Introduction
The WW domain-containing oxidoreductase (WWOX) gene
is located at a chromosome region, 16q23.3-24.1, that spans the
second most common human fragile site, FRA16D (1). The WWOX gene has nine exons and
encodes a Wwox protein with a molecular weight for 46 kDa. The Wwox
protein contains two N-terminal WW domains and one C-terminal
short-chain dehydrogenase domain known as SDR (1,2). WW
domains are characterized by their interactions with
proline-containing ligands, which is a basis for protein-protein
interactions (3), while the SDR
domain is located in the central region of the Wwox protein and is
shared by steroid-hormone-metabolizing enzymes and characterized by
its high frequency loss or alteration implicated in numerous types
of carcinomas (3,4).
To date, numerous studies have shown either a
reduction or loss of WWOX expression in a variety of carcinomas,
including breast, esophagus, pancreas and thyroid cancer (5–9).
Significantly, a restoration or upregulation of WWOX in tumors such
as lung and breast cancer can sensitize them to apoptosis both
in vitro and in vivo(10,11).
Studies of WWOX animal models suggest that an inactivation of one
allele for WWOX accelerates the predisposition of normal cells to
malignant transformation (12). In
addition, WWOX knockout mice also showed a shortened life span and
defects in bone metabolism, as well as other deficiencies (11,13).
These studies indicate that WWOX may function as a tumor suppressor
gene.
All the results cited above verified the functional
role of WWOX or its protein product in tumorigenesis in leukemia,
particularly aberration or absence of WWOX expression in primary
hematopoietic malignancies has also been reported (14,15).
This prompted us to hypothesize that WWOX may function as a tumor
suppressor in leukemia, also due to its special location on FRA16D
which is susceptible to activation by carcinogens. Studies on the
biological role of WWOX in tumorigenesis have shown that its
function in tumor cellular metabolism is likely to modulate cell
viability by interacting with factors involved in cell apoptosis
control such as Bcl-2, Bcl-xL and caspase (16–19),
which suggests a close relationship between WWOX and the apoptosis
signal pathways. However, this relationship remains to be
confirmed.
In the present study, we first evaluated the
expression of WWOX in 38 cases of primary leukemia patients and 10
leukemia cell lines. With a reduced or absent expression of WWOX
observed in our results, we restored Wwox expression in its
negative cell lines as Jurkat and K562 cells by transfecting them
with pGC-FU-WWOX lentiviral plasmid, and we observed that the
Jurkat and K562 cells all underwent viability inhibition and
apoptosis following the restoration in vitro. To elucidate
the underlying mechanisms, we further examined whether WWOX
regulates cell death via the mitochondrial pathway, and we found an
activation of the mitochondrial pathway in WWOX-mediated apoptosis
in human leukemia.
Materials and methods
Samples, cell lines and cell culture
Thirty-eight diagnosed leukemia patients at Fujian
Medical University Affiliated Xiehe Hospital from September 2010 to
June 2011 were enrolled in the study. These patients included 9
cases of acute lymphoblastic leukemia (ALL), 18 cases of acute
myelogenous leukemia (AML) and 11 cases of chronic myelogenous
leukemia (CML). Mononuclear cells purified from 20 healthy
volunteers were used as controls. Human Jurkat (acute
T-lymphoblastic leukemia), K562 (chronic myelogenous leukemia in
erythroid crisis), NB4 (promyelocytic leukemia), HL-60
(acute myelogenous leukemia), U937 (promonocytic leukemia), CEM
(acute T-lymphocyte leukemia), U266 (human myeloma), KAR (K562
resistance to doxorubicin), ADR (HL-60 resistance to doxorubicin)
and CA46 (human Burkitt’s lymphoma) cell lines were all purchased
from the cell bank of the Chinese Academy of Medical Sciences and
maintained in RPMI-1640 supplemented with 10% fetal bovine serum
(FBS) (Gibco, USA), and cultured at 37°C in a 5% CO2
humidified atmosphere.
Reverse transcription-PCR analysis
Total RNA was extracted with TRIzol Reagent
(Invitrogen, USA) and was reverse transcribed into cDNA using a
commercial kit (Fermentas, USA). We then amplified 6–8 exons of
WWOX with the primers (20). The
primers of an internal control gene, glyceraldehyde 3-phosphate
dehydrogenase (GAPDH), were: forward, 5′-CAAGGTCATCCATGACAACTTTG-3′
and reverse, 5′-GTCCACCACCCTGTTGCTGTAG-3′. PCR was performed using
a PCR kit (Biomed, Beijing, China) under the recommended conditions
at an initial denaturation for 5 min at 94°C followed by 30 cycles
of 94°C for 30 sec, 55°C for 30 sec, 72°C for 30 sec, and a final
extension for 7 min at 72°C. Quantified data were normalized to
GAPDH.
In vitro lentiviral transduction and
growth condition observation
The pGC-FU-WWOX lentiviral vector was constructed by
our laboratory, amplified and titrated based on the manufacturer’s
instructions (Genechem, Shanghai, China) (21). A pGC-FU-GFP vector served as
negative control (mock lentiviral vector only encoding GFP). Cells
were seeded at a density of 1×105/ml in 6-well plates
and infected with pGC-FU-WWOX at appropriate multiplicities of
infection (MOI). The transduction efficiency and growth conditions
were evaluated by visualization of green fluorescent protein
(Wwox-GFP fusion protein) and cell morphological features every 24
h following infection using a fluorescence microscope (Nikon,
Tokyo, Japan).
Proliferation assay
Proliferation inhibition ratio was assessed by CCK-8
assay (Dojindo, Japan). Briefly, 5×103 cells/well were
seeded with a total volume of 100 μl in 96-well plates. Cell
infection followed the procedures above. Every 24 h following
incubation, 10 μl CCK-8 was added to each well followed by
incubation for another 3 h at 37°C in a 5% CO2
humidified atmosphere. The optical density (OD) value was measured
by a microculture plate reader (BioTek Instruments, USA) using
double-wavelength (450 and 630 nm). Growth inhibition ratio (%) =
(1 − Mean OD value of lentivirus infected cells/Mean OD value of
non-treated cells) × 100%. Each assay was performed in
triplicate.
Colony-forming assay
Briefly, GFP-expressing cells were sorted by flow
cytometry using Vi-Cell counter (Beckman Coulter, Fullerton, CA,
USA), and 300 cells/ml were seeded in 24-well plates.
Methylcellulose dissolved in RPMI-1640 containing 30% FBS was added
to each well with a concentration of 0.8 g/l. The colonies
containing >50 cells were counted on Day 14. Each assay was
performed in triplicate.
DAPI fluorescence staining and DNA
fragmentation analysis
For DAPI fluorescence staining, cells were collected
and washed 2 times with PBS and stained by DAPI (Beyotime,
Shanghai, China) in a concentration of 2 mg/ml for 3–5 min, then
washed 2 times with PBS. Morphological changes of the stained cells
were examined using the fluorescence microscope. DNA fragmentation
analysis was performed according to the protocols of a commercial
DNA Fragmentation Kit (Beyotime), followed by agarose gel
electrophoresis: each sample containing equal amounts of extracted
DNA (2 μg) in 1.0% agarose gel with a constant voltage 18 V for 4–6
h.
Immunofluorescence staining
In brief, cell monolayers were fixed with 4%
paraformaldehyde, and incubated at 4°C overnight with rabbit
anti-human Wwox, 1:500, and mouse monoclonal anti-human cytochrome
c, 1:500 (Abcam, USA). FITC-conjugated goat anti-rabbit and
Cy3-conjugated goat anti-mouse IgG (Beyotime) were all diluted in
1:1,000. DAPI was used to stain the nucleus. Stained cell
monolayers were washed 2 times with PBS and observed under the
fluorescence microscope.
Real-time PCR analysis
Real-time PCR was enabled using a SYBR-Green PCR
Master Mix (Roche Diagnostics GmbH, Germany) under the recommended
conditions. The primer sequences for Bcl-2 and Bax were described
previously (22), and for GAPDH
they were: forward, 5′-GAAGGTGAAGGTCGGAGT-3′ and reverse,
5′-GAAGATGGTGATGGGATTTC-3′. The comparative Ct method to GAPDH was
used to calculate the relative expression level of Bcl-2 and
Bax.
Western blot analysis
A Cell Mitochondria Isolation Kit (Beyotime) was
used in the isolation of proteins from the nucleus, cytoplasm and
mitochondria of the cells. The primary antibodies and the dilutions
were: rabbit anti-human Wwox, 1:1,000; anti-Bcl-2 (1:500; Abcam,
USA); anti-caspase-9 (1:300; Santa Cruz, Biotechnology, Inc., Santa
Cruz, CA, USA); anti-caspase-3 (1:500; Beyotime). Mouse monoclonal
anti-human β-actin and anti-tubulin (Beyotime) were all diluted in
1:1,000 and used as controls.
Statistical analysis
Data are expressed as the means ± standard deviation
(SD), and analyzed by Student’s t-test, or non-parametric test
using the SPSS 13.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of WWOX is reduced or absent
in leukemia
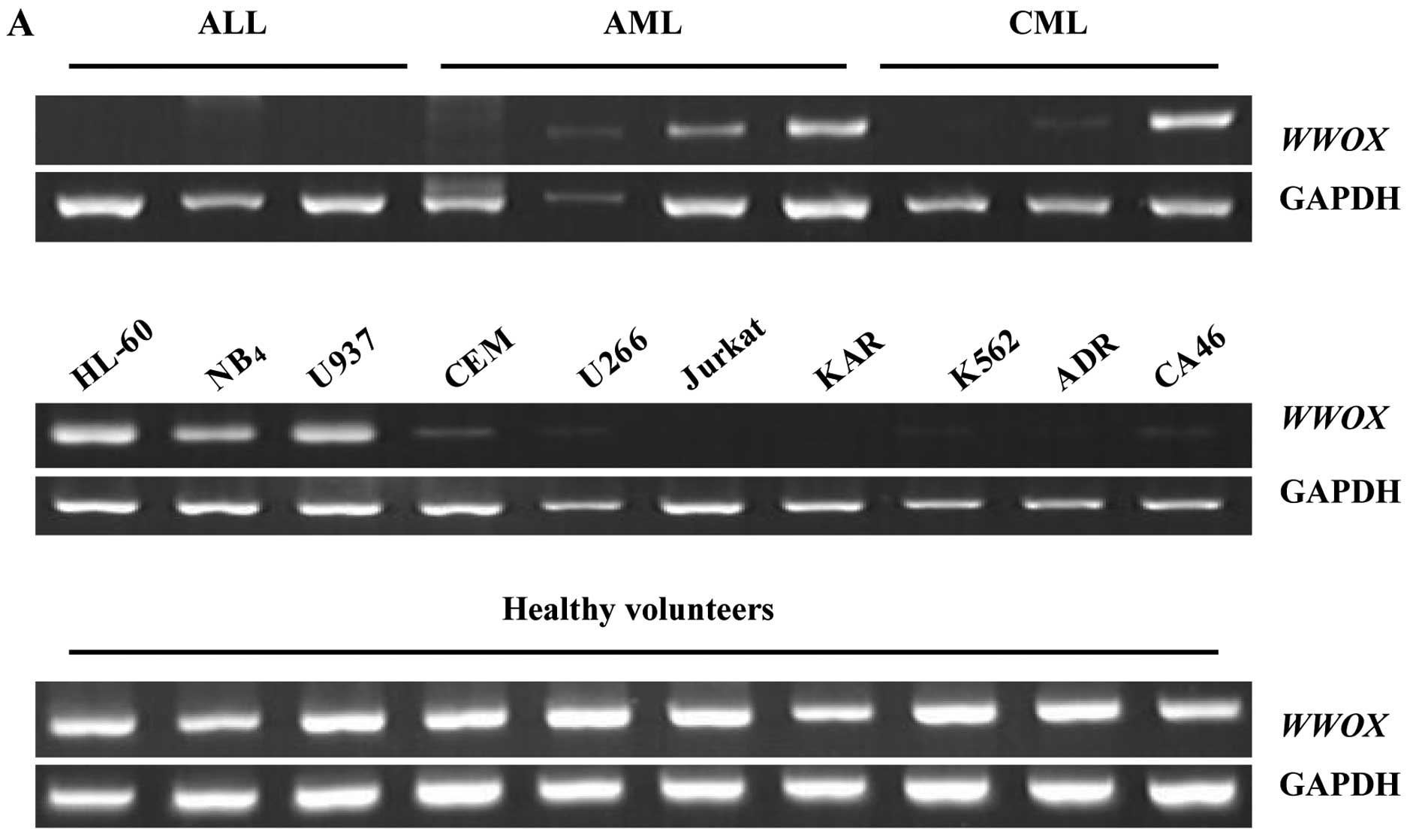
We examined the expression level of WWOX mRNA
and the Wwox protein in leukemia patients and cell lines via RT-PCR
and western blot analysis. Twenty-eight of the 38 leukemia cases
(74%) showed reduced or lost expression of WWOX, and
included 11 AML (39%), 8 ALL (29%) and 9 CML (32%) patients
(Fig. 1A). For Wwox, only 6/38
cases (16%) had a positive expression (Fig. 1B). Similarly, 7/10 leukemic cell
lines (70%) showed absent or reduced WWOX expression; Jurkat
and KAR cells were negative, while K562, CEM, U266, CA46 and ADR
cells had a low expression level, and only HL-60, NB4 as
well as U937 cells exhibited a high expression level similar to the
normal controls (Fig. 1A).
Endogenous Wwox in all leukemic cells was undetectable (Fig. 1B). All controls exhibited a high
expression level of both WWOX mRNA and Wwox protein
(Fig. 1).
Wwox restoration suppresses cell
proliferation and colony formation in Jurkat and K562 cells
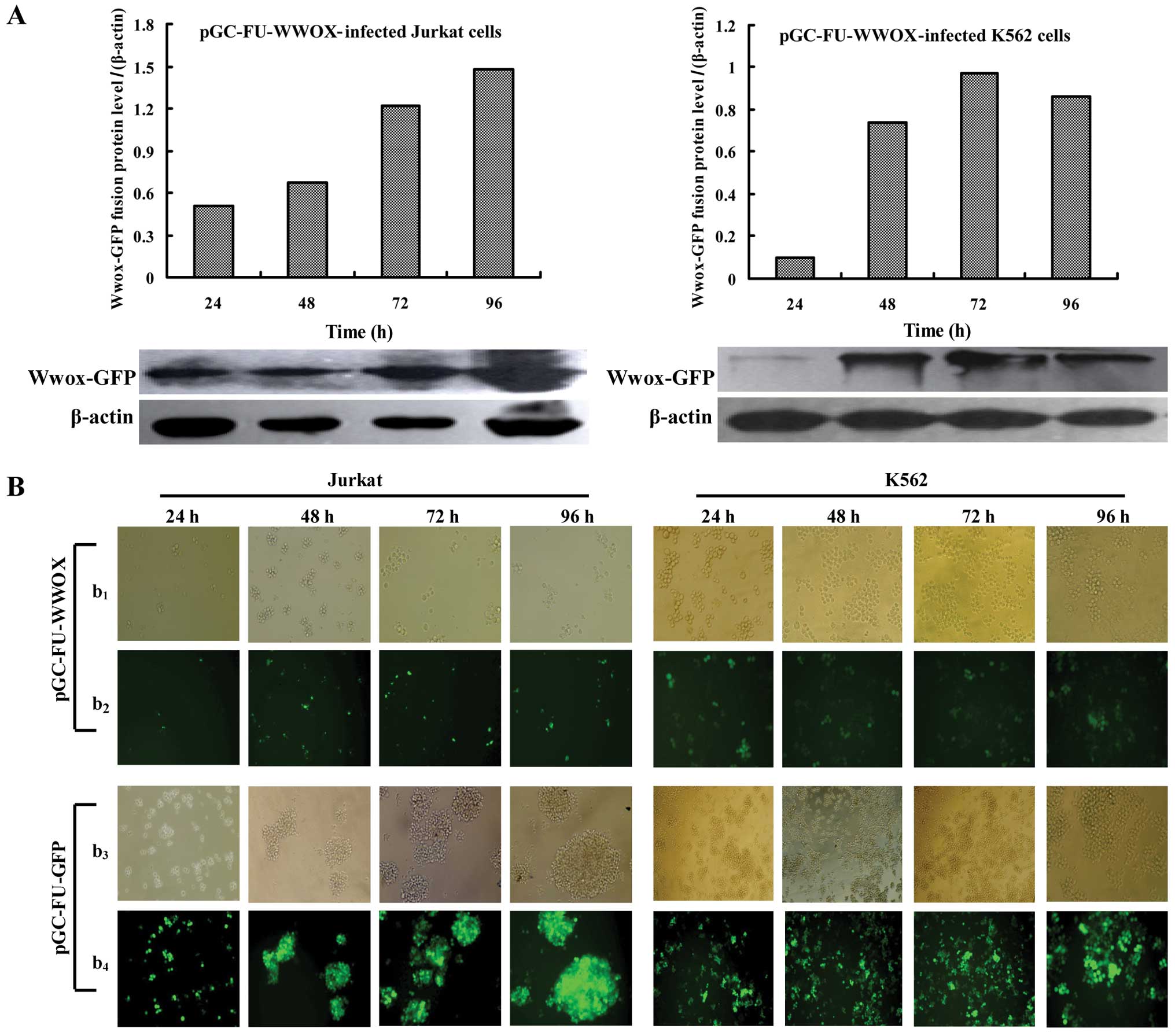
We first examined whether Wwox was successfully
restored in Jurkat and K562 cells via fluorescence microscope and
western blot analysis. The results showed that pGC-FU-WWOX- and
pGC-FU-GFP-infected cells all expressed GFP at 24 h following
infection (Fig. 2B). Cells infected
with pGC-FU-WWOX exhibited an increased expression level of Wwox
with time lapse (Fig. 2A), while
pGC-FU-GFP-infected cells had no expression (data not shown),
indicating that Wwox was successfully restored in the Jurkat and
K562 cells. In addition, pGC-FU-WWOX-infected cells presented
anti-proliferation morphological features characterized by cell
shrinkage and colonies decreased with time lapse (Fig. 2B). Wwox restoration resulted in
significantly reduced cell proliferation with the proliferation
inhibition rate increasing from 9.1% at 48 h to 74.62% at 96 h for
Jurkat cells, and from 3.23% at 48 h to 40.68% at 96 h for K562
cells, all with P<0.05 when vs. pGC-FU-GFP-infected cells
(Fig. 2C). Moreover, colony
formation numbers of pGC-FU-WWOX-infected cell lines were
significantly lower than those of non-treated cells (P<0.01, for
both Jurkat and K562 cells), while pGC-FU-GFP-infected cells showed
no statistical significance vs. non-treated cells with P>0.05
(Fig. 2D).
Wwox restoration promotes apoptosis in
Jurkat and K562 cells
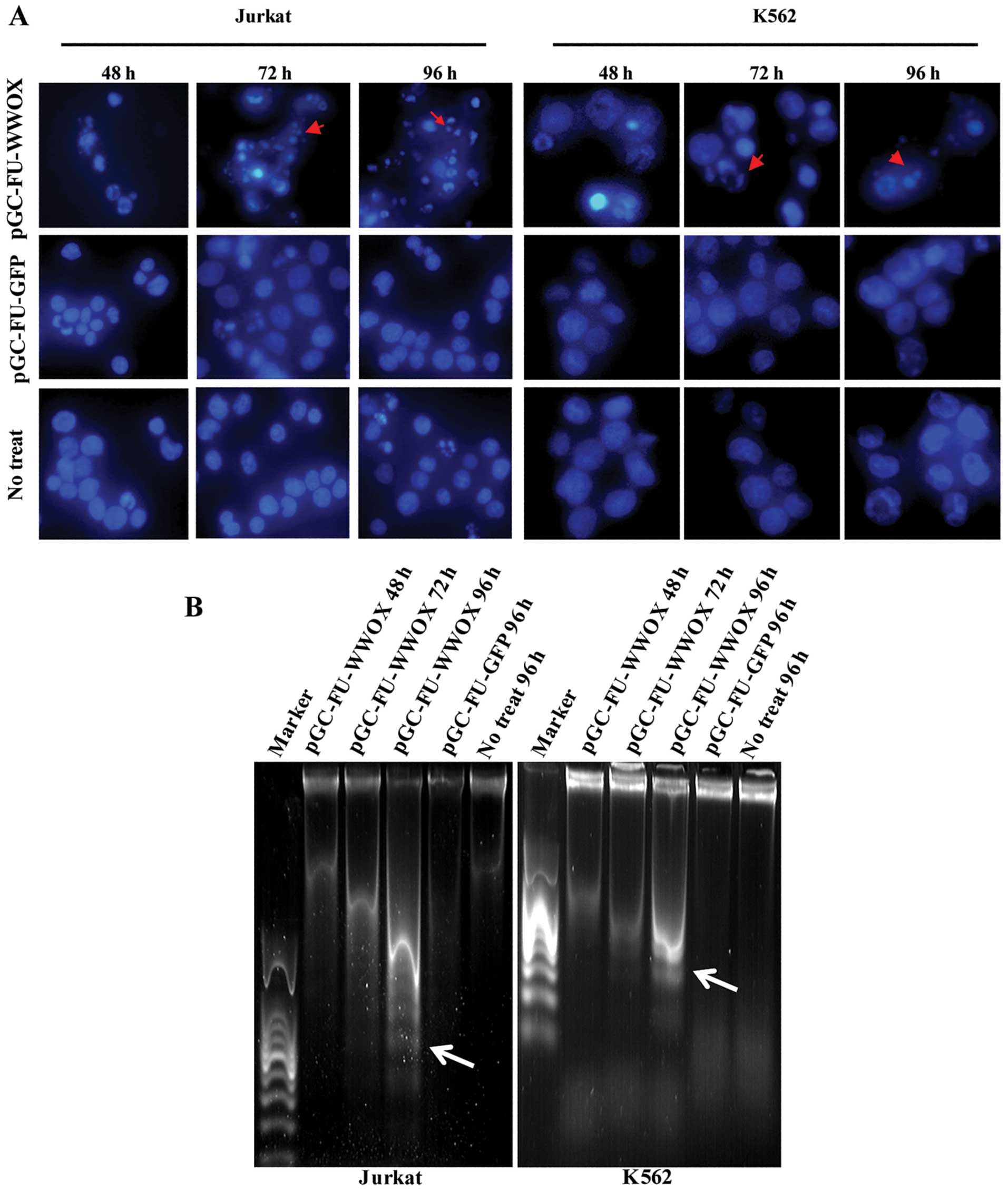
As shown in Fig. 3A,
there were apparent microscopic changes of nucleus in
pGC-FU-WWOX-infected cells with a morphology of chromatin
condensation and shrinkage in phase IIb of apoptosis exhibited by
DAPI staining. DNA degradative fragments were detected by DNA
ladder electrophoresis, which also showed typical increased
apoptosis ‘DNA ladders’ in pGC-FU-WWOX-infected cells with time
lapse when compared with the controls (Fig. 3B).
Bcl-2, Bax, caspase-3 and -9 are involved
in WWOX-mediated apoptosis
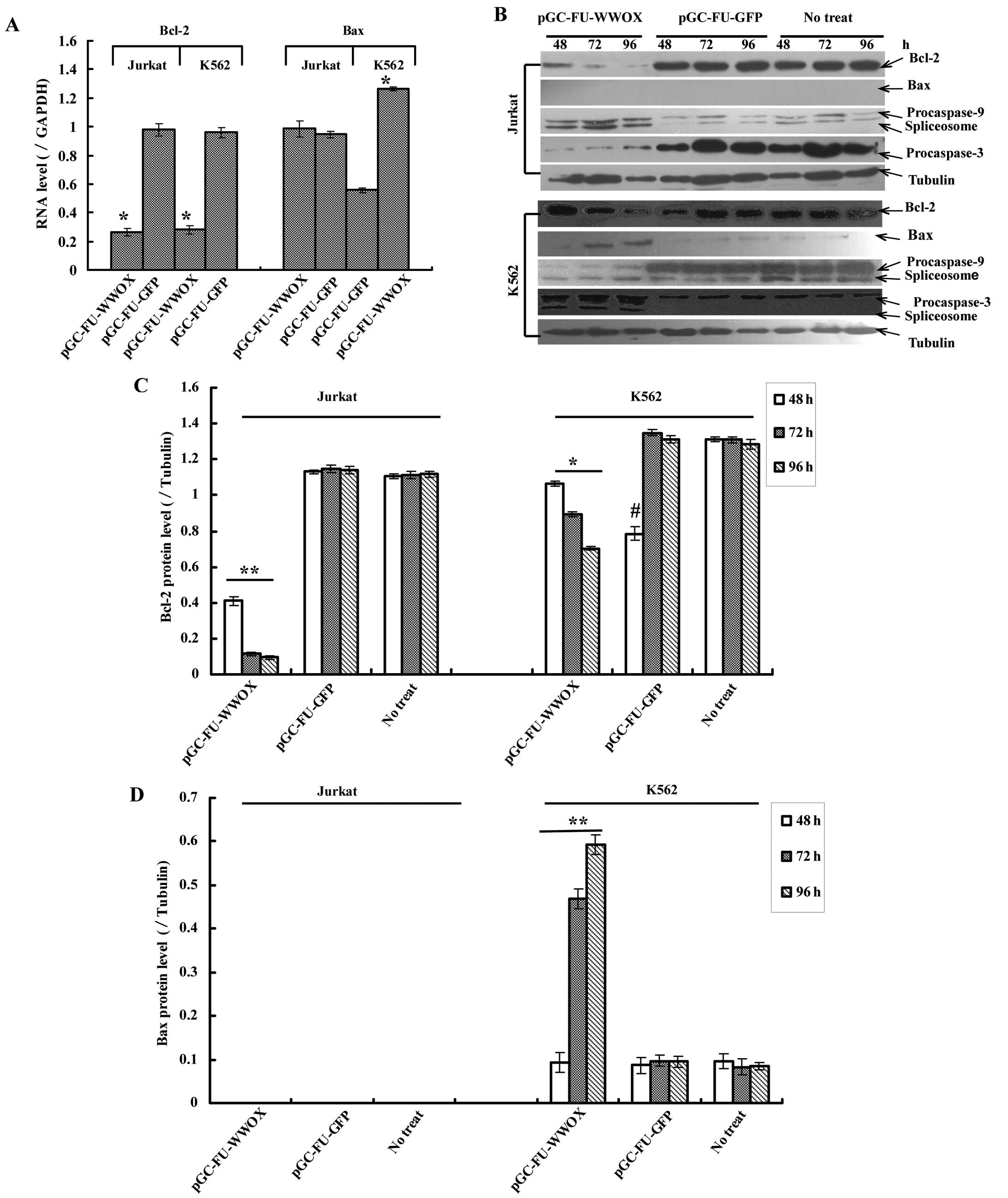
To investigate whether WWOX restoration could induce
apoptosis-related factors, we assessed the changes of Bcl-2, Bax,
caspase-3 and -9 in both protein and mRNA levels. Real-time PCR
results indicated that Bcl-2 mRNA in pGC-FU-WWOX-infected cells
decreased, while Bax mRNA (only in K562 cells) increased with time
lapse, all with P<0.05 when vs. pGC-FU-GFP-infected cells
(Fig. 4A). Western blot analysis
revealed similar results for Bcl-2 and Bax. In addition, the Bax
protein in Jurkat cells was undetectable although Bax mRNA showed
positively in it (Fig. 4A and B).
Furthermore, western blot analysis displayed both procaspase-9 and
-3 were cleaved by presenting their spliceosomes: 37 kDa
spliceosome for procaspase-9 and 17 kDa for procaspase-3 were
observed in pGC-FU-WWOX-infected cells (Fig. 4B). Although the 17 kDa spliceosome
of procaspase-3 in pGC-FU-WWOX-infected Jurkat cells was not
exposed, procaspase-3 decreased with time lapse (Fig. 4B).
Release of cytochrome c is a result of
WWOX-mediated apoptosis in Jurkat and K562 cells
To explore whether WWOX restoration could trigger
the release of cytochrome c from mitochondria, we employed
immunofluorescence staining and western blot assay. Our results
revealed that cytochrome c for pGC-FU-WWOX-infected cells
located in the cytosol with an increased, dispersive or block-like
distribution (Fig. 5A). Moreover,
total cytochrome c protein as well as in the cytosol for
pGC-FU-WWOX-infected cells increased and in mitochondria it
decreased with time lapse, all with P<0.05 when compared with
pGC-FU-GFP-infected cells (Fig.
5B).
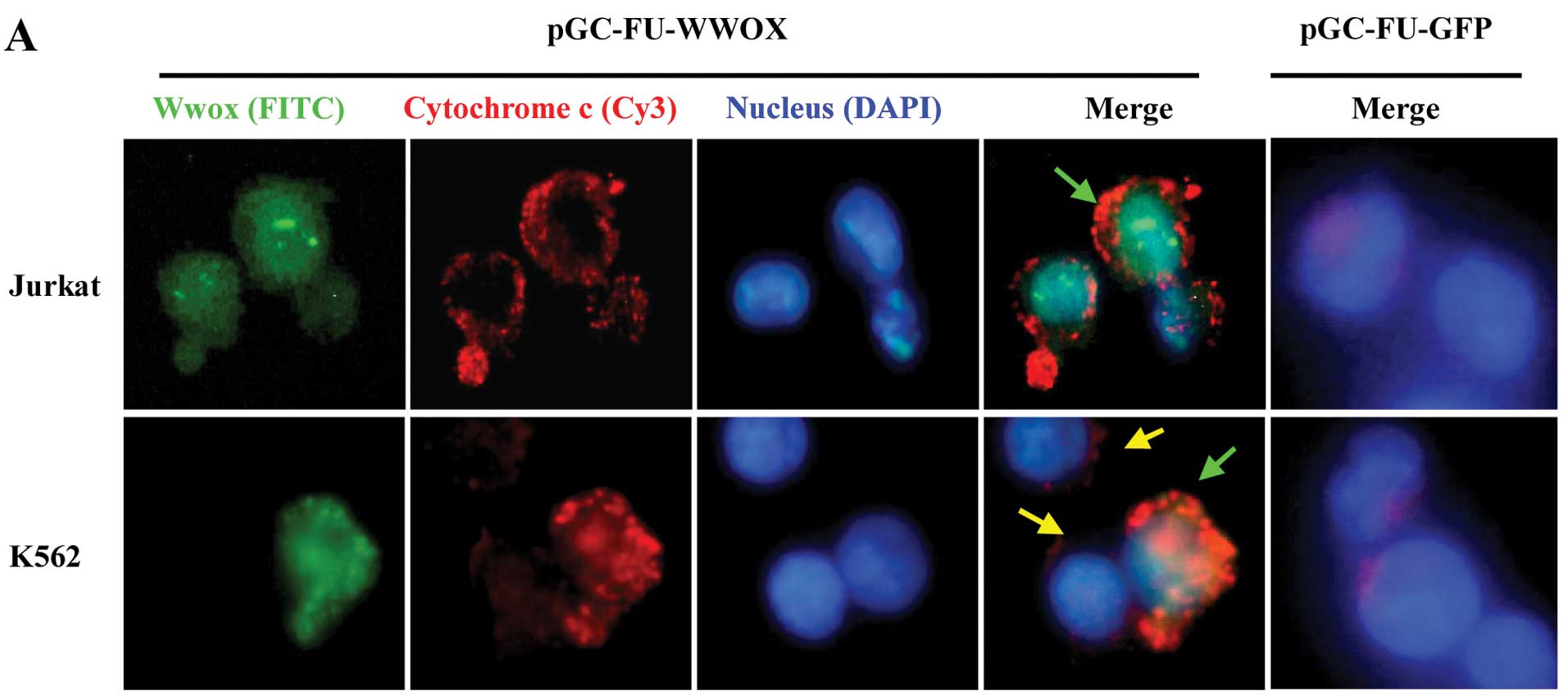 | Figure 5Release of cytochrome c caused
by Wwox restoration was measured by immunofluorescence staining
assay and western blot analysis. (A) Immunofluorescence staining
assay, observed by fluorescence microscopy at 60 h after infection,
×400. As GFP could be easily destroyed by the denaturant while
staining, green fluorescence FITC-conjugated anti-rabbit IgG was
reapplied to remark Wwox, and cytochrome c was marked by red
fluorescence Cy3-conjugated anti-mouse IgG. The nucleus was stained
by DAPI. Green arrows show the domain where Wwox and cytochrome
c merged in an orange patch in pGC-FU-WWOX-infected cells,
and yellow arrows show the lentivirus-uninfected cells with no
green fluorescence Wwox-GFP expression as well as low expression of
cytochrome c. (B) Western blot analysis. Proteins from
different organelles were extracted at 48, 72 and 96 h after
infection, and normalized to tubulin. Data shown are the means ± SD
(n=3). *P<0.05, vs. non-treated cells; cyto, cytosol;
mito, mitochondria; total, total protein. |
Discussion
In the present study, we first evaluated the
expression of WWOX in 38 cases of primary leukemia patients and in
10 leukemic cell lines, and observed a low expression level of WWOX
in leukemia. Then, we restored Wwox protein expression in
endogenous Wwox-negative cell lines Jurkat and K562 by utilizing
the lentiviral vector pGC-FU-WWOX, and explored the effects of Wwox
expression on biological properties of these cell lines. Our
results exhibited that Wwox restoration resulted in significant
cell viability inhibition and apoptosis in both Jurkat and K562
cells. We further investigated the possible mechanism underlying
this process and whether WWOX regulates cell death via the
mitochondrial pathway in leukemia. Finally, we found that restored
Wwox promoted cytochrome c release from the mitochondria and
also activated caspase-9 and -3, indicating that WWOX could
activate the mitochondrial pathway in its antitumor activities in
leukemia.
Leukemia is an oligoclonal or monoclonal disease,
and its molecular basis remains poorly understood. The WWOX gene
was previously identified from chromosome region 16q23.3-24.1 which
spans the common fragile site FRA16D (1), and low expression of WWOX has also
been reported in various types of cancer (5–9). The
present study also showed that expression of WWOX (mRNA and
protein) was frequently reduced or lost in different types of
primary leukemia patients and cell lines, but exhibited a high
expression level in healthy volunteers. Recent evidence suggests
that loss of heterozygosity, as well as epigenetic modification of
the promoter by methylation, can reduce WWOX expression in various
types of tumor (7,23,24).
Apoptosis plays a key role in tumor viability or
development, and a lack or failure of cell apoptosis in tumors
leads to their development; thus, inducing cell apoptosis in tumors
could be a candidate strategy for a new therapeutic approach for
oncotherapy. Bednarek et al(1,25)
first reported that ectopic expression of WWOX could inhibit breast
cancer viability both in vitro and in vivo. Gourley
et al(26) revealed that
WWOX expression abolishes ovarian cancer tumorigenicity in
vivo, and Fabbri et al(10) also found an increase of WWOX in lung
cancer cells exhibits marked suppression of tumorigenicity. Recent
publications demonstrated that ectopic expression of WWOX leads to
cell apoptosis in tumors such as lung cancer, glioblastoma
multiforme, hepatoma, ovarian and prostate cancer as well (17,19,24,27,28).
Consistent with these findings, we also observed a restoration of
Wwox in its absent leukemic cells led to a marked inhibition of
cell growth and colony formation, and apoptosis effects were
exhibited by the microscopic changes of nucleus in phase IIb of
apoptosis accompanied by DNA ladders appearing in
pGC-FU-WWOX-infected cells. Although there are opposing views
regarding the function of WWOX as a tumor suppressor gene (29), the role of ectopic expression of
WWOX in prohibiting proliferation and promoting apoptosis in
leukemia is examined in this study.
WWOX is closely related to apoptosis-associated
factors including Bcl-2, Bcl-xL and caspase in its antitumor
activities (10,16–19),
suggesting that WWOX may play a role in triggering
apoptosis-associated pathways. Our study demonstrated that Wwox
restoration could activate the mitochondrial pathway in Jurkat and
K562 cells, as our results displayed ectopic expression of WWOX
resulted in a promotion of cytochrome c release from the
mitochondria, even a downregulation of Bcl-2 and upregulation of
Bax (only in K562 cells). Furthermore, both procaspase-3 and -9
were cleaved as their spliceosomes were detected in
pGC-FU-WWOX-infected cells, although the 17 kDa spliced caspase-3
in Jurkat cells was difficult to detect, perhaps due to limitations
in our experiment.
Supporting evidence of a possible association
between WWOX and the mitochondrial pathway from Chang’s review
showed that overexpression of WWOX in L929 cells leads to an
upregulation of the proapoptotic p53, as well as a downregulation
of Bcl-2 and Bcl-xL (18). Fabbri
et al(10) reported that
restoration of WWOX in lung cancer cell lines enhances apoptosis by
activating the intrinsic apoptotic caspase cascade. Zhang et
al(17) recently transfected
A549 cells with pcDNA3.0-WWOX, and found that the ectopic
expression of WWOX not only caused apoptosis in A549 cells, but it
also triggered caspase cascade, as well as a release of cytochrome
c from the mitochondria in A549 cells, indicating that the
mitochondrial pathway is mainly involved in WWOX-mediated apoptosis
in A549 cells. Hu et al(24)
recently presented similar results. Our findings are consistent
with these data.
In summary, this study reveals a functional role of
WWOX in human hematopoietic malignancies as well as the molecular
mechanisms of its proapoptotic activities. However, the number of
cases examined in our study may not be sufficient, and we also did
not evaluate the functional role of WWOX in leukemia in
vivo. Clinical characterization of WWOX in hematopoietic
malignancies warrants further study. Further investigations are
also required to provide additional insights into the mechanism
underlying the molecular action of WWOX in leukemia.
Acknowledgements
This study was supported by the Provincial Natural
Science Fund of Fujian, grant no. 2010J01181.
References
|
1
|
Bednarek AK, Laflin KJ, Daniel RL, Liao Q,
Hawkins KA and Aldaz CM: WWOX, a novel WW domain-containing protein
mapping to human chromosome 16q23.3-24.1, a region frequently
affected in breast cancer. Cancer Res. 60:2140–2145.
2000.PubMed/NCBI
|
|
2
|
Ried K, Finnis M, Hobson L, et al: Common
chromosomal fragile site FRA16D sequence: identification of the FOR
gene spanning FRA16D and homozygous deletions and translocation
breakpoints in cancer cells. Hum Mol Genet. 9:1651–1663. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Del Mare S, Salah Z and Aqeilan RI: WWOX:
Its genomics, partners, and functions. J Cell Biochem. 108:737–745.
2009.PubMed/NCBI
|
|
4
|
Macias MJ, Wiesner S and Sudol M: WW and
SH3 domains, two different scaffolds to recognize proline-rich
ligands. FEBS Lett. 513:30–37. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X, Chao L, Jin G, Ma G, Zang Y and
Sun J: Association between CpG island methylation of the WWOX gene
and its expression in breast cancers. Tumour Biol. 30:8–14. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo W, Wang G, Dong Y, Guo Y, Kuang G and
Dong Z: Decreased expression of WWOX in the development of
esophageal squamous cell carcinoma. Mol Carcinog. Dec 27–2011.(Epub
ahead of print). View
Article : Google Scholar
|
|
7
|
Nakayama S, Semba S, Maeda N, Matsushita
M, Kuroda Y and Yokozaki H: Hypermethylation-mediated reduction of
WWOX expression in intraductal papillary mucinous neoplasms of the
pancreas. Br J Cancer. 100:1438–1443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dias EP, Pimenta FJ, Sarquis MS, et al:
Association between decreased WWOX protein expression and thyroid
cancer development. Thyroid. 17:1055–1059. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang J, Cogdell D, Yang D, et al: Deletion
of the WWOX gene and frequent loss of its protein expression in
human osteosarcoma. Cancer Lett. 291:31–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fabbri M, Iliopoulos D, Trapasso F, et al:
WWOX gene restoration prevents lung cancer growth in vitro and in
vivo. Proc Natl Acad Sci USA. 102:15611–15616. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iliopoulos D, Fabbri M, Druck T, Qin HR,
Han SY and Huebner K: Inhibition of breast cancer cell growth in
vitro and in vivo: effect of restoration of Wwox expression. Clin
Cancer Res. 13:268–274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aqeilan RI, Hagan JP, Aqeilan HA,
Pichiorri F, Fong Ly and Croce CM: Inactivation of the WWOX Gene
accelerates forestomach tumor progression in vivo. Cancer Res.
67:5606–5610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aqeilan RI, Trapasso F, Hussain S, et al:
Targeted deletion of Wwox reveals a tumor suppressor function. Proc
Natl Acad Sci USA. 104:3949–3954. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishii H, Vecchione A, Furukawa Y, et al:
Expression of FRA16D/WWOX and FRA3B/FHIT genes in hematopoietic
malignancies. Mol Cancer Res. 1:940–947. 2003.PubMed/NCBI
|
|
15
|
Ishii H and Furukawa Y: Alterations of
common chromosome fragile sites in hematopoietic malignancies. Int
J Hematol. 79:238–242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang JL and Zhang W: WWOX tumor suppressor
gene. Histol Histopathol. 23:877–882. 2008.
|
|
17
|
Zhang P, Jia R, Ying L, Liu B, Qian G, Fan
X and Ge S: WWOX-mediated apoptosis in A549 cells mainly involves
the mitochondrial pathway. Mol Med Rep. 6:121–124. 2012.PubMed/NCBI
|
|
18
|
Chang NS: A potential role of p53 and WOX1
in mitochondrial apoptosis (review). Int J Mol Med. 9:19–24.
2002.PubMed/NCBI
|
|
19
|
Kosla K, Pluciennik E, Kurzyk A,
Jesionek-Kupnicka D, Kordek R, Potemski P and Bednarek AK:
Molecular analysis of WWOX expression correlation with
proliferation and apoptosis in glioblastoma multiforme. J
Neurooncol. 101:207–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou YL, Xu YJ and Zhang ZX: A study on
expression changes of a tumor suppressor WWOX in human lung
adenocarcinoma cell line A549. China Oncology. 15:234–237.
2005.
|
|
21
|
Wang XF and He YL: Construction of a
lentiviral vector carrying human bcl-2 gene and its expression in
human ovarian granulosa cells. Nan Fang Yi Ke Da Xue Xue Bao.
28:1856–1859. 2008.(In Chinese).
|
|
22
|
Reagan-Shaw S, Nihal M, Ahsan H, Mukhtar H
and Ahmad N: Combination of vitamin E and selenium causes an
induction of apoptosis of human prostate cancer cells by enhancing
Bax/Bcl-2 ratio. Prostate. 68:1624–1634. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iliopoulos D, Guler G, Han SY, et al:
Fragile genes as biomarkers: epigenetic control of WWOX and FHIT in
lung, breast and bladder cancer. Oncogene. 24:1625–1633. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu BS, Tan JW, Zhu GH, Wang DF, Zhou X and
Sun ZQ: WWOX induces apoptosis and inhibits proliferation of human
hepatoma cell line SMMC-7721. World J Gastroenterol. 18:3020–3026.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bednarek AK, Keck-Waggoner CL, Daniel RL,
et al: WWOX, the FRA16D gene, behaves as a suppressor of tumor
growth. Cancer Res. 61:8068–8073. 2001.PubMed/NCBI
|
|
26
|
Gourley C, Paige AJ, Taylor KJ, et al:
WWOX gene expression abolishes ovarian cancer tumorigenicity in
vivo and decreases attachment to fibronectin via integrin alpha3.
Cancer Res. 69:4835–4842. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiong Z, Hu S and Wang Z: Cloning of WWOX
gene and its growth-inhibiting effects on ovarian cancer cells. J
Huazhong Univ Sci Technolog Med Sci. 30:365–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qin HR, Iliopoulos D, Semba S, et al: A
Role for the WWOX gene in prostate cancer. Cancer Res.
66:6477–6480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Watanabe A, Hippo Y, Taniguchi H, et al:
An opposing view on WWOX protein function as a tumor suppressor.
Cancer Res. 63:8629–8633. 2003.PubMed/NCBI
|