Introduction
Acute promyelocytic leukemia (APL) is characterized
by a balanced reciprocal translocation between chromosome 15 and
17, generating the PML/RARα fusion gene, which is thought to play a
central role in the initiation of leukemogenesis (1–3).
Conventional therapy comprising all-trans retinoic acid
(ATRA), alone or in combination with chemotherapy, has dramatically
improved the clinical outcome of this disease (4,5).
Nevertheless, ~30% of patients relapse and often become resistant
to conventional therapy (5). In
this regard, a new breakthrough arsenic-based regimen has been
established as a result of successful clinical outcomes showing
that 90% of relapsed patients have been remitted by treatment with
arsenic trioxide (ATO) (6–8). These results have led to the
exploration of potential treatment applications for other
malignancies, including solid tumors (9,10). In
order to understand the mode of actions of ATO and provide an
effective treatment protocol for individual APL patients, detailed
studies concerning the pharmacokinetics of ATO in APL patients have
been conducted (7,11). In fact, we clarified the
distribution of arsenic metabolites, not only in peripheral blood
and cerebrospinal fluid, but also in bone marrow from APL patients
who received consecutive administration of ATO (12–14).
These findings concerning the pharmacokinetics of ATO in APL
patients provide a new insight into the clinical applications of
ATO, and may contribute to better therapeutic protocols (15).
It is quite logical to consider that intracellular
arsenic accumulation (As[i]) is critical for the control of various
biological functions, and that its levels are tightly associated
with arsenic uptake and efflux (16–21).
Of note, aquaporin 9 (AQP9), a member of the aquaporin superfamily,
has been proposed to be responsible for arsenite transport
(16,18–21).
It was demonstrated that As[i] in the K562 chronic myeloid leukemia
cell line, transfected with AQP9, was significantly higher than the
level in untransfected K562 cells, resulting in increased
ATO-induced cytotoxicity (16,18).
Moreover, a retrospective study demonstrated that the expression
level of AQP9 was significantly higher in bone marrow aspirate
samples from APL patients compared to other subtypes of acute
myeloid leukemia (AML) (18). We
recently demonstrated that AQP9 and multidrug resistance-associated
protein 2 contributed to the differential sensitivity of primary
human-derived normal cells to arsenite (20) using a unique in vitro primary
cell culture system (22–24). These findings indicate that
sensitivity to ATO correlates with the expression levels of AQP9 in
leukemia cells as well as normal cells, suggesting a close
relationship between the cytocidal effect of ATO and the expression
levels of AQP9. However, direct evidence for this correlation in
primary APL cells from newly diagnosed and relapsed APL patients
who have never received ATO therapy has not yet been provided.
ATO exerts a dual effect on APL cells (15,25).
Under high concentrations ranging from 1 to 2 μM, ATO induces
apoptosis mainly through activation of a mitochondrial-mediated
intrinsic apoptotic pathway. While under low concentrations ranging
from 0.1 to 0.5 μM with a longer treatment period, ATO tends to
promote differentiation of APL cells. In order to evaluate the
clinical efficiency of ATO in APL patients, flow cytometric
analysis has been routinely performed to assess the expression
levels of cell surface markers related to differentiation status,
such as CD11b, CD15 and CD34 (12,26,27).
It is well known that CD13 and CD56 are positively associated with
the poor prognosis of APL patients treated with ATRA and/or
chemotherapy (28–32). However, whether these biological
markers are appropriate to predict the efficacy of ATO in APL
patients without a medication history of ATO still remains
unclear.
In the present study, we hypothesized that AQP9 is a
promising candidate biomarker with which to predict the efficacy of
ATO in APL patients prior to ATO-based treatment. Our results
provide initial direct evidence that the expression level of AQP9,
rather than other biomarkers, correlates closely with sensitivity
to ATO in both APL cell lines and primary blasts. Our findings
suggest that the AQP9 status of patients with APL may be predictive
of the success of ATO treatment.
Materials and methods
Materials
ATO was purchased from Sigma (St. Louis, MO, USA)
and dissolved in 1 M sodium hydroxide solution, diluted with
phosphate-buffered saline (PBS), sterilized by filtration (0.22
μm), and stored as a stock solution. The Apoptosis Detection Kit I,
including Annexin V-fluorescein isothiocyanate (FITC), propidium
iodide (PI), and 10X Annexin V binding buffer [0.1 M HEPES/NaOH (pH
7.4), 1.4 M NaCl, 25 mM CaCl2], was purchased from
Becton-Dickinson (San Jose, CA, USA). Rabbit anti-rat AQP9 antibody
(primary antibody) and FITC-labeled goat anti-rabbit IgG (secondary
antibody) were purchased from Alpha Diagnostic (San Antonio, TX,
USA) and Kirkegaard and Perry Laboratories (Gaithersburg, MD, USA),
respectively, and dissolved in PBS as a stock solution and stored
at 4°C until use.
Cell cultures and treatment
Two APL (NB4, HT93A), a myeloid leukemia (HL-60), a
chronic myeloid leukemia (K562), and a T-cell leukemia (Jurkat)
cell line were studied. The cells were cultured in RPMI-1640 medium
(Gibco-BRL, Rockville, MD, USA) supplemented with 10%
heat-inactivated fetal bovine serum (FBS) (Gibco-BRL) and
antibiotics [100 U/ml of penicillin and 100 μg/ml of streptomycin
(Gibco-BRL)] at 37°C in a humidified atmosphere (5% CO2
in air). Cells were cultured at a density of <1×106
cells/ml.
Bone marrow aspirates and peripheral blood were
obtained from 5 newly diagnosed APL patients and 1 relapsed APL
patient, who had never received ATO therapy. Prior to clinical
therapy, mononuclear cell fractions were isolated from freshly
collected bone marrow aspirates or peripheral blood using
Lymphoprep™ (Cosmo Bio Co., Ltd., Tokyo, Japan), and the cell
fractions were then cryopreserved until use. Prior to conducting
the experiment, aliquots of the APL cells were rapidly thawed at
37°C, washed with RPMI-1640, and resuspended at 1×106
cells/ml in RPMI-1640 supplemented with 10% FBS. Cell viability was
assessed by trypan blue exclusion assay to ensure that cells were
>80% viable before the experiment. A written informed consent
was obtained from the study patients before cell collection, and
the study was approved by the Internal Review Committee of Nihon
University Itabashi Hospital, Tokyo, Japan.
Determination of apoptosis
Induction of apoptosis was analyzed using the
Apoptosis Detection Kit I according to the manufacturer's
instructions. Briefly, cells were washed twice with cold PBS and
then resuspended in 1X Annexin V binding buffer. Cells were then
double stained with Annexin V-FITC and PI for 15 min at room
temperature in the dark, followed by analysis within 1 h. Apoptotic
cells were determined by flow cytometry (Cyto ACE-150) with a
minimum acquisition of 10,000 events for NB4 and HT93A cells, and
2,000–5,000 events for APL cells prepared from patients. The ratio
of the number of early apoptotic cells [Annexin V(+)PI(−)] plus the
late apoptotic cells [Annexin V(+)PI(+)] to the total cell number
was consider as the total apoptotic cell percentage. Cells were
considered viable if they lacked both Annexin V and PI
staining.
Analysis of intracellular As[i]
After exposure to 0.5 μM ATO for the indicated time
periods, cells were harvested and counted to provide accurate
viable cell numbers, which were used to normalize As[i]. After
washing three times with PBS, the cells were pelleted by
centrifugation and stored at −20°C until analysis. After transfer
to 15-ml polypropylene centrifuge tubes, cell pellets were mixed
with HNO3 (0.1 ml) at room temperature for 10 min, and
incubated at 80°C on a hot plate for 90 min. The samples were
diluted with Milli-Q water to 3 ml, followed by analysis using
inductively coupled plasma mass spectrometry (ICP-MS)
(ELAN® DRC-e; Perkin-Elmer SCIEX, Concord, ON, Canada)
for total arsenic determination, as described previously (14).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis for AQP9 mRNA expression
Total RNA and complementary DNA were prepared as
described previously (22). Total
RNA was extracted from cells using an RNA extraction kit (Isogen).
Complementary DNA was synthesized from 1 μg of RNA using 100 pmol
random hexamers and 100 units Moloney murine leukemia virus reverse
transcriptase (Invitrogen, Carlsbad, CA, USA) in a total volume of
20 μl, according to the manufacturer's instructions. PCR was
performed according to the method described previously (33), using a Takara Thermal Cycler MP
(Takara Shuzo, Osaka, Japan). DNA primers for RT-PCR were purchased
from Sigma-Aldrich (Tokyo, Japan): sense (5′-CTC AGT GTC ATC ATG
TAG TG-3′) and antisense primer (5′-GAC TAT CGT CAA GAT GCC G-3′)
for AQP9 mRNA; sense primer (5′-CCT TCC TGG GCA TGG AGT CCT G-3′)
and antisense primer (5′-GGA GCA ATG ATC TTG ATC TTC-3′) for
β-actin mRNA. PCR was carried out at the indicated cycles for AQP9
mRNA (30 sec at 94°C for denaturation, 30 sec at 60°C for annealing
and 40 sec at 72°C for extension); and 21 cycles for β-actin mRNA
(1 min at 94°C for denaturation, 1 min at 55°C for annealing and 1
min at 72°C for extension) using a Takara Thermal Cycler MP. PCR
products and Ready-Load™ 100-bp DNA ladder marker were
electrophoresed, respectively, on a 2.0% UltraPure™ agarose gel
(both from Invitrogen), and visualized by ethidium bromide
staining, followed by viewing under UV Light Printgraph (ATTO Corp,
Tokyo, Japan).
Expression profiles of AQP9 protein
expression
After washing with PBS containing 2.5% FBS and 0.5%
NaN3 (PBSF), APL cells from patients and non-stimulated
cultured leukemia cell lines were stained with rabbit anti-rat AQP9
antibody at a concentration of 10 μg/ml for 30 min at 4°C in the
dark. After washing three times with PBSF, the cells were further
stained with FITC-labeled goat anti-rabbit antibody, followed by
analysis using flow cytometry (Cyto ACE-150). The relative
expression level of AQP9 was shown as mean fluorescence intensity
(MFI) and was calculated by subtracting the mean fluorescence of
the unstained cells from that of the stained cells, as previously
described (34). The specificity of
FITC-labeled secondary antibody for AQP9 analysis was confirmed
based on no apparent observations of non-specific immunostaining by
omitting the primary antibody.
Flow cytometric immunophenotypic analysis
of APL cells from patients and NB4 and HT93A cell lines
A flow cytometric immunophenotypic analysis of the
expression levels of CD11b, CD13, CD15, CD34 and CD56 in APL cells
from patients and unstimulated cultured NB4 and HT93A cells was
routinely conducted at Bio Medical Laboratories (BML, Tokyo,
Japan).
Cytogenetic studies
Metaphase chromosomes were prepared and G-banded
using trypsin and Giemsa (GTG) on bone marrow cell after a 48-h
unstimulated culture. Twenty metaphases were counted and analyzed
routinely according to International System for Human Cytogenetic
Nomenclature (ISCN 2005) at our hospital.
Statistical analysis
Experiments were independently repeated three times,
and the results are shown as means ± standard deviation (SD) of
three assays. A two-tailed, paired Student's t-test was applied,
and a P-value <0.05 was considered to indicate a statistically
significant result. The correlation in two factors was evaluated
with Pearson product-moment correlation coefficient.
Results
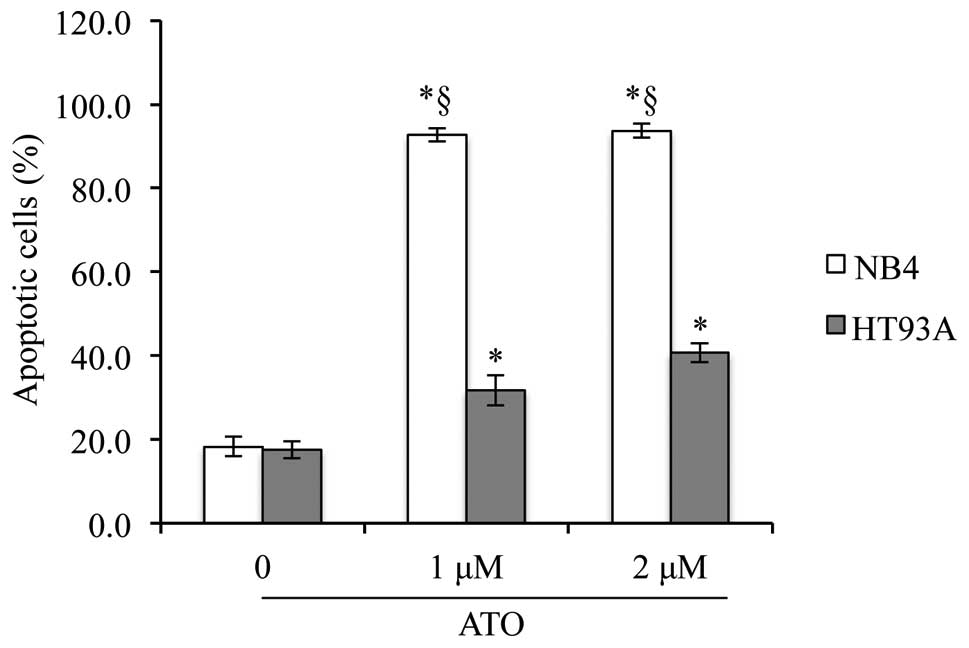
Induction of apoptosis in the NB4 and
HT93A cells by ATO
After treatment with ATO (1 or 2 μM) for 48 h, a
significant induction in apoptosis was observed in both NB4 and
HT93A cells (Fig. 1). Furthermore,
after treatment with 1 μM ATO, a much higher percentage (~90%) of
apoptotic cells was observed in the NB4 cells when compared with
the apoptotic rate of ~30% cells in the HT93A cells (Fig. 1). These results indicate that NB4
cells are more sensitive to the apoptotic activity of ATO as
compared to HT93A cells.
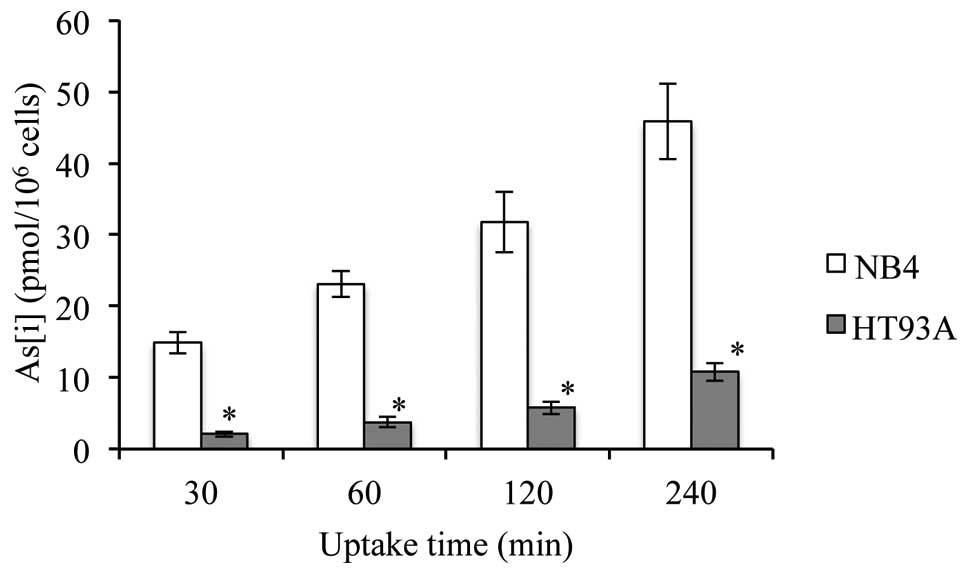
As[i] in NB4 and HT93A cells
After exposure to 0.5 μM ATO for 30, 60, 120 and 240
min, As[i] was measured by ICP-MS. As shown in Fig. 2, the levels of As[i] increased with
time in both cell lines. Furthermore, the levels of As[i] were ~4–7
times higher in the NB4 cells when compared to levels in the HT93A
cells. Furthermore, the levels of As[i] in both untreated cell
lines were less than the detection threshold (data not shown).
Expression profiles of AQP9 mRNA in NB4
and HT93A cells
The expression profile of AQP9 mRNA was analyzed by
RT-PCR. PCR was carried out for 21, 24, 27, 30, 33, 36 and 39
cycles, and the respective PCR products were harvested and
subjected to agarose gel electrophoresis analysis. In NB4 cells,
the expression of AQP9 mRNA was observed at a low but measurable
level at cycle 33, and increased quickly and linearly with the
amplification (Fig. 3). In
contrast, only a measurable level of AQP9 was observed in the HT93A
cells at cycle 39, indicating much higher expression levels of AQP9
mRNA in NB4 cells when compared with levels in the HT93A cells
(Fig. 3).
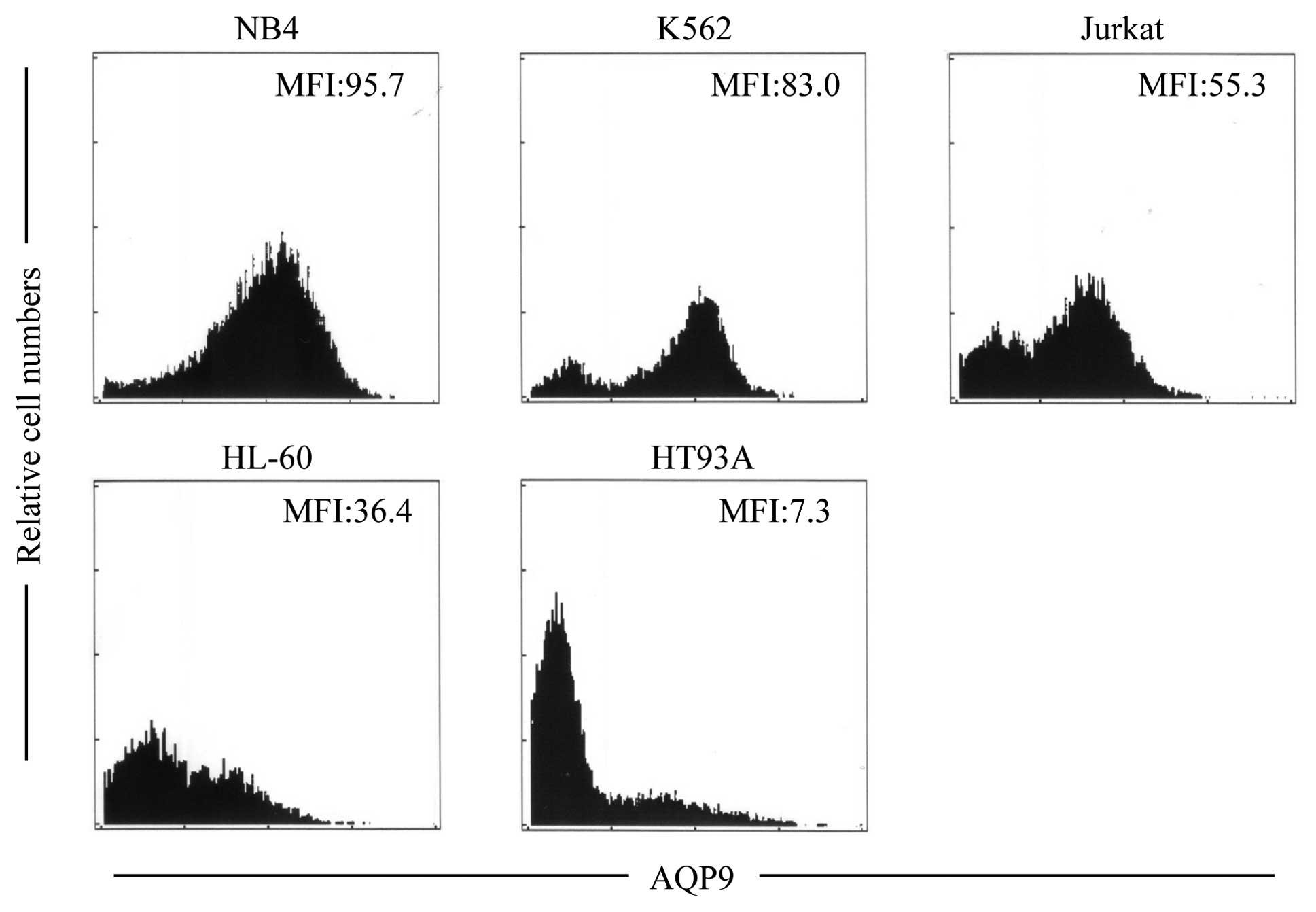
Expression profiles of AQP9 protein in
NB4 and HT93A and other leukemia cell lines
The expression levels of AQP9 protein in NB4 and
HT93A cells were assessed using flow cytometry. Consistent with the
expression level of AQP9 mRNA (Fig.
3), a much higher expression level of its protein was also
observed in the NB4 cells when compared to the level in the HT93A
cells (Fig. 4). Furthermore, the
expression level of AQP9 was investigated in other leukemia cell
lines, such as K562, Jurkat and HL-60. The rank order for the
expression levels of AQP9 protein was NB4 > K562 > Jurkat
> HL-60 > HT93A (Fig. 4), a
result which is similar to previous experimental results determined
by western blotting (18).
Furthermore, the expression levels of AQP9 in primary APL cells
from patients were evaluated as described above and are summarized
in Table I.
 | Table ICharacteristics of the NB4 and HT93A
cells and APL patient-derived cells. |
Table I
Characteristics of the NB4 and HT93A
cells and APL patient-derived cells.
| Samples | AQP9 (MFI) | CD11b (%) | CD13 (%) | CD15 (%) | CD34 (%) | CD56 (%) | Additive
chromosome |
|---|
| NB4 | 95.7 | 48.2 | 97.8 | 61.7 | 0.5 | 8.2 | Complex |
| HT93A | 7.3 | 1.2 | 6.4 | 24.3 | 88.1 | 99.5 |
t(1;21)(q25;p13) |
| Patient 1 | 54.8 | 0.5 | 12.7 | 65.2 | 0.1 | 0.1 | Add(11)q(25) |
| Patient 2 | 48.6 | 0.5 | 42.8 | 52.7 | 0.1 | 0.6 | (−) |
| Patient 3 | 39.5 | 0.3 | 43.1 | 19.9 | 0.1 | 1.8 | (−) |
| Patient 4 | 36.9 | 1.9 | 90.0 | 13.0 | 0.3 | 0.1 | (−) |
| Patient 5 | 33.1 | 1.6 | 97.5 | 78.8 | 8.2 | 86.2 | −10, −12 |
| Patient 6 | 94.3 | 23.7 | 97.5 | 19.1 | 0.1 | 27.8 | (−) |
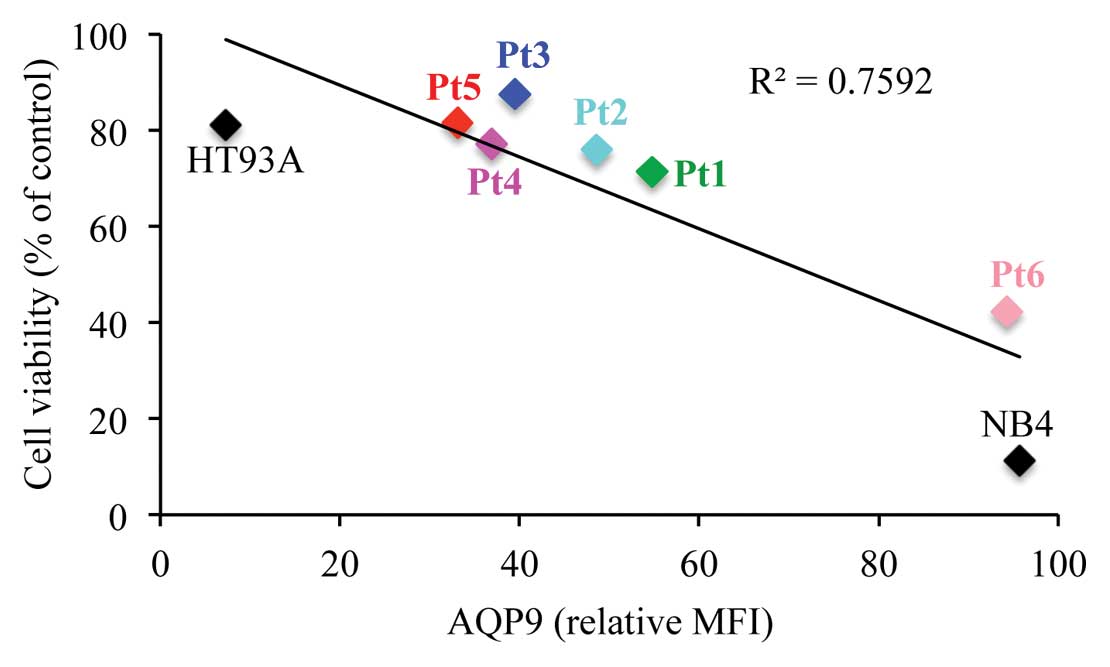
Correlation of AQP9 expression with ATO
sensitivity
The expression levels of AQP9 protein in NB4, HT93A
and primary APL cells from patients (Pt) showed the following
trend: NB4 > Pt6 > Pt1 > Pt2 > Pt3 > Pt4 > Pt5
> HT93A (Table I). Furthermore,
the cytocidal effects of ATO on these cells were evaluated after
treatment with 1 μM ATO for 48 h. As expected, the expression
levels of AQP9 were found to be positively correlated with
ATO-induced cytotoxicity not only in the APL cell lines but also in
the primary APL cells from patients (P=0.0049) (Fig. 5).
Immunophenotypic analysis of NB4 and
HT93A cells and APL patient-derived cells
As summarized in Table
I, NB4 cells demonstrated CD11b+CD15+,
whereas HT93A cells showed CD11b− and the number of
CD15+ cells was as low as 24.3%. Moreover, HT93A cells
exhibited an expression level of CD34 as high as 88.1%; however,
NB4 cells were CD34−, indicating the high
differentiation degree of NB4 cells when compared to HT93A. A
diverse range of expression profiles of CD11b, CD13, CD15, CD34 and
CD56 was observed in the primary blasts from individual patients;
however, unlike the strong correlations between AQP9 expression
levels and ATO sensitivity, no correlation was observed between the
expression profiles of these cell surface markers and ATO
sensitivity. Apart from t(15;17), other additional chromosomal
changes were also identified in individual patients, as shown in
Table I. Again, no correlation was
observed between chromosomal alterations and ATO sensitivity
associated with AQP9 expression level.
Discussion
The present study clarified that relatively high
concentrations of ATO (1 to 2 μM) triggered the induction of
apoptosis in APL cell lines, NB4 and HT93A, as previously
demonstrated by other researchers (26,27,35,36).
Furthermore, a substantially higher sensitivity to the apoptotic
activity of ATO, along with more As[i], was observed in NB4 cells
when compared to these values in HT93A cells. These results suggest
that the sensitivity to ATO correlates closely with As[i].
Substantially high expression levels of AQP9 have
been observed in NB4 cells, which results in high sensitivity to
ATO (18). In agreement with this
previous finding, we found markedly higher expression levels of
AQP9 in NB4 cells when compared to levels in HT93A cells. It is
noteworthy that in the present study, flow cytometry was used to
investigate the expression profiles of AQP9 in leukemia cells,
whereas PCR and/or western blotting have been used in the past. In
fact, we recently applied this technique successfully to
demonstrate time- and dose-dependent increases in the expression of
AQP9 induced by ATRA in HT93A cells (37), similar to the phenomena observed in
NB4 and HL-60 cells (18,21). Moreover, flow cytometric analysis
showed that the expression levels of AQP9 protein in all leukemia
cells revealed the following trend: NB4 > K562 > Jurkat >
HL-60 > HT93A, the results of which are in good agreement with
previous experimental results determined by western blotting
(18). Therefore, flow cytometry is
a convenient and valuable tool for analyzing the AQP9 status of
patients with APL, and the results of this analysis can be used to
predict the response of patients with cancer to ATO.
Although a close relationship between the cytocidal
effect of ATO and the expression level of AQP9 has been suggested
(15,18), there is no direct evidence to date
concerning a correlation between the AQP9 expression level and the
sensitivity to ATO based on research using primary APL cells. In
this regard, our findings revealed a strong positive correlation
between the AQP9 expression level and ATO sensitivity in primary
APL cells from 6 patients, as well as in NB4 and HT93A cells. To
the best of our knowledge, this is the first report to correlate
the sensitivity to ATO with the AQP9 status of APL patients who
have never received ATO therapy.
Furthermore, our results clearly demonstrated a
relatively high degree of differentiation in the NB4 cells when
compared to HT93A cells. Based on the results and the differences
in the expression level of AQP9 and the sensitivity to ATO between
the two cell lines, we suggest that there is a good correlation
between the degree of differentiation associated with the
expression level of AQP9 and ATO sensitivity. In other words, the
cells with a relatively high degree of differentiation are more
likely to express AQP9, which ultimately affects their sensitivity
to ATO. However, a similar correlation was not observed in primary
APL cells from patients. In addition, the expression levels of CD13
and CD56, known as prognostic factors for APL patient treated with
ATRA and/or chemotherapy (28–32),
did not appear to be correlated with ATO sensitivity. Similarly, it
appears that there is no correlation between chromosomal
alterations and ATO sensitivity in all tested cells. Taken
together, AQP9 plays a vital role in the differential sensitivity
to ATO of APL cell lines and primary blasts. Therefore, monitoring
both the expression level of AQP9 and the distribution of arsenic
metabolites should be essential for better clinical outcome.
In conclusion, AQP9 is a vital clinical parameter
with which to predict clinical achievement in APL patients prior to
ATO-based treatment, although a larger scale study must be launched
in order to draw a solid conclusion. Indeed, our hypothesis was
strongly supported by a previous study (18), showing that APL patients expressed
significantly higher AQP9 levels than patients with all other
subtypes of AML among 80 cases of leukemia, yet the authors did not
test the sensitivity of these primary APL cells to ATO.
Furthermore, we recently demonstrated the contribution of AQP9 and
multidrug resistance-associated protein 2 to the differential
sensitivity of primary human-derived normal cells to arsenite
(20). Given that these arsenic
transporters play pivotal roles in various arsenite-mediated
biological effects on normal and cancer cells, monitoring their
expression levels has important implications for predicting not
only clinical efficacy but also the risks of side effects in
patients with cancer prior to ATO therapy.
Acknowledgements
This study was supported in part by grants from the
Japan China Medical Association to B.Y. This study was also
supported in part by grants from the Ministry of Education,
Culture, Sports, Science and Technology and by the Promotion and
Mutual Aid Corporation for Private Schools of Japan. The authors
also thank Ms. Eiko Ishizuka for her technical assistance.
References
|
1
|
de Thé H, Chomienne C, Lanotte M, Degos L
and Dejean A: The t(15;17) translocation of acute promyelocytic
leukaemia fuses the retinoic acid receptor alpha gene to a novel
transcribed locus. Nature. 347:558–561. 1990.PubMed/NCBI
|
|
2
|
Goddard AD, Borrow J, Freemont PS and
Solomon E: Characterization of a zinc finger gene disrupted by the
t(15;17) in acute promyelocytic leukemia. Science. 254:1371–1374.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tong JH, Dong S, Geng JP, Huang W, Wang
ZY, Sun GL, Chen SJ, Chen Z, Larsen CJ and Berger R: Molecular
rearrangements of the MYL gene in acute promyelocytic leukemia
(APL, M3) define a breakpoint cluster region as well as some
molecular variants. Oncogene. 7:311–316. 1992.PubMed/NCBI
|
|
4
|
Burnett AK, Grimwade D, Solomon E,
Wheatley K and Goldstone AH: Presenting white blood cell count and
kinetics of molecular remission predict prognosis in acute
promyelocytic leukemia treated with all-trans retinoic acid:
result of the Randomized MRC Trial. Blood. 93:4131–4143.
1999.PubMed/NCBI
|
|
5
|
Melnick A and Licht JD: Deconstructing a
disease: RARalpha, its fusion partners, and their roles in the
pathogenesis of acute promyelocytic leukemia. Blood. 93:3167–3215.
1999.PubMed/NCBI
|
|
6
|
Cohen MH, Hirschfeld S, Flamm Honig S,
Ibrahim A, Johnson JR, O'Leary JJ, White RM, Williams GA and Pazdur
R: Drug approval summaries: arsenic trioxide, tamoxifen citrate,
anastrazole, paclitaxel, bexarotene. Oncologist. 6:4–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM,
Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, Chen Y, Zhou L, Fang ZW,
Wang YT, Ma J, Zhang P, Zhang TD, Chen SJ, Chen Z and Wang ZY: Use
of arsenic trioxide (As2O3) in the treatment
of acute promyelocytic leukemia (APL): II. Clinical efficacy and
pharmacokinetics in relapsed patients. Blood. 89:3354–3360.
1997.PubMed/NCBI
|
|
8
|
Soignet SL, Maslak P, Wang ZG, Jhanwar S,
Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J,
Scheinberg DA, Pandolfi PP and Warrell RP Jr: Complete remission
after treatment of acute promyelocytic leukemia with arsenic
trioxide. N Engl J Med. 339:1341–1348. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dilda PJ and Hogg PJ: Arsenical-based
cancer drugs. Cancer Treat Rev. 33:542–564. 2007. View Article : Google Scholar
|
|
10
|
Litzow MR: Arsenic trioxide. Expert Opin
Pharmacother. 9:1773–1785. 2008. View Article : Google Scholar
|
|
11
|
Fujisawa S, Ohno R, Shigeno K, Sahara N,
Nakamura S, Naito K, Kobayashi M, Shinjo K, Takeshita A, Suzuki Y,
Hashimoto H, Kinoshita K, Shimoya M, Kaise T and Ohnishi K:
Pharmacokinetics of arsenic species in Japanese patients with
relapsed or refractory acute promyelocytic leukemia treated with
arsenic trioxide. Cancer Chemother Pharmacol. 59:485–493. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iriyama N, Yoshino Y, Yuan B, Horikoshi A,
Hirabayashi Y, Hatta Y, Toyoda H and Takeuchi J: Speciation of
arsenic trioxide metabolites in peripheral blood and bone marrow
from an acute promyelocytic leukemia patient. J Hematol Oncol.
5:1–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kiguchi T, Yoshino Y, Yuan B, Yoshizawa S,
Kitahara T, Akahane D, Gotoh M, Kaise T, Toyoda H and Ohyashiki K:
Speciation of arsenic trioxide penetrates into cerebrospinal fluid
in patients with acute promyelocytic leukemia. Leuk Res.
34:403–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshino Y, Yuan B, Miyashita SI, Iriyama
N, Horikoshi A, Shikino O, Toyoda H and Kaise T: Speciation of
arsenic trioxide metabolites in blood cells and plasma of a patient
with acute promyelocytic leukemia. Anal Bioanal Chem. 393:689–697.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan B, Yoshino Y, Kaise T and Toyoda H:
Application of arsenic trioxide therapy for patients with
leukaemia. Biological Chemistry of As, Sb and Bi. Sun HZ: John
Wiley and Sons, Ltd; New York: pp. 263–292. 2011
|
|
16
|
Bhattacharjee H, Carbrey J, Rosen BP and
Mukhopadhyay R: Drug uptake and pharmacological modulation of drug
sensitivity in leukemia by AQP9. Biochem Biophys Res Commun.
322:836–841. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee TC, Ho IC, Lu WJ and Huang JD:
Enhanced expression of multidrug resistance-associated protein 2
and reduced expression of aquaglyceroporin 3 in an
arsenic-resistant human cell line. J Biol Chem. 281:18401–18407.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leung J, Pang A, Yuen WH, Kwong YL and Tse
EW: Relationship of expression of aquaglyceroporin 9 with arsenic
uptake and sensitivity in leukemia cells. Blood. 109:740–746. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Z, Shen J, Carbrey JM, Mukhopadhyay R,
Agre P and Rosen BP: Arsenite transport by mammalian
aquaglyceroporins AQP7 and AQP9. Proc Natl Acad Sci USA.
99:6053–6058. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshino Y, Yuan B, Kaise T, Takeichi M,
Tanaka S, Hirano T, Kroetz DL and Toyoda H: Contribution of
aquaporin 9 and multidrug resistance-associated protein 2 to
differential sensitivity to arsenite between primary cultured
chorion and amnion cells prepared from human fetal membranes.
Toxicol Appl Pharmacol. 257:198–208. 2011. View Article : Google Scholar
|
|
21
|
Hu J, Liu YF, Wu CF, Xu F, Shen ZX, Zhu
YM, Li JM, Tang W, Zhao WL, Wu W, Sun HP, Chen QS, Chen B, Zhou GB,
Zelent A, Waxman S, Wang ZY, Chen SJ and Chen Z: Long-term efficacy
and safety of all-trans retinoic acid/arsenic trioxide-based
therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl
Acad Sci USA. 106:3342–3347. 2009.PubMed/NCBI
|
|
22
|
Yuan B, Ohyama K, Bessho T and Toyoda H:
Contribution of inducible nitric oxide synthase and
cyclooxygenase-2 to apoptosis induction in smooth chorion
trophoblast cells of human fetal membrane tissues. Biochem Biophys
Res Commun. 341:822–827. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan B, Ohyama K, Bessho T, Uchide N and
Toyoda H: Imbalance between ROS production and elimination results
in apoptosis induction in primary smooth chorion trophoblast cells
prepared from human fetal membrane tissues. Life Sci. 82:623–630.
2008. View Article : Google Scholar
|
|
24
|
Yuan B, Ohyama K, Takeichi M and Toyoda H:
Direct contribution of inducible nitric oxide synthase expression
to apoptosis induction in primary smooth chorion trophoblast cells
of human fetal membrane tissues. Int J Biochem Cell Biol.
41:1062–1069. 2009. View Article : Google Scholar
|
|
25
|
Wang ZY and Chen Z: Acute promyelocytic
leukemia: from highly fatal to highly curable. Blood.
111:2505–2515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J,
Cai X, Han ZG, Ni JH, Shi GY, Jia PM, Liu MM, He KL, Niu C, Ma J,
Zhang P, Zhang TD, Paul P, Naoe T, Kitamura K, Miller W, Waxman S,
Wang ZY, de The H, Chen SJ and Chen Z: Use of arsenic trioxide
(As2O3) in the treatment of acute
promyelocytic leukemia (APL): I. As2O3 exerts
dose-dependent dual effects on APL cells. Blood. 89:3345–3353.
1997.PubMed/NCBI
|
|
27
|
Zhang TD, Chen GQ, Wang ZG, Wang ZY, Chen
SJ and Chen Z: Arsenic trioxide, a therapeutic agent for APL.
Oncogene. 20:7146–7153. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Di Bona E, Sartori R, Zambello R, Guercini
N, Madeo D and Rodeghiero F: Prognostic significance of CD56
antigen expression in acute myeloid leukemia. Haematologica.
87:250–256. 2002.
|
|
29
|
Ferrara F, Morabito F, Martino B, Specchia
G, Liso V, Nobile F, Boccuni P, Di Noto R, Pane F, Annunziata M,
Schiavone EM, De Simone M, Guglielmi C, Del Vecchio L and Lo Coco
F: CD56 expression is an indicator of poor clinical outcome in
patients with acute promyelocytic leukemia treated with
simultaneous all-trans-retinoic acid and chemotherapy. J
Clin Oncol. 18:1295–1300. 2000.PubMed/NCBI
|
|
30
|
Montesinos P, Rayon C, Vellenga E, Brunet
S, Gonzalez J, Gonzalez M, Holowiecka A, Esteve J, Bergua J,
Gonzalez JD, Rivas C, Tormo M, Rubio V, Bueno J, Manso F, Milone G,
de la Serna J, Perez I, Perez-Encinas M, Krsnik I, Ribera JM,
Escoda L, Lowenberg B and Sanz MA: Clinical significance of CD56
expression in patients with acute promyelocytic leukemia treated
with all-trans retinoic acid and anthracycline-based regimens.
Blood. 117:1799–1805. 2011. View Article : Google Scholar
|
|
31
|
Schwarzinger I, Valent P, Koller U, Marosi
C, Schneider B, Haas O, Knapp W, Lechner K and Bettelheim P:
Prognostic significance of surface marker expression on blasts of
patients with de novo acute myeloblastic leukemia. J Clin Oncol.
8:423–430. 1990.PubMed/NCBI
|
|
32
|
Vahdat L, Maslak P, Miller WH Jr, Eardley
A, Heller G, Scheinberg DA and Warrell RP Jr: Early mortality and
the retinoic acid syndrome in acute promyelocytic leukemia: impact
of leukocytosis, low-dose chemotherapy, PMN/RAR-alpha isoform, and
CD13 expression in patients treated with all-trans retinoic acid.
Blood. 84:3843–3849. 1994.PubMed/NCBI
|
|
33
|
Andus T, Targan SR, Deem R and Toyoda H:
Measurement of tumor necrosis factor alpha mRNA in small numbers of
cells by quantitative polymerase chain reaction. Reg Immunol.
5:11–17. 1993.PubMed/NCBI
|
|
34
|
Ruan XZ, Varghese Z, Powis SH and Moorhead
JF: Human mesangial cells express inducible macrophage scavenger
receptor. Kidney Int. 56:440–451. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cai X, Shen YL, Zhu Q, Jia PM, Yu Y, Zhou
L, Huang Y, Zhang JW, Xiong SM, Chen SJ, Wang ZY, Chen Z and Chen
GQ: Arsenic trioxide-induced apoptosis and differentiation are
associated respectively with mitochondrial transmembrane potential
collapse and retinoic acid signaling pathways in acute
promyelocytic leukemia. Leukemia. 14:262–270. 2000. View Article : Google Scholar
|
|
36
|
Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ,
Si GY, Jin XL, Tang W, Li XS, Xong SM, Shen ZX, Sun GL, Ma J, Zhang
P, Zhang TD, Gazin C, Naoe T, Chen SJ, Wang ZY and Chen Z: In vitro
studies on cellular and molecular mechanisms of arsenic trioxide
(As2O3) in the treatment of acute
promyelocytic leukemia: As2O3 induces NB4
cell apoptosis with downregulation of Bcl-2 expression and
modulation of PML-RAR alpha/PML proteins. Blood. 88:1052–1061.
1996.PubMed/NCBI
|
|
37
|
Iriyama N, Yuan B, Hatta Y, Horikoshi A,
Yoshino Y, Toyoda H, Aizawa S and Takeuchi J: Granulocyte
colony-stimulating factor potentiates differentiation induction by
all-trans retinoic acid and arsenic trioxide and enhances
arsenic uptake in the acute promyelocytic leukemia cell line HT93A.
Oncol Rep. 28:1875–1882. 2012.PubMed/NCBI
|