Introduction
Head and neck squamous cell carcinomas (HNSCCs)
remain a significant cause of morbidity worldwide, with as many as
466,831 and 168,368 cases diagnosed in 2008 among men and women,
respectively (1–3). HNSCC patients with early clinical
stage disease (stages I and II) have similar survival rates, with a
5-year survival rate between 70 and 90%, independent of the
sublocation or the treatment (surgery vs. radiotherapy) (4). In contrast, HNSCC patients with
advanced clinical stage disease (stages III and IV) display
different survival rates depending on the histological type of the
tumor and its sublocation (4,5). In
this group, the combination of chemotherapy and radiotherapy allows
for a better local-regional control rate of up to 65% (6,7).
However, the obvious benefit of chemotherapy is associated with
higher (grade III and IV) toxicity and mortality (8). It is, therefore, crucial to predict
which patients will not benefit from concomitant chemoradiotherapy
(CCR).
During the last 30 years, we have observed a clear
increase in the incidence of carcinomas arising from the oral
cavity and the oropharynx in the United States and in Europe,
whereas the incidence of laryngeal carcinoma has been stable or has
decreased slightly (9). This
observation led us to propose that human papillomavirus (HPV)
infection is a new risk factor for HNSCC in younger, non-smoking
and non-drinking patients. In this subpopulation of HNSCC patients,
HPV+ tumors occur more frequently in the oropharynx than
in other sites and appear to have a more favorable prognosis than
HPV− carcinomas (10,11).
The better prognosis of HPV+ tumors was also reported
for advanced oropharyngeal carcinomas treated by CCR (12–20).
However, HPV-associated tumors have a different pathogenesis with
less chromosomal aberrations than tumors caused by alcohol and
tobacco abuse. In Belgium, the situation is more complex since our
HNSCC patients present with a higher incidence of HPV positivity
associated with alcohol and tobacco abuse. In this context, we
recently described that oral cavity HPV+ carcinomas are
associated with a worse prognosis that of HPV−
carcinomas (21). The aim of the
present study was to assess the impact of HPV positivity on the
response to CCR in a series of 72 HNSCC patients.
Materials and methods
Histopathological and clinical data
Formalin-fixed, paraffin-embedded HNSCC specimens
were obtained from 72 patients (57 males, 15 females) who underwent
concomitant chemoradiotherapy at the Saint-Pieter Hospital
(Brussels) and Epicura (Baudour). The clinical data collected from
this series of 72 HNSCC patients are described in Table I. This prospective and retrospective
study was approved by the Institutional Review Board
(AK/09-09-47/3805AD).
 | Table IClinical data of the HNSCC
patients. |
Table I
Clinical data of the HNSCC
patients.
| Variable | Patients
(n=72) |
|---|
| Gender |
| Male | 57 |
| Female | 15 |
| Age (years) |
| Mean | 57.9 |
| Range | 42–83 |
| Localization |
| Oral cavity | 19 |
| Oropharynx | 29 |
| Hypopharynx | 12 |
| Larynx | 12 |
| Grade
(differentiation) |
| Well | 29 |
| Moderate | 15 |
| Poor | 13 |
| In
situ | 2 |
| Not recorded | 13 |
| TNM stage |
| T1N2 | 4 |
| T1N3 | 1 |
| T2N0 | 1 |
| T2N1 | 3 |
| T2N2 | 5 |
| T3N0 | 8 |
| T3N1 | 9 |
| T3N2 | 10 |
| T4N0 | 5 |
| T4N1 | 6 |
| T4N2 | 19 |
| T4N3 | 1 |
| TNM stage I–IV |
| I | 0 |
| II | 1 |
| III | 20 |
| IV | 51 |
| Risk factors |
| Tobacco |
| Smoker | 54 |
| Non-smoker | 12 |
| Former
smoker | 6 |
| Alcohol |
| Drinker | 55 |
| Non-drinker | 7 |
| Former
drinker | 10 |
| Treatment |
| Cisplatin 100
mg/m2 (Day 1–21–42) |
| Two doses | 12 |
| Three doses | 23 |
| Cisplatin 40
mg/m2 (weekly) | 7 |
| Carboplatin
(weekly) | 7 |
| Cisplatin 100
mg/m2 (1 cycle) and carboplatin 40 mg/m2
(weekly) | 2 |
| Erbitux | 18 |
| Erbitux (2 cycles)
and cisplatin 40 mg/m2 (weekly) | 1 |
| Carboplatin +
5FU | 1 |
| Cisplatin 100
mg/m2 (2 cycles) and erbitux (1 cycle) | 1 |
| Radiotherapy
(n=70) |
| 70 Gy | 66 |
| >70 Gy | 1 |
| <70 Gy | 3 |
| Responders |
| Yes | 38 |
| No | 34 |
| Lymph node
dissection |
| Yes | 10 |
| Positive node | 3 |
| Negative node | 7 |
| No | 62 |
| Recurrence |
| Local | 10 |
| Nodal | 5 |
| Distant
metastases | 4 |
| Local + distant
metastases | 1 |
| Nodal + distant
metastases | 1 |
| Follow-upa |
| Range
(months) | 1–106 |
| Mean (months) | 30 |
DNA extraction
The formalin-fixed, paraffin-embedded tissue samples
(n=72) were sectioned (10×5 μm), de-paraffinized and digested with
proteinase K by overnight incubation at 56°C. DNA was purified
using the QIAamp DNA Mini Kit (Qiagen, Benelux, Belgium) according
to the manufacturer's recommended protocol.
Detection of HPV by polymerase chain
reaction (PCR) amplification
HPV detection was performed using PCR with
GP5+/GP6+ primers (synthesized by Eurogentec,
Liege, Belgium). The GP5+/GP6+ primers
amplify a consensus region located within the L1 region of the HPV
genome. The PCR amplification of the HPV-L1 DNA was performed in a
25-μl reaction mixture containing 2 μl of extracted DNA, 2.5 μl 1X
PCR buffer, 0.025 U Taq DNA polymerase (Roche, Mannheim, Germany),
200 μM DNTPs and 0.5 pmol of each primer. The cycling conditions
for the PCR were as follows: denaturation was performed at 94°C for
1 min, annealing was performed at 55°C for 1 min and 30 sec, and
extension was performed at 72°C for 2 min, for a total of 45
amplification cycles. The first cycle was preceded by a 7-min
denaturation step at 94°C, and the last cycle was followed by an
additional 10-min extension step at 72°C. Aliquots (10 μl) of each
PCR product were electrophoresed through a 1.8% agarose gel and
stained with ethidium bromide to visualize the amplified HPV-L1 DNA
fragments.
Real-time quantitative PCR amplification
of the HPV type-specific DNA
All DNA extracts were tested for the presence of 18
different HPV genotypes using TaqMan-based real-time quantitative
PCR that targeted type-specific sequences of the following viral
genes: 6 E6, 11 E6, 16 E7, 18 E7, 31 E6, 33 E6, 35 E6, 39 E7, 45
E7, 51 E6, 52 E7, 53 E6, 56 E7, 58 E6, 59 E7, 66 E6, 67 L1 and 68
E7 (22). For the various real-time
quantitative PCR assays, the analytical sensitivity ranged from 1
to 100 copies and was calculated using standard curves generated
with plasmids containing the entire genome of the different HPV
types (23). Real-time quantitative
PCR for the detection of β-globin was performed in each PCR assay
to verify the quality of DNA in the samples and to measure the
amount of input DNA (23,24). The following HPV types tested were
considered high-risk (HR): 16, 18, 31, 33, 35, 39, 45, 51, 52, 53,
56, 58, 59 and 66.
Results
HPV status in our clinical series of
HNSCC patients treated by CCR
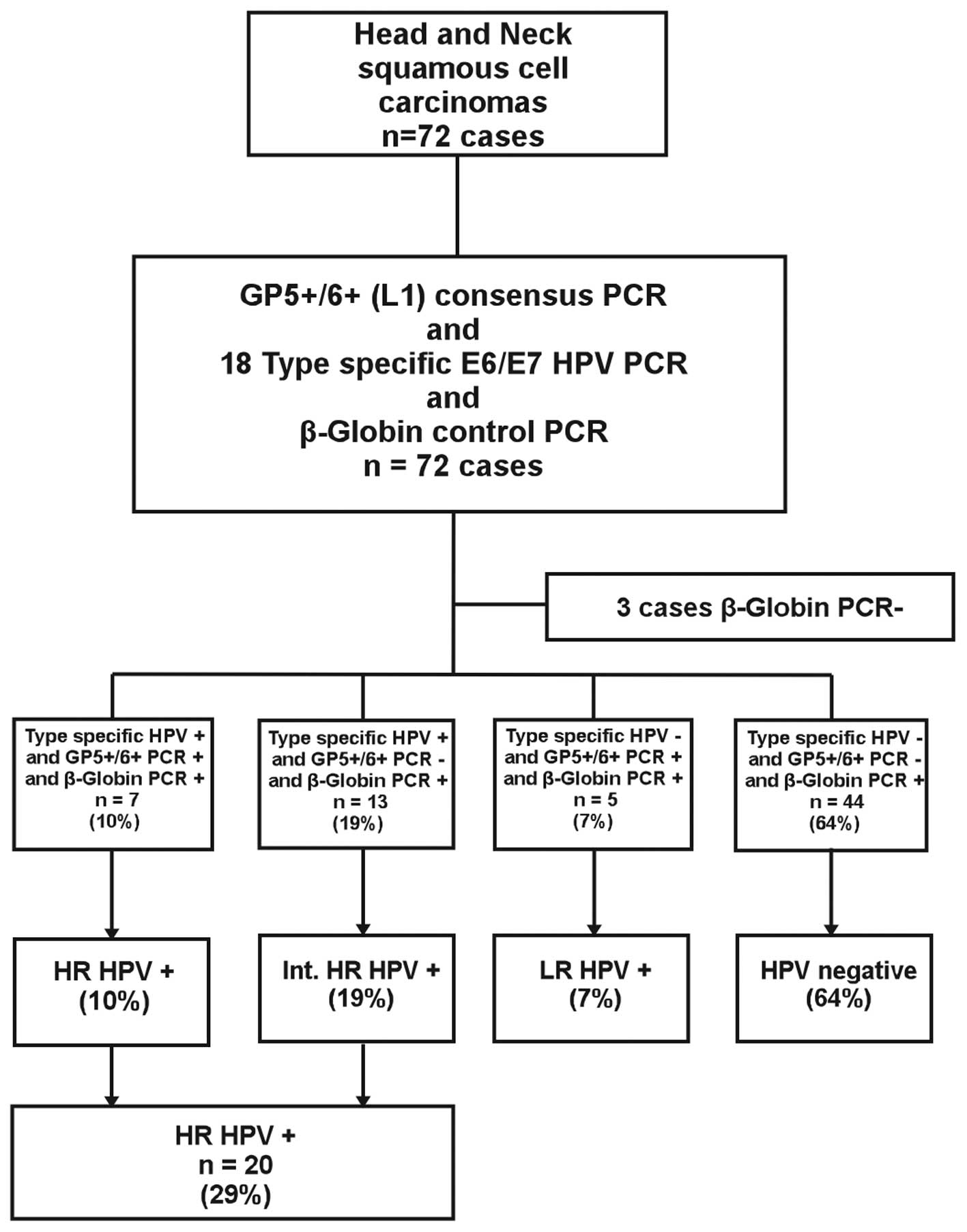
Of the 72 cases, three were excluded from further
analysis since β-globin could not be amplified (Fig. 1). Ultimately, 69 β-globin
PCR-positive specimens were typed by quantitative real-time PCR
using primers for 18 different HPV types. We identified 20 (29%)
patients whose tumors tested positive for the following HR HPV
types: 16 (15 cases), 59 (2 cases), 53 (2 cases), 58 (1 case), 66
(1 case) and 67 (1 case). One patient was infected with multiple
types of HR HPV (HPV 59 E7 and HPV 33, 52, 58, 67 L1). Among the 20
patients with HR HPV+ tumors, 7 tumors were both
GP5+/GP6+ positive (L1 detection) and
type-specific HPV positive (HR HPV+ group). However, 13
tumors were GP5+/GP6+ negative and
type-specific HPV positive, corresponding to an integrated
HPV+ group (int. HR HPV+). In the HR
HPV-negative group (n=49), 5 patients tested positive for HPV using
the GP5+/GP6+ consensus primers and were
considered to be infected with low-risk (LR) HPV types. Forty-four
tumors (64%) were negative for both GP5+/GP6+
and type-specific HPV upon PCR analysis (Fig. 1).
Correlation between HPV detection and
clinical data
The HPV+ group was composed of more men
(n=20, 77%) than women (n=6, 23%). The age ranged from 43 to 78
years, and most patients had stage IV disease (4 had stage III and
22 had stage IV). There was a clear predominance of smokers (n=19,
73%) or former smokers (n=6, 23%) and drinkers (n=23, 88%) or
former drinkers (n=1, 4%) compared to patients who did not consume
tobacco (n=1, 4%) or alcohol (n=2, 8%) (Table I). However, no statistical
correlation was found between the HPV status and the following
clinical data: gender, smoking status, alcohol status, sublocation,
differentiation, T and N stage.
Correlation between HPV detection and of
response rate to CCR
When analyzing the impact of HPV positivity on the
rate of response and non-response to CCR, we tested the potential
correlation using the two tests (GP5+/GP6+
PCR and qPCR) separately and also in combination. We investigated
whether one of these two tests or their combination could predict
the response to CCR. Interestingly, using the PCR
GP5+/GP6+ as a tool for HPV detection, the
rate of response to CCR was statistically lower, with 23% of
responders in the HPV+ group against 59% in the
HPV− group (P=0.02, Fisher's exact test). There was no
statistical correlation between the type of chemotherapy
administered and the number of responders according to HPV status
determined through consensus PCR or qPCR. Using the qPCR for HPV
detection, no statistical correlation was observed, and the rate of
response was lower in the HPV+ group (50%) than in the
HPV− group (57%).
Correlation between HPV infection and
prognosis in HNSCC patients
Based on the GP5+/GP6+ PCR
detection, we observed that the HPV+ group exhibited a
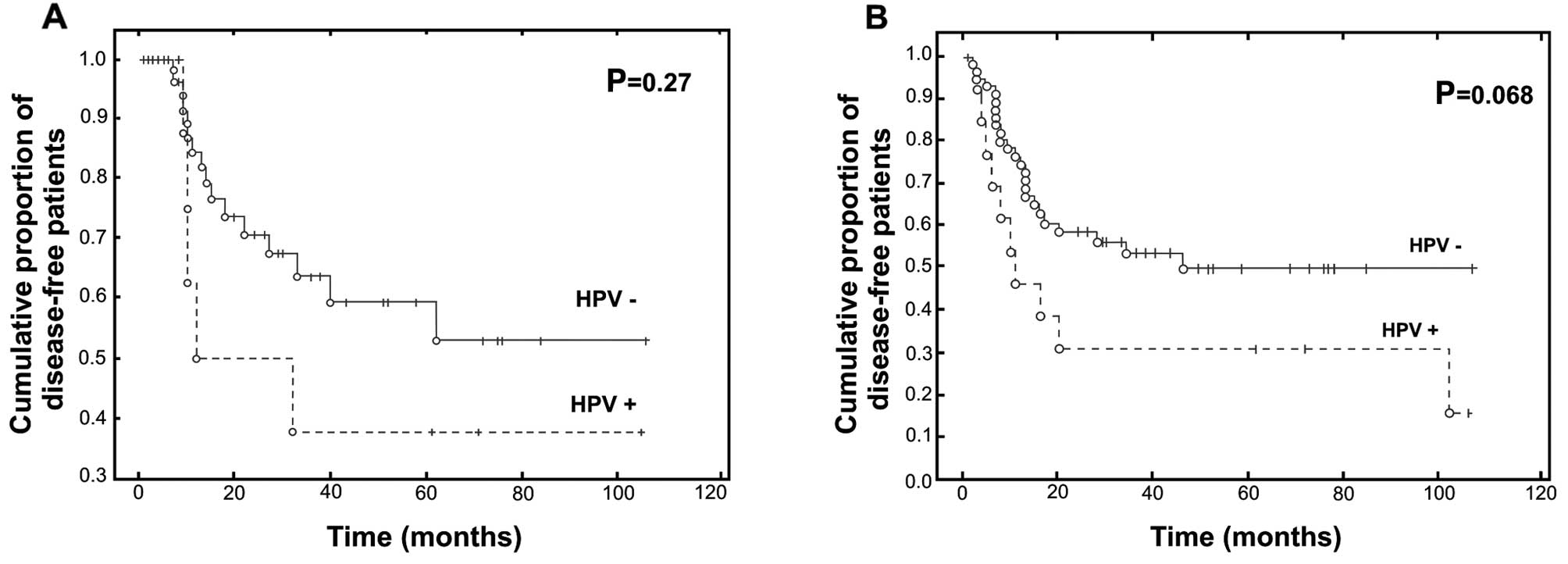
worse prognosis in terms of survival. In fact, the recurrence rate
was higher in patients with HPV+ carcinomas, at 38%,
while it reached only 27% among patients with HPV−
carcinomas (log-rank test, P=0.27) (Fig. 2A). However, the treatment did not
influence the recurrence rate; there was no significant difference
between patients who received platin or erbitux. Therefore, the
disease-free survival rate at 5 years was 57% for patients with
HPV− carcinoma vs. 23% for patients with HPV+
carcinoma (Gehan-Wilcoxon test, P=0.068) (Fig. 2B).
The percentages shown in the two curves do not
represent the total number of patients. The follow-up of several
patients was shorter than the first event of recurrence or
death.
Discussion
In the early 2000s, with the advent of CCR, we
observed a clear increase in the 5-year disease-free survival of
HNSCC stage IV patients, from 45 to 66% (25). However, CCR was also associated with
significant morbidity and mortality (notably, a higher incidence of
dysphonia and dysphagia) (26).
Therefore, clinicians are searching for new reliable prognostic
markers of CCR response and are considering the growing interest in
HPV infection in the biology of HNSCC. We decided to investigate
whether a correlation exists between HPV positivity and the
response to CCR in a series of 72 HNSCC patients with a history of
tobacco and alcohol use (in more than 90% of our population).
In our study, the prevalence of HPV positivity
reached 36%, with 29% of samples containing HR HPV DNA and 7% of
samples containing LR HPV DNA. Moreover, we observed that the rate
of response was statistically lower in the HPV+ group.
Several studies have reported that HPV DNA detection was closely
correlated to a more favorable prognosis in HNSCC patients treated
with CCR (Table II) (12–20).
 | Table IIStudies that found a positive
correlation between HPV and response to chemoradiotherapy in
HNSCCs. |
Table II
Studies that found a positive
correlation between HPV and response to chemoradiotherapy in
HNSCCs.
| Authors (ref.) | No. of
patients | HPV prevalence
(%) | Anatomical
site | Smokers (n) | Drinkers (n) | Detection
methods |
|---|
| Kumar et
al(12) | 42 | 64 | Oropharynx | 34 | Not listed | qPCR |
| Chung et
al(17) | 46 | 50 | Oropharynx | Not listed | Not listed | PCR in situ
hybridization |
| Nichols et
al(18) | 44 | 61 | Oropharynx | Not listed | Not listed | In situ
hybridization |
| Fallai et
al(20) | 22 | 23 | Oropharynx | Not listed | Not listed | qPCR |
| de Jong et
al(19) | 75 | 49 | Pharynx
Oral cavity | Not listed | Not listed | Genetic
signature |
| Rischin et
al(13) | 172 | 65 | Oropharynx | 111 | Not listed | PCR in situ
hybridization |
| Hong et
al(15) | 35 | 24 | Head and neck
squamous cell carcinomas | Not listed | Not listed | qPCR |
| Lill et
al(14) | 29 | 38 | Head and neck
squamous cell carcinomas | Not listed | Not listed | PCR in situ
hybridization |
We recently revealed, in a large clinical series of
162 oral cavity carcinoma patients, that HPV+ tumors
were significantly associated with a poorer prognosis (27). The association between HPV
positivity and poor prognosis was also previously reported in two
Swedish studies in which oral HPV infection was associated with a
dramatically increased risk of oral squamous cell carcinoma (OSCC)
development (28,29). Clayman et al also showed that
HPV+ carcinomas were significantly correlated with a
decreased survival rate (30). In
fact, our results could be explained by the fact that our series
was mainly composed of smokers and/or drinkers (Table I), which is contrary to the majority
of studies describing a favorable prognosis for patients with
HPV+ tumors (Table II).
It should also be emphasized that HPV+ tumors related to
tobacco and alcohol consumption constitute a distinct biological
and clinical entity from HPV+ tumors without classical
risk factors, which are associated with a better outcome. In this
regard, trends in smoking behavior in Europe present some
significant differences (31). A
greater decline in smoking habits was observed among Norwegian,
Finnish and Dutch populations, highlighting that individuals in
Northern European countries are less exposed to classical risk
factors than those residing in Southern European countries
(31). Therefore, all studies
investigating the HPV status in HNSCC need to be interpreted with
caution since many are small clinical series without information on
the alcohol consumption and smoking status of their patients.
Moreover, our clinical series was composed of patients with an
extremely long-term follow-up (ranging from 0 to 106 months), which
is a crucial point for assessing the prognostic implications of HPV
infection.
A persistent HPV infection that can lead to the
development of epithelial cancer requires immune tolerance. Thus,
HPV has also developed several mechanisms to avoid detection by the
host immune defense system, such as downregulation of INF-α and
toll-like receptor 9, production of TGF-β and maintenance of low
viremia (viral protein synthesis is confined to keratinocytes
without an increase in cell death) (32–34).
In the absence of cell lysis, there is little or no release of the
pro-inflammatory cytokines that are crucial for the activation and
migration of dendritic cells (32,35).
There are limited data describing the interaction between the host
immune system during HPV infection in the context of HNSCC, which
means that the role of innate and adaptive immunity in this context
is largely unknown. As mentioned previously, in several studies,
HPV+ HNSCC was associated with an unfavorable outcome.
From these results, some authors supported the hypothesis that
immunosuppression favors HPV infection and that a failing immune
response may be negative in terms of prognosis for HPV+
HNSCC. In fact, Tung et al reported the presence of HPV-16
or HPV-18 and the Epstein Barr virus in 80% of nasopharyngeal
carcinoma samples (36). Another
study showed that herpes simplex virus-2 infection was associated
with an increased risk of HPV infection (37). In 2004, Kreimer et al
demonstrated that tonsillar HPV infection was strongly associated
with HIV co-infection and immunosuppression (38). More recently, we studied different
markers of the immune system in a large series of 110 HNSCC cases
(36 HPV+ cases vs. 74 HPV− cases) to study
the involvement of HPV infection in the alterations of the immune
system in a population of smokers and drinkers. We observed a
significant decrease in the number of natural killer cells and
dendritic cells in HPV+ samples compared to
HPV− samples (unpublished data).
In conclusion, we showed for the first time, in a
clinical series of 72 HNSCC patients, that the rate of response to
CCR was statistically lower in the HPV+ group. Notably,
the association between HPV positivity and an unfavorable prognosis
was discovered in a population of smokers and drinkers with HNSCC.
Our study also highlights the need for prospective, controlled
studies with larger numbers of patients, a detailed history of
tobacco and alcohol use among patients, and homogeneous treatments
and anatomical sites in order to confirm the impact of HPV
infection in HNSCCs treated with CCR.
Acknowledgements
Anaëlle Duray and Géraldine Descamps are PhD
students supported by a grant from the FNRS (Bourse Televie).
Christine Decaestecker is a Senior Researcher of the Belgian
National Fund for Scientific Research (FNRS, Brussels,
Belgium).
References
|
1
|
World Health Organization. International
Agency for Research on Cancer. Globocan. 2008, Available from:
http://globocan.iarc.fr/.
|
|
2
|
Grandis JR, Pietenpol JA, Greenberger JS,
Pelroy RA and Mohla S: Head and neck cancer: meeting summary and
research opportunities. Cancer Res. 64:8126–8129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shah JP and Patel SG: Head and Neck
Surgery and Oncology. 3rd edition. Mosby; New York: pp. 232–236.
352. 2003
|
|
4
|
Forastiere AA and Trotti A: Radiotherapy
and concurrent chemotherapy: a strategy that improves locoregional
control and survival in oropharyngeal cancer. J Natl Cancer Inst.
91:2065–2066. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Denis F, Garaud P, Bardet E, et al: Final
results of the 94-01 French Head and Neck Oncology and Radiotherapy
Group randomized trial comparing radiotherapy alone with
concomitant radiochemotherapy in advanced-stage oropharynx
carcinoma. J Clin Oncol. 22:69–76. 2004. View Article : Google Scholar
|
|
6
|
Stenson KM, Kunnavakkam R, Cohen EE, et
al: Chemoradiation for patients with advanced oral cavity cancer.
Laryngoscope. 120:93–99. 2010.PubMed/NCBI
|
|
7
|
Milano MT, Vokes EE, Kao J, et al:
Intensity-modulated radiation therapy in advanced head and neck
patients treated with intensive chemoradiotherapy: preliminary
experience and future directions. Int J Oncol. 28:1141–1151.
2006.
|
|
8
|
Hu M, Ampil F, Clark C, Sonavane K,
Caldito G and Nathan CA: Comorbid predictors of poor response to
chemoradiotherapy for laryngeal squamous cell carcinoma.
Laryngoscope. 122:565–571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sturgis EM and Cinciripini PM: Trends in
head and neck cancer incidence in relation to smoking prevalence:
an emerging epidemic of human papillomavirus-associated cancers?
Cancer. 110:1429–1435. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dahlstrand H, Nasman A, Romanitan M,
Lindquist D, Ramqvist T and Dalianis T: Human papillomavirus
accounts both for increased incidence and better prognosis in
tonsillar cancer. Anticancer Res. 28:1133–1138. 2008.PubMed/NCBI
|
|
11
|
Reimers N, Kasper HU, Weissenborn SJ, et
al: Combined analysis of HPV-DNA, p16 and EGFR expression to
predict prognosis in oropharyngeal cancer. Int J Cancer.
120:1731–1738. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kumar B, Cordell KG, Lee JS, et al:
Response to therapy and outcomes in oropharyngeal cancer are
associated with biomarkers including human papillomavirus,
epidermal growth factor receptor, gender, and smoking. Int J Radiat
Oncol Biol Phys. 69:109–111. 2007. View Article : Google Scholar
|
|
13
|
Rischin D, Young RJ, Fisher R, et al:
Prognostic significance of p16INK4A and human papillomavirus in
patients with oropharyngeal cancer treated on TROG 02.02 phase III
trial. J Clin Oncol. 28:4142–4148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lill C, Kornek G, Bachtiary B, et al:
Survival of patients with HPV-positive oropharyngeal cancer after
radiochemotherapy is significantly enhanced. Wien Klin Wochenschr.
123:215–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hong AM, Dobbins TA, Lee CS, et al: Human
papillomavirus predicts outcome in oropharyngeal cancer in patients
treated primarily with surgery or radiation therapy. Br J Cancer.
103:1510–1517. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sedaghat AR, Zhang Z, Begum S, et al:
Prognostic significance of human papillomavirus in oropharyngeal
squamous cell carcinomas. Laryngoscope. 119:1542–1549. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chung YL, Lee MY, Horng CF, et al: Use of
combined molecular biomarkers for prediction of clinical outcomes
in locally advanced tonsillar cancers treated with
chemoradiotherapy alone. Head Neck. 31:9–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nichols AC, Faquin WC, Westra WH, et al:
HPV-16 infection predicts treatment outcome in oropharyngeal
squamous cell carcinoma. Otolaryngol Head Neck Surg. 140:228–234.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Jong MC, Pramana J, Knegjens JL, et al:
HPV and high-risk gene expression profiles predict response to
chemoradiotherapy in head and neck cancer, independent of clinical
factors. Radiother Oncol. 95:365–370. 2010.PubMed/NCBI
|
|
20
|
Fallai C, Perrone F, Licitra L, et al:
Oropharyngeal squamous cell carcinoma treated with radiotherapy or
radiochemotherapy: prognostic role of TP53 and HPV status. Int J
Radiat Oncol Biol Phys. 75:1053–1059. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pim D, Collins M and Banks L: Human
papillomavirus type 16 E5 gene stimulates the transforming activity
of the epidermal growth factor receptor. Oncogene. 7:27–32.
1992.PubMed/NCBI
|
|
22
|
Depuydt CE, Benoy IH, Bailleul EJ,
Vandepitte J, Vereecken AJ and Bogers JJ: Improved endocervical
sampling and HPV viral load detection by Cervex-Brush Combi.
Cytopathology. 17:374–381. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Depuydt CE, Boulet GA, Horvath CA, Benoy
IH, Vereecken AJ and Bogers JJ: Comparison of MY09/11 consensus PCR
and type-specific PCRs in the detection of oncogenic HPV types. J
Cell Mol Med. 11:881–891. 2007. View Article : Google Scholar
|
|
24
|
Arbyn M, Benoy I, Simoens C, Bogers J,
Beutels P and Depuydt C: Prevaccination distribution of human
papillomavirus types in women attending at cervical cancer
screening in Belgium. Cancer Epidemiol Biomarkers Prev. 18:321–330.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Filleul O, Preillon J, Crompot E, Lechien
J and Saussez S: Incidence of head and neck cancers in Belgium:
comparison with worldwide and French data. Bulletin du Cancer.
98:1173–1183. 2011.
|
|
26
|
Saussez S: Cancer of the upper
aero-digestive tract: elevated incidence in Belgium, new risk
factors and therapeutic perspectives. Bull Mem Acad R Med Belg.
165:453–461. 2010.PubMed/NCBI
|
|
27
|
Duray A, Descamps G, Decaestecker C, et
al: Human papillomavirus DNA strongly correlates with a poorer
prognosis in oral cavity carcinoma. Laryngoscope. 122:1558–1565.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rosenquist K, Wennerberg J, Annertz K, et
al: Recurrence in patients with oral and oropharyngeal squamous
cell carcinoma: human papillomavirus and other risk factors. Acta
Otolaryngol. 127:980–987. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hansson BG, Rosenquist K, Antonsson A, et
al: Strong association between infection with human papillomavirus
and oral and oropharyngeal squamous cell carcinoma: a
population-based case-control study in southern Sweden. Acta
Otolaryngol. 125:1337–1344. 2005. View Article : Google Scholar
|
|
30
|
Clayman GL, Stewart MG, Weber RS,
el-Naggar AK and Grimm EA: Human papillomavirus in laryngeal and
hypopharyngeal carcinomas. Relationship to survival. Arch
Otolaryngol. 120:743–748. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Giskes K, Kunst AE, Benach J, et al:
Trends in smoking behavior between 1985 and 2000 in nine European
countries by education. J Epidemiol Community Health. 59:395–401.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stanley MA: Immune responses to human
papillomaviruses. Indian J Med Res. 130:266–276. 2009.PubMed/NCBI
|
|
33
|
Hasan UA, Bates E, Takeshita F, et al:
TLR9 expression and function is abolished by the cervical
cancer-associated human papillomavirus type 16. J Immunol.
178:3186–3197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lepique AP, Daghastanli KR, Cuccovia IM
and Villa LL: HPV16 tumor associated macrophages suppress antitumor
T cell responses. Clin Cancer Res. 15:4391–4400. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stanley MA: Immune responses to human
papillomavirus. Vaccine. 24:16–22. 2006. View Article : Google Scholar
|
|
36
|
Tung YC, Lin KH, Chu PY, Hsu CC and Kuo
WR: Detection of human papillomavirus and Epstein Barr virus DNA in
nasopharyngeal carcinoma by polymerase chain reaction. Kaohsiung J
Med Sci. 15:256–262. 1999.PubMed/NCBI
|
|
37
|
Moscicki AB, Hills N, Shiboski S, et al:
Risks for incident human papillomavirus infection and low-grade
squamous intraepithelial lesion development in young females. JAMA.
285:2995–3002. 2001. View Article : Google Scholar
|
|
38
|
Kreimer AR, Alberg AJ, Daniel R, et al:
Oral human papillomavirus infection in adults is associated with
sexual behavior and HIV serostatus. J Infect Dis. 189:686–698.
2004. View
Article : Google Scholar : PubMed/NCBI
|