Introduction
Bone metastases (BM) are a frequent event in breast
cancer and are difficult to detect. The assessment of skeletal
metastases primarily depends on imaging techniques. Routinely, bone
scintigraphy is used for detection of BM. Although bone
scintigraphs have high sensitivity, they lack specificity in the
detection of skeletal metastases (1,2). Thus,
additional available imaging techniques such as radiography,
computer tomography or magnetic resonance imaging are necessary to
verify the bone scan, and these techniques are highly expensive,
particularly for monitoring therapeutic response of skeletal
metastases during palliative systemic therapy.
Malignant bone disease is associated with increased
levels of bone resorption markers (3,4).
Osteoblasts and osteoclasts are affected by malignant cells in
skeletal metastases causing increased number, activity and survival
of these bone remodelling cells, a phenomenon known as the vicious
cycle (5). In this regard, series
of biochemical serum markers of bone turnover, such as
aminoterminal procollagen propeptide of type I collagen (PINP) and
carboxyterminal telopeptide of type I collagen (ICTP), have been
evaluated for the early detection of skeletal metastases (6,7).
Bisphosphonates inhibit osteoclast-mediated
osteolysis and reduce bone marker levels (8,9).
Zoledronic acid (ZOL) reduces the risk of skeletal-related events
and suppresses levels of bone turnover markers in patients with
multiple myeloma or bone metastases from solid tumors, including
breast cancer (10,11). Assuming that bone metastasis
requires a modification of bone formation and resorption, it seems
likely that measurement of bone markers during palliative therapy
in metastatic breast cancer (MBC) patients could be useful to
monitor the therapeutic response. In contrast to imaging
techniques, bone marker measurements are easy to perform, minimally
invasive and inexpensive.
Therefore, the objective of this study was to assess
PINP and ICTP levels in MBC patients receiving standard systemic
therapy with ZOL over the course of time for one year and analyze
these findings in relation to response rates assessed by imaging
techniques and to the serum levels of tumor markers CEA and CA
15-3.
Patients and methods
Patients and study design
This prospective study included MBC patients with
bone metastases. The study was conducted at the Department of
Obstetrics and Gynecology at the University Hospital in Essen.
Forty MBC patients (mean age 60 years; range, 36–85) with confirmed
BM who received systemic therapy including chemotherapy or hormonal
therapy and ZOL 4 mg i.v. q4 weeks participated in our study
(Table I).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Study group n
(%) | Comparison group n
(%) |
|---|
| Overall | 40 (100) | 11 (100) |
| Histology | | |
| Ductal | 24 (60.0) | 6 (54.5) |
| Lobular | 11 (27.5) | 4 (36.4) |
| Others | 5 (12.5) | 1 (9.1) |
| ER status | | |
| Negative | 6 (15) | 4 (36.4) |
| Positive | 34 (85) | 7 (63.6) |
| PR status | | |
| Negative | 16 (40) | 6 (54.5) |
| Positive | 24 (60) | 5 (45.5) |
| HER2 status | | |
| Negative 1 | 33 (82.5) | 9 (81.8) |
| Positive 2 | 6 (15.0) | 1 (9.1) |
| Unknown 3 | 1 (2.5) | 1 (9.1) |
| Metastatic site | | |
| Bone | 21 (52.5) | 0 (0) |
| Visceral and
bone | 19 (47.5) | 11 (100) |
| Metastatic site | | |
| One site | 7 (17.5) | 2 (9.1) |
| Multiple
sites | 33 (82.5) | 9 (81.8) |
| No. of bone
metastases | | |
| ≤3 | 11 (27.5) | 0 (0) |
| ≥3 | 29 (72.5) | 0 (0) |
| Therapeutic
setting | | |
| First-line | 34 (85) | 6 (54.5) |
| Second-line | 4 (10) | 3 (18.2) |
| Third-line or
more | 2 (5) | 2 (18.2) |
| Clinical
response | | |
| CR/PR/SD | 30 (75) | |
| PD | 10 (25) | |
Blood (5 ml) was collected at the start of the study
and every 3 months for one year for the analysis of levels of PINP,
ICTP, CEA and CA 15-3 using radioimmunoassays and ELISA technique.
Imaging of BM was performed at the same time points.
The trial was initiated after the approval of the
Institutional Ethics Committee Review Board.
Eligibility criteria
The eligibility criteria were as follows:
histologically confirmed breast cancer disease; patient age ≥18
years; measurable or evaluable BM; predicted life expectancy ≥3
months; Eastern Cooperative Oncology Group (ECOG) scores for
performance status of 0–2; no severe uncontrolled co-morbidities or
medical conditions; no second malignancies.
Patients had either a relapse of breast cancer
diagnosed years before and were to start a new systemic therapy or
they had a documented progressive breast cancer before receiving a
new endocrine, chemo- or experimental therapy in combination with
ZOL. Prior adjuvant treatment, radiation or any other treatment of
metastatic disease were permitted. ZOL in the metastatic setting
was not allowed. Further exclusion criteria were other malignancies
except breast cancer.
Response criteria
Before starting a new treatment, patients underwent
an evaluation of metastatic sites, particularly BM by bone
scintigraphy, X-ray and/or computer tomography. Blood samples were
collected for laboratory evaluations, including assaying of plasma
CEA and CA 15-3 levels as well as for the assessment of PINP and
ICTP. Re-evaluation of disease status was carried out using the
same techniques every 12 weeks for one year.
Response to therapy was evaluated according to the
Response Evaluation Criteria in Solid Tumors (RECIST); complete
response (CR), disappearance of all target lesions; partial
response (PR), at least 30% decrease in the sum of the LD (longest
diameter) of target lesions, taking as reference the baseline sum
of the LDs; progressive disease (PD), at least 20% increase in the
sum of the LDs of the target lesions, taking as reference the
smallest sum of the LDs recorded since the treatment started or the
appearance of one or more new lesions; stable disease (SD), neither
sufficient shrinkage to qualify for PR nor sufficient increase to
qualify for PD, taking as reference the smallest sum of the LDs
since the treatment started.
Sampling of serum
Blood (2×5 ml) was collected with an
S-Monovette® (Sarstedt AG & Co.) at the start of the
study and every 3 months for one year for the analysis of PINP,
ICTP, CEA and CA 15-3. The samples were processed immediately and
the serum was stored at −80°C until further examination.
Determination of markers of bone
turnover
PINP and ICTP were assayed by radioimmunoassay (RIA)
(Orion Diagnostica, Oulunsalo, Finland). The reference range for
PINP was 19–84 μg/l and the reference range for ICTP was 1.8–5
μg/l. The samples for bone marker measurement were sent to and
analyzed by an extern laboratory (Department of Medical
Microbiology and Infectious Diseases, Medical Care Centre, Dr Stein
and colleagues, Mönchengladbach, Germany).
Determination of serum tumor markers
CEA and CA 15-3 were determined using the Elecsys
CEA/CA 15-3 immunoassays (Roche, Mannheim, Germany) for the
quantitative determination in human serum and plasma. The serial
measurement of CEA/CA 15-3 is intended to aid in the management of
cancer patients. These assays were performed in the central
laboratory of our University Hospital on Cobas®
immunoassay analyzers according to the manufacturer’s instructions.
The central laboratory has a valid certification for the
performance of these assays following international guidelines. The
reference range for CEA was <5 ng/ml, and the reference range
for CA 15-3 was <35 U/ml.
Statistical consideration
Statistical analyses were carried out using SAS v.
9.2 (SAS Institute Inc., Cary, NC). Differences in continuous
parameters between the groups were tested by the non-parametric
Mann-Whitney U test, and changes within groups were evaluated using
the Wilcoxon sign rank test. Differences in proportions were tested
using Chi-square statistics or Fisher’s exact test, as appropriate.
A P-value of <0.05 was regarded as statistically significant.
The statistical significance of the differences in
receiver-operating characteristic (ROC) analysis was determined
using the method developed by DeLong, DeLong and
Clarke-Pearson.
Results
Characteristics of the study
population
Patient ages ranged from 36 to 85 years (median 60).
A total of 40 patients were enrolled since January 2008. Ten out of
40 (25%) were premenopausal and 30 out of 40 (75%) were
postmenopausal. The patient characteristics are documented in
Table I. Thirty-four out of 40
patients (85%) had a primary metastatic breast cancer, 4 out of 40
(10%) received second-line therapy and 2 out of 40 (5%) received
third-line or more in the metastatic setting. Most patients (60%)
had ductal breast cancer. Moderately and poorly differentiated
tumors were predominant (data not shown). Twenty-four out of 40
(76.5%) of the primary tumors were ER- and PR-positive,
respectively, and only 15% (6 out of 40) had an overexpression of
HER2 (Dako score 3+). Nineteen out of 40 patients (47.5%) had
visceral and non-visceral metastases, 21 out of 40 patients (52.5%)
only BM. In 29 out of 40 patients (72.5%) >3 BM were confirmed
by imaging and 11 out of 40 patients (27.5%) presented with ≤3 BM.
Patients received different chemotherapeutic treatments including
anthracyclines, taxanes, capecitabine, vinorelbine and 5-FU (data
not shown). After starting measurements of PINP, ICTP and tumor
markers, patients underwent individual chemotherapeutic or hormonal
treatment depending on their pretreatment and the addition of
ZOL.
The control group consisted of 11 patients who had
visceral metastases only and no BM. Median age of the control group
was 66 (43–85) years.
Stratification of results
In total, 40 patients were monitored for PINP, ICTP,
CA 15-3 and CEA during chemotherapy over a time period of 12
months. Blood samples were collected and imaging techniques were
performed every three months. Based on the total number of
collected blood samples, only baseline and the data evaluated at
the end of the study were statistically analyzed. The results for
PINP and ICTP were analyzed in relation to clinical response
(stratified into responders and non-responders), the number of BM
(≤3 or >3) and tumor markers. Responders were defined as
patients with complete remission (CR), partial remission (PR) and
stable disease (SD). Non-responders included patients with
progressive disease (PD).
Comparison group
The median value of ICTP was 5.44 μg/l (IQR 3–12.8)
and the median value of PINP was 55.67 μg/l (IQR 17.9–112.3). The
CA 15-3 level was 123.27 U/ml (IQR 19–682) and the CEA level was
9.23 ng/ml (IQR 1.5–61.8).
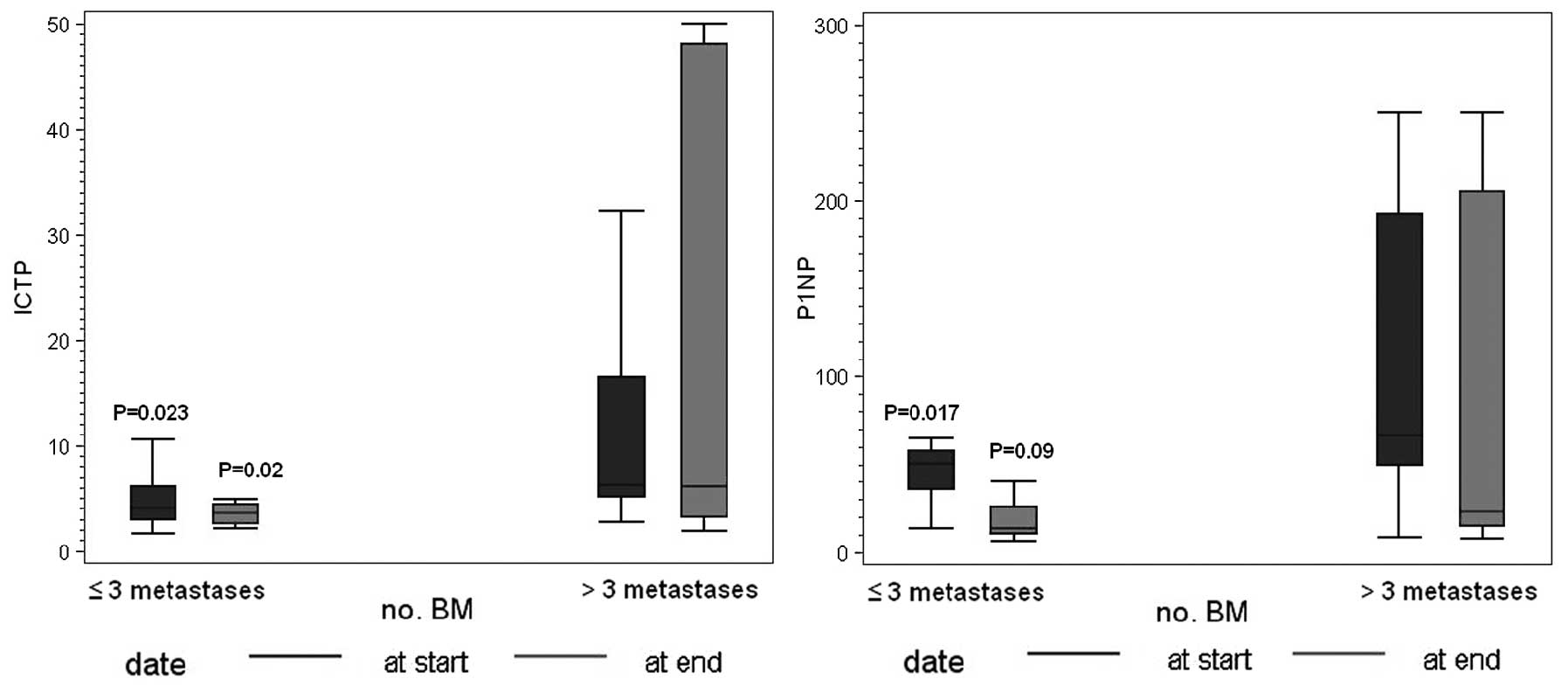
Correlation of PINP and ICTP with number
of BM
The relationship among PINP, ICTP and the number of
BM is shown in Fig. 1. Twenty-nine
patients had >3 BM, 11 patients has ≤3. Serum PINP and ICTP
concentrations were significantly different when patients were
stratified into groups according to whose with >3 BM (median
value ICTP, 6.2 μg/l; IQR 4.8–16.8 and PINP, 66.6 μg/l; IQR
49.4–221) and ≤3 BM (median value ICTP, 4 μg/l; IQR 3–6.1 and PINP,
50.4 μg/l; IQR 35.6–54.2).
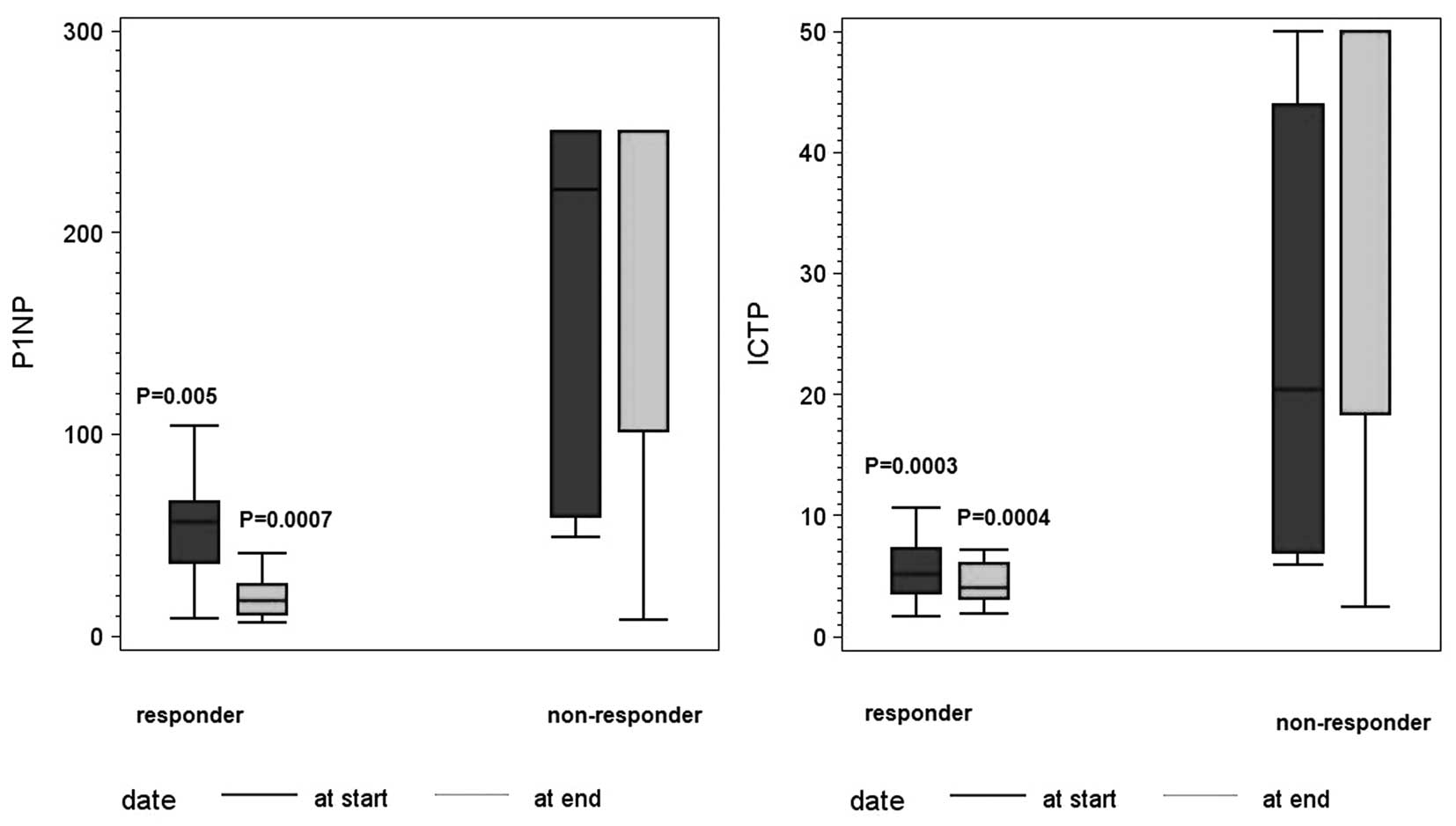
Correlation of PINP and ICTP with the
response to therapy
The levels of PINP and ICTP, determined at the start
and the end of the follow-up period, were analyzed with respect to
clinical follow-up results. At the start of the study, the median
value for ICTP was 6 μg/l (IQR 3.9–12.7) and the median value for
PINP was 58.7 μg/l (IQR 43.5–164). Thirty out of 40 patients showed
response to therapy, and 10 out of 40 patients had progressive
disease. The median value of ICTP at the start of the study for the
responders was 5.1 μg/l (IQR 3.6–7.2) vs. 20.4 μg/l (IQR 6.9–43.9)
for non-responders. The median value of PINP at the start of the
study for the responders was 50.4 μg/l (IQR 36.4–66.6) vs. 221 μg/l
(IQR 59–250) for the non-responders (P=0.0003 for ICTP and P=0.005
for PINP) (Fig. 2). At the end of
the study, the mean value of ICTP was 4.1 μg/l (IQR 3.1–6) for the
responders vs. 50 μg/l (IQR 18.4–50) for non-responders. In
contrast, the median value of PINP at the study end was 17.3 μg/l
(IQR 10.8–25.6) for responders vs. 250 μg/l (IQR 101.2–250) for
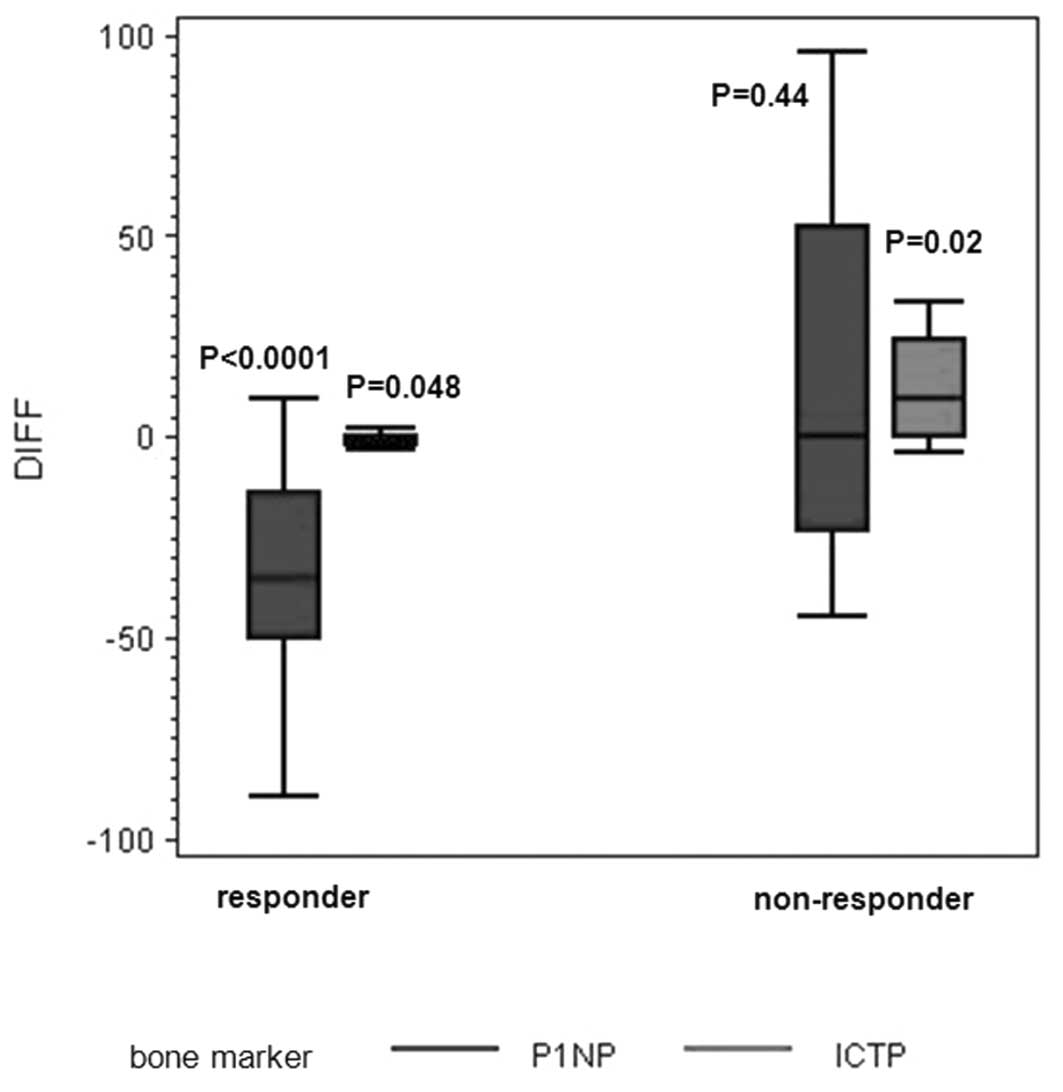
non-responders (P=0.0004 for ICTP and P=0.0007 for PINP) (Fig. 2). Changes in expression levels of
the markers from the start to the end of the study were
significantly different from zero for PINP (P<0.0001) and ICTP
(P=0.048) (decrease) for responders and ICTP (P=0.02) for
non-responders (increase, Fig.
3).
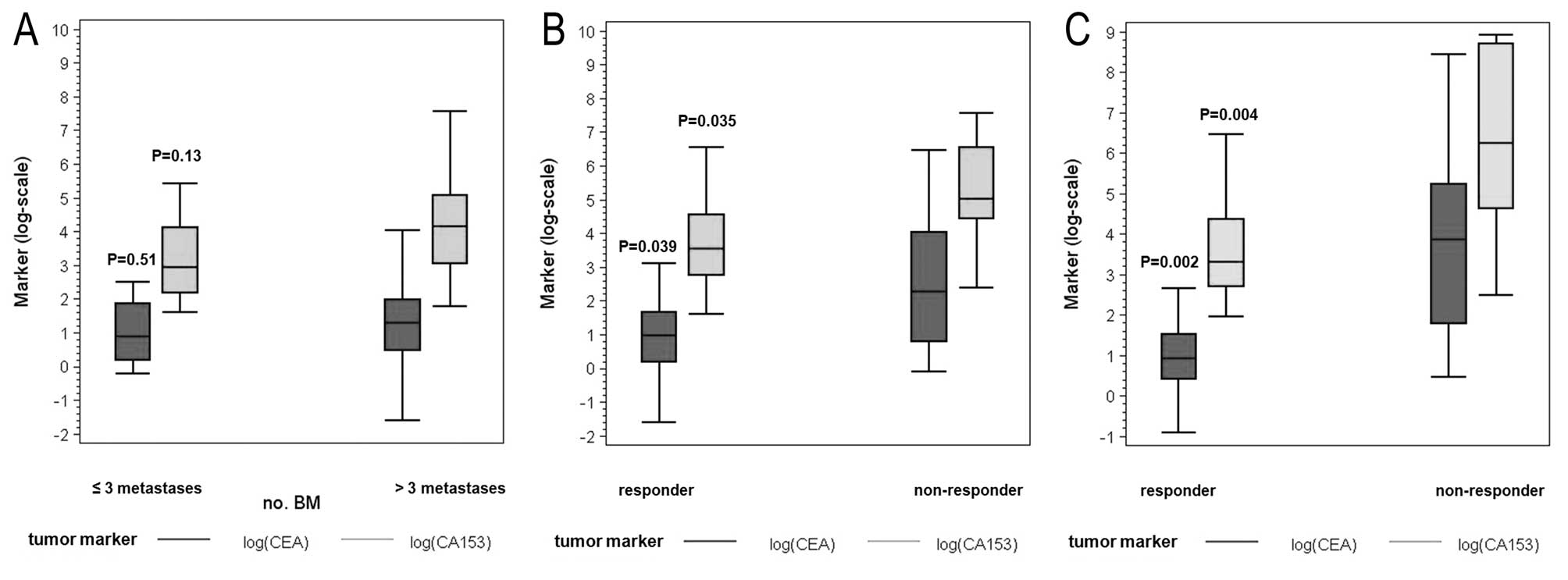
Correlation between tumor markers and
number of bone metastases
As shown in Fig. 4A
no significant differences were noted when tumor marker levels were
analyzed with respect to the number of BM (CEA P=0.51 and CA 15-3
P=0.13). Median value of CEA for patients with >3 BM was 3.6
ng/ml (IQR 1.6–7.3) and the median value of CEA for patients with
≤3 BM was 2.4 ng/ml (IQR 1.2–6.5). Median value of CA 15-3 for
patients with >3 BM was 64 U/ml (IQR 21–160) vs. 19 U/ml (IQR
9–61) for patients with ≤3 BM.
Correlation between tumor markers and
response to therapy
Median value for CEA determined at the start of the
therapy was 2.7 ng/ml (IQR 1.2–5.2) for responders and 11.7 ng/ml
(IQR 2.2–57.1) for non-responders (P=0.039). The value for CA 15-3
was 34.5 U/ml (IQR 16.0–94) for responders and 149 U/ml (IQR
85–698) for non-responders (P=0.035). The values determined at the
end of the follow-up period were 2.5 ng/ml (IQR 1.5–4.6) for
responders vs. 62.7 ng/ml (IQR 6–187) for non-responders for CEA
and 27.5 U/ml (IQR 15–79) for responders vs. 603 U/ml (IQR
102–6000) for non-responders for CA 15-3 (P=0.002 for CEA and
P=0.004 for CA 15-3). Tumor marker levels significantly
distinguished responders from non-responders (Fig. 4B and C).
Comparison of markers of bone turnover
and tumor markers
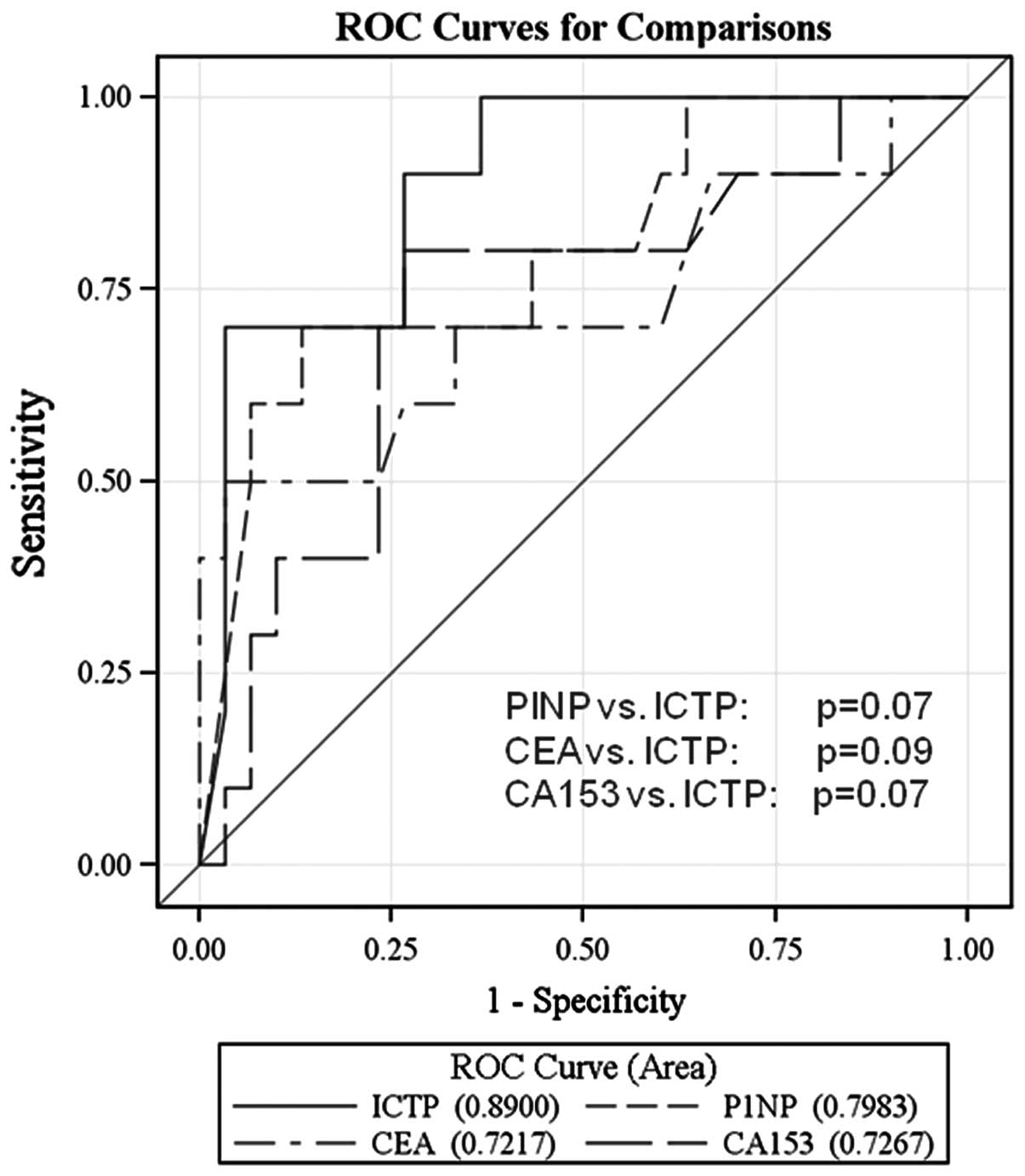
As shown in Fig. 5,
ROC analyses demonstrated that measurement of PINP and ICTP
revealed more specificity and sensitivity than the determination of
CA 15-3 and CEA. Although statistical significance was not reached,
we observed a tendency for best discrimination with ICTP (ICTP vs.
PINP, P=0.07; ICTP vs. CEA, P=0.09 and ICTP vs. CA 15-3; P=0.07,
respectively).
Correlation between the number of BM and
response to therapy
Twenty-nine out of 40 patients (72.5%) showed >3
BM confirmed by imaging and 11 out of 40 patients (27.5%) presented
with ≤3 BM. Thirty out of the 40 patients demonstrated response to
therapy, while 10 out of 40 patients did not. All non-responders
had >3 BM at baseline (P=0.04).
Correlation of the results with
menopausal status
Thirty out of 40 patients were postmenopausal, 23
out of 30 were responders and 7 out of 30 non-responders,
respectively. The premenopausal cohort showed 7 out of 10 responses
and 3 out of 10 had no response, comparable to the postmenopausal
group (P=0.69). While expression of the markers at the start of the
study did not differ between the premenopausal and postmenopausal
groups (data not shown), they were noted in the findings of the
postmenopausal patients of the full study group, being higher
non-responders. The median value of ICTP at the start of the study
for the responders was 5.7 vs. 23.8 μg/l for non-responders. The
median value of PINP was 63.4 vs. 192.0 μg/l, respectively, with
P=0.006 for ICTP and P=0.04 for PINP. For tumor markers, the median
CEA was 2.8 ng/ml for responders and 18.2 ng/ml for non-responders
(P=0.014) and the values for CA 15-3 were 35 U/ml and 150 U/ml,
respectively (P=0.08).
Discussion
Several studies have shown that the serum of
patients with metastatic bone disease contains significantly higher
concentrations of markers of bone resorption and formation than
age-matched controls (12). In our
study, patients with more than three involved sites of BM had
significantly higher serum levels of PINP and ICTP than patients
with three or fewer sites of involved skeletal metastases as
compared to a group without any BM. Furthermore, we demonstrated a
significant relationship between PINP, ICTP and clinical outcome.
When patients were stratified into responders and non-responders,
significant differences in the expression levels of the markers
were obtained for both groups. Non-responders had significantly
more than three BM. Comparing markers of bone turnover and tumor
markers, with regard to the best diagnostic efficacy using
ROC-analysis, ICTP and PINP revealed more specificity and
sensitivity than CA 15-3 and CEA. No correlation between menopausal
status and response to therapy was determined.
At present, the detection and monitoring of BM
relies on imaging studies such as bone scan, radiography, CT scan
or MRT scan. Besides being limited by low sensitivity and
specificity, bone scans may be unable to detect osteolytic
metastasis and are affected by the flare phenomenon (13,14).
Furthermore, these imaging techniques are time-consuming and costly
for patients. To date, none of the biochemical markers of bone
turnover are able to monitor the development and progression of
metastatic bone disease as accurately as do well-established bone
imaging techniques (15). When used
alone, they do not possess sufficient diagnostic and prognostic
specificity to assess the overall treatment outcome for patients
with cancer (16). However, in
combination with other diagnostic techniques and prognostic
markers, these biochemical markers of bone turnover may be useful
tools in the assessment of cancer with metastatic bone disease
(15–20).
In our study, levels of PINP and ICTP were elevated
in patients with osseous MBC as compared to patients without bone
involvement. Similar results were previously confirmed by Pollmann
et al, who showed elevated PINP levels for osseous MBC
patients (21). The quality of life
of MBC patients could be preserved by determining the expression of
bone marker levels before indicating imaging techniques to follow
up therapeutic response. Furthermore, our data revealed that PINP
and ICTP reflect the therapeutic response of BM. Thus, assessing
serum PINP and ICTP may replace primary techniques in the
future.
In our analyses, there was a strong relationship
between serum levels of PINP and ICTP and the extent of BM but no
association with the presence of visceral metastases only. Similar
data were shown by Costa et al, who found an association
between bone marker concentrations (N-telopeptides and bone
alkaline phosphatase) and the extent of BM in cancer patients but
not with the presence of extraskeletal metastases (22). Berruti et al offered another
possible use of these markers in patients with metastatic bone
disease, i.e., to assess both tumor burden and extent of true bone
pain (23). In their retrospective
study, they showed that markers of bone turnover were significantly
associated with the extent of disease or tumor burden by
demonstrating a relationship between the concentrations of markers
(serum bone alkaline phosphatase, the C-terminal telopeptide of
type I collagen, and urine deoxypyridinoline) and the number of
skeletal sites involved (skull, spine, femur, ribs, pelvis, and
others). The more skeletal involvement of the disease, the higher
marker concentration they found (24). Our data define this association more
precisely, showing that the serum marker expression levels of PINP
and ICTP were significantly higher at baseline when more than three
skeletal sites were involved.
Other reports have also shown that resorption and
formation markers such as urinary pyridinoline (Py) and
deoxypyridinoline (dPy) correlate with the number of lesions and/or
the number of skeletal segments involved in patients with prostate
cancer (25). Recent studies
reported that bone resorption markers may detect an early
progression in patients with stable disease in imaging studies
(26,27). Furthermore, a high level of
biochemical markers as NTX (N-terminal telopeptide) have been
associated with increased skeletal-related events, disease
progression and death compared with normal NTX levels in patients
with malignant skeletal metastases (17,28).
Our data confirm that there is a high evidence to suggest that ICTP
and PINP can be used to determine the degree of skeletal
involvement in MBC. In our analysis, PINP and ICTP demonstrated
utility for the differentiation of patients with BM from patients
without metastatic spread to the bone, while tumor marker
expression was not as effective. Measurement of the biochemical
markers to determine the extension of skeletal involvement could
change and improve strategies in the clinical management of
metastatic breast cancer. More than three BM correlated
significantly with no response to therapy. High levels of PINP and
ICTP at baseline or at any time during the course of the disease
may indicate that more aggressive intervention strategies in
addition to ZOL therapy are needed to prevent skeletal morbidity.
It may be appropriate, therefore, for marker levels to be assessed
at regular intervals during the course of metastatic bone disease.
In our analysis, PINP and ICTP presented a relationship with
clinical outcome in patients with osseous MBC.
In clinical practice, measurement of tumor markers
such as CEA and CA 15-3 are commonly used for monitoring therapy
response to a variety of breast cancer-related therapies. CA 15-3
appears to be more sensitive than CEA when the primary tumor is
diagnosed and when metastasis is discovered. In this last
situation, it allowed monitoring in two-thirds of the cases
(29). Other studies recommend the
combination of both markers to improve diagnostic sensitivity
(30,31). We demonstrated no correlation
between tumor marker levels and extent of metastatic spread,
particularly no correlation to the number of BM. In contrast, tumor
marker levels significantly distinguished responders from
non-responders during systemic therapy added to ZOL.
In conclusion, in contrast to serum tumor markers,
the determination of levels of PINP and ICTP allows inferences in
regards to the number of BM and appears to be a useful tool for
monitoring metastatic breast cancer patients undergoing
bisphosphonate therapy with ZOL for treatment of BM.
PINP and ICTP, as well as the number of BM were
significantly associated with clinical outcome and therapeutic
response. Thus, increased levels of PINP or ICTP may be an early
marker for therapy failure in patients with MBC. This could provide
an opportunity for early therapeutic management that could extend
the overall survival of patients and provide meaningful information
concerning patients with MBC in daily practice.
Acknowledgements
This study was supported by a research grant from
Novartis Pharma GmbH, Germany. We thank Gisela Koestner and Jens
Rasch for their excellent technical assistance.
Abbreviations:
|
MBC
|
metastatic breast cancer
|
|
BM
|
bone metastases
|
|
PINP
|
aminoterminal procollagen propeptide
of type I collagen
|
|
ICTP
|
carboxyterminal telopeptide of type I
collagen
|
|
PD
|
progressive disease
|
|
PR
|
partial remission
|
|
SD
|
stable disease
|
|
CR
|
complete remission
|
|
ZOL
|
zoledronic acid
|
|
BP
|
bisphosphonate
|
|
CEA
|
carcinoembryonic antigen
|
|
CA 15-3
|
cancer antigen 15-3
|
|
IQR
|
interquartile range
|
References
|
1
|
Plebani M, Bernardi D, Zanninotto M, De
Paoli M, Secchiero S and Sciacovelli L: New and traditional serum
markers of bone metabolism in the detection of skeletal metastases.
Clin Biochem. 29:67–72. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosenthal DI: Radiologic diagnosis of bone
metastases. Cancer. 80:1595–1607. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jung K, Lein M, Stephan C, Von HK,
Semjonow A and Sinha P: Comparison of 10 serum bone turnover
markers in prostate carcinoma patients with bone metastatic spread:
diagnostic and prognostic implications. Int J Cancer. 111:783–791.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O’Sullivan JM and Cook GJ: A review of the
efficacy of bone scanning in prostate and breast cancer. Q J Nucl
Med. 46:152–159. 2002.PubMed/NCBI
|
|
5
|
Costa L, Demers LM, Gouveia-Oliveira A,
Schaller J, Costa EB, Moura MC and Lipton A: Prospective evaluation
of the peptide-bound collagen type I cross-links N-telopeptide and
C-telopeptide in predicting bone metastases status. J Clin Oncol.
20:850–856. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lipton A, Costa L, Ali S and Demers L: Use
of markers of bone turnover for monitoring bone metastases and the
response to therapy. Semin Oncol. 28(4 Suppl 11): 54–59. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Green JR: Bisphosphonates: preclinical
review. Oncologist. 9(Suppl 4): 3–13. 2004. View Article : Google Scholar
|
|
8
|
Coleman RE: The clinical use of bone
resorption markers in patients with malignant bone disease. Cancer.
94:2521–2533. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roodman GD: Mechanisms of bone metastases.
N Engl J Med. 350:1655–1664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosen LS, Gordon D, Kaminski M, Howell A,
Belch A, Mackey J, Apffelstaedt J, Hussein MA, Coleman RE, Reitsma
DJ, Chen BL and Seaman JJ: Long-term efficacy and safety of
zoledronic acid in the treatment of skeletal metastases in patients
with non-small cell lung carcinoma and other solid tumors: a
randomized, phase III, double-blind, placebo-controlled trial.
Cancer. 98:1735–1744. 2003.PubMed/NCBI
|
|
11
|
Saad F, Gleason D, Murray R, Tchekmedyian
S, Venner P, Lacombe L, Chin J, Vinholes J, Goas A and Zheng M; for
the Zoledronic Acid Prostate Cancer Study Group. Long-term efficacy
of zoledronic acid for the prevention of skeletal complications in
patients with metastatic hormone-refractory prostate cancer. J Natl
Cancer Inst. 96:879–882. 2004. View Article : Google Scholar
|
|
12
|
Pecherstorfer M, Zimmer-Roth I, Schilling
T, Woitge HW, Schmidt H, Baumgartner G, et al: The diagnostic value
of pyridinium cross-links of collagen, serum total alkaline
phosphatase, and urinary calcium excretion in neoplastic bone
disease. J Clin Endocrinol Metab. 80:97–103. 1995.
|
|
13
|
Vogel CL, Schoenfelder J, Shemano I, Hayes
DF and Gams RA: Worsening bone scan in the evaluation of antitumor
response during hormonal therapy of breast cancer. J Clin Oncol.
13:1123–1128. 1995.PubMed/NCBI
|
|
14
|
Pollen JF, Witztum KF and Ashburn WL: The
flare phenomenon on radionuclide bone scan in metastatic prostate
cancer. AJR Am J Roentgenol. 142:773–776. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang Q and Ouyang X: Biochemical-markers
for the diagnosis of bone metastasis: a clinical review. Cancer
Epidemiol. 6:94–98. 2011.
|
|
16
|
Seibel MJ: Clinical use of markers of bone
turnover in metastatic bone disease. Nat Clin Pract Oncol.
2:504–517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Coleman R, Brown J, Terpos E, Lipton A,
Smith MR, Cook R and Major P: Bone markers and their prognostic
value in metastatic bone disease: clinical evidence and future
directions. Cancer Treat Rev. 34:629–639. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seibel MJ: The use of molecular markers of
bone turnover in the management of patients with metastatic bone
disease. Clin Endocrinol. 68:839–849. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cremers S and Garnero P: Biochemical
markers of bone turnover in the clinical development of drugs for
osteoporosis and metastatic bone disease: potential uses and
pitfalls. Drugs. 66:2031–2058. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brown JE, Cook RJ, Major P, Lipton A, Saad
F, Smith M, Lee KA, Zheng M, Hei YJ and Colemann RE: Bone turnover
markers as predictors of skeletal complications in prostate cancer,
lung cancer, and other solid tumors. J Natl Cancer Inst. 97:59–69.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pollmann D, Siepmann S, Geppert R,
Wernecke KD, Possinger K and Lüftner D: The amino-terminal
propeptide (PINP) of type I collagen is a clinically valid
indicator of bone turnover and extent of metastatic spread in
osseous metastatic breast cancer. Anticancer Res. 27:1853–1862.
2007.PubMed/NCBI
|
|
22
|
Costa L, Demers LM, Speicher T, Gouveia
Curley E, Harvey H, et al: Biochemical markers of bone turnover
correlate with the extent of metastatic bone disease. In: American
Society of Clinical Oncology 35th Annual Meeting; Philadelphia.
1999
|
|
23
|
Berruti A, Dogliotti L, Gorzegno G, Torta
M, Tampellini M, Tucci M, Cerutti S, Frezet MM, Stivanello M,
Sacchetto G and Angeli A: Differential patterns of bone turnover in
relation to bone pain and disease extent in bone in cancer patients
with skeletal metastases. Clin Chem. 45:1240–1247. 1999.PubMed/NCBI
|
|
24
|
Demers LM: Biochemical markers in the
management of patients with metastatic bone disease. Clin Chem.
45:1131–1132. 1999.PubMed/NCBI
|
|
25
|
Ikeda I, Miura T and Kondo I: Pyridinium
cross-links as urinary markers of bone metastases in patients with
prostate cancer. Br J Urol. 77:102–106. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Costa L, Demers LM, Gouveia A, Schalla J,
Costa EB, Demoura MC and Lipton A: Correlation of urinary
N-telopeptide levels with progression of bone metastases: a
prospective study (Abstract 12). Cancer. 88(Suppl): 30942000.
|
|
27
|
Zafeirakis A: Collagenous and
non-collagenous biochemical markers of bone metastases from
prostate cancer. Hippokratia. 14:164–169. 2010.PubMed/NCBI
|
|
28
|
Lipton A, Cook R, Saad F, Major P, Garnero
P, Terpos E, Brown JE and Coleman RE: Normalization of bone markers
is associated with improved survival and elevated bone resorption
receiving zoledronic acid. Cancer. 113:193–201. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pons-Anicet DMF, Krebs BP, Mira R and
Namer M: Value of CA 15:3 in the follow-up of breast cancer
patients. Br J Cancer. 55:567–569. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yildiz M, Oral B, Bozkurt M and Cobaner A:
Relationship between bone scintigraphy and tumor markers in
patients with breast cancer. Ann Nucl Med. 18:501–505. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Buamah PK, Bent DJ, Bodger WAH and Skillen
AW: A profile of serum CA 15-3, carcinoembryonic antigen, alkaline
phosphatase, and γ-glutamyl transferase levels in patients with
breast cancer. J Surg Oncol. 53:84–87. 1993.PubMed/NCBI
|