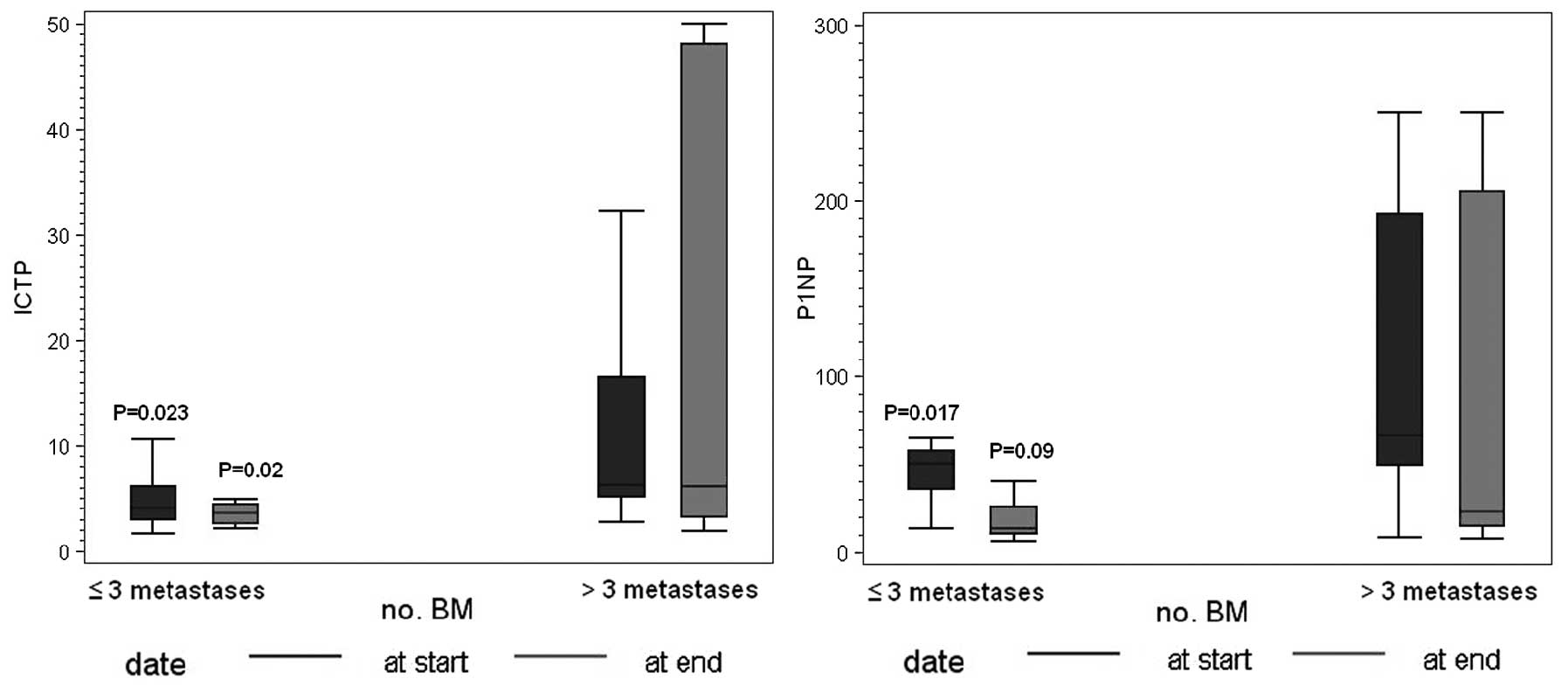
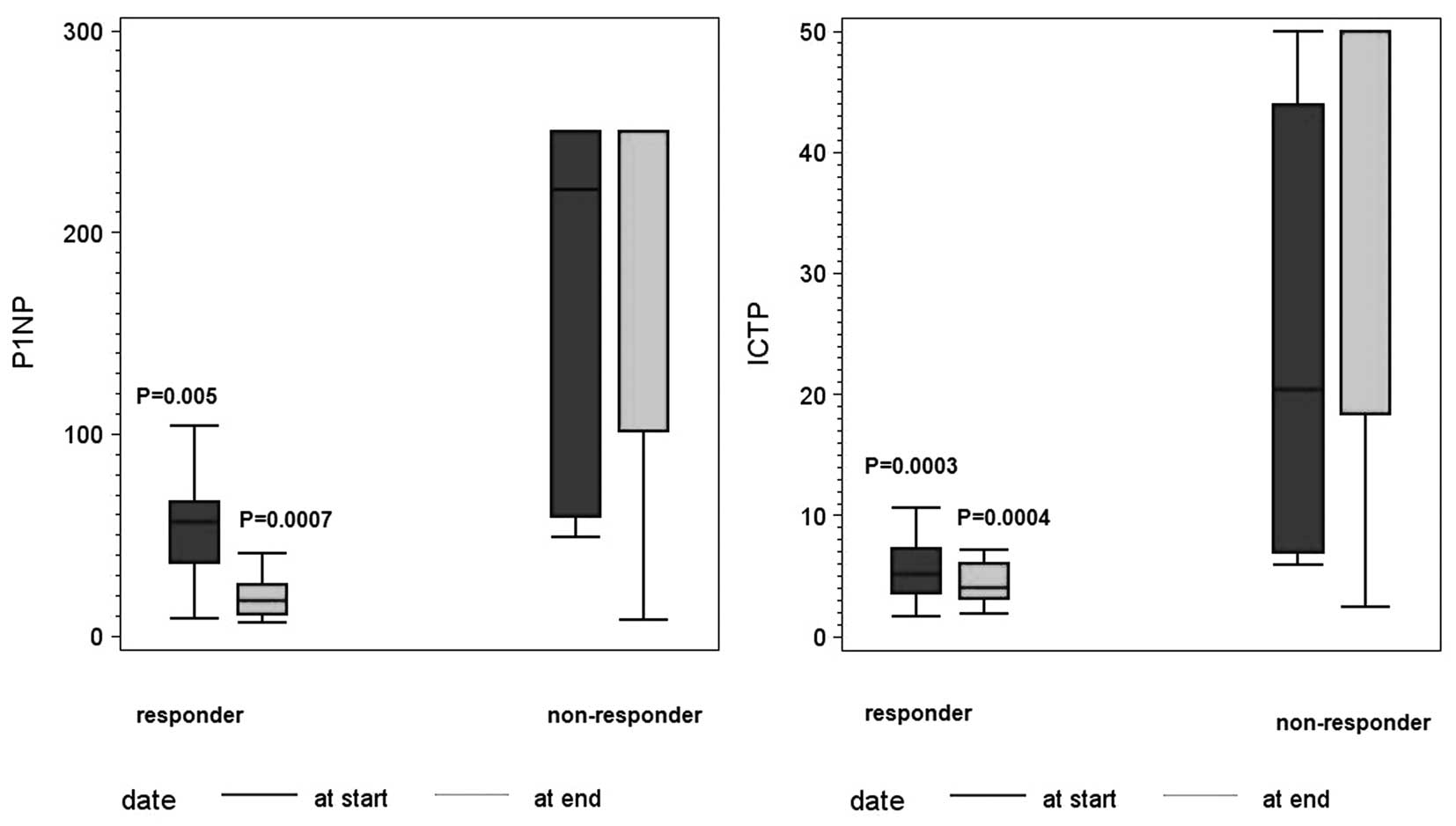
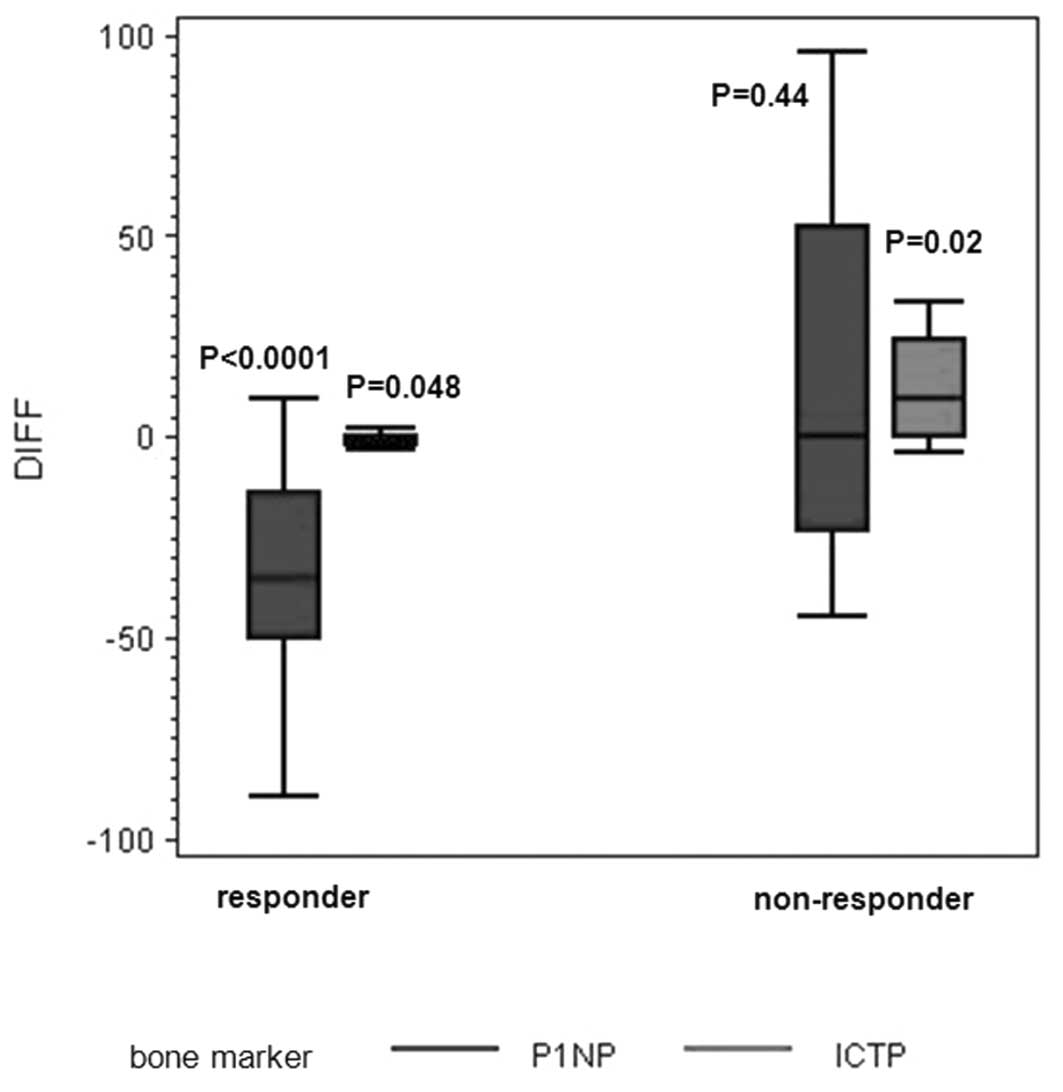
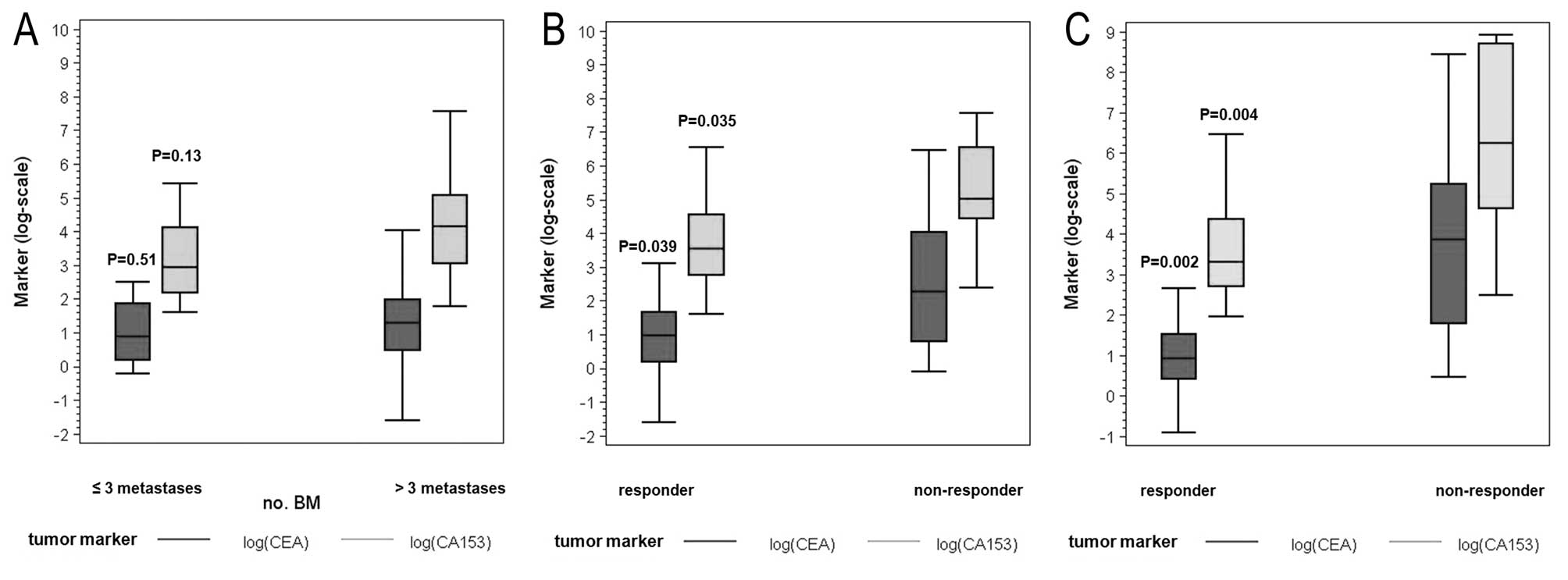
|
1
|
Plebani M, Bernardi D, Zanninotto M, De
Paoli M, Secchiero S and Sciacovelli L: New and traditional serum
markers of bone metabolism in the detection of skeletal metastases.
Clin Biochem. 29:67–72. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosenthal DI: Radiologic diagnosis of bone
metastases. Cancer. 80:1595–1607. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jung K, Lein M, Stephan C, Von HK,
Semjonow A and Sinha P: Comparison of 10 serum bone turnover
markers in prostate carcinoma patients with bone metastatic spread:
diagnostic and prognostic implications. Int J Cancer. 111:783–791.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O’Sullivan JM and Cook GJ: A review of the
efficacy of bone scanning in prostate and breast cancer. Q J Nucl
Med. 46:152–159. 2002.PubMed/NCBI
|
|
5
|
Costa L, Demers LM, Gouveia-Oliveira A,
Schaller J, Costa EB, Moura MC and Lipton A: Prospective evaluation
of the peptide-bound collagen type I cross-links N-telopeptide and
C-telopeptide in predicting bone metastases status. J Clin Oncol.
20:850–856. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lipton A, Costa L, Ali S and Demers L: Use
of markers of bone turnover for monitoring bone metastases and the
response to therapy. Semin Oncol. 28(4 Suppl 11): 54–59. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Green JR: Bisphosphonates: preclinical
review. Oncologist. 9(Suppl 4): 3–13. 2004. View Article : Google Scholar
|
|
8
|
Coleman RE: The clinical use of bone
resorption markers in patients with malignant bone disease. Cancer.
94:2521–2533. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roodman GD: Mechanisms of bone metastases.
N Engl J Med. 350:1655–1664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosen LS, Gordon D, Kaminski M, Howell A,
Belch A, Mackey J, Apffelstaedt J, Hussein MA, Coleman RE, Reitsma
DJ, Chen BL and Seaman JJ: Long-term efficacy and safety of
zoledronic acid in the treatment of skeletal metastases in patients
with non-small cell lung carcinoma and other solid tumors: a
randomized, phase III, double-blind, placebo-controlled trial.
Cancer. 98:1735–1744. 2003.PubMed/NCBI
|
|
11
|
Saad F, Gleason D, Murray R, Tchekmedyian
S, Venner P, Lacombe L, Chin J, Vinholes J, Goas A and Zheng M; for
the Zoledronic Acid Prostate Cancer Study Group. Long-term efficacy
of zoledronic acid for the prevention of skeletal complications in
patients with metastatic hormone-refractory prostate cancer. J Natl
Cancer Inst. 96:879–882. 2004. View Article : Google Scholar
|
|
12
|
Pecherstorfer M, Zimmer-Roth I, Schilling
T, Woitge HW, Schmidt H, Baumgartner G, et al: The diagnostic value
of pyridinium cross-links of collagen, serum total alkaline
phosphatase, and urinary calcium excretion in neoplastic bone
disease. J Clin Endocrinol Metab. 80:97–103. 1995.
|
|
13
|
Vogel CL, Schoenfelder J, Shemano I, Hayes
DF and Gams RA: Worsening bone scan in the evaluation of antitumor
response during hormonal therapy of breast cancer. J Clin Oncol.
13:1123–1128. 1995.PubMed/NCBI
|
|
14
|
Pollen JF, Witztum KF and Ashburn WL: The
flare phenomenon on radionuclide bone scan in metastatic prostate
cancer. AJR Am J Roentgenol. 142:773–776. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang Q and Ouyang X: Biochemical-markers
for the diagnosis of bone metastasis: a clinical review. Cancer
Epidemiol. 6:94–98. 2011.
|
|
16
|
Seibel MJ: Clinical use of markers of bone
turnover in metastatic bone disease. Nat Clin Pract Oncol.
2:504–517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Coleman R, Brown J, Terpos E, Lipton A,
Smith MR, Cook R and Major P: Bone markers and their prognostic
value in metastatic bone disease: clinical evidence and future
directions. Cancer Treat Rev. 34:629–639. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seibel MJ: The use of molecular markers of
bone turnover in the management of patients with metastatic bone
disease. Clin Endocrinol. 68:839–849. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cremers S and Garnero P: Biochemical
markers of bone turnover in the clinical development of drugs for
osteoporosis and metastatic bone disease: potential uses and
pitfalls. Drugs. 66:2031–2058. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brown JE, Cook RJ, Major P, Lipton A, Saad
F, Smith M, Lee KA, Zheng M, Hei YJ and Colemann RE: Bone turnover
markers as predictors of skeletal complications in prostate cancer,
lung cancer, and other solid tumors. J Natl Cancer Inst. 97:59–69.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pollmann D, Siepmann S, Geppert R,
Wernecke KD, Possinger K and Lüftner D: The amino-terminal
propeptide (PINP) of type I collagen is a clinically valid
indicator of bone turnover and extent of metastatic spread in
osseous metastatic breast cancer. Anticancer Res. 27:1853–1862.
2007.PubMed/NCBI
|
|
22
|
Costa L, Demers LM, Speicher T, Gouveia
Curley E, Harvey H, et al: Biochemical markers of bone turnover
correlate with the extent of metastatic bone disease. In: American
Society of Clinical Oncology 35th Annual Meeting; Philadelphia.
1999
|
|
23
|
Berruti A, Dogliotti L, Gorzegno G, Torta
M, Tampellini M, Tucci M, Cerutti S, Frezet MM, Stivanello M,
Sacchetto G and Angeli A: Differential patterns of bone turnover in
relation to bone pain and disease extent in bone in cancer patients
with skeletal metastases. Clin Chem. 45:1240–1247. 1999.PubMed/NCBI
|
|
24
|
Demers LM: Biochemical markers in the
management of patients with metastatic bone disease. Clin Chem.
45:1131–1132. 1999.PubMed/NCBI
|
|
25
|
Ikeda I, Miura T and Kondo I: Pyridinium
cross-links as urinary markers of bone metastases in patients with
prostate cancer. Br J Urol. 77:102–106. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Costa L, Demers LM, Gouveia A, Schalla J,
Costa EB, Demoura MC and Lipton A: Correlation of urinary
N-telopeptide levels with progression of bone metastases: a
prospective study (Abstract 12). Cancer. 88(Suppl): 30942000.
|
|
27
|
Zafeirakis A: Collagenous and
non-collagenous biochemical markers of bone metastases from
prostate cancer. Hippokratia. 14:164–169. 2010.PubMed/NCBI
|
|
28
|
Lipton A, Cook R, Saad F, Major P, Garnero
P, Terpos E, Brown JE and Coleman RE: Normalization of bone markers
is associated with improved survival and elevated bone resorption
receiving zoledronic acid. Cancer. 113:193–201. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pons-Anicet DMF, Krebs BP, Mira R and
Namer M: Value of CA 15:3 in the follow-up of breast cancer
patients. Br J Cancer. 55:567–569. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yildiz M, Oral B, Bozkurt M and Cobaner A:
Relationship between bone scintigraphy and tumor markers in
patients with breast cancer. Ann Nucl Med. 18:501–505. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Buamah PK, Bent DJ, Bodger WAH and Skillen
AW: A profile of serum CA 15-3, carcinoembryonic antigen, alkaline
phosphatase, and γ-glutamyl transferase levels in patients with
breast cancer. J Surg Oncol. 53:84–87. 1993.PubMed/NCBI
|