subtracted cDNA libraries
constructed by suppression subtractive hybridization. Eur J Cancer
Prev. 17:561–571. 2008. View Article : Google Scholar
|
|
19
|
Kuo FC, Wu DC, Yuan SS, et al: Effects of
arecoline in relaxing human umbilical vessels and inhibiting
endothelial cell growth. J Perinat Med. 33:399–405. 2005.PubMed/NCBI
|
|
20
|
Bartek J, Lukas J and Bartkova J:
Perspective: defects in cell cycle control and cancer. J Pathol.
187:95–99. 1999. View Article : Google Scholar
|
|
21
|
Malumbres M: Cyclins and related kinases
in cancer cells. J BUON. 12(Suppl 1): S45–S52. 2007.
|
|
22
|
van den Heuvel S: Cell-cycle regulation.
WormBook. 1–16. 2005.
|
|
23
|
Chen HZ, Tsai SY and Leone G: Emerging
roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev
Cancer. 9:785–797. 2009.PubMed/NCBI
|
|
24
|
Kwong RA, Nguyen TV, Bova RJ, et al:
Overexpression of E2F-1 is associated with increased disease-free
survival in squamous cell carcinoma of the anterior tongue. Clin
Cancer Res. 9:3705–3711. 2003.
|
|
25
|
Luo X, Pan Q, Liu L, et al: Genomic and
proteomic profiling II: comparative assessment of gene expression
profiles in leiomyomas, keloids and surgically-induced scars.
Reprod Biol Endocrinol. 5:352007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Calonge TM and O'Connell MJ: Turning off
the G2 DNA damage checkpoint. DNA Repair. 7:136–140.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miyazaki T and Arai S: Two distinct
controls of mitotic cdk1/cyclin B1 activity requisite for cell
growth prior to cell division. Cell Cycle. 6:1419–1425. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu Y, Kovacevic Z and Richardson DR:
Tuning cell cycle regulation with an iron key. Cell Cycle.
6:1982–1994. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakayama KI and Nakayama K: Ubiquitin
ligases: cell-cycle control and cancer. Nat Rev Cancer. 6:369–381.
2006. View Article : Google Scholar : PubMed/NCBI
|