Introduction
Tissue factor (TF) is known as the primary initiator
of the blood coagulation cascade (1–4).
Transcription of the TF gene (F3) leads to the generation of
TF pre-messenger RNA (pre-mRNA) consisting of six coding exonic
sequences, which are separated by five non-coding introns (5,6).
Splicing of this primary transcript results in the removal of all
introns, thereby, enabling translation of the remaining six exonic
sequences (5). This process yields
what is considered to be the mature ‘full length’ TF (flTF) mRNA
(4,5). However, by alternative splicing the
5th exon may be removed from the TF pre-mRNA, resulting in creation
of the mature alternatively spliced TF (asTF) mRNA (5,7,8).
Ribosomal translation of both mRNA splice variants finally leads to
the generation of two individual TF protein isoforms (5,6). The
membrane-bound flTF was found to be essential for the
thrombogenicity of cells and tissues (2,9). In
contrast, the function of asTF is not well studied. Although asTF
is able to promote FXa generation in the presence of phospholipids,
its pro-coagulant activity is much lower than that of flTF
(4,5). Some studies have suggested that asTF
may be linked to increased tissue growth and angiogenesis (10–13).
Cancer is a leading cause of hospitalization and
death in the world (14). Both
angiogenesis and cell proliferation, which are important for
tumorigenesis and growth, have been shown to be promoted by hypoxic
conditions. Other factors stimulating these processes are monocyte
chemotactic protein-1 (MCP-1, CCL-2), cysteine-rich 61 (Cyr61,
CCN1) or vascular endothelial growth factor (VEGF) (15–17).
Integrin interaction with these factors is often involved in
pro-angiogenic and proliferation-facilitating processes and has
been associated with the pathogenesis of cancer in general
(18–20). Several studies have demonstrated
that the expression of asTF is significantly increased in a variety
of cancer cells (3,14,21).
Moreover, elevated asTF levels were found to be associated with
increased angiogenesis in vivo and to accelerate tumor
growth in mice (12). Recently, van
den Berg et al(13) reported
asTF to boost angiogenesis via integrin signaling in endothelial
cells. Yet, it remains unclear how asTF induces integrin-mediated
angiogenesis and tumor growth.
In the present study, we set out to characterize the
impact of post-transcriptional splicing regulation by Cdc2-like
kinases (Clks) on the modulation of pro-angiogenic properties of
A549 lung cancer cells under hypoxic conditions. In this context,
we investigated the effect of stable asTF overexpression on
expression levels of pro-angiogenic and proliferation-promoting
factors, such as MCP-1, Cyr61 and VEGF in A549 cells. We also
determined the impact of asTF on cell number and angiogenesis.
Overall, our results suggest that
post-transcriptional splicing regulation is able to modulate the
pro-angiogenic potential of A549 cancer cells. Furthermore, the
data imply that this effect is, at least in part, caused by
differential isoform expression of TF and induction of
proliferation-facilitating pro-angiogenic factors, such as MCP-1,
Cyr61 and VEGF, as well as integrin-mediated signaling.
Materials and methods
Cell culture
Human lung carcinoma cells (A549) (PromoCell GmbH,
Heidelberg, Germany) were cultured in RPMI medium [containing 10%
fetal calf serum (FCS); PAA Laboratories GmbH, Pasching, Austria]
at 37°C in a humidified incubator (5% CO2, 95% air).
Human microvascular endothelial cells (HMEC-1) (PromoCell GmbH)
were cultured in endothelial cell growth medium (containing 5% FCS;
PromoCell GmbH). For antibody-mediated inhibition, A549 cells or
cell supernatant were incubated with 5 μg/ml of specific
neutralizing antibodies against Cyr61 (a kind gift from Lester F.
Lau, University of Illinois, Chicago, IL, USA); MCP-1 and VEGF were
provided by R&D Systems (Minneapolis, MN, USA), and flTF
(#4501) was from American Diagnostica Inc. (Greenwich, CT, USA).
Compound-based experiments were performed using the
αvβ3 inhibitor cyclic RGD (cRGD, H-4772 from
Bachem AG, Bubendorf, Switzerland; 10-μM final concentration) or
the Clk1- and Clk4-specific inhibitor KH-CB19 (a generous gift from
Professor Franz Bracher, Department of Pharmacy, Center for Drug
Research, Ludwig Maximilians University of Munich, Munich, Germany)
(22). Controls were stimulated
with 100 nM recombinant human MCP-1 (R&D Systems) or 50 μg/ml
recombinant human asTF (a kind gift from Vladimir Y. Bogdanov,
University of Cincinnati College of Medicine, Cincinnati, OH, USA);
negative controls were not treated. Hypoxia was induced by
incubation of cells at 37°C in a humidified incubator (5%
CO2, 95% air) in a reduced O2 environment
(3%). siRNA-mediated inhibition was conducted by transfection with
Clk1- or Clk4-specific siRNAs (100 pmol) or nonsense control siRNA
(100 pmol; Applied Biosystems, Darmstadt, Germany). Transfection
was performed using Lipofectamine™ 2000 (Invitrogen GmbH,
Karlsruhe, Germany). For expression analyses (mRNA and protein
level), time courses from 0 to 24 h were performed in preliminary
tests to assess the optimal time points (data not shown). These
time points were then used for expression analyses as indicated in
the results.
Overexpression plasmids
For transfection experiments, a cDNA construct of
asTF was cloned into the multiple cloning site of the pVITRO2
vector (InvivoGen, San Diego, CA, USA). The quality, orientation
and identity of the overexpression vector and the insert were
confirmed by automated sequencing.
Stable transfection of A549 cells
A594 cells (5×106/well) were seeded in
6-well plates. Transfection of pVITRO2_asTF or pVITRO2_LV was
conducted using Lipofectamine™ 2000 following the manufacturer’s
protocol. Selection of stable transfectants was performed by
incubating transfected cells with 250 ng/ml hygromycin (PAA
Laboratories GmbH). Stable overexpression was continuously verified
by real-time PCR.
Semi-quantitative RT-PCR
Total RNA was reverse transcribed using AMV (Roche
Diagnostics GmbH, Mannheim, Germany), and cDNAs encoding MCP-1,
Cyr61 and VEGF, were amplified. Primers used for detection were:
MCP-1 forward, 5′-TCTGTGCCTGCTGCTCATAG-3′ and MCP-1 reverse,
5′-CAGATCTCCTTGGCCACAAT-3′; Cyr61 forward,
5′-TCCTCTGTGTCCCCAAGAAC-3′ and Cyr61 reverse,
5′-TTCAGGCTGCTGTACACTGG-3′; VEGF forward, 5′-TCC
AGGAGTACCCTGATGAGA-3′ and VEGF reverse, 5′-GCT
TGTCACATCTGCAAGTACG-3′ (Ocimum Biosolutions Ltd., Hyderabad,
India). GAPDH-, Clk1-, Clk-4-, flTF- and asTF-specific primers were
as previously described (4). RT-PCR
conditions consisted of 94°C for 2 min, and 36 cycles of 94°C for
30 sec; 60°C for 25 sec; and 72°C for 1 min. Additionally, HIF-1α
mRNA expression was determined as control for the induction of the
hypoxia response. Primers used for detection were: HIF-1α forward,
5′-CACCTCTGGACT GCCTTTC-3′ and HIF-1α reverse, 5′-GAAGTGGCAACT
GATGAGCA-3′. RT-PCR conditions for HIF-1α consisted of 94°C for 2
min, and 36 cycles of 94°C for 30 sec; 58°C for 25 sec; and 72°C
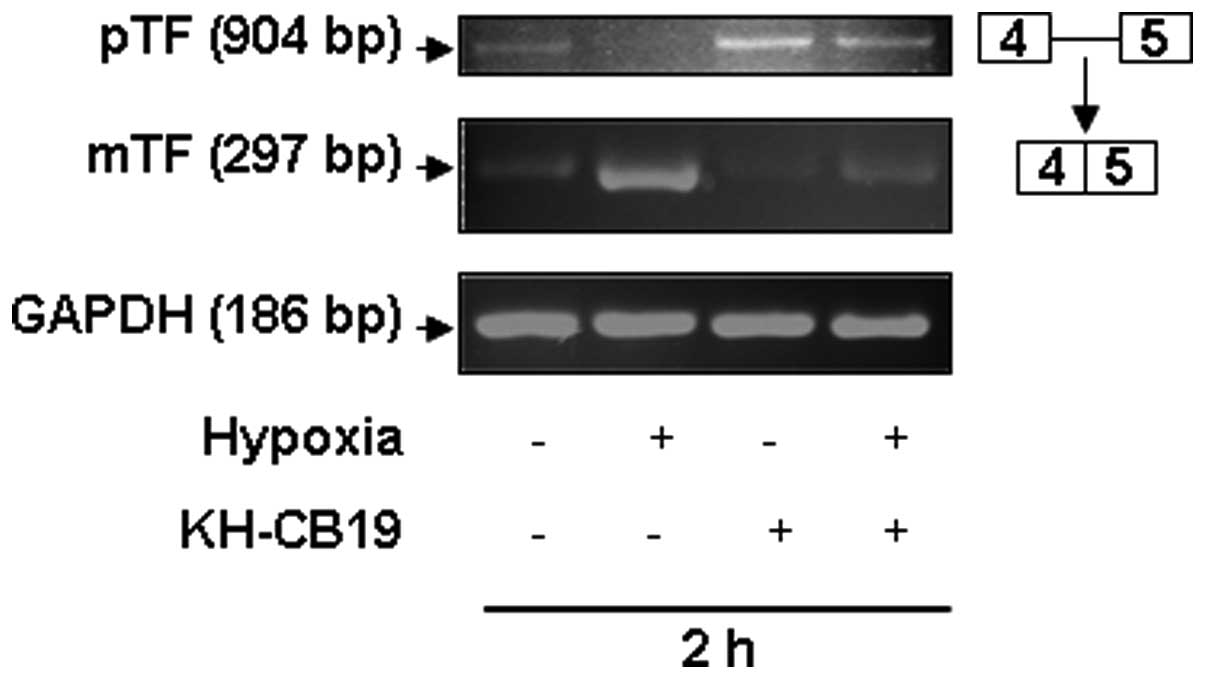
for 30 sec. The impact of Clk inhibition on TF splicing was
assessed at the mRNA level following the protocol by Schwertz et
al(23). Post-transcriptional
processing of unspliced human TF pre-mRNA (pTF) to spliced mature
human TF mRNA (mTF) was measured using the specific primers:
TF-exon 4 forward, 5′-CTCGGACAGCCA ACAATTCAG-3′ and TF-exon 5
reverse, 5′-CGGGCTGTCT GTACTCTTCC-3′ (23). The corresponding RT-PCR product pTF
(904 bp) includes a part of exon 4, intron 4, and a part of exon 5.
In contrast to pTF, the mTF amplicon (297 bp) only includes exon 4
and exon 5 but not the intronic sequence due to the exclusion of
this intron via post-transcriptional splicing. Since the expression
level of pTF is much lower than that of mTF, the cycle numbers for
the RT-PCRs were adjusted. RT-PCR conditions for pTF (and mTF)
consisted of 94°C for 2 min, and 40 cycles (27 cycles for mTF) of
94°C for 30 sec; 60°C for 25 sec; and 72°C for 1 min.
Tube formation assay
To analyze angiogenesis, the In Vitro Angiogenesis
Assay kit from Millipore (Billerica, MA, USA) was used. The assay
was performed following the manufacturer’s protocol. In brief,
HMEC-1 cells (7.5×103/well) were resuspended in the
supernatant of asTF-overexpressing A549 cells or cells transfected
with a control plasmid (LV). Fresh RPMI containing 100 nM
recombinant MCP-1 or 50 μg/ml recombinant asTF was used as positive
controls. The impact of hypoxia was determined using the
supernatant of A549 cells incubated for 24 h under hypoxic (3%
O2) or normoxic conditions (20% O2),
identical to the conditions used for protein expression analyses
via western blotting. Subsequently, HMEC-1 cells were seeded on 50
μl of the ECMatrix™ in 96-well plates in 150 μl of the A549
supernatants as mentioned above and incubated for 7 h at 37°C in a
humidified air incubator with 5% CO2. This time point
was found to be optimal for the measurement of tube formation by
HMEC-1 cells in this experimental setting. After 7 h, the number of
rings formed by endothelial cells was counted and compared to the
corresponding controls. Tube formation by endothelial cells was
analyzed using a Leica DMIL light microscope (Leica, Wetzlar,
Germany).
MTT assay
Cell proliferation of A549 cells was determined by
MTT assay. This colorimetric assay determines the activity of
cellular enzymes that reduce the yellow tetrazolium salt
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
to a purple formazan dye in living cells. The MTT assay measures
the cell proliferation via determination of the cellular metabolic
activity of NAD(P)H-dependent oxidoreductase enzymes. Here,
2.5×104 cells in 100 μl RPMI medium/well were seeded in
96-well plates. Cells were incubated for 48 h with or without
inhibitory antibodies or cRGD at 37°C in humidified air with 5%
CO2. After the incubation, 25 μl of MTT (5 mg/ml) was
added to each well and incubated at 37°C for 3 h. The reaction was
stopped by adding solubilization solution. The plates were further
incubated for 1 h at 37°C. Subsequently, the concentration of the
generated formazan was determined by UV absorption at 570 nm in a
VersaMax Microplate Reader (Molecular Devices GmbH, Ismaning,
Germany). Cell number was determined using a standard curve from 0
to 10,000 cells/100 μl of media and well.
Western blotting
Western blot analysis of samples from the A549 cell
lysates was performed as previously described (4). For analysis of asTF in the supernatant
of A549 cells, the medium was collected after 24 h, respectively.
Soluble proteins in the supernatant were precipitated by treatment
with trichloroacetic acid (TCA) overnight at 4°C. Since there is no
established protein loading control for secreted proteins, equal
protein loading was confirmed by determining the amount of total
protein via a BCA assay. For western blot experiments, 20 μg of
whole protein was used. For detection, specific antibodies against
flTF, asTF and GAPDH as previously described (4), MCP-1 (Santa Cruz Biotechnology, Santa
Cruz, CA, USA) VEGF (Abnova GmbH, Heidelberg, Germany), Clk1 and
Clk4 (Aviva Systems Biology, San Diego, CA, USA) and Cyr61 (a kind
gift from Lester F. Lau, University of Illinois, Chicago, IL, USA)
were used.
Quantification of western blots and
RT-PCR
The results of western blot and RT-PCR experiments
were quantified using Gel-Pro Analyzer™ software version 4.0.00.001
(Media Cybernetics, Bethesda, MD, USA).
TF isoform-specific real-time PCR
(TaqMan)
Real-time PCR employing flTF-, asTF- and
GAPDH-specific primers and probes was performed as previously
described (4). Quantification of
real-time PCR data was performed by employing standard curves of
double-stranded cDNA fragments covering the complete coding
sequence for each target (asTF, flTF and GAPDH). Results of
real-time PCRs for flTF and asTF were normalized against GAPDH.
Statistical analyses were carried out using GraphPad Prism 4
version 4.03 (GraphPad Software Inc., La Jolla, CA, USA).
Measurement of TF activity
Determination of total pro-coagulant activity of
A549 cells was performed as previously described (4).
Statistical analysis
All data are expressed as means ± SEM. Data were
analyzed by the Student’s t-test or one-way ANOVA. A probability
value ≤0.05 was considered to indicate a statistically significant
result.
Results
Hypoxia induces a modulated TF expression
pattern in A549 cells
To determine the impact of hypoxia on TF isoform
expression and other angiogenic factors, A549 cells were incubated
under hypoxic conditions (3% O2) for 2, 4 or 24 h,
respectively. Compared to cells cultured under normoxic conditions,
hypoxia increased the mRNA expression of both TF isoforms, flTF and
asTF, as well as the pro-angiogenic mediators Cyr61, HIF-1α, and
other factors involved in alternative splicing, such as Clk1 and
Clk4 (Fig. 1A). Consistent with
these data, protein levels of TF isoforms and Cyr61 were also found
to be increased under hypoxic conditions (Fig. 1B). Moreover, this setting also led
to a significant increase in the protein expression of the
alternative splicing-modulating kinases, Clk1 and Clk4, and
strongly affected the phosphorylation of the known Clk downstream
effector SRp75. Furthermore, treatment of microvascular endothelial
cells (HMEC-1) with the supernatant of A549 cells incubated for 24
h under hypoxic conditions induced pro-angiogenic tube formation of
HMEC-1 in vitro (Fig. 1C).
Together these data indicate that hypoxia triggers the switch from
a normal to a pro-angiogenic state.
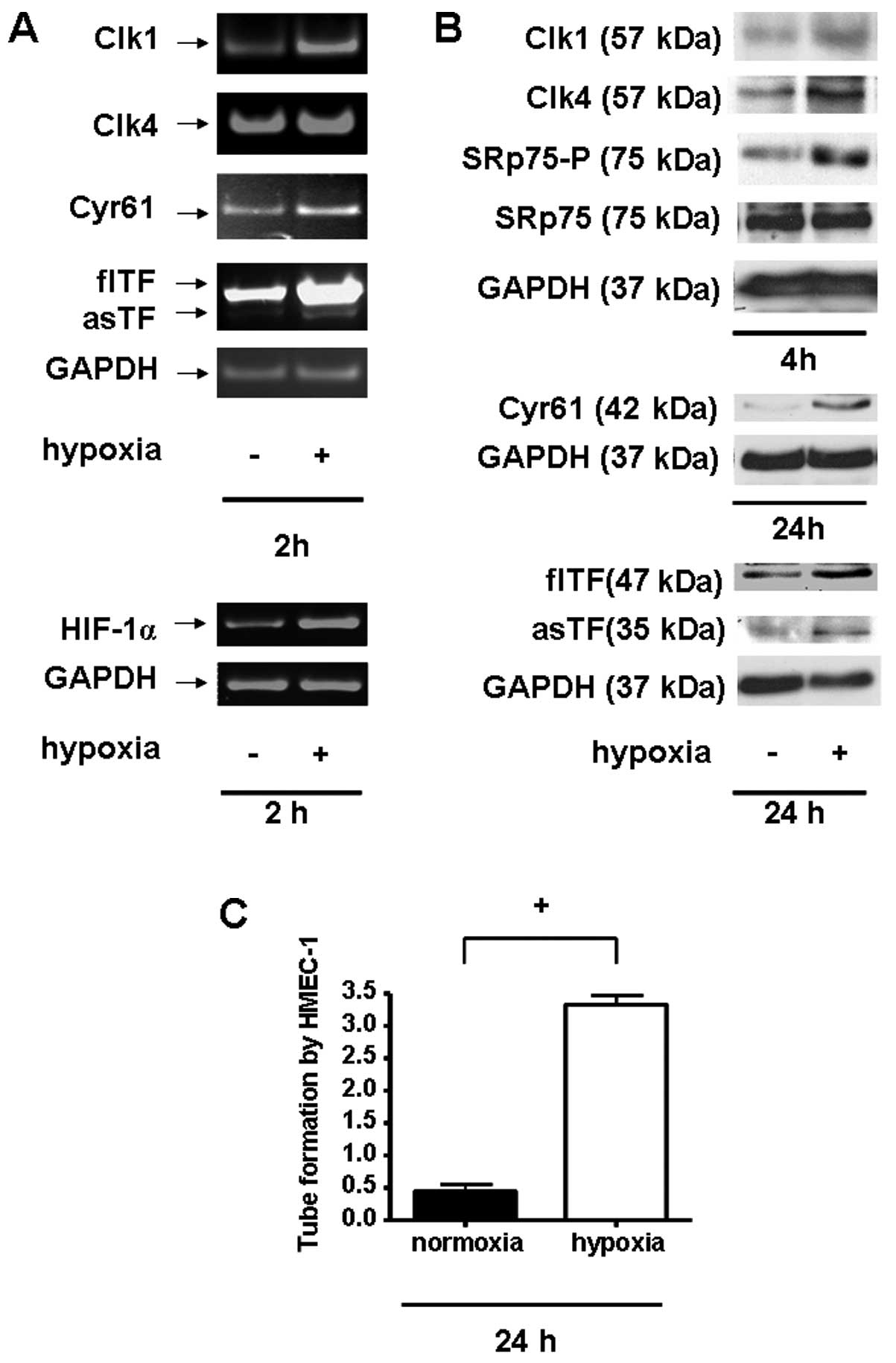 | Figure 1Hypoxia alters the expression pattern
and the pro-angiogenic potential of A549 cells. (A) mRNA expression
of Clk1 (347 bp), Clk4 (440 bp), Cyr61 (450 bp), flTF (931 bp),
asTF (771 bp), HIF-1α (252 bp) and GAPDH (185 bp) in A549 cells 2 h
post hypoxia induction; n ≥3. (B) Western blot analysis of
intracellular expression of Clk1 (57 kDa), Clk4 (57 kDa),
phosphorylated SRp75 (75 kDa), Cyr61 (42 kDa), flTF (47 kDa), asTF
(35 kDa) and GAPDH (37 kDa) in A549 cells 4 or 24 h, respectively,
after induction of hypoxic conditions; n ≥3. (C) Tube formation
assay of HMEC-1 cells treated with the supernatant of
hypoxia-treated A549 cells post 5 h. +P<0.01, n
≥3. |
Effect of asTF overexpression on mRNA and
protein levels in A549 cells
To assess whether asTF mediates pro-angiogenic
effects, asTF was stably overexpressed in A549 cells followed by
evaluation of the expression of pro-angiogenic factors as well as
cell number and angiogenesis induction. Compared to cells
transfected with the empty control plasmid (LV), stable asTF
overexpression (asTF) was associated with a significant increase in
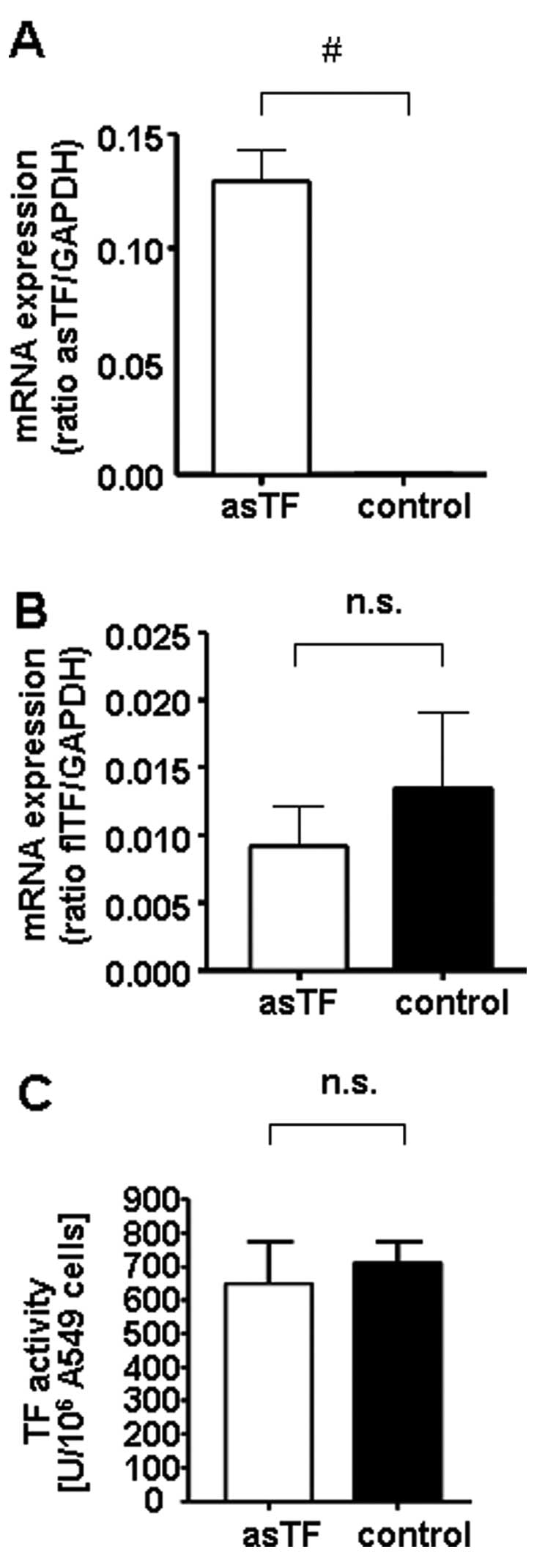
asTF, but not flTF, at the mRNA level (Fig. 2A and B). Overall, expression levels
of asTF and flTF mRNA were comparable in asTF-overexpressing cells.
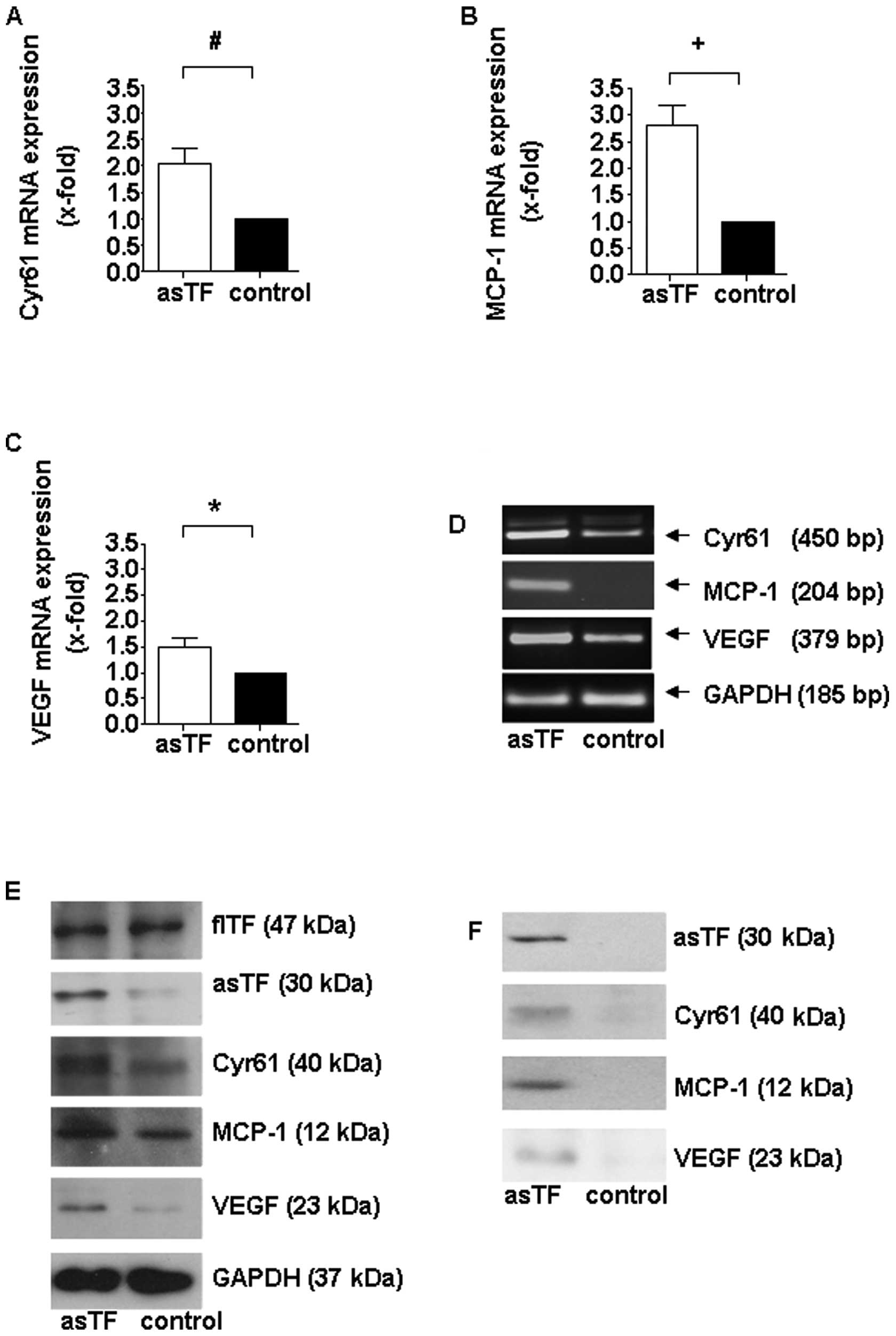
Stable asTF overexpression also significantly induced the
expression of the pro-angiogenic factors Cyr61, MCP-1 and VEGF
(Fig. 3A-E). In addition, the total
amount of asTF and the other above-mentioned pro-angiogenic factors
secreted into the supernatant was increased compared to cells
transfected with the control plasmid (Fig. 3F).
Overexpression of the soluble TF isoform
had no influence on cellular thrombogenicity
AsTF is assumed to exhibit a low pro-thrombogenic
potential yet flTF appears to be the major contributor to
thrombogenicity. To confirm these data in our experimental setting,
the impact of asTF overexpression on the pro-coagulant activity of
A549 cells was analyzed using a chromogenic TF activity assay. As
shown in Fig. 2C, TF activity of
A549 cells was not significantly influenced by stable asTF
overexpression in comparison to the corresponding controls
(Fig. 2C).
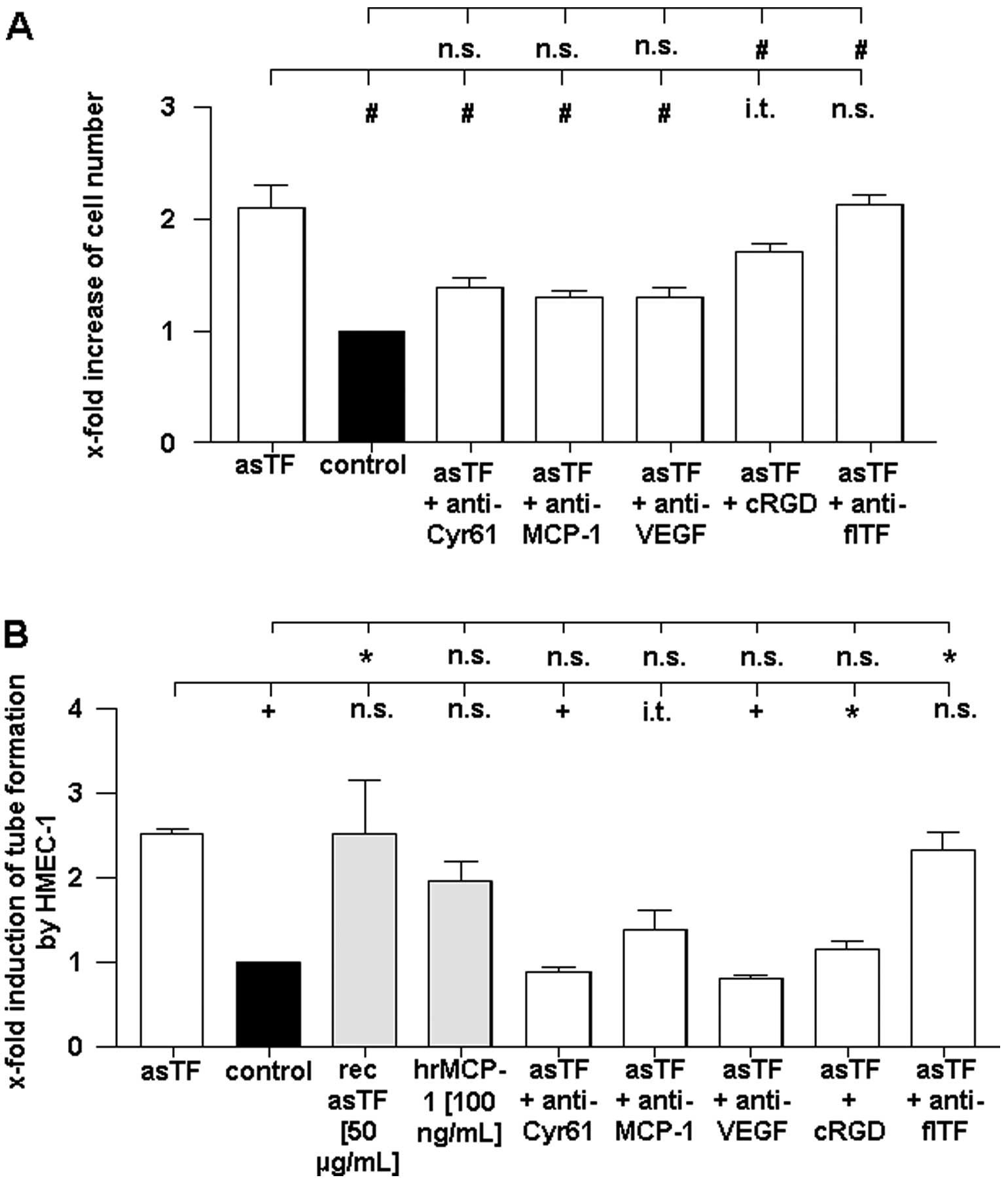
Impact of asTF overexpression on cell
number
Overexpression of asTF increased the number of A549
cells by ~2-fold compared to cells transfected with the empty
control vector (Fig. 4A). However,
inhibition of the pro-angiogenic factors Cyr61, MCP-1, as well as
VEGF by specific neutralizing antibodies significantly reduced the
pro-proliferative effect mediated by asTF. Moreover, pharmacologic
inhibition of integrin αvβ3 also led to a
significant reduction in the number of asTF-overexpressing cells.
In contrast to the inhibition of integrin
αvβ3 and the above mentioned pro-angiogenic
factors, the neutralization of flTF by specific inhibitory
antibodies had no influence on cell number in the
asTF-overexpressing A549 cells (Fig.
4A).
Supernatant of asTF-overexpressing A549
cells induces endothelial tube formation
To characterize the pro-angiogenic potential of asTF
overexpression, we investigated the effect of the cell supernatant
on in vitro tube formation of human endothelial cells
(HMEC-1) (Fig. 4B). To elucidate
the individual effects of different components within the
supernatant, we used neutralizing antibodies and a pharmacologic
integrin αvβ3 inhibitor. MCP-1 as well as
recombinant asTF were applied as positive controls for the
induction of tube formation by HMEC-1.
As shown in Fig. 4B,
the supernatant of asTF-overexpressing A549 cells increased the
tube formation by HMEC-1 compared to the supernatant of cells
transfected with a control plasmid. The degree of the
pro-angiogenic potential of the asTF-containing supernatant was
comparable to that of recombinant asTF or MCP-1 on endothelial tube
formation (Fig. 4B).
Antibody-mediated depletion of Cyr61 and VEGF significantly reduced
the pro-angiogenic effect of the supernatant of asTF-overexpressing
A549 cells. Similarly, blocking of MCP-1 led to a slight decrease
in tube formation. Pharmacologic inhibition of integrin
αvβ3 also reduced the effect of the
asTF-containing supernatant of HMEC-1 in this assay. In contrast,
inhibition of flTF by neutralizing antibodies had no effect on the
increase in endothelial tube formation (Fig. 4B).
Together, these data indicate that asTF induces
proliferation as well as the pro-angiogenic potential of A549 cells
without affecting the pro-thrombogenicity of these cells. Based on
these results, we conclude that asTF mediates its effects via
several proliferation-modulating and pro-angiogenic factors
including Cyr61 and MCP-1, as well as via integrin
αvβ3 signaling.
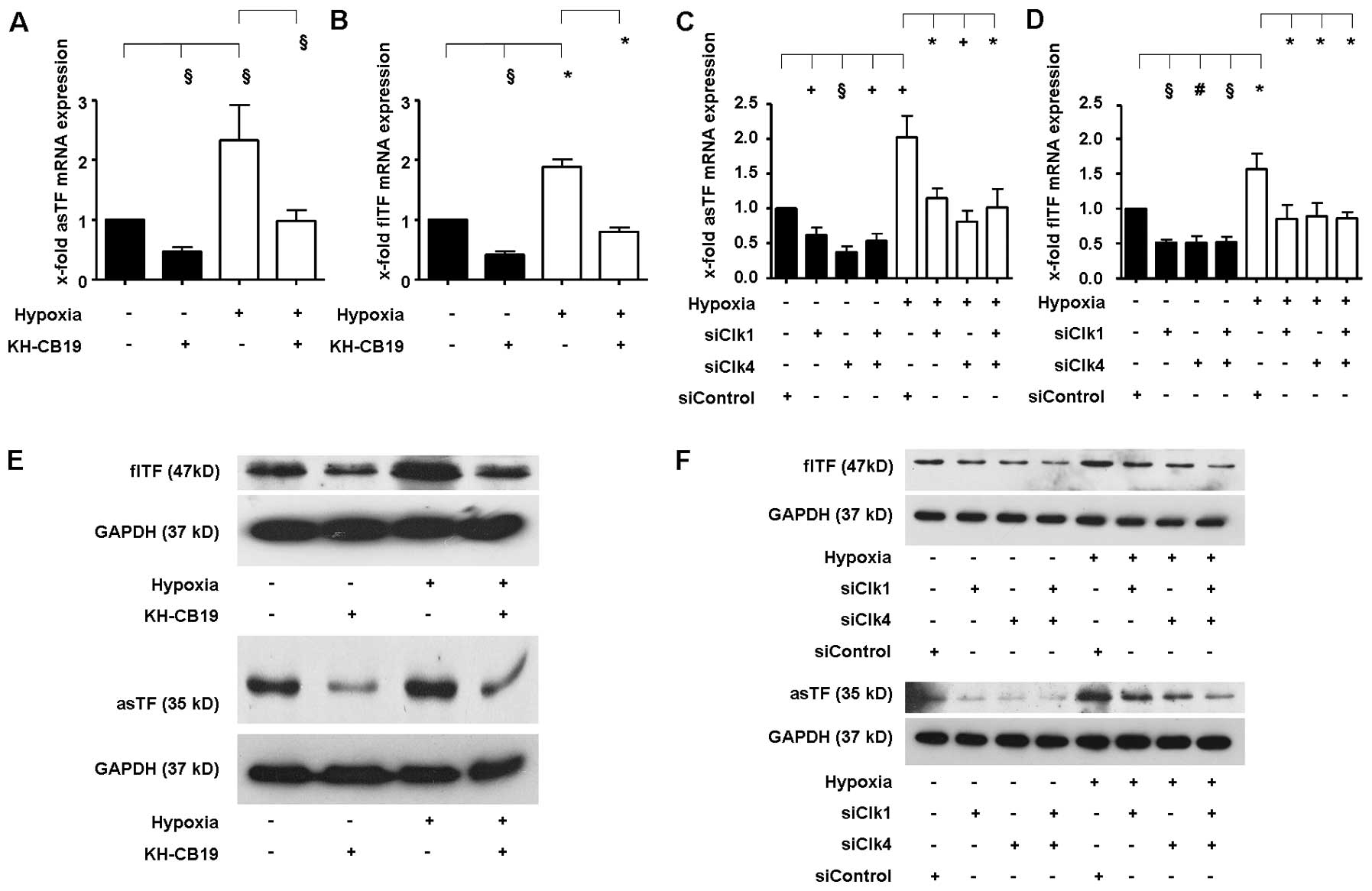
Clks modulate the TF isoform expression
under hypoxic conditions
Next, we aimed to ascertain how TF isoform
expression is regulated at the post-transcriptional level by
alternative splicing when hypoxic conditions are applied.
As expected, we found an induction of flTF and asTF
mRNA expression in A549 cells when comparing hypoxic vs. normoxic
conditions (Fig. 5A and B).
Treatment of cells with the Clk1- and Clk4-specific inhibitor
KH-CB19 (22) led to a significant
reduction in both flTF and asTF independent of the amount of oxygen
provided during incubation. To verify these data, we transfected
A549 cells with specific siRNAs targeting Clk1 and Clk4. Consistent
with the results obtained with KH-CB19, treatment of cells with
siRNA either against Clk1 and Clk4 alone or in combination
significantly reduced mRNA expression of flTF and asTF under
normoxic as well as under hypoxic conditions (Fig. 5C and D).
To determine whether pharmacologic inhibition of
Clk1 and Clk4 also influences the protein expression of the TF
isoforms, we performed western blot analyses. In agreement with the
previous mRNA data, hypoxia induced protein expression of both TF
isoforms after 24 h (Fig. 5E and
F). Treatment with the Clk1 and Clk4 inhibitor (Fig. 5E) or siRNAs against Clk1 and Clk4
(Fig. 5F) reduced protein
expression of both TF isoforms in both high and low oxygen
setting.
In order to further validate the impact of Clks on
TF splicing, we also investigated the post-transcriptional
processing of unspliced human TF pre-mRNA (pTF) to spliced mature
human TF mRNA (mTF) by semi-quantitative RT-PCR as described by
Schwertz et al (Fig. 6)
(23). Under normoxic conditions
both pTF as well as mTF were detected at the mRNA level in A549
cells (Fig. 6). As shown, hypoxia
increased the amount of the spliced mTF form and reduced the pTF
level. Treatment of cells with the specific Clk inhibitor KH-CB19
(10 μM) led to a significant reduction in the spliced mTF. In
contrast, the level of the unspliced pTF form was increased in
these cells. The same results were observed both under normoxic as
well as under hypoxic conditions.
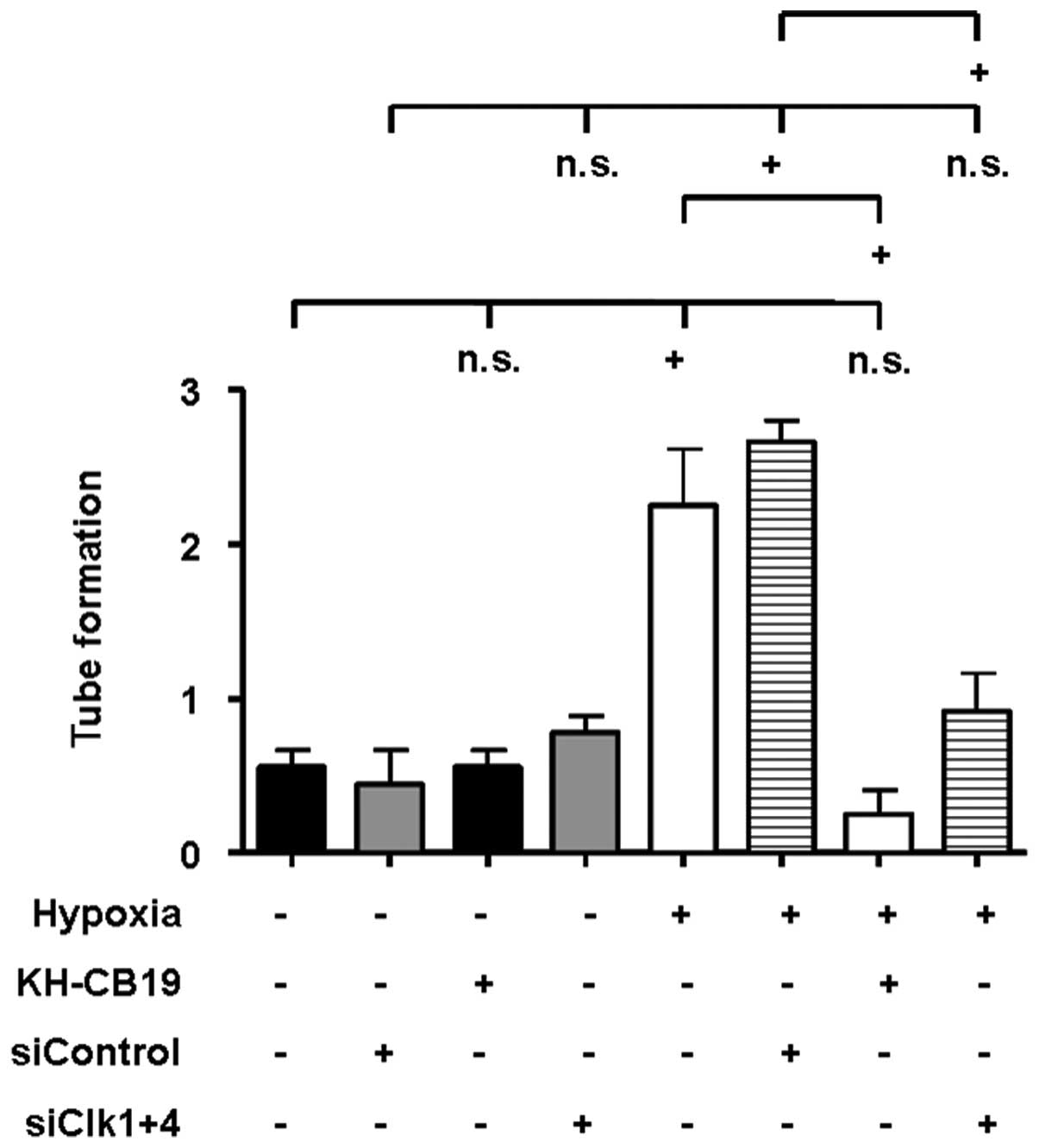
Impact of Clk inhibition on the
pro-angiogenic potential of A549 cells
We performed HMEC-1 tube formation assays to
elucidate the impact of Clk inhibition on the pro-angiogenic
potential of A549 cells. HMEC-1 cells treated with the supernatant
of A549 cells which had been incubated for 24 h under hypoxic
conditions displayed an induction in tube formation (Fig. 7). Inhibition of Clk1 and Clk4 by
KH-CB19 as well as treatment with the siRNA targeted against Clk1
and Clk4 significantly reduced the hypoxia-induced tube formation
by HMEC-1 cells, whereas nonsense siRNA controls had no influence
on this effect. The low pro-angiogenic potential of the supernatant
of A549 cells incubated under normoxic conditions was not altered
by Clk inhibition (Fig. 7).
In summary, these data indicate that Clks play an
important role in the regulation of the pro-angiogenic properties
of A549 cells under hypoxic conditions.
Discussion
Our results show that hypoxia induces the expression
of the TF isoforms as well as of alternative splicing factors and
the pro-angiogenic factor Cyr61. Moreover, we found that asTF
overexpression in human A549 lung cancer cells increased the
proliferative and pro-angiogenic properties of these cells without
affecting cellular thrombogenicity. Our data further suggest that
these effects are, at least in part, mediated by the induction of
the pro-migratory and pro-angiogenic factors Cyr61, MCP-1 and VEGF,
as well as integrin αvβ3. Furthermore, we
showed for the first time that TF isoform expression is modulated
at the post-transcriptional level via Clk-mediated splicing
processes under hypoxic conditions and that this modulation can
directly alter the pro-angiogenic potential of A549 cells.
Hypoxia induces the pro-angiogenic
potential
The transcription factor HIF-1α plays an essential
role in the hypoxic response in cancer as well as in other cells
and tissues (24). As reported in
the literature, hypoxia induces the expression of HIF-1α (25). Consistent with this, we also found
hypoxia to induce the expression of HIF-1α in our experiments
indicating an adequate hypoxic response of A549 cells in our
experimental setting. Moreover, we demonstrated that hypoxic
conditions (O2 concentration 3%) induces the expression
of alternative splicing-regulating factors, such as Clk1 and Clk4
as well as the phosphorylation of splicing factors including SRp75.
This is in line with previous results showing that hypoxia can
modulate alternative splicing and expression of alternatively
spliced isoforms (26,27). The regulation of alternative
splicing plays an essential role in several types of cancer cells
as well as in the pathophysiology of malignant diseases (27,28).
In our study, we found hypoxia to induce the expression of both TF
isoforms asTF and flTF. In line with previous data, total asTF mRNA
levels are generally lower than that of flTF (5,13).
Furthermore, hypoxia induced the expression of the pro-angiogenic
factor Cyr61 in our A549 model system. This was associated with an
increased pro-angiogenic potential of the cells. Cyr61 and flTF are
known to modulate angiogenesis in cancer (17,21).
AsTF was shown to be a pro-angiogenic factor in non-cancer
settings, such as endothelial cells or cardiomyocytes (10,11,13).
However, the role of asTF in cancer-associated angiogenesis is
largely unknown. Assuming that asTF also mediates pro-angiogenic
effects under hypoxic conditions in lung cancer cells, we evaluated
the impact of asTF on the pro-angiogenic potential of A549 lung
cancer cells.
Effects of asTF overexpression on
angiogenesis and cell number
Data obtained by Hobbs et al(12) indicated that asTF may play a role in
tumor growth and tumor-associated angiogenesis in vitro and
in vivo. Those experiments demonstrated that asTF
overexpression in pancreatic cancer cells increases tumor cell
proliferation. In line with these data, stable asTF overexpression
also led to an increased cell number in human A549 lung cancer
cells in our study. Moreover, we found asTF overexpression to be
associated with higher expression levels of the pro-angiogenic and
proliferation-promoting factors Cyr61 (17,20),
MCP-1 (15,29) and VEGF (16,30).
In murine cardiomyocytic cells, asTF was also shown to induce
murine VEGF and Cyr61 (10). Here,
we showed that antibody-mediated inhibition of these factors
reduced both the asTF-mediated increase in cell number as well as
the pro-angiogenic effect of asTF overexpression in A549 cells.
This is in line with a recently published study of Arderiu et
al(31) who demonstrated that
TF regulates the generation of MCP-1 in endothelial cells which in
turn mediates the pro-angiogenic effect by recruitment of smooth
muscle cells to endothelial cells. TF isoforms were also shown to
induce the expression of the pro-angiogenic factors VEGF and Cyr61
(10,32). Moreover, A549 cells were found to
express VEGF receptors (33).
Furthermore, VEGF treatment increased A549 cell survival (33). Therefore, it is possible that asTF
mediates its effects on cell number and tube formation via the
induction of pro-angiogenic and proliferation-promoting factors,
such as MCP-1, VEGF and Cyr61.
In 2009, van den Berg et al(13) showed asTF to induce angiogenesis in
endothelial cells. The authors found blockade of β1 and
β3 integrins as well as the treatment with a TF antibody
inhibits the pro-angiogenic effect of asTF. It was suggested that
the pro-angiogenic effects of asTF may be mediated directly via
asTF-integrin signaling (13). In
our experiments, pharmacologic inhibition of
αvβ3 integrins by cRGD peptides also reduced
the cell number of A549 cells as well as the pro-angiogenic effect
on endothelial tube formation. Consistent with this, Loges et
al(34) demonstrated that
pharmacologic inhibition of integrin αvβ3 by
cRGD peptides (cilengitide) reduces the proliferation of human
endothelial progenitor cells in vitro. Notably,
αvβ3 was reported to be an important mediator
of Cyr61 and VEGF functions (18,19).
Total TF was found to trigger the proliferation of
human vascular cells (35). Yet, no
asTF-specific inhibitory siRNA or pharmacologic inhibitor has been
reported. It is noteworthy that neutralization of flTF had no
influence on the asTF-mediated increase in the cell number of A549
cells or endothelial cell tube formation in our experiments. This
may be due to the fact that overexpression of the soluble TF
isoform had no impact on flTF expression in A549 cells (Fig. 2). Therefore, the asTF-induced
increase in cell number and the pro-angiogenic effect were
independent of flTF and probably not mediated via flTF
upregulation. Based on our results, we hypothesize that the
increase in cell number as well as the pro-angiogenic effect of
asTF overexpression in A549 lung carcinoma cells is, at least in
part, directly mediated by asTF-integrin αvβ3
signaling or indirectly via an asTF-induced increase in the
expression of the proliferation-promoting factors Cyr61, MCP-1 and
VEGF, which may also be associated with integrin
αvβ3 signaling.
Role of Clk-regulated alternative
splicing under hypoxic conditions
Alternative splicing is essential for the regulation
of the functional diversity and the plasticity of the proteome at
the post-transcriptional level in response to environmental changes
(11,28). Serine/arginine-rich (SR) protein
kinases, such as the Cdc2-like kinases, DNA topoisomerase I and
protein kinase B are known to control alternative splicing
processes via modulation of SR protein phosphorylation (7,36–38).
Hypoxia was also shown to modulate alternative splicing in normal
tissues as well as in cancer cells (9,27). In
line with this, we found that hypoxic conditions induce the
expression of Clk1 and Clk4, as well as the phosphorylation of SR
proteins, such as SRp75. Moreover, we found that a low oxygen
environment promotes the expression of both flTF as well as of the
alternatively spliced TF isoform. This implies that TF isoform
expression may be modulated at the post-transcriptional level by
alternative splicing. In this context, we investigated the role of
Clks in hypoxia-induced TF isoform expression. We found that both
pharmacologic suppression using specific inhibitors as well as
siRNA-mediated gene-silencing of Clk1 and Clk4 reduced the flTF and
asTF expression in A549 cells under hypoxic as well as normoxic
conditions. In line with this, we and others have previously shown
that Clks modulate TF isoform expression in TNF-α-stimulated human
endothelial cells and in thrombin-treated platelets (4,23). In
addition, Schwertz et al (23) demonstrated that the
thrombin-induced activation of platelets results in
post-transcriptional processing of the TF pre-mRNA (pTF) to the
spliced mature form of the TF mRNA (mTF). It was also shown that
thrombin-induced splicing led to a reduced level of the pTF form
and an increased amount of the mature mTF form in platelets.
Moreover, pharmacologic inhibition of Clks prevented
thrombin-induced processing of the TF pre-mRNA in activated
platelets (23). Consistent with
these data, we found hypoxia to induce processing of the unspliced
TF pre-mRNA (pTF) to the spliced mature mTF form as assessed by
RT-PCR, indicated by reduced levels of the pTF form and an increase
in mTF mRNA under hypoxic conditions compared to normoxic
conditions. Inhibition of Clk1 and Clk4 by KH-CB19 completely
abolished this hypoxia-induced effect on TF splicing, which is also
in line with the data of Schwertz et al(23). Under normoxic conditions, inhibition
of Clk1 and Clk4 also led to an increased amount of pTF and reduced
mTF mRNA compared to control cells incubated under normoxic
conditions. This may be due to the fact that A549 cells also
express TF under basal conditions (Fig.
6). Therefore, TF pre-mRNA can also be processed under normal
conditions. Since Clks are involved in alternative as well as in
constitutive splicing (23,39), inhibition of Clks may also affect
mRNA splicing under basal normoxic conditions in A549 cells
supporting the hypothesis that Clks modulate splicing of the TF
pre-mRNA under both normoxic as well as hypoxic conditions.
Together, our data demonstrated that asTF is a
pro-angiogenic factor and increases cell proliferation in A549
cells. We identified Clk1 and Clk4 as modulators of TF isoform
expression affecting both splicing of TF pre-mRNA and protein
expression of the TF isoforms under hypoxic as well as normoxic
conditions in A549 lung carcinoma cells. Furthermore, inhibition of
Clk1 and Clk4 was found to influence the pro-angiogenic potential
of A549 cells under hypoxic conditions.
Therefore, we conclude that the TF isoform
expression is modulated by Clk-mediated alternative splicing at the
post-transcriptional level in A549 lung carcinoma cells under
hypoxia as well as under normoxia. This in turn controls the
pro-angiogenic potential of these cells, which is, at least in
part, mediated via the asTF-mediated induction of the downstream
effectors Cyr61, MCP-1 and VEGF.
Acknowledgements
This study was supported by grants from the Deutsche
Forschungsgemeinschaft (DFG) (SFB-TR19 to U.R. and H.-P.S.), and
the Else Kröner-Fresenius-Stiftung (P09/08//A129/07 to U.R.).
References
|
1
|
Eisenreich A, Celebi O, Goldin-Lang P,
Schultheiss HP and Rauch U: Upregulation of tissue factor
expression and thrombogenic activity in human aortic smooth muscle
cells by irradiation, rapamycin and paclitaxel. Int
Immunopharmacol. 8:307–311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giesen PL, Rauch U, Bohrman B, et al:
Blood-borne tissue factor: another view of thrombosis. Proc Natl
Acad Sci USA. 96:2311–2315. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rauch U, Antoniak S, Boots M, et al:
Association of tissue-factor upregulation in squamous-cell
carcinoma of the lung with increased tissue factor in circulating
blood. Lancet Oncol. 6:2542005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Szotowski B, Antoniak S, Poller W,
Schultheiss HP and Rauch U: Procoagulant soluble tissue factor is
released from endothelial cells in response to inflammatory
cytokines. Circ Res. 96:1233–1239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bogdanov VY, Balasubramanian V, Hathcock
J, Vele O, Lieb M and Nemerson Y: Alternatively spliced human
tissue factor: a circulating, soluble, thrombogenic protein. Nat
Med. 9:458–462. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eisenreich A, Bogdanov VY, Zakrzewicz A,
et al: Cdc2-like kinases and DNA topoisomerase I regulate
alternative splicing of tissue factor in human endothelial cells.
Circ Res. 104:589–599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eisenreich A, Malz R, Pepke W, Ayral Y,
Poller W, Schultheiss HP and Rauch U: Role of the
phosphatidylinositol 3-kinase/protein kinase B pathway in
regulating alternative splicing of tissue factor mRNA in human
endothelial cells. Circ J. 73:1746–1752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tardos JG, Eisenreich A, Deikus G, et al:
SR proteins ASF/SF2 and SRp55 participate in tissue factor
biosynthesis in human monocytic cells. J Thromb Haemost. 6:877–884.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rauch U and Antoniak S: Tissue
factor-positive microparticles in blood associated with
coagulopathy in cancer. Thromb Haemost. 97:9–10. 2007.PubMed/NCBI
|
|
10
|
Eisenreich A, Boltzen U, Malz R,
Schultheiss HP and Rauch U: Overexpression of alternatively spliced
tissue factor induces the pro-angiogenic properties of murine
cardiomyocytic HL-1 cells. Circ J. 75:1235–1242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eisenreich A and Rauch U: Regulation and
differential role of the tissue factor isoforms in cardiovascular
biology. Trends Cardiovasc Med. 20:199–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hobbs JE, Zakarija A, Cundiff DL, et al:
Alternatively spliced human tissue factor promotes tumor growth and
angiogenesis in a pancreatic cancer tumor model. Thromb Res.
120(Suppl 2): S13–S21. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van den Berg YW, van den Hengel LG, Myers
HR, et al: Alternatively spliced tissue factor induces angiogenesis
through integrin ligation. Proc Natl Acad Sci USA. 106:19497–19502.
2009.PubMed/NCBI
|
|
14
|
Goldin-Lang P, Tran QV, Fichtner I, et al:
Tissue factor expression pattern in human non-small cell lung
cancer tissues indicate increased blood thrombogenicity and tumor
metastasis. Oncol Rep. 20:123–128. 2008.
|
|
15
|
Zhang T, Koide N, Wada Y, et al:
Significance of monocyte chemotactic protein-1 and thymidine
phosphorylase in angiogenesis of human cardiac myxoma. Circ J.
67:54–60. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Han J, Yang X, et al: Pigment
epithelium-derived factor inhibits angiogenesis and growth of
gastric carcinoma by down-regulation of VEGF. Oncol Rep.
26:681–686. 2011.PubMed/NCBI
|
|
17
|
Shimizu T, Okayama A, Inoue T and Takeda
K: Analysis of gene expression during staurosporine-induced
neuronal differentiation of human prostate cancer cells. Oncol Rep.
14:441–448. 2005.PubMed/NCBI
|
|
18
|
Chen N, Leu SJ, Todorovic V, Lam SC and
Lau LF: Identification of a novel integrin
αvβ3 binding site in CCN1 (CYR61) critical
for pro-angiogenic activities in vascular endothelial cells. J Biol
Chem. 279:44166–44176. 2004.
|
|
19
|
Hutchings H, Ortega N and Plouët J:
Extracellular matrix-bound vascular endothelial growth factor
promotes endothelial cell adhesion, migration, and survival through
integrin ligation. FASEB J. 17:1520–1522. 2003.
|
|
20
|
Löbel M, Bauer S, Meisel C, et al: CCN1:
CCN1: a novel inflammation-regulated biphasic immune cell migration
modulator. Cell Mol Life Sci. 69:3101–3113. 2012.PubMed/NCBI
|
|
21
|
Schaffner F, Versteeg HH, Schillert A,
Yokota N, Petersen LC, Mueller BM and Ruf W: Cooperation of tissue
factor cytoplasmic domain and PAR2 signaling in breast cancer
development. Blood. 116:6106–6113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fedorov O, Huber K, Eisenreich A, et al:
Specific CLK inhibitors from a novel chemotype for regulation of
alternative splicing. Chem Biol. 18:67–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schwertz H, Tolley ND, Foulks JM, et al:
Signal-dependent splicing of tissue factor pre-mRNA modulates the
thrombogenicity of human platelets. J Exp Med. 203:2433–2440. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim SH, Kim KW and Jeong JW: Inhibition of
hypoxia-induced angiogenesis by sodium butyrate, a histone
deacetylase inhibitor, through hypoxia-inducible factor-1α
suppression. Oncol Rep. 17:793–797. 2007.PubMed/NCBI
|
|
25
|
Łuczak MW, Roszak A, Pawlik P, Kędzia H,
Lianeri M and Jagodziński PP: Increased expression of HIF-1A and
its implication in the hypoxia pathway in primary advanced uterine
cervical carcinoma. Oncol Rep. 26:1259–1264. 2011.PubMed/NCBI
|
|
26
|
Gang H, Hai Y, Dhingra R, et al: A novel
hypoxia-inducible spliced variant of mitochondrial death gene Bnip3
promotes survival of ventricular myocytes. Circ Res. 108:1084–1092.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hirschfeld M, zur Hausen A, Bettendorf H,
Jäger M and Stickeler E: Alternative splicing of Cyr61 is regulated
by hypoxia and significantly changed in breast cancer. Cancer Res.
69:2082–2090. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tazi J, Bakkour N and Stamm S: Alternative
splicing and disease. Biochim Biophys Acta. 1792:14–26. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu F, Shi J, Yu B, Ni W, Wu X and Gu Z:
Chemokines mediate mesenchymal stem cell migration toward gliomas
in vitro. Oncol Rep. 23:1561–1567. 2010.PubMed/NCBI
|
|
30
|
Zhao X, Li DC, Zhao H, et al: A study of
the suppressive effect on human pancreatic adenocarcinoma cell
proliferation and angiogenesis by stable plasmid-based siRNA
silencing of c-Src gene expression. Oncol Rep. 27:628–636.
2012.PubMed/NCBI
|
|
31
|
Arderiu G, Peña E, Aledo R, Juan-Babot O
and Badimon L: Tissue factor regulates microvessel formation and
stabilization by induction of chemokine (C-C motif) ligand 2
expression. Arterioscler Thromb Vasc Biol. 31:2607–2615. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ollivier V, Bentolila S, Chabbat J, Hakim
J and de Prost D: Tissue factor-dependent vascular endothelial
growth factor production by human fibroblasts in response to
activated factor VII. Blood. 91:2698–2703. 1998.PubMed/NCBI
|
|
33
|
Roberts JR, Perkins GD, Fujisawa T,
Pettigrew KA, Gao F, Ahmed A and Trickett DR: Vascular endothelial
growth factor promotes physical wound repair and is anti-apoptotic
in primary distal lung epithelial and A549 cells. Crit Care Med.
35:2164–2170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Loges S, Butzal M, Otten J, et al:
Cilengitide inhibits proliferation and differentiation of human
endothelial progenitor cells in vitro. Biochem Biophys Res Commun.
357:1016–1020. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cirillo P, Calì G, Golino P, et al: Tissue
factor binding of activated factor VII triggers smooth muscle cell
proliferation via extracellular signal-regulated kinase activation.
Circulation. 109:2911–2916. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bourgeois CF, Lejeune F and Stévenin J:
Broad specificity of SR (serine/arginine) proteins in the
regulation of alternative splicing of pre-messenger RNA. Prog
Nucleic Acids Res Mol Biol. 78:37–88. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eisenreich A, Boltzen U, Poller W,
Schultheiss HP and Rauch U: Effects of the Cdc2-like kinase-family
and DNA topoisomerase I on the alternative splicing of eNOS in
TNF-α-stimulated human endothelial cells. Biol Chem. 389:1333–1338.
2008.PubMed/NCBI
|
|
38
|
Eisenreich A and Rauch U: PI3K inhibitors
in cardiovascular disease. Cardiovasc Ther. 29:29–36. 2011.
View Article : Google Scholar
|
|
39
|
Prasad J, Colwill K, Pawson T and Manley
JL: The protein kinase Clk/Sty directly modulates SR protein
activity: both hyper- and hypophosphorylation inhibit splicing. Mol
Cell Biol. 19:6991–7000. 1999.PubMed/NCBI
|