Introduction
Endometrial cancer is the most common malignancy of
the female genital system, and in 2011 it led to an estimated 8,120
deaths in the USA (1). The majority
of endometrial cancers (72%) are detected at an early stage (stage
I-II); however, 28% of patients have regional or distant metastasis
at diagnosis (20% in stage III and 8% in stage IV) (2). Endometrial endometrioid carcinoma
(EEC) accounts for 80–90% of the cases of endometrial cancers.
Although EEC has a relatively low mortality rate, some tumors are
aggressive and insensitive to surgery, chemotherapy or radiation
therapy. For patients with localized disease, hysterectomy and
bilateral salpingo-oophorectomy remain the primary and most
effective treatment. However, the etiology of endometrial cancer
remains unclear. Therefore, there is an urgent need for new
therapeutic targets and strategies, both of which may be obtained
through an increased understanding of the molecular mechanisms
involved in endometrial tumorigenesis.
microRNAs (miRNAs) are a group of non-coding nucleic
acids which regulate gene expression by facilitating the
degradation or translational inhibition of their target mRNAs.
miRNAs are reported to play important roles in cancer by regulating
basic cellular functions including proliferation, differentiation
and cell death (3,4). Recently, it has been revealed that the
expression of miR-205 is altered in human EEC (5,6);
however, the function of miR-205 in EEC remains unclear.
In this study, we investigated the potential roles
of miR-205 in EEC. Moreover, we identified estrogen-related
receptor-γ (ESRRG) to be a novel target of miR-205.
Materials and methods
Tissue collection
Fifty-three samples of endometrial endometrioid
carcinoma were obtained from patients who underwent surgical
therapy at the International Peace Maternity and Child Health
Hospital of the China Welfare Institute from February 2008 to March
2011. The stages and histological grades of these tumors were
established according to the FIGO criteria (2009)(7). The characteristics of the patients are
shown in Table I. Twenty-two normal
endometrial samples were obtained from patients who underwent
hysterectomy to treat other diseases including uterine myoma and
adenomyosis. The research project was approved by the local ethics
committee, and informed consent for the experimental use of the
surgical samples was obtained from all patients.
 | Table ICorrelation between miR-205 expression
and clinicopathological variables of the endometrial endometrioid
carcinoma cases. |
Table I
Correlation between miR-205 expression
and clinicopathological variables of the endometrial endometrioid
carcinoma cases.
| No. of patients
(%) | miR-205
expression | |
|---|
|
|
| |
|---|
| Clinicopathological
data | n | % | No. of low (%) | No. of high (%) | P-valuea |
|---|
| FIGO stage |
| Stage I | 41 | 77.4 | 23 | 18 | 0.496 |
| Stages II and
III | 12 | 22.6 | 8 | 4 | |
| Grade |
| G1 | 25 | 66.0 | 14 | 6 | 0.246 |
| G2/3 | 28 | 34.0 | 15 | 14 | |
| Myometrial
invasion |
| <1/2 | 40 | 75.5 | 22 | 18 | 0.277 |
| ≥1/2 | 13 | 24.5 | 7 | 2 | |
| Nodal metastasis |
| Negative | 49 | 92.5 | 29 | 20 | 0.162 |
| Positive | 4 | 7.5 | 2 | 2 | |
| Estrogen receptor
status |
| Negative | 9 | 17.0 | 5 | 4 | 0.358 |
| Positive | 44 | 83.0 | 26 | 18 | |
RNA isolation and quantitative reverse
transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA). The expression
of miR-205 was quantified by qRT-PCR using TaqMan microRNA assays
(Applied Biosystems, Carlsbad, CA, USA) and normalized to U6B. The
expression of ESRRG was quantified by qRT-PCR using SYBR-Green
assays (Applied Biosystems) and normalized to β-actin. The primers
used for qRT-PCR are listed in Table
II. Gene expression was calculated using the 2−ΔCt
method (8).
 | Table IIPrimers used in the study. |
Table II
Primers used in the study.
| Identifier | Sense primer
sequences | Antisense primer
sequences |
|---|
| miR-205
inhibitor |
5′-CAGACUCCGGUGGAAUGAAGGA-3′ (a) | |
| miR-205 inhibitor
(negative control) |
5′-CAGUACUUUUGUGUAGUACAA-3′ (b) | |
| miR-205 mimics |
5′-UCCUUCAUUCCACCGGAGUCUG-3′ |
5′-GACUCCGGUGGAAUGAAGAAUU-3′ |
| miR-205 mimics
(negative control) |
5′-UUCUCCGAACGUGUCACGUTT-3′ (b) |
5′-ACGUGACACGUUCGGAGAATT-3′ |
| ESRRG |
5′-CTACCCTTCTGCTCCTATCCTG-3′ |
5′-AGCGATGTCACCACACACTAAA-3′ |
| β-actin |
5′-CAGCCATGTACGTTGCTATCCAGG-3′ |
5′-AGGTCCAGACGCAGGATGGCATG-3′ |
| ESRRG 3′UTR |
5′-CGGactagtGATGGAAGCCAGCCCTGCCA-3′ |
5′-AGGgtttaaacGGCTCACAGCTCCTTCTGAGGC-3′ |
Cell culture and transfection
The human endometrial carcinoma cell lines Ishikawa,
KLE and AN3CA, and human embryonic kidney 293T cells were obtained
from the American Type Culture Collection (ATCC, Manassas, VA,
USA). All cells were maintained in Dulbecco’s modified Eagle’s
medium (DMEM)/F12 media (Gibco, Auckland, NZ, USA) supplemented
with 10% fetal bovine serum (Biowest, Nuaillé, France) at 37°C in
5% CO2.
The miR-205 mimics, miR-205 inhibitors and negative
control molecules were synthesized by GenePharma Co., Ltd.
(Shanghai, China), added to culture media at a final concentration
of 100 nM and transfected into cells using Lipofectamine™ 2000
(Invitrogen Life Technologies) according to the manufacturer’s
instructions.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Ishikawa (or AN3CA) cells were seeded in 96-well
plates (4×103 cells/well) and transfected with miR-205
inhibitor (or miR-205 mimics) duplexes using Lipofectamine™ 2000.
After overnight incubation, the media were removed and cell
proliferation was evaluated using the MTT assay as previously
described (9).
Wound healing assay
Ishikawa (or AN3CA) cells were seeded in 6-well
plates (4×105 cells/well) the day before transfection,
and then transfected with miR-205 inhibitor (or miR-205 mimics)
duplexes using Lipofectamine™ 2000. The wound healing assay was
performed 24 h post-transfection as previously described (10).
Transwell migration assay
Ishikawa (or AN3CA cells) (5×104/well)
were placed in serum-free medium in the top chamber of Transwell
migration chambers (Corning, Inc., Corning, NY, USA). After a 24 h
incubation at 37°C, the cells adhering to the lower membrane were
fixed in 4% paraformaldehyde at room temperature for 20 min and
then stained with crystal violet staining solution (Beyotime
Institute Biotech, Shanghai, China) overnight. The stained cells
were visualized under a microscope and counted.
Western blotting
Proteins were separated on 10% SDS-PAGE gels,
transferred to PVDF membranes, blocked with 5% non-fat milk and
incubated with the mouse anti-human ESRRG monoclonal antibody
(R&D Systems, Minneapolis, MN, USA) or mouse anti-β-actin
monoclonal antibody (Proteintech Group, Chicago, IL, USA).
Immunoreactivity was visualized using SuperSignal West Pico
Chemiluminescent Substrate (Thermo Scientific, Rockford, IL, USA)
and Kodak XAR-5 film (Sigma-Aldrich, St. Louis, MO, USA).
Construction of reporter plasmids and
luciferase assays
The partial 3′ untranslated region (3′UTR) of human
ESRRG mRNA was cloned between the SpeI and PmeI sites
of the pMIR-REPORT™ vector (Applied Biosystems). The length cloned
was 869 bp. A 60-bp mutant ESRRG 3′UTR was also cloned into the
pMIR-REPORT™ vector, which was mutated by the principle of
complementary base pairing to disrupt the miR-205 binding
sites.
The day prior to transfection, 293T cells were
seeded in 96-well plates (4×103 cells/well) to achieve
~70% confluency. The cells were co-transfected with pMIR-REPORT™
constructs containing the wild-type or mutated ESRRG 3′UTR (50
ng/well), pRL-SV40 Renilla luciferase (5 ng/well) and
miR-205 mimics (38 nM/well). The luciferase assay was performed 48
h later as previously described (11).
Statistical analysis
All values are presented as means ± SE where
appropriate. Categorical data were analyzed using the χ2
test. Quantitative values were evaluated by t-tests or one-way
ANOVA using SPSS 16.0 (SPSS, Chicago, IL, USA); P<0.05 was
considered to indicate a statistically significant result.
Results
MiR-205 is overexpressed in EEC
tissues
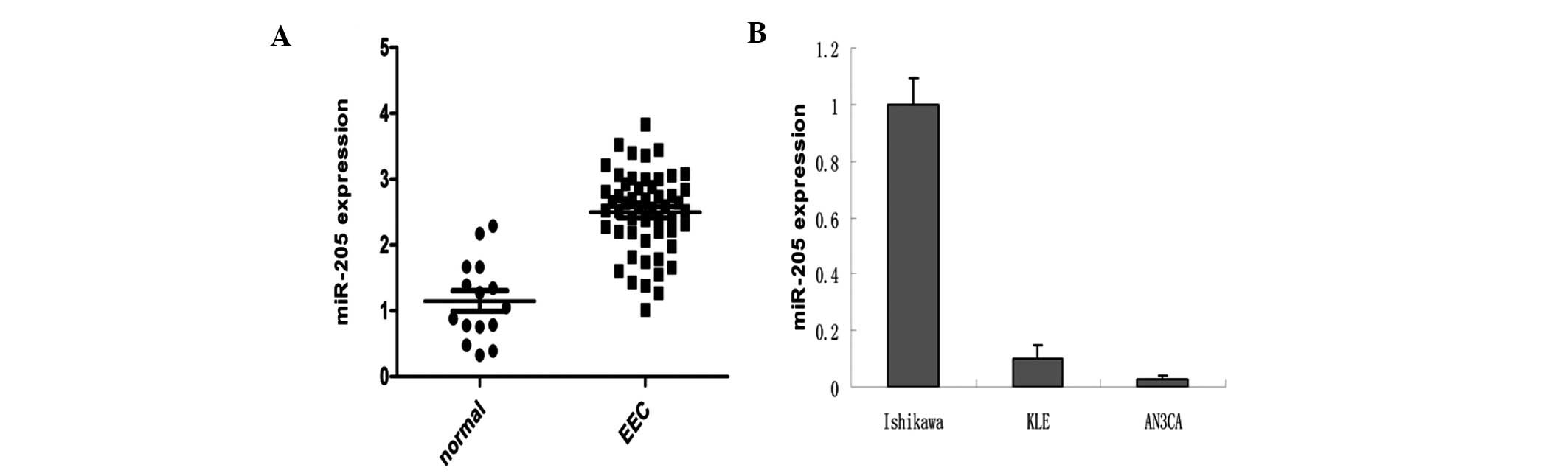
We found that miR-205 was significantly
overexpressed in EEC samples when compared with that in the normal
control (χ2=7.146, P=0.008) (Fig. 1). miR-205 was also expressed at high
levels in Ishikawa, moderate levels in KLE, and very low levels in
AN3CA cells (Fig. 1B).
We analyzed the relationship between miR-205 and
FIGO stage, histological grade, myometrial invasion, nodal
metastasis and estrogen receptor (ER) status in the EEC cases.
However, no association was observed between the expression of
miR-205 and these clinicopathological features of the EEC cases
(Table I).
Inhibition of miR-205 suppresses cellular
proliferation, migration and invasion in Ishikawa cells
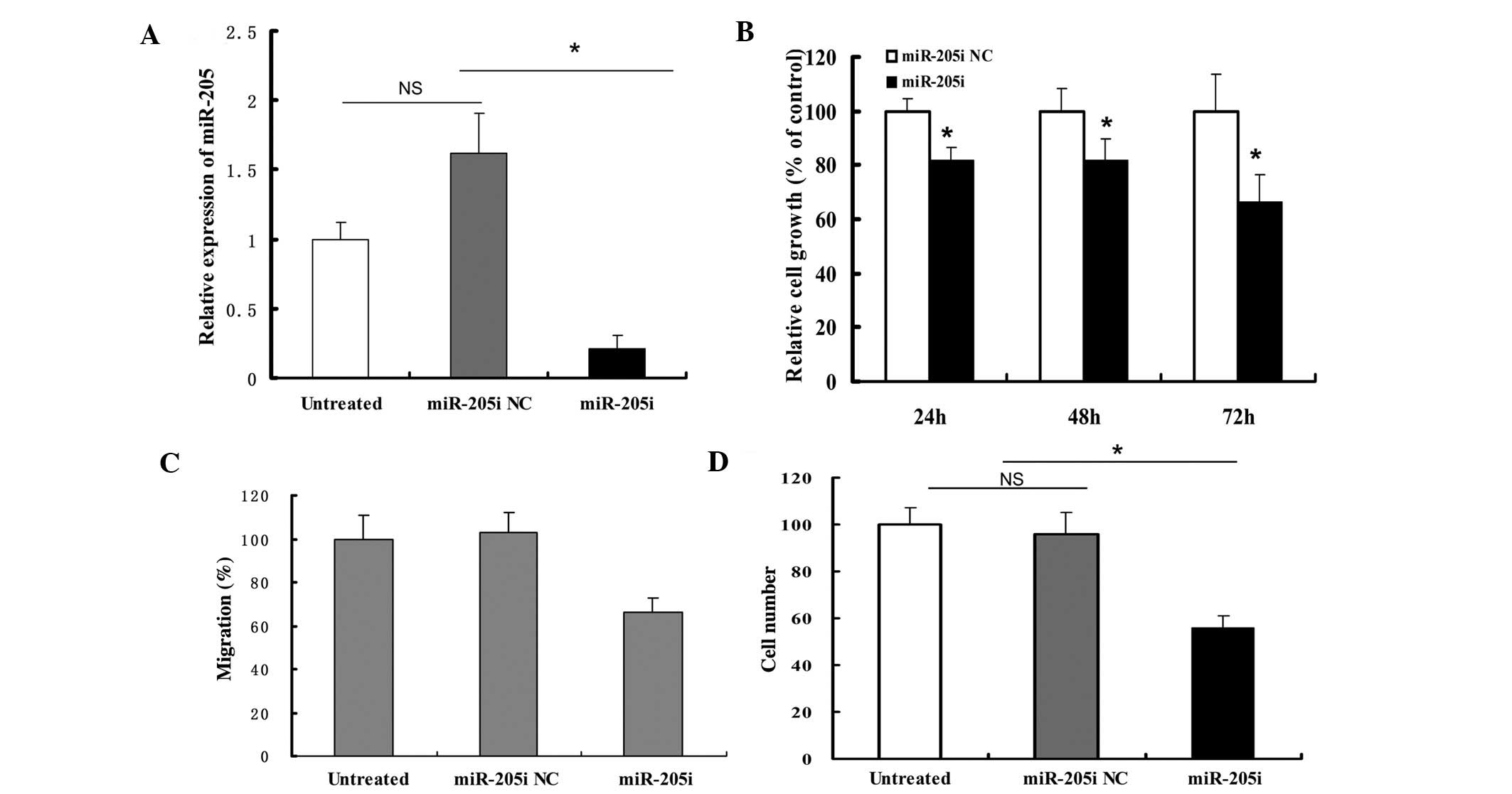
We transfected the miR-205 inhibitors into Ishikawa
cells for loss-of-function assays. The expression of miR-205 was
significantly reduced 72 h after transfection (Fig. 2A). The MTT assay demonstrated that
inhibition of miR-205 inhibited the growth of Ishikawa cells at 24,
48 and 72 h (Fig. 2B).
Downregulation of miR-205 also significantly reduced the migratory
and invasive abilities of Ishikawa cells (Fig. 2C and D).
Restoration of miR-205 promotes
proliferation, migration and invasion of AN3CA cells
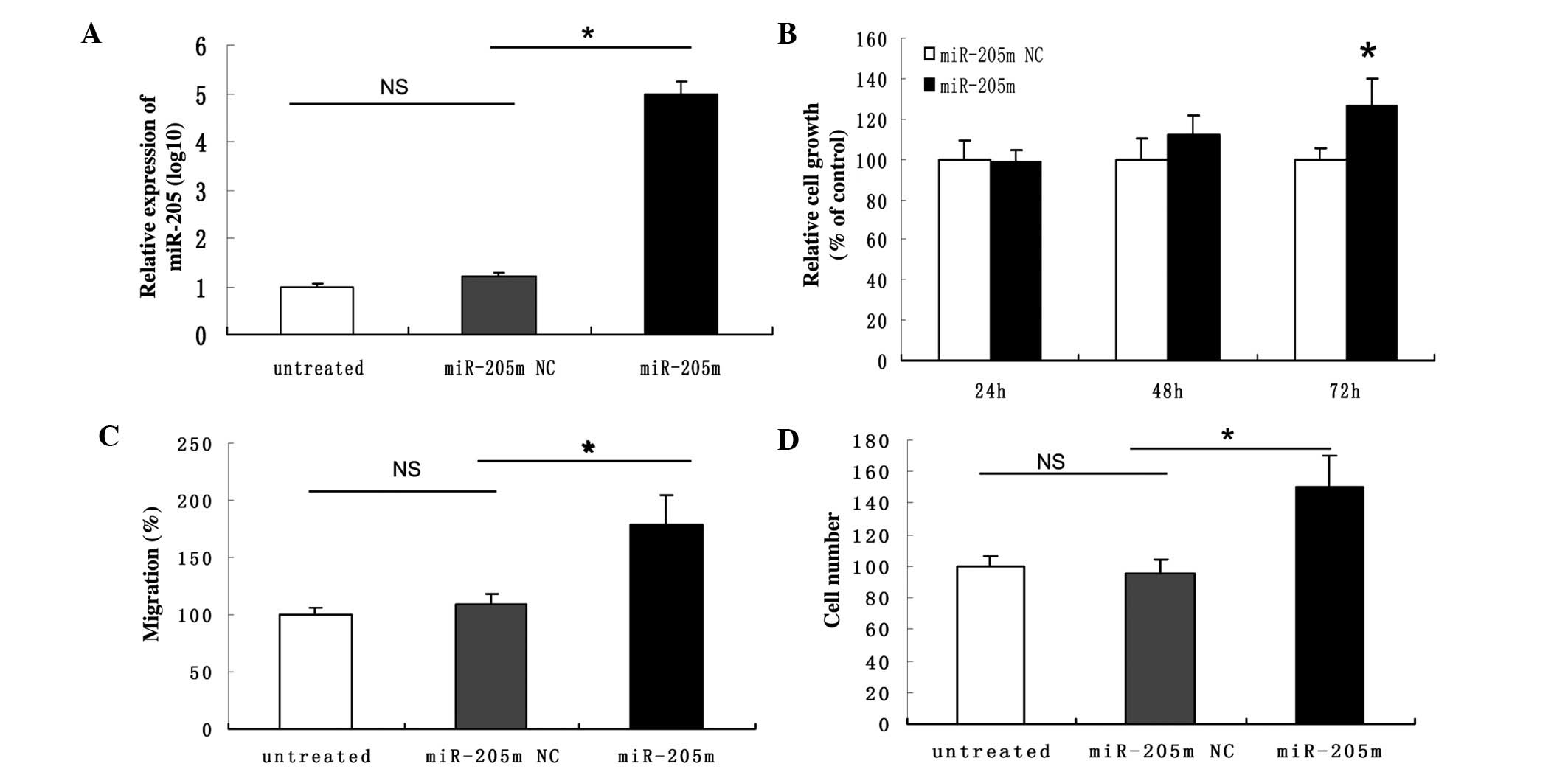
We transfected the miR-205 mimics into AN3CA cells
for the gain-of-function assays. After transfection, AN3CA cells
showed enhanced proliferation, migration and invasion properties
(Fig. 3).
miR-205 directly targets ESRRG
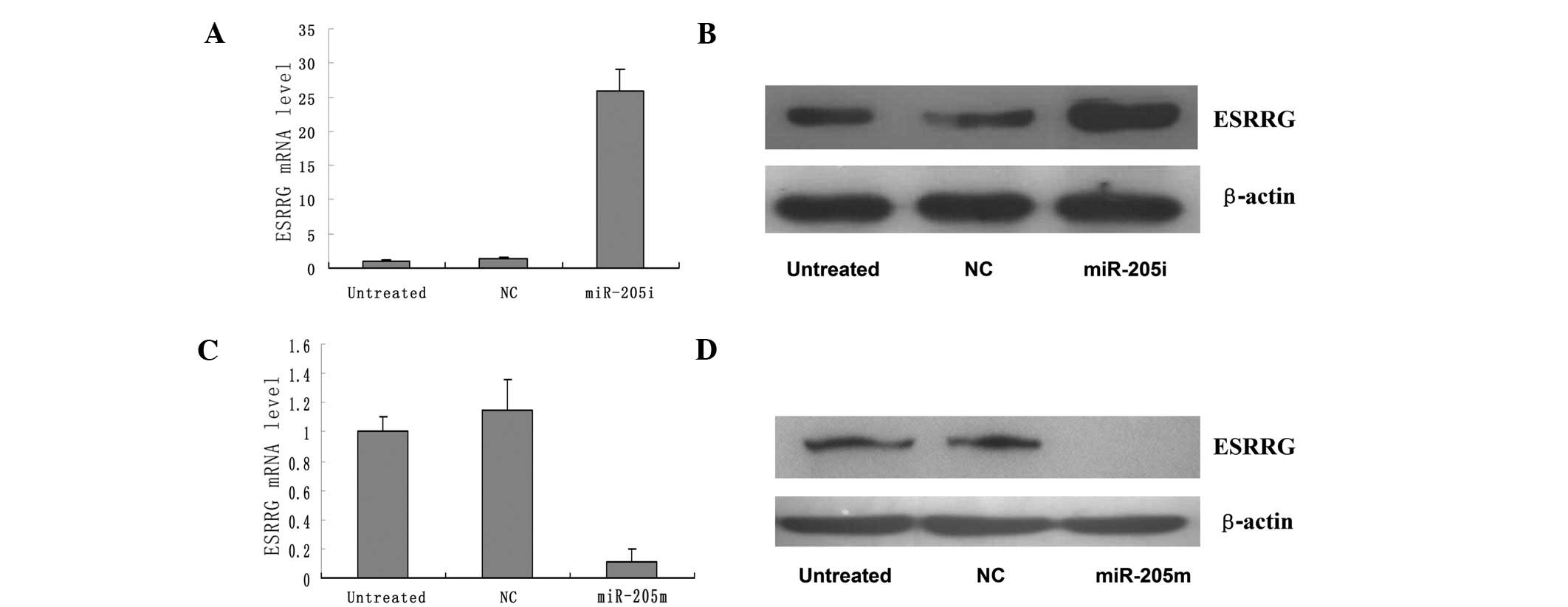
Human ESRRG was predicted to be a target of miR-205
by TargetScanHuman 6.0 (12).
Additionally, ESRRG mRNA and protein expression was negatively
correlated with miR-205 levels in the EEC cell lines (Fig. 4).
To determine whether miR-205 regulates expression of
ESRRG via the miR-205 binding sites in its 3′UTR, we cloned a
fragment of the ESRRG 3′UTR containing the two predicted miR-205
binding sites into a luciferase reporter vector to generate
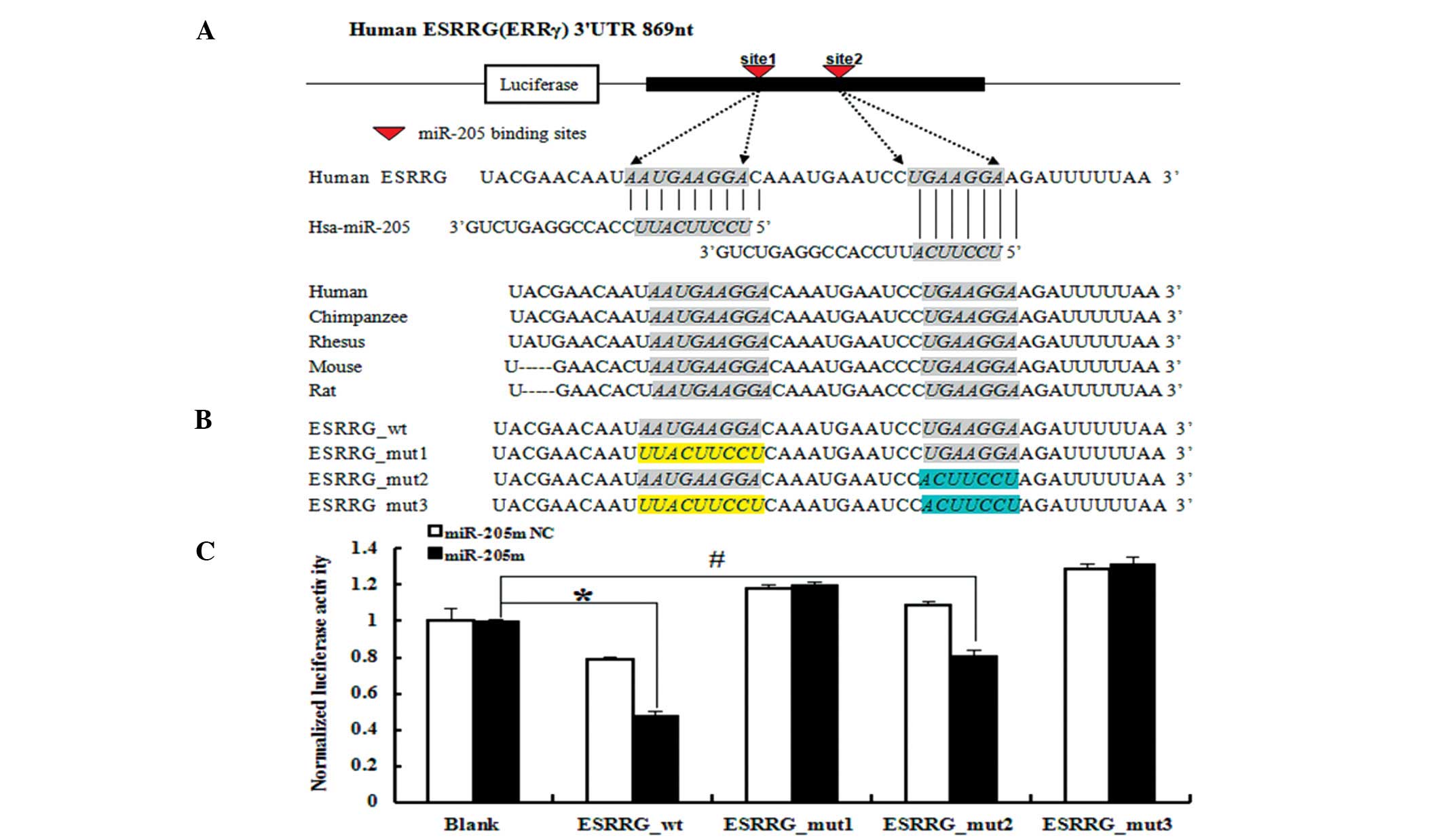
ESRRG_wt (Fig. 5A). The luciferase
activity of ESRRG_wt was significantly reduced (~50%) in 293T cells
treated with the miR-205 mimics (Fig.
5C) (P<0.01), indicating that miR-205 targets ESRRG via the
predicted miR-205 binding sites in the ESRRG 3′UTR.
As the 3′UTR of ESRRG mRNA contains two putative
miR-205-binding sites, we mutated the miRNA-binding seed regions of
these sites to prevent miRNA binding and created ESRRG_mut1,
ESRRG_mut2 and ESRRG_mut3 (Fig.
5B). When the first miR-205-binding site was mutated
(ESRRG_mut1) or both miR-205-binding sites were mutated
(ESRRG_mut3), the ability of miR-205 mimics to silence reporter
gene expression was completely inhibited (Fig. 5C). Mutation of the second
miR-205-binding site (ESRRG_mut2) had no effect, suggesting that
the first one is the real binding site for miR-205 (Fig. 5C). Collectively, these results
indicate that ESRRG is a direct target gene of miR-205.
Discussion
Recent studies have indicated that miR-205 is
dysregulated in many types of cancer, such as head and neck cancer
(13), squamous cell carcinoma
(11) and bladder cancer (14). By TaqMan PCR, we observed that
miR-205 was upregulated in EEC compared to normal endometrium
(P<0.001), as well as in the two EEC cell lines, Ishikawa and
KLE.
In the gain- and loss-of-function assay, our data
indicated that downregulation of miR-205 suppressed the
proliferation and migration of Ishikawa cells; conversely,
upregulation of miR-205 promoted these properties of AN3CA cells.
Therefore, we suggest that miR-205 plays a role as an oncomiR in
EEC.
To date, many genes have been identified to be
targets of miR-205, including the tumor suppressors SHIP2 (11) and MED1 (15), the oncogenes E2F1, E2F5 and PKCɛ
(16), and the pro-metastatic genes
Zeb1 and Zeb2 (17). By microRNA
target prediction software, we proposed ESRRG as a target of
miR-205, which was confirmed in the luciferase reporter assays.
These results suggest that miR-205 acts as an oncomiR through
targeting ESRRG.
ESRRG (ERRγ), a member of the estrogen-related
receptors (ERRs), shares a significant homology with the classical
estrogen receptors (ERs) at the amino acid level, and is
constitutively activated even in the absence of estrogen. Recently,
ESRRG was shown to function as a tumor suppressor in several types
of cancers. Tiraby et al reported that increased expression
of ESRRG upregulated E-cadherin, promoted the
mesenchymal-to-epithelial transition and suppressed breast cancer
growth in vivo(18).
Likewise, transfection of ESRRG into the LnCaP and DU145 prostate
cancer cell lines significantly suppressed proliferation in
vitro and tumorigenicity in vivo(19). In the present study, inhibition of
miR-205 increased the protein expression of ESRRG, and suppressed
cell proliferation, migration and invasion.
In summary, we found frequent upregulation of
miR-205 in EEC. In the gain- and loss-of-functions assays,
inhibition of miR-205 reduced cellular proliferation, migration and
invasion; conversely, upregulated levels of miR-205 led to
increased cellular proliferation, migration and invasion. We
identified the ESRRG gene to be a novel target, which could be
helpful to elucidate mechanisms underlying the tumorigenesis of
EEC.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 30872760 and 81072139).
We would like to thanks Dr Wei Bao, Wen Lu and Guiqiang Du for the
technical assistance and Cong Lu and Yuhong Xia for manuscript
revision.
Abbreviations:
|
EEC
|
endometrial endometrioid carcinoma
|
|
ESRRG
|
estrogen-related receptor-γ
|
|
ER
|
estrogen receptor
|
|
qRT-PCR
|
quantitative reverse
transcription-polymerase chain reaction
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
3′UTR
|
3′untranslated region
|
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Souza Nunes LS, De Oliveira RV, Holgado
LA, Nary Filho H, Ribeiro DA and Matsumoto MA: Use of bovine
hydroxyapatite with or without biomembrane in sinus lift in
rabbits: histopathologic analysis and immune expression of core
binding factor 1 and vascular endothelium growth factor. J Oral
Maxillofac Surg. 69:1064–1069. 2011.
|
|
3
|
Miska EA: How microRNAs control cell
division, differentiation and death. Curr Opin Genet Dev.
15:563–568. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar
|
|
5
|
Chung TK, Cheung TH, Huen NY, Wong KW, Lo
KW, Yim SF, Siu NS, Wong YM, Tsang PT, Pang MW, Yu MY, To KF, Mok
SC, Wang VW, Li C, Cheung AY, Doran G, Birrer MJ, Smith DI and Wong
YF: Dysregulated microRNAs and their predicted targets associated
with endometrioid endometrial adenocarcinoma in Hong Kong women.
Int J Cancer. 124:1358–1365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu W, Lin Z, Zhuang Z and Liang X:
Expression profile of mammalian microRNAs in endometrioid
adenocarcinoma. Eur J Cancer Prev. 18:50–55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Creasman W: Revised FIGO staging for
carcinoma of the endometrium. Int J Gynaecol Obstet. 105:1092009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee JW, Choi CH, Choi JJ, Park YA, Kim SJ,
Hwang SY, Kim WY, Kim TJ, Lee JH, Kim BG and Bae DS: Altered
microRNA expression in cervical carcinomas. Clin Cancer Res.
14:2535–2542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang F, Liu T, He Y, Yan Q, Chen X, Wang
H and Wan X: MiR-125b promotes proliferation and migration of type
II endometrial carcinoma cells through targeting TP53INP1 tumor
suppressor in vitro and in vivo. BMC Cancer. 11:4252011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu J, Ryan DG, Getsios S,
Oliveira-Fernandes M, Fatima A and Lavker RM: microRNA-184
antagonizes microRNA-205 to maintain SHIP2 levels in epithelia. Pro
Natl Acad Sci USA. 105:19300–19305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lewis BP, Shih IH, Jones-Rhoades MW,
Bartel DP and Burge CB: Prediction of mammalian microRNA targets.
Cell. 115:787–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang J, Lee EJ, Gusev Y and Schmittgen
TD: Real-time expression profiling of microRNA precursors in human
cancer cell lines. Nucleic Acids Res. 33:5394–5403. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gottardo F, Liu CG, Ferracin M, Calin GA,
Fassan M, Bassi P, Sevignani C, Byrne D, Negrini M, Pagano F,
Gomella LG, Croce CM and Baffa R: Micro-RNA profiling in kidney and
bladder cancers. Urol Oncol. 25:387–392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mouillet JF, Chu T, Nelson DM, Mishima T
and Sadovsky Y: MiR-205 silences MED1 in hypoxic primary human
trophoblasts. FASEB J. 24:2030–2039. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gandellini P, Folini M, Longoni N, Pennati
M, Binda M, Colecchia M, Salvioni R, Supino R, Moretti R, Limonta
P, Valdagni R, Daidone MG and Zaffaroni N: miR-205 exerts
tumor-suppressive functions in human prostate through
down-regulation of protein kinase Cepsilon. Cancer Res.
69:2287–2295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tiraby C, Hazen BC, Gantner ML and Kralli
A: Estrogen-related receptor gamma promotes
mesenchymal-to-epithelial transition and suppresses breast tumor
growth. Cancer Res. 71:2518–2528. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu S, Wang X, Ng CF, Chen S and Chan FL:
ERRγ suppresses cell proliferation and tumor growth of
androgen-sensitive and androgen-insensitive prostate cancer cells
and its implication as a therapeutic target for prostate cancer.
Cancer Res. 67:4904–4914. 2007.
|