Introduction
Gastric carcinoma is one of the most common
malignant tumors with high incidence and mortality rates. The
5-year overall survival rate for patients with early stage gastric
carcinoma following treatment is higher than 90%. However, the
5-year postoperative survival rate for patients with gastric
carcinoma in progressive stages is only 30–40%, and the overall
rate of disease relapse is 50–70%. Current treatments for gastric
carcinoma include the removal of the primary tumors and affected
lymph nodes in combination with radiotherapy, chemotherapy,
biological therapy and gene therapy (1).
Radical gastric cancer therapy is only applicable to
early stage tumors, whereas radiotherapy and chemotherapy lack
selectivity, have marked toxic side effects, and are often not
tolerated by patients. Thus, there is a need for novel tumor
therapies. Gene therapy aims to introduce an exogenous normal gene
or sequence into target cells to replace or compensate for the
function of a defective gene or to suppress the overexpression of
an abnormal gene, thereby treating disease (2). As the understanding of the molecular
mechanisms of tumor pathology has advanced, gene therapy as a novel
strategy has been shown to have a therapeutic advantage for
treating several types of tumors, including gastric carcinoma
(3–6).
Proliferin-related protein (PRP), a prolactin (PRL)
family member (7–10), is secreted by mammalian placenta
(11–15). Placenta-secreted proliferin (PLF)
can induce the formation of decidual new blood vessels, whereas PRP
inhibits the effects of PLF and other angiogenic factors, thereby
blocking the formation of new blood vessels. Jackson et
al(12) found that PRP inhibits
endothelial cell migration and blood vessel formation. This
inhibitory role may affect tumorigenesis and cancer
progression.
Our previous studies demonstrated that the PRP gene
is not only expressed in the female reproductive system but is also
expressed in the male reproductive system, where it is located in
Leydig cells, and its expression increases with age (16). To elucidate the function of PRP, we
performed experiments to examine the consequences of
lentiviral-mediated PRP knockdown on cell growth, cell cycle,
testosterone production and the expression of several Leydig genes.
Our results revealed that PRP indirectly participates in
testosterone production. In addition, we found that another marked
change in TM3 Leydig cells, which was induced by PRP silencing, was
related to the growth rate. Cell growth and cell cycle analyses
demonstrated that the inhibition of PRP expression resulted in a
decrease in the TM3 cell doubling time and an increase in the
number of cells in S-phase, suggesting that PRP plays a negative
role in cell proliferation.
Based on the anti-proliferative effects of PRP, we
aimed to ascertain whether PRP also inhibits the growth of SGC-7901
gastric carcinoma cells. Thus, we stably expressed PRP in SGC-7901
cells to evaluate the effects of PRP on proliferation and invasion.
We also established an in vivo xenograft tumor model by
subcutaneously injecting SGC-7901 cells in nude mice to evaluate
the effects of PRP on xenograft tumor growth.
Materials and methods
Materials
RPMI-1640 medium and fetal bovine serum (FBS) were
purchased from HyClone (Logan, UT, USA). A protein extraction kit
and BeyoECL Plus western blotting detection reagent were purchased
from Beyotime Biotechnology (Jiangsu, China). Anti-PRP (rat
monoclonal IgG2a, sc-80531), anti-MMP-9 (sc-6840) and anti-TIMP-1
(sc-5538) antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). All other chemicals were purchased from
Sangon Biotech (Shanghai, China).
Cell culture
The human gastric cancer cell line SGC-7901 was
obtained from the Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China) and maintained in RPMI-1640 medium
supplemented with 10% FBS at 37°C in a humidified atmosphere
containing 5% CO2.
EGFP-C1-PRP eukaryotic expression vector
construction and transfection
The PRP full-length open reading frame was cloned
into the pEGFP-C1 vector to generate the pEGFP-C1-PRP expression
vector. Following the manufacturer’s instructions, the pEGFP-C1-PRP
vector or the empty vector control was transfected into SGC-7901
cells using Lipofectamine 2000, and the cells were subsequently
selected with G418 to screen for stably transfected cell lines,
which were named SGC-7901-PRP and SGC-7901-C1, respectively.
Calculation of the cell doubling
time
Parental SGC-7901, SGC-7901-PRP and SGC-7901-C1
cells in the log growth phase were trypsinized, separated into
single-cell suspensions and seeded into 24-well plates. Three wells
were randomly selected at 24, 48, 72, 96, 120, 144 and 168 h before
being trypsinized. Cell numbers were counted, and an average was
obtained from three calculations. A growth curve was graphed with
the cell number plotted on the y-axis and time on the x-axis.
Doubling time was calculated from the viable cell number versus
time (linear zone) graph using the following formula: Doubling time
(TD) = t * lg2/(1gNt - lgN0),
where t is the time of culture and IgN0 and
1gNt are the cell numbers at the time of seeding and at
t, respectively.
Invasion and migration assay
Cell invasion assays were performed using Transwells
(8-mm pore size; Corning-Costar Corp., Acton, MA, USA). Filters
were coated with 120 μl Matrigel, and the upper chambers were
seeded with 200 μl of the cell suspension containing
1×105 cells/ml, and the lower chambers were seeded with
500 μl of complete media. The Transwell plates were incubated at
37°C in the presence of 5% CO2 for 24 h. Cells that
infiltrated and adhered to the filter membrane surface facing the
lower chamber were fixed with 4% paraformaldehyde and stained with
0.1% crystal violet. Five to 10 views were randomly selected, and
an average cell number per view was counted to evaluate the cancer
cell invasive ability. Cell migration assays were performed using
the same protocol with non-coated filters.
Cell cycle analysis
Synchronized SGC-7901, SGC-7901-PRP and SGC-7901-C1
cells were trypsinized, separated into single-cell suspensions and
seeded into 6-well plates at 1×105 cells/ml. Cells were
cultured at 37°C in the presence of 5% CO2 for 24 h to
allow the cells to adhere to the plates prior to replacement with
fresh media. After 48 h, cells were trypsinized, collected by
centrifugation at 1,000 rpm for 5 min, washed twice with
phosphate-buffered saline (PBS) and fixed in 1 ml pre-chilled 70%
ethanol at 4°C overnight. The next day, the cells were washed 3
times with PBS to remove the ethanol, collected by centrifugation
at 1,000 rpm for 5 min and resuspended in 0.5 ml PBS. Subsequently,
protease inhibitor and RNase inhibitor were added at a final
concentration of 50 μg/ml. The cells were then incubated in a 37°C
waterbath for 30 min, filtered through a 75-nm filter and analyzed
by flow cytometry for cell cycle analysis. Three independent
experiments were performed.
Immunofluorescence
To determine the PRP expression level in the
pEGFP-C1-PRP-transfected stable SGC-7901 cells and to detect PRP
protein in the xenografts, an immunofluorescence assay was
performed using a rat anti-PRP primary monoclonal antibody. Slides
were analyzed by observation under a fluorescence microscope.
Western blotting
For protein isolation, tissues or cells were lysed
with RIPA buffer. Insoluble material was removed by centrifugation
at 12,000 × g for 10 min at 4°C, and the supernatants were
collected. The protein concentration was determined using a
bicinchoninic acid (BCA) assay. Proteins were separated in 10%
SDS-PAGE gels and transferred to polyvinylidene difluoride (PVDF)
membranes followed by incubation with the primary antibody. After
washing 3 times with Tris-buffered saline and Tween-20 (TBST), the
membranes were incubated with a secondary IgG antibody for 2 h (at
room temperature) and washed again. The antigen-antibody complex
was detected using the BeyoECL Kit following the manufacturer’s
protocol.
Nude mouse xenograft experiments
Nude mice were grouped to receive an injection of
parental SGC-7901, SGC-7901-C1 or SGC-7901-PRP cells. Cells in the
log growth phase were collected in serum-free RPMI-1640, spun down
and resuspended in PBS at 3×106 cells/ml. Cell
suspensions (0.2 ml per animal) were injected into the right front
armpit of nude mice. The xenografts were observed every 4 days for
32 days.
The maximum (A) and minimal diameters (B) of the
xengrafts were measured. The volume of the tumors was calculated as
Volume = A × B2/2 and plotted onto a xenograft growth
curve. Mice were euthanized by cervical dislocation at the end of
the experiment. The tumors were removed under sterile conditions
and weighed. The tumor inhibitory rate was calculated as follows:
Tumor inhibitory rate = (1 - experimental group average
weights/control group average weights) × 100%. The tumors were
fixed in methanol and stored in liquid nitrogen for subsequent
immunofluorescence staining and protein extraction. The animal
protocols conformed to those approved by the Chongqing Medical
University Animal Care and Use Committee.
Statistical analysis
All data are presented as means ± SE. For
comparisons between two groups, the Student’s t-test was performed.
For comparisons among multiple groups, an ANOVA test was performed
followed by a Student-Newman-Keuls test. Differences were
considered significant at P<0.05.
Results
PRP is expressed in the SGC-7901-PRP
cells
To investigate the potential effects of the mouse
PRP activity on cell growth and proliferation, pEGFP-C1-PRP or the
pEGFP-C1 empty vector (as a control) were transfected into SGC-7901
cells. Stably transfected cells were selected using G418 and
subsequently analyzed for PRP protein expression. Western blot
analysis results confirmed the detectable expression of PRP in
cells stably transfected with the pEGFP-C1-PRP vector (Fig. 1).
Immunofluorescence staining demonstrates
that PRP is expressed in the cytoplasm of SGC-7901-PRP cells
(Fig. 2A)
Immunofluorescence staining of tumor slices revealed
that PRP was expressed in the cytoplasm of tumor cells in the
SGC-7901-PRP group, while there was no PRP expression in tumors
from the SGC-7901-C1 and SGC-7901 groups (Fig. 2B-D).
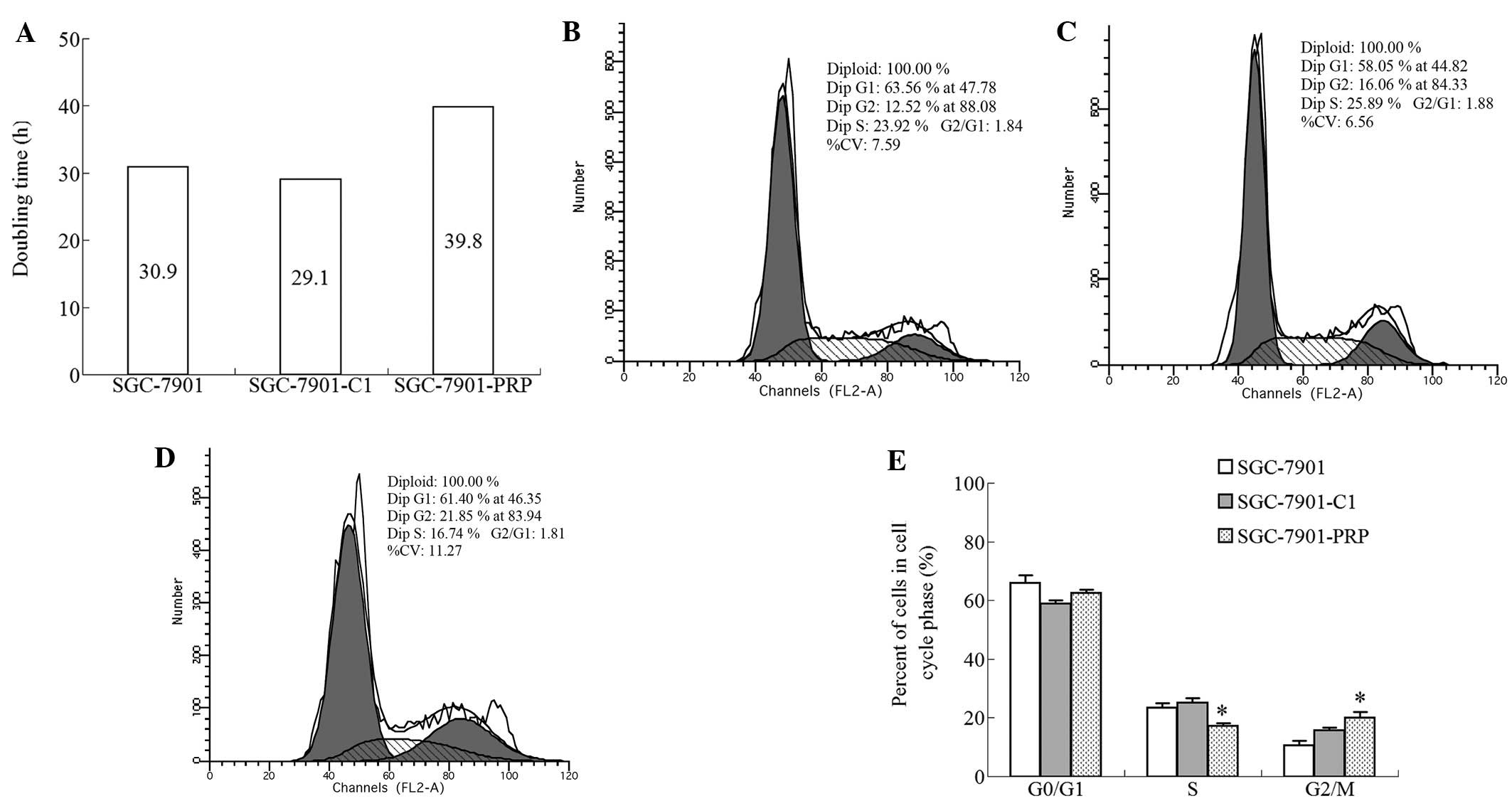
PRP overexpression alters the doubling
time and cell cycle distribution of SGC-7901 cells in vitro
The doubling times were calculated by cell counting.
Compared with the SGC-7901-C1 and SGC-7901 cells, the SGC-7901-PRP
cells exhibited a longer doubling time, suggesting a role for PRP
in human tumor cell growth (Fig.
3A). To address this observation further, the cell cycle
profiles were assessed by flow cytometric analysis. The data
demonstrated that PRP overexpression caused a marked decrease in
the number of cells in the S-phase (Fig. 3B-E).
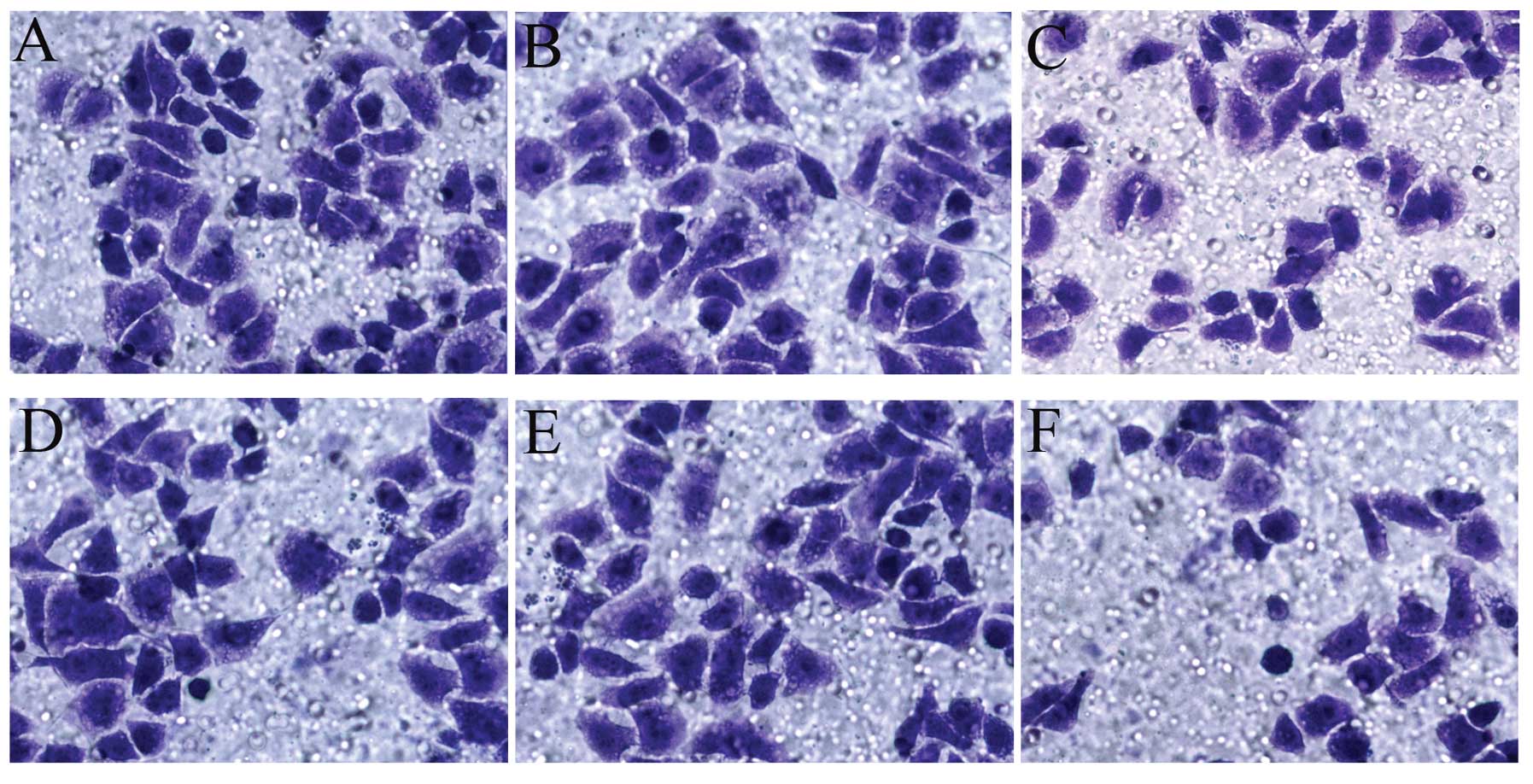
Effect of PRP overexpression on SGC-7901
cell migration and invasion
The results of the Transwell cell migration and
invasion assays are shown in Fig. 4
and Table I. PRP overexpression
resulted in significant suppression of SGC-7901 cell migration and
invasion.
 | Table IEffect of the overexpression of PRP on
SGC-7901 cell migration and invasion. |
Table I
Effect of the overexpression of PRP on
SGC-7901 cell migration and invasion.
| Cell group | No. of invading
cells | No. of migrating
cells |
|---|
| SGC-7901 | 65.6±4.0 | 70.2±4.9 |
| SGC-7901-C1 | 60.0±3.5 | 68.4±7.2 |
| SGC-7901-PRP | 31.8±6.1a | 46.6±7.6a |
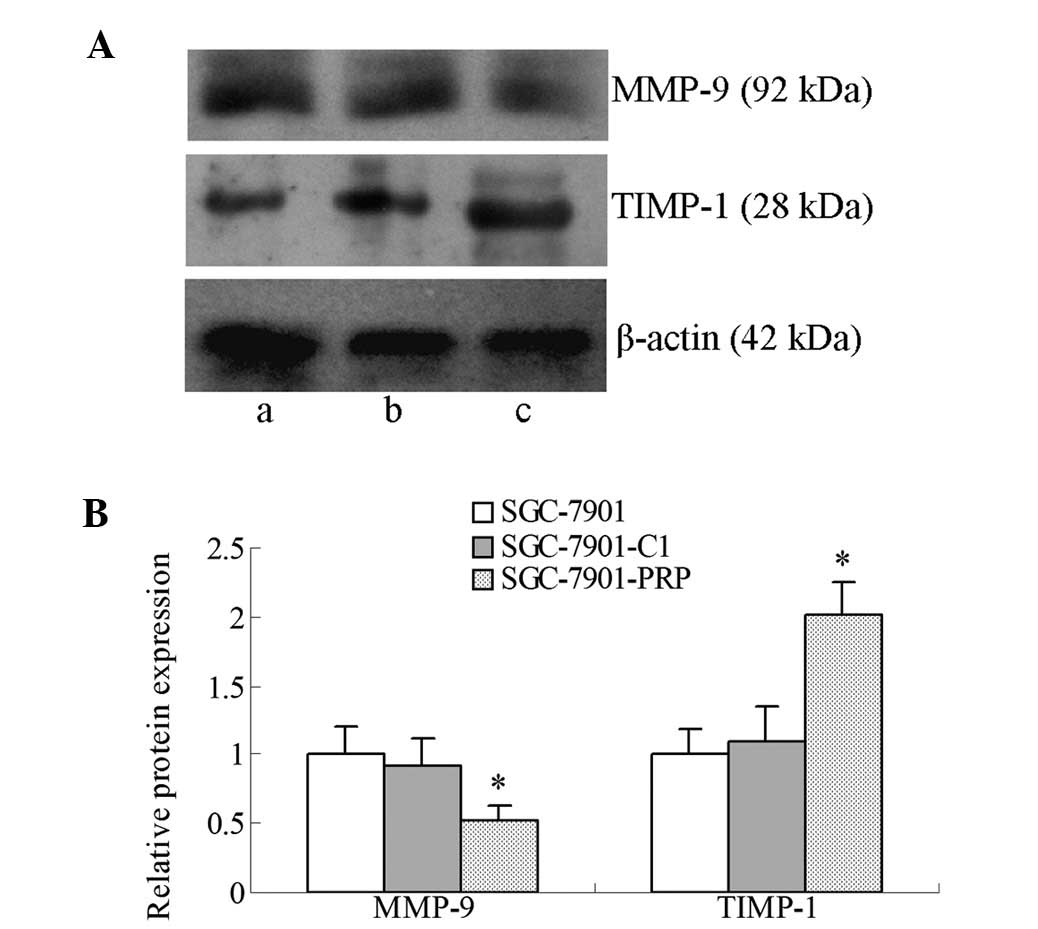
Matrix metalloproteinase-9 (MMP-9) and
tissue inhibitor of metalloproteinases-1 (TIMP-1) are involved in
PRP-regulated SGC-7901 cell invasion
Our finding that PRP has an inhibitory effect on
cell invasion prompted us to examine its effects on MMP-9 and
TIMP-1 expression in SGC-7901 cells. As shown in Fig. 5, western blot analysis revealed that
MMP-9 expression was significantly decreased (P<0.05), while
TIMP-1 expression was significantly increased (P<0.05) in the
SGC-7901-PRP cells. These results suggest that the inhibitory
effect of PRP on the invasiveness of SGC-7901 cells was at least
partially mediated by MMP-9 downregulation and TIMP-1 upregulation,
which may contribute to degradation of the extracellular
matrix.
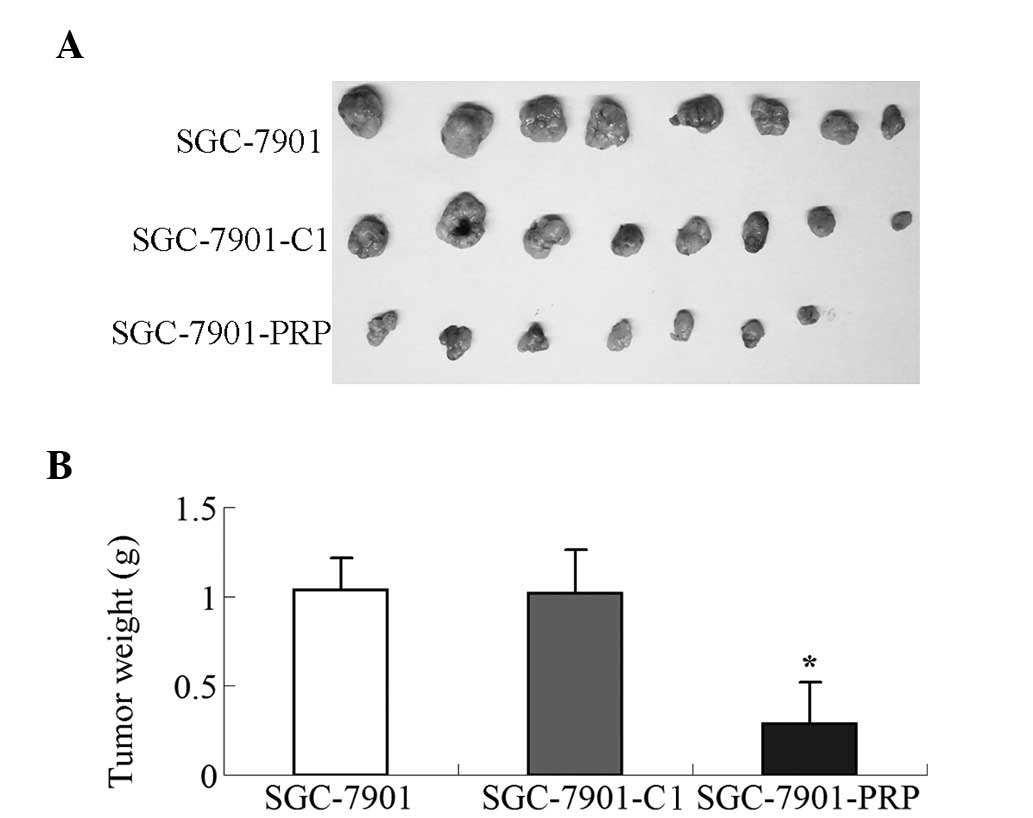
Growth-inhibitory effect of the
overexpression of PRP in gastric tumor xenografts
We performed a subcutaneous tumor formation assay in
nude mice to evaluate the growth suppressive effects of PRP in
vivo. Nude mice were subcutaneously injected with parental
SGC-7901, SGC-7901-C1 or SGC-7901-PRP cells. The tumor volume and
the tumor weight at 32 days were measured after subcutaneous
injection. Among the 8 mice that were injected with SGC-7901-PRP
cells, one did not develop a tumor. The tumor formation rate was
87.5% for all mice. The tumor formation rate for the groups of mice
that received parental SGC-7901 or SGC-7901-C1 cells was 100%. As
shown in Fig. 6, the tumor volume
and tumor weight induced by PRP-overexpressing SGC-7901 cells were
significantly reduced when compared with the tumor xenografts from
SGC-7901-C or parental SGC-7901 cells (P<0.01). The inhibitory
rate of tumor growth of the mouse group injected with
PRP-overexpressing SGC-7901 cells was 71.9%.
Discussion
PRP has been previously reported to be expressed
only in female mammals. The main function of PRP was believed to be
anti-angiogenesis, and it was reported to play a role in pregnancy
(12). However, the present study
provides evidence demonstrating that in addition to the
anti-angiogenesis function (12,17),
PRP also possesses in vitro and in vivo antitumor
effects through the inhibition of cell growth, proliferation,
migration and invasion.
Our results demonstrated that PRP significantly
inhibited cell proliferation. Compared with the controls,
SGC7901-PRP cells exhibited a longer doubling time, suggesting a
role for PRP in human tumor cell growth. Cell proliferation
proceeds through a series of events in which the synthesis and
organization of DNA and other cellular components occur prior to
mitosis. Since PRP inhibits cell proliferation and an altered cell
cycle is a common cancer characteristic, we aimed to ascertain
whether PRP plays a role in regulating cell cycle progression. Flow
cytometric results demonstrated that PRP overexpression caused a
significant decrease in the cell number in the S-phase of the cell
cycle.
Numerous studies have demonstrated an association
between cell cycle progression and cancer, and targeting the cell
cycle has become a recognized strategy for tumor treatment
(18,19). The potency of PRP in inducing a
decrease in the number of S-phase cells in human tumor cells
suggests that it may lead to new strategies for treating human
gastric cancer.
Metastasis is a major cause of the high mortality
rate of patients with gastric cancer, and gastric cancer has a high
frequency of recurrence, even after treatment. Cancer cell
migration and invasion are the major events that occur during
metastasis. Thus, blocking cancer cell migration and invasion may
enhance the efficacy of gastric cancer treatments. Targeting
particular mechanisms in these events may become a novel strategy
to treat gastric cancer. By evaluating the migration and invasion
capabilities of SGC-7901 cells using a Transwell chamber assay, our
study demonstrated that PRP significantly decreased the migration
and invasion of SGC-7901 cells. These data suggest that PRP not
only inhibits cell proliferation, it also antagonizes cancer cell
migration and invasion.
A major event that occurs during invasion and
metastasis is the degradation of the extracellular matrix (ECM).
MMP-9 degrades type-IV collagen, which is the major component of
the ECM; therefore, it plays an important role in tumor invasion
into the surrounding tissue and the subsequent distal metastasis
through blood vessels and the lymph vascular system. TIMP-1, an
MMP-9 inhibitor (20–22), inhibits metastasis.
To investigate further the molecular mechanism
underlying the anti-migratory and anti-invasive properties of PRP,
we assessed the effect of the overexpression of PRP on MMP-9 and
TIMP-1 expression in SGC-7901 cells. Our results demonstrated that
PRP overexpression resulted in a significant decrease in MMP-9
expression and an increased in TIMP-1 expression. Sier et
al(23) and Bando et
al(24) confirmed that the
MMP-9 expression level significantly increases in gastric tumors.
Patients with higher MMP-9 expression have a lower survival rate,
and MMP-9 expression may be used as an independent prognostic
indicator for gastric cancer. The downregulation of MMP-9
expression inhibits tumor invasion, angiogenesis and growth
(25). The orally available MMP
inhibitor marimastat has demonstrated a trend toward survival
benefit in a small phase III gastric cancer study and was proposed
as a maintenance therapy for patients with advanced gastric cancer
(4). PRP overexpression decreased
the MMP-9 and TIMP-1 ratio, suggesting that PRP could antagonize
metastasis by modulating the expression of MMP-9 and TIMP-1.
Our assessment of the biological effects of PRP on
human gastric carcinoma cells focused on the tumorigenicity of
SGC-7901 cells in a xenotransplantation nude mouse model. Our
results demonstrated that tumors from the PRP-overexpressing group
had a lower growth rate. There were significant differences in the
volume and weight of tumors between the PRP and control groups,
suggesting that PRP was able to reverse the tumorigenic effects of
SGC-7901 cells in vivo, which was also supported by in
vitro experiments in which PRP overexpression caused a
decreased in proliferation, motility and invasion.
Theoretically, gene therapies should effectively
inhibit tumor growth to various extents; however, the clinical
outcomes of these therapies are not ideal, which may be because
tumor formation is a multistep, multistage, complex process.
Abnormalities in the function of multiple genes have been
implicated in cancer and when these genes interact with each other,
they synergistically stimulate proliferation and inhibit apoptosis,
altering the ability of cells to adhere and migrate. To achieve an
optimal therapeutic effect, multiple tumorigenesis-related factors
must be targeted. In addition to its previously reported
anti-angiogenic role during pregnancy, we provide evidence that PRP
has multiple inhibitory effects on gastric tumor growth, including
suppressing cell proliferation and reducing proliferative activity
by blocking the cell cycle and inhibiting cell migration and
invasion. Furthermore, we revealed that, at least partially, the
anti-invasive and anti-migratory effects of PRP are mediated via
MMP-9 downregulation and TIMP-1 upregulation. The in vitro
and in vivo antitumor effects of PRP suggest that it may be
an effective target for the gene therapy of gastric carcinoma.
Acknowledgements
This study was supported by the National Nature
Science Foundation of China (30901062) and the Foundation of
Chongqing Municipal Health Bureau (2011-2-040), China.
References
|
1
|
Foukakis T, Lundell L, Gubanski M and Lind
PA: Advances in the treatment of patients with gastric
adenocarcinoma. Acta Oncol. 46:277–285. 2007. View Article : Google Scholar
|
|
2
|
Robins D: Gene Therapy Protocols. Humana
Press Inc; New Jersey: pp. 102–105. 1997
|
|
3
|
Meza-Junco J, Au HJ and Sawyer MB:
Critical appraisal of trastuzumab in treatment of advanced stomach
cancer. Cancer Manag Res. 3:57–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bramhall SR, Hallissey MT, Whiting J, et
al: Marimastat as maintenance therapy for patients with advanced
gastric cancer: a randomised trial. Br J Cancer. 86:1864–1870.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McNamara MJ and Adelstein DJ: Current
developments in the management of locally advanced esophageal
cancer. Curr Oncol Rep. 14:342–349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Vita F, Giuliani F, Silvestris N, et
al: Current status of targeted therapies in advanced gastric
cancer. Expert Opin Ther Targets. 16(Suppl 2): S29–S34.
2012.PubMed/NCBI
|
|
7
|
Bachelot A and Binart N: Reproductive role
of prolactin. Reproduction. 133:361–369. 2007. View Article : Google Scholar
|
|
8
|
Soares MJ, Konno T and Alam SM: The
prolactin family: effectors of pregnancy-dependent adaptations.
Trends Endocrinol Metab. 18:114–121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ben-Jonathan N, LaPensee CR and LaPensee
EW: What can we learn from rodents about prolactin in humans?
Endocr Rev. 29:1–41. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haig D: Placental growth hormone-related
proteins and prolactin-related proteins. Placenta. 29(Suppl A):
S36–S41. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Linzer DI and Nathans D: A new member of
the prolactin-growth hormone gene family expressed in mouse
placenta. EMBO J. 4:1419–1423. 1985.PubMed/NCBI
|
|
12
|
Jackson D, Volpert OV, Bouck N and Linzer
DI: Stimulation and inhibition of angiogenesis by placental
proliferin and proliferin-related protein. Science. 266:1581–1584.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Colosi P, Swiergiel JJ, Wilder EL, Oviedo
A and Linzer DI: Characterization of proliferin-related protein.
Mol Endocrinol. 2:579–586. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Toft DJ and Linzer DI: Identification of
three prolactin-related hormones as markers of invasive
trophoblasts in the rat. Biol Reprod. 63:519–525. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee SJ, Talamantes F, Wilder E, Linzer DI
and Nathans D: Trophoblastic giant cells of the mouse placenta as
the site of proliferin synthesis. Endocrinology. 122:1761–1768.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao L, Hao J, Hu J, et al: Expression of
proliferin-related protein in testis and the biological
significance in testosterone production. Mol Cell Endocrinol.
343:25–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bengtson NW and Linzer DI: Inhibition of
tumor growth by the antiangiogenic placental hormone,
proliferin-related protein. Mol Endocrinol. 14:1934–1943. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwartz GK and Shah MA: Targeting the
cell cycle: a new approach to cancer therapy. J Clin Oncol.
23:9408–9421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dickson MA and Schwartz GK: Development of
cell-cycle inhibitors for cancer therapy. Curr Oncol. 16:36–43.
2009.PubMed/NCBI
|
|
20
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Klein T and Bischoff R: Physiology and
pathophysiology of matrix metalloproteases. Amino Acids.
41:271–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cruz-Munoz W and Khokha R: The role of
tissue inhibitors of metalloproteinases in tumorigenesis and
metastasis. Crit Rev Clin Lab Sci. 45:291–338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sier CF, Kubben FJ, Ganesh S, et al:
Tissue levels of matrix metalloproteinases MMP-2 and MMP-9 are
related to the overall survival of patients with gastric carcinoma.
Br J Cancer. 74:413–417. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bando E, Yonemura Y, Endou Y, et al:
Immunohistochemical study of MT-MMP tissue status in gastric
carcinoma and correlation with survival analyzed by univariate and
multivariate analysis. Oncol Rep. 5:1483–1488. 1998.PubMed/NCBI
|
|
25
|
Zhao F, Zhang Q, Kang C, et al:
Suppression of matrix metalloproteinase-9 expression by RNA
interference inhibits SGC7901 gastric adenocarcinoma cell growth
and invasion in vitro and in vivo. Med Oncol. 27:774–784. 2010.
View Article : Google Scholar : PubMed/NCBI
|