Introduction
Malignant tumors develop initially as localized
clones of tumor stem cells. These subsequently become invasive and
migrate through the blood and lymphatic vessels to generate
metastatic tumors in other organs (1). Since mortality due to malignant tumor
is most often induced by metastatic rather than primary tumors,
studies that are concerned with elucidating the metastatic process
are critical to cancer research. The results of previous studies
have demonstrated that various molecular mechanisms are involved in
the progression to the metastatic state, which results in tumor
cells that differ from their progenitors in several ways (2–4). At
present, tissue factor (TF) has been implicated in the metastatic
process. TF is a transmembrane protein that can function as a
receptor for clotting factor VII (FVII) and form a complex with
FVII/FVIIa (zymogen and protease forms of clotting FVII) to
initiate blood coagulation (5).
Blood coagulation occurs at the end of a cascade of serial zymogen
activations, which lead to the formation of a fibrin network
(5). Various early clinical
observations have shown a close connection between cancer and blood
coagulation (6,7). In addition, some experimental studies
have found high-level expression of TF in metastatic human melanoma
cells relative to their nonmetastatic counterparts (8,9).
Blocking coagulation activity by monoclonal anti-TF antibodies, TF
pathway inhibitor, or in vivo delivery of anti-TF short
interfering RNA (siRNA) has been reported to inhibit experimental
lung metastasis in some preclinical studies (10–12).
However, the exact mechanism underlying the relationship between TF
and tumor metastasis is not solely dependent on the blood
coagulation cascade; other intracellular signal pathways are also
involved in the progression of tumor metastasis (13).
Cytochalasins are major metabolites that are
extracted from various fungi abundant in tropical regions.
Cytochalasins can permeate cell membranes, bind to actin and alter
its polymerization, thereby altering cellular morphology, gene
expression, and even cellular functions such as cell division and
apoptosis. Of the various types of cytochalasins, cytochalasins B
and E have been shown to possess the most evident anticancer
effects (14,15). However, cytochalasin D (CytD) is
considered the most specific to the actin cytoskeleton (16). Functionally, CytD binds to the
barbed end of growing actin microfilaments and at last causes
inhibition or disruption of actin microfilaments by altering actin
polymerization (17). Several
previous studies have been carried out to determine the effects of
CytD on cell and tissue morphology and its functions in
vitro and in vivo. These include comparisons of normal
and cancer cells (18–20). The effects of CytD on cellular
functions, such as adherence, motility, secretion, and drug efflux,
indicate that CytD may induce important responses in experimental
cancer chemotherapy model systems, either as an individual drug or,
more likely, as an amplifier of known antitumor agents. In
addition, a study by Milsom and Rak (21) showed that CytD treatment can alter
cellular architecture and increase expression of TF and multiple
angiogenic effectors, such as VEGF, TSP-1, TSP-2 and Ang-1.
Recently, we isolated abundant CytD from a strain of endophytic
fungus in an endangered plant (Cephalotaxus hainanensis). We
found it to have antitumor effects in some tumor cell lines
(22). However, we also found
something that we had not anticipated; in a B16 melanoma model CytD
did not induce anti-metastatic effects but rather promoted tumor
metastasis. We further investigated the possible mechanisms by
which this may occur and found that CytD can stimulate TF
expression in melanoma cells. This promotes melanoma metastasis in
combination with FVIIa by activation of the mitogen-activated
protein kinase (MAPK) p38 signal pathway.
Materials and methods
Cell culture and preparation for tail
vein injections
Murine melanoma cell line B16 was purchased from the
American Type Culture Collection (ATCC, Manassas, VA, USA). The B16
cells were cultured in DMEM medium (Gibco) and supplemented with
10% fetal bovine serum (FBS), 2 mmol/l glutamine, 100 IU/ml
penicillin, and 100 μg/ml streptomycin at 37°C in a humidified
atmosphere with 5% CO2. The logarithmic phase cells were
washed twice and detached with trypsin. Following serum
inactivation of the trypsin, cells were washed twice in PBS and
finally resuspended in PBS at a concentration of
2×106/ml. Viability of the injected cells was assessed
by trypan blue staining and was typically >95% for subsequent
experiments.
Establishment of lung metastatic model
and CytD treatment in vivo
A lung metastatic model was established as in our
previous study (23). Female
C57BL/6N mice 4 weeks old were obtained from Hainan Provincial
Animal Center (Hainan, China) and housed (5 mice/cage) in macrolon
cages in a laminar flow cabinet. They were provided with food and
water ad libitum prior to and during the experiments. To
establish the lung metastatic model, 6-week-old mice were injected
with 200 μl 2×106/ml B16 cells into the tail vein. On
the second day after injection, the mice were randomly divided into
two groups (n=5–10 in each group), a CytD treatment group and a
dimethyl sulfoxide (DMSO) control group. CytD group mice were i.v.
injected with CytD 50 mg/kg dissolved in 100 μl DMSO every other
day, and DMSO group mice were i.v. injected with DMSO 100 μl only.
Eighteen days after injection with B16 cells, all mice were
euthanized by cervical dislocation to measure the weight of the
lungs and to count the number of metastatic nodules on the lung
surfaces. The animal protocols used in this study were approved by
the College’s Animal Care and Use Committee (approval ID:
HNMCE10012-4).
Cell treatment with CytD in vitro
Stock concentration (1 mg/ml) of CytD
(Sigma-Aldrich, USA) was prepared in DMSO. B16 cells cultured in
logarithmic growth were treated with CytD (5 μg/ml) for 24 h. This
was established through extensive dose-response and time course
testing (data not shown). For control, B16 cells were also treated
with DMSO only for 24 h. Thereafter, cells were detached with
trypsin and washed twice in PBS following serum inactivation of the
trypsin. These cells were used to isolate the total RNA for
subsequent experiments.
Western blot analysis
Western blot analysis was performed as previously
described (23,24). In brief, lysates of cells treated
with chemical agents (CytD and DMSO) or tumor-tissue homogenate
proteins were separated by 12% SDS-PAGE. Gels were further
transported onto a polyvinylidene difluoride membrane (Bio-Rad,
USA) by a Mini Trans-Blot system (Bio-Rad). The membrane blots were
blocked at 4°C in 5% nonfat dry milk, washed, and probed with
antibodies against corresponding target molecules [TF, MAPK p38 and
phosphorylated-p38 (P-p38) MAPK, all purchased from Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA] at 1:500. They were then
detected using the enhanced chemiluminescence system (Amersham) as
previously reported (25). Digital
images were acquired and analyzed with a gel imaging system
(Bio-Rad Gel Doc 1000; Bio-Rad). The resultant protein levels of
age-comparable normal mice were set as 1 and those of mice in other
groups were compared against this standard. Data are expressed as
fold values.
RNA isolation and northern blot
analysis
Total RNA was isolated directly from cultured cells
and lung tumor masses using TRIzol reagent (Gibco-BRL/Invitrogen,
Gaithersburg, MD, USA) as recommended by the manufacturer. For
northern blot analysis, RNA was transferred to Hybond-N+
membranes and then hybridized with full-length cDNA probes for
murine TF (mTF) and glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) in PerfectHyb™ Plus hybridization buffer (Sigma-Aldrich)
according to the manufacturer’s instructions.
Real-time quantitative PCR
The total RNA extracted from B16 cells and lung
tumor masses was stored in ethanol at −80°C and cDNA synthesis was
performed with First Strand cDNA Synthesis Kit (Pharmacia Biotech,
Inc.). Real-time quantitative PCR was performed in a Multi-Color
Real-Time PCR Detection System (Bio-Rad) to detect of mTF and GAPDH
levels using TaqMan Gene Expression Assay reagents and Universal
PCR Master Mix (Takara). The total reaction volume in each well of
the 96-well MicroAmp Optical reaction plates was 10 μl, including 1
μl of cDNA. The reaction was carried out using a standard two-step
protocol (step 1, 10 min at 95°C; step 2, 40 cycles of 15 sec at
95°C plus 1 min at 60°C). The Ct value of each amplification
reaction was determined using a threshold value 0.03. For data
analysis, mTF-specific Ct values were normalized against the
house-keeping gene GAPDH, whose expression was determined in a
similar manner. The experiments were performed in triplicate and
the results were averaged. The resultant mRNA levels of
age-comparable normal mice were set as 1 and those of other
experimental mice were compared against this standard. Data are
expressed as fold values.
Preparation of lentiviruses expressing
siRNA against TF and tumor cell infection
A siRNA known as mTF223i
(5′-GCAUUCCAGAGAAAGCGUUUA-3′) has been shown to have a potent
interfering effect (10). We chose
mTF223i to construct the plasmid that we would use to produce
lentiviral vectors expressing siRNA against mTF (termed iTF in this
study). We prepared the lentivirus as in a previous study (26). Briefly, the sequences of mTF223i and
control negative siRNA (5′-GUCAGAGUGUGCCUUGACUTG-3′) were cloned
into pTY-linker plasmids for the lentiviral delivering system.
These pTY-linker vectors and three other packaging plasmids
(transfer vector, packaging vector, and envelope vector) were mixed
at ratios of 3, 3, 2 and 1 and co-transfected into 293T cells
(Invitrogen). Forty-eight hours after transfection, lentiviral
particles in the supernatant were concentrated with Microcon YM-100
Centrifugal Filter Unit (Millipore, USA) and stored at −80°C for
subsequent experiments.
For infection of tumor cells, B16 cells at the
logarithmic phase were cultured in DMEM supplemented with 10% FBS.
Fresh medium was replaced after 2 h and the lentiviral particles
were added at a multiplicity of infection (MOI) of 50 overnight
before being injected into the recipient mice to establish tumor
models.
Cell treatment by FVIIa in vitro
CytD (5 μg/ml) was preincubated with the resuspended
B16 cells for 60 min at 37°C and then stimulated with recombinant
FVIIa (Novo Nordisk). Subsequently, cells were washed and extracted
in cold lysis buffer, normalized for protein content, and subjected
to western blot analysis to detect total p38 MAPK and P-p38
MAPK.
Data and statistical analysis
All experiments were conducted in triplicate (at
least) with similar results. Representative images are included.
Student’s t-test for independent samples was performed for pairwise
comparisons of mean values of variables. In calculating two-tailed
significance levels for equality of means, equal variances were
assumed for the two populations. P-values <0.05 were considered
to indicate statistically significant differences.
Results
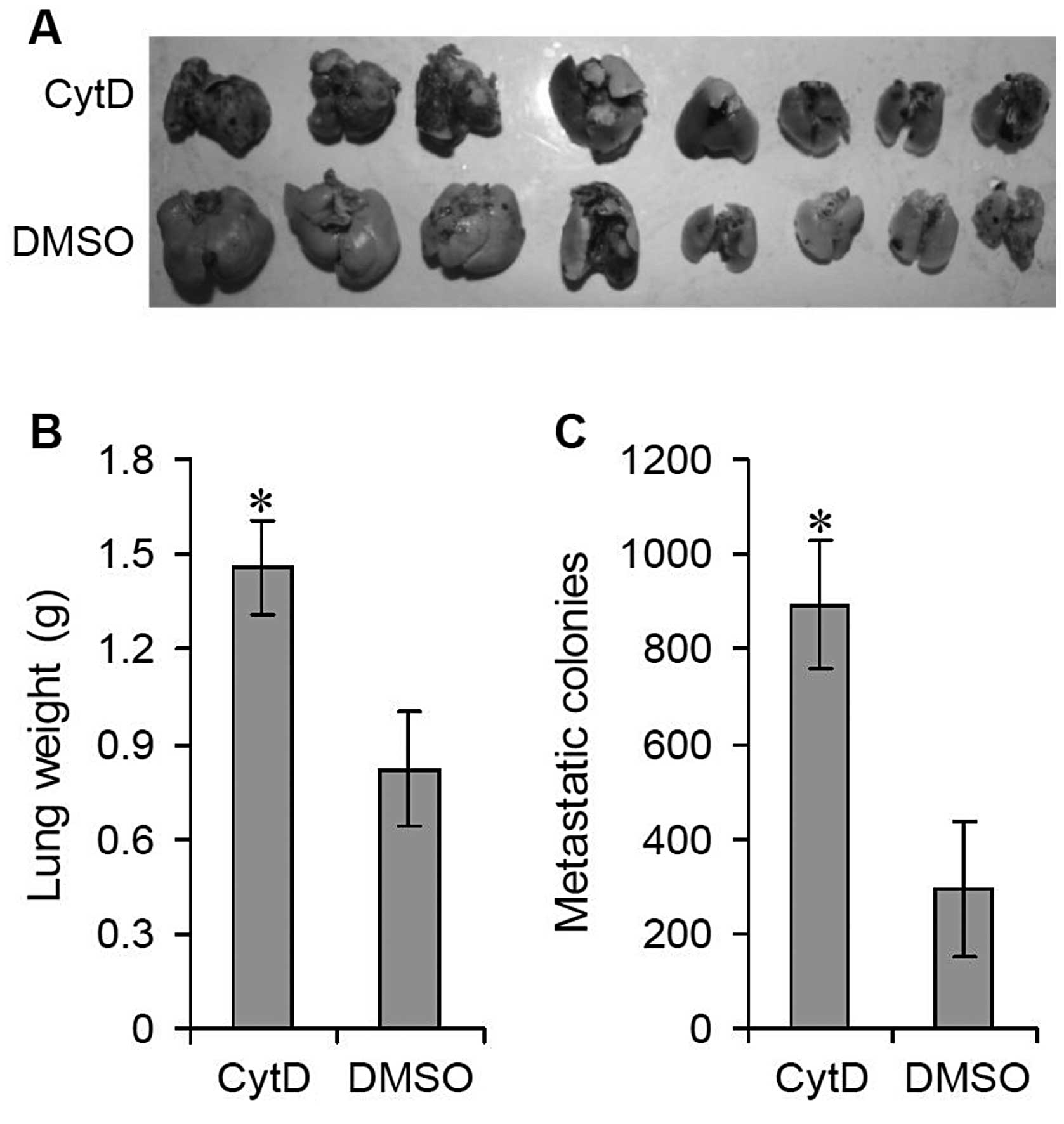
Effects of CytD treatment on lung
metastasis in vivo
C57BL/6N mice were injected with B16 cells into the
tail vein to establish a lung metastatic model. These mice were
randomly divided into two groups (n=5–10 in each group) and i.v.
injected with CytD (50 mg/kg dissolved in 100 μl DMSO) or DMSO (100
μl) every other day. Eighteen days after injection with B16 cells,
all mice were sacrificed by cervical dislocation. Relative to the
DMSO-treated control group, significant lung metastatic colonies
were observed on the lung surfaces of the mice treated with CytD
(Fig. 1A). The average lung weight
of the CytD-treated mice was significantly greater than that of the
DMSO-treated mice, 1.46±0.15 vs. 0.82±0.18 g (Fig. 1B) (P<0.001). In addition, the
number of surface metastatic colonies was also significantly
increased in CytD-treated mice relative to controls, 893.47±135.69
vs. 294.63±143.29 (Fig. 1C)
(P<0.001).
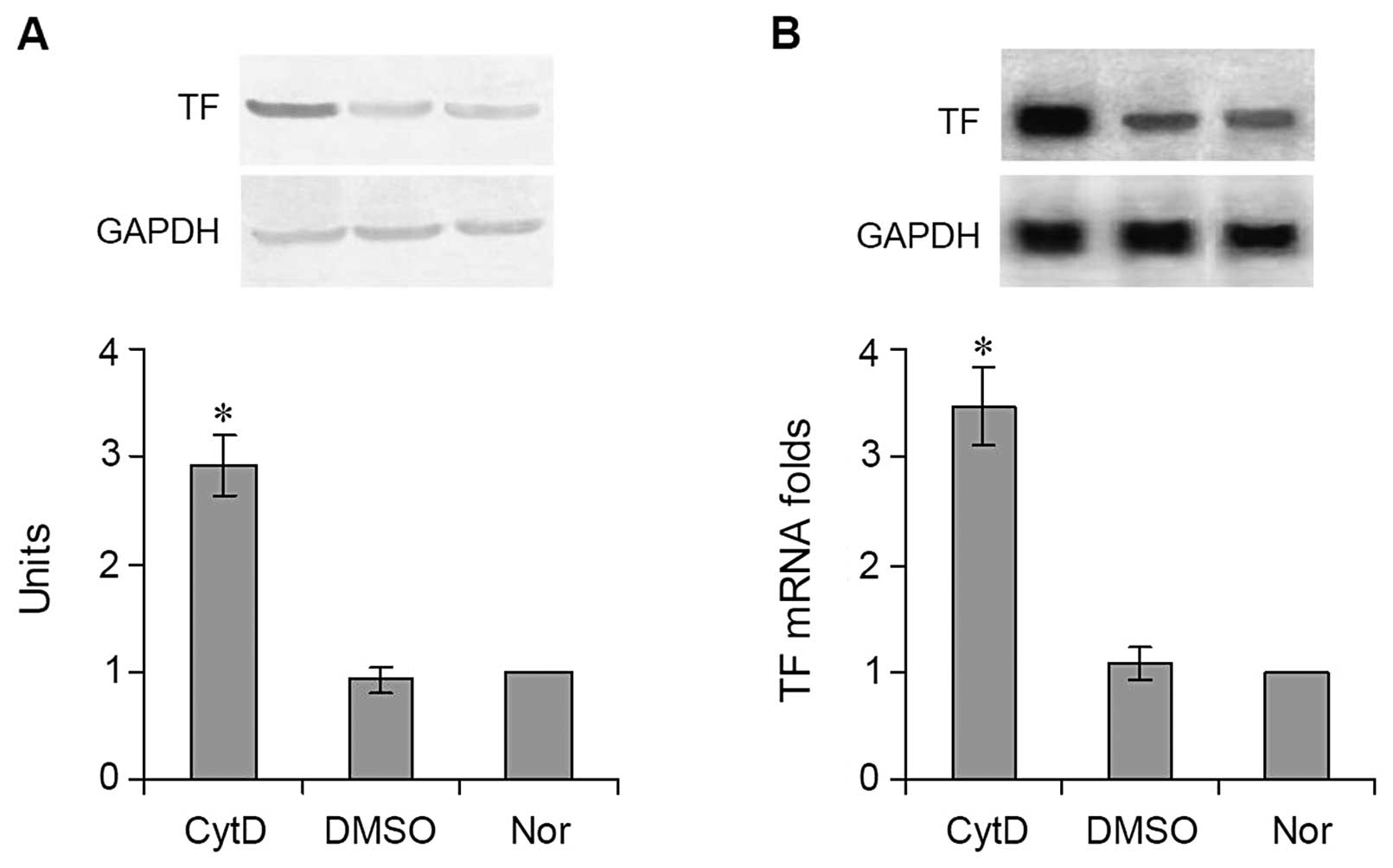
Effects of CytD treatment on the
expression of TF in vitro
CytD and DMSO were added to DMEM medium to culture
the logarithmic-phase B16 cells for 24 h. Thereafter, the cells
(including normal cultured cells not treated with CytD or DMSO)
were collected to isolate total RNA for northern blot analysis and
real-time quantitative PCR. B16 cells treated with CytD and B16
cells treated with DMSO were also subjected to western blot
analysis to determine TF protein expression. Both the results of
western blot analysis (Fig. 2A) and
northern blot analysis (Fig. 2B)
showed that TF protein and mRNA levels expressed in the
CytD-treated cells were significantly higher than in the
DMSO-treated cells. The quantitative value of the TF protein in the
cells treated with CytD was 2.91±0.28 vs. 0.92±0.12 in the cells
treated with DMSO (Fig. 2A)
(P<0.001). The results of real-time quantitative PCR showed that
the level of TF mRNA expression in the cells treated with DMSO
(1.08±0.15-folds) was similar to that in the normal untreated cells
(1-fold), whereas the level of TF mRNA in the cells treated with
CytD was >3-fold (3.47±0.36) that of DMSO-treated and normal
cells (Fig. 2B) (P<0.001).
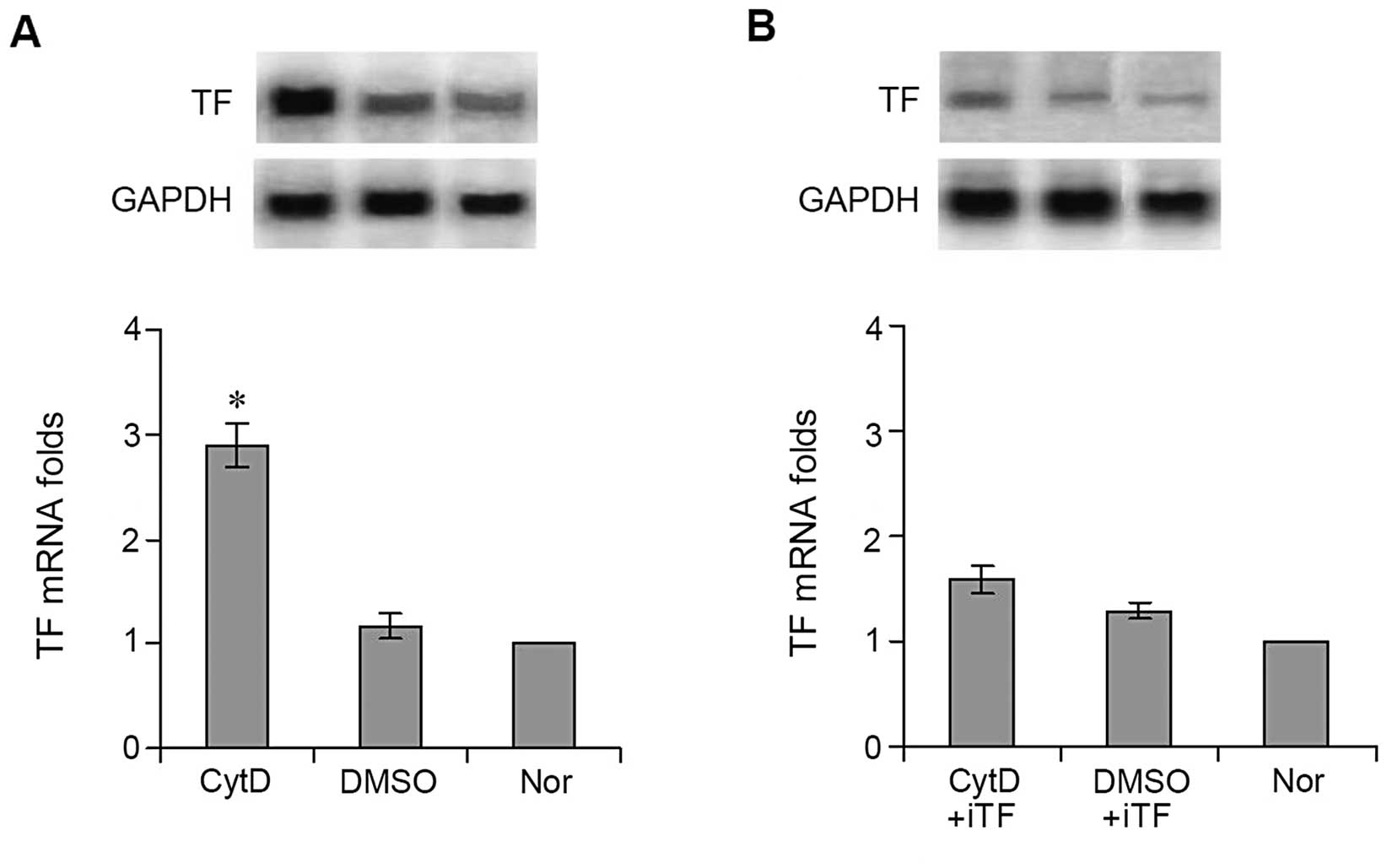
Effects of CytD treatment on the
expression of TF by tumor tissues in vivo
Total RNA was isolated from tumor tissues treated
with CytD or DMSO and used to perform northern blot analysis and
real-time quantitative PCR. Results similar to those observed in
cultured cells were found. TF mRNA levels expressed in tumor
tissues treated with CytD were significantly higher than those
expressed in tumor tissues treated with DMSO. TF mRNA expression in
the tumor tissues treated with CytD was almost 3-fold (2.89±0.21)
that (1.16±0.12) of tumor tissues treated with DMSO when mRNA
expression in normal lung tissue was set as 1 (Fig. 3A) (P<0.001).
Effects of TF interference on TF
expression and lung metastasis
In order to determine whether high levels of TF
expression in the CytD-treated cells were involved in B16 tumor
metastasis, we constructed a lentivirus expressing siRNA against TF
(iTF-lentivirus) to interfere with TF expression both in
vitro and in vivo. After B16 cells were transfected with
the iTF-lentiviruses, CytD was added to the culture medium. As
expected, results were opposite to those found with cultured cells
not exposed to TF interference. TF mRNA expression in the cells
treated with CytD was significantly decreased following
interference, reaching a level almost identical to that of cells
treated with DMSO, 1.58±0.13 vs. 1.29±0.07 (Fig. 3B) (P>0.05).
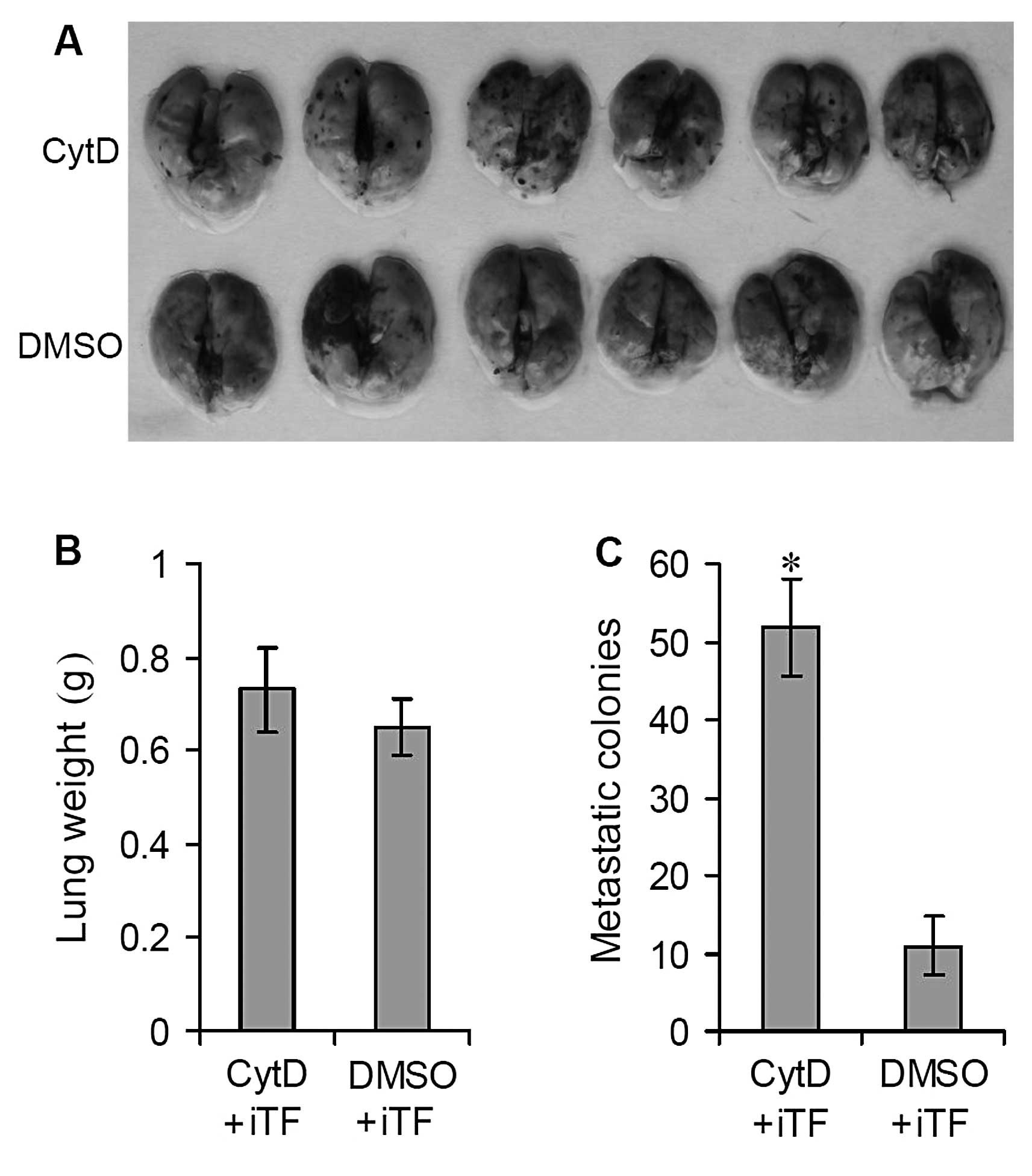
B16 cells were then further transfected with
iTF-lentiviruses and injected by tail vein into C57BL/6N mice to
establish a lung metastatic model. The mice were then treated with
CytD or DMSO as above. Relative to the results of the in
vivo experiments detailed above (Fig. 1), TF interference with
iTF-lentiviruses significantly decreased the number of lung
metastatic colonies on the surfaces of the lungs of CytD-treated
mice (Fig. 4A) (P<0.0001).
Following TF interference, treatment with CytD significantly
suppressed the growth and formation of metastatic colonies on the
lung surfaces. The average lung weight of the CytD-treated mice was
similar to that of the DMSO-treated mice, 0.73±0.09 vs. 0.65 ± 0.06
g (Fig. 4B) (P>0.05). However,
the number of surface metastatic colonies showed a significant
decrease relative to the DMSO-treated controls, 51.73±6.32 vs.
10.86±3.74 (Fig. 4C) (P<0.05).
These results indicate that TF interference can prevent B16 lung
metastasis and that the promotion of TF expression by CytD may play
an important role in B16 lung metastasis.
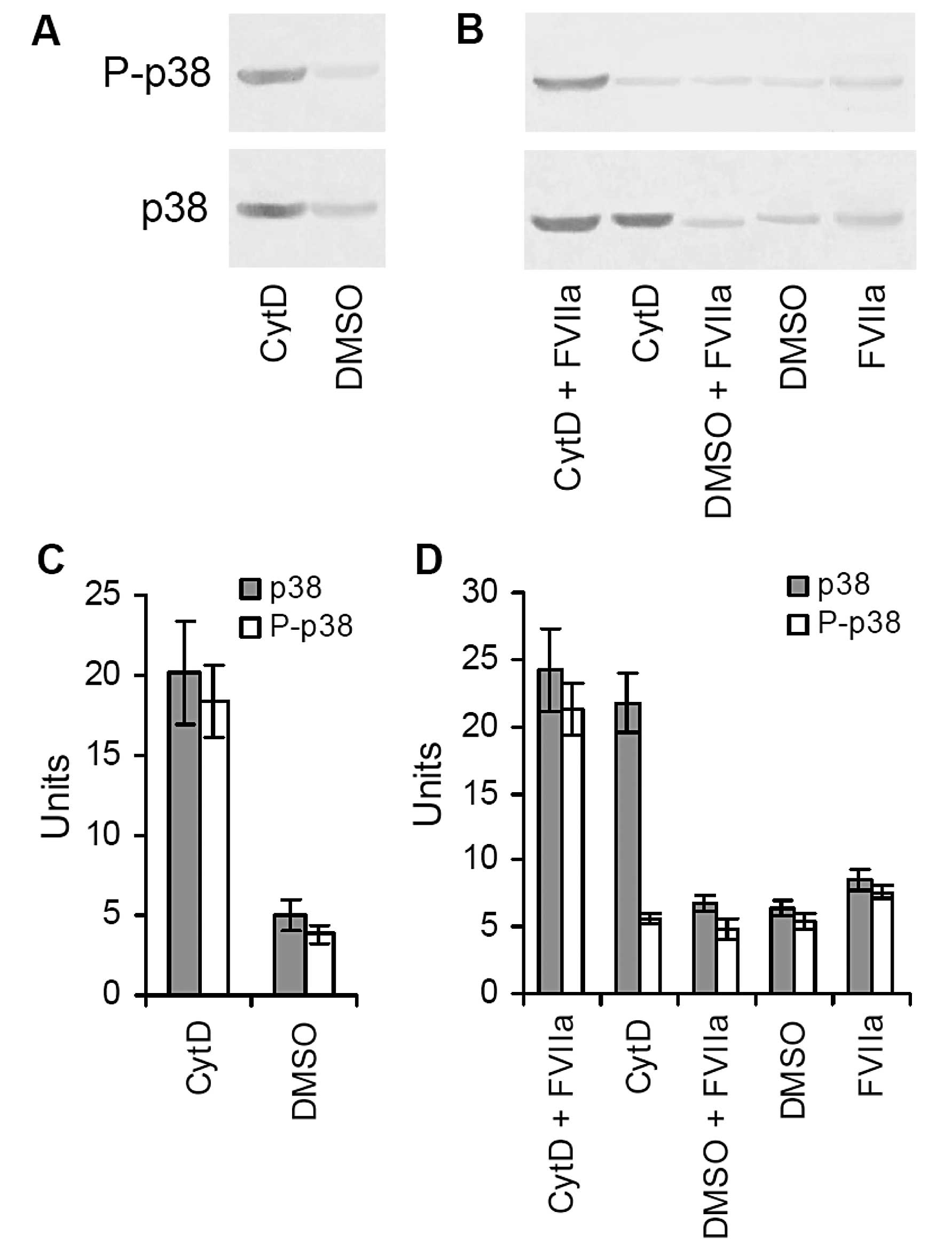
Effects of TF activation of the MAPK p38
pathway on lung metastasis
In order to determine the possible mechanisms by
which upregulation of TF expression increases metastatic potential,
we detected MAPK p38 (p38) and P-p38 in tumor tissues treated with
CytD or DMSO by western blot analysis. Our results showed that both
p38 and P-p38 were proportionally increased in the CytD-treated
tumor tissues relative to those in the DMSO-treated tumor tissues
(Fig. 5A). The quantitative units
of both p38 and P-p38 in the CytD-treated tumor tissues were
20.17±3.27 and 18.39±2.19, whereas the corresponding quantitative
units of both p38 and P-p38 in the DMSO-treated tumor tissues were
4.96±0.93 and 3.82±0.57 (Fig. 5C).
This suggests that the p38 signaling pathway became activated in
the CytD-treated tumor tissues.
We cultured B16 cells in vitro and added
various doses of FVIIa accompanied by CytD or DMSO to the culture
medium to detect p38 and P-p38. We found that B16 cells cultured
with CytD or FVIIa alone did not induce P-p38, but B16 cells
co-cultured with CytD and FVIIa not only significantly promoted TF
expression but they also significantly induced P-p38 (Fig. 5B). The quantitative units of both
p38 and P-p38 were 24.19±3.14 and 21.26±1.95 in the cells treated
with both CytD and FVIIa, 21.73±2.19 and 5.62±0.42 in the cells
treated with only CytD, 6.74±0.58 and 4.83±0.83 in the cells
treated with both DMSO and FVIIa, 6.38±0.63 and 5.49±0.57 in the
cells treated with only DMSO, and 8.52±0.69 and 7.64±0.53 in the
cells treated with only FVIIa (Fig.
5D). These results indicate that TF promotes pulmonary
metastasis of B16 melanoma cells through ligation with FVIIa
(TF/FVIIa), which further activates the MAPK p38 signal
pathway.
Discussion
CytD is a fungal metabolite that is capable of
permeating cell membranes, binding to actin and altering its
polymerization. At present, CytD is thought to be the cytochalasin
most specific to the actin cytoskeleton (16). In addition, a number of studies have
indicated that actin filaments transduce signals into cells by
connection to focal adhesion molecules such as integrins, vinculin,
and talin (27–30). Thus, disruption of actin filaments
by CytD leads to severe impairment in cell function, even cell
death (14,15,30,31).
This also indicates that CytD may be able to induce significant
responses in cancer chemotherapy model systems, either as an
individual agent or, more likely, as an amplifier of known
antitumor drugs. We previously investigated the anti-metastatic
effects induced by CytD or by its modified formula, with unexpected
results. In a previous study, we found that CytD did not inhibit
melanoma lung metastasis but rather promoted the process, as shown
in our murine metastatic model induced by B16 melanoma cells. We
designed the current study to investigate possible molecular
mechanisms behind this process.
TF is a transmembrane protease receptor for the
zymogen FVII and the active enzyme form FVIIa. Binding of TF to
FVIIa (TF/FVIIa) not only initiates the extrinsic coagulation
cascade, which ultimately leads to thrombin generation, fibrin
deposition, and platelet aggregation, but also mediates activation
of various intrinsic cell signal pathways. These pathways influence
various cell functions, including embryonic vessel development,
tumor metastasis, and proinflammatory responses (11,13,21).
Previous findings have indicated that TF is involved in tumor
growth and metastasis in various types of cancer. Early clinical
observations have shown that TF expression is strongly correlated
with tumor progression (8,9,32,33).
TF may promote tumor dissemination and metastasis through
coagulation-dependent or -independent mechanisms (34). Activation of coagulation can capture
tumor cells in fibrin-platelet clots and promote local tumor
growth. This is the first important step in tumor dissemination and
metastasis (35). Several products
of the coagulation system, including thrombin and fibrin, can
stimulate and promote tumor angiogenesis (35–37).
This is also necessary for tumor growth and metastasis. Independent
of the downstream coagulation factors, TF/FVIIa can also stimulate
tumor-related angiogenesis by upregulating expression of certain
angiogenic factors, such as vascular endothelial growth factor and
IL-8 (38–40). Previous studies have indicated that
the TF/FVIIa signal pathway resulting from the proteolytic activity
of the complex is necessary for expression of such angiogenic
factors and for TF-dependent metastasis (41–43).
Scientists previously found evidence explaining why TF promotes
melanoma metastasis by a pathway independent of blood coagulation,
specifically the discovery of TF/FVIIa-induced calcium signal
activation (44). Independent of
the coagulation cascade, the binding of TF to FVIIa can lead to
upregulation of growth factors, cytokines, and even induction of
antiapoptosis, which may contribute to tumor growth and metastasis
(45,46). Blocking TF function by
administration of TF antibody, TF pathway inhibitor, inactivated
FVIIa, or in vivo delivery of anti-TF siRNA has been found
to decrease the rate of metastasis in murine metastasis models
(10,11,47,48).
The results of our present study show that B16 melanoma cells
treated with CytD can increase expression of TF mRNA and protein
both in vitro and in vivo (Figs. 2 and 4A) and significantly inhibit their
expression by RNA interference against TF constructed in a
recombinant lentivirus (Fig. 4B).
In a murine lung metastatic model established by B16 melanoma
cells, significantly increased lung metastasis was observed in mice
treated with CytD (Fig. 1). This
was almost completely suppressed by RNA interference against TF
(Fig. 3). Western blot analyses
demonstrated upregulation and phosphorylation of MAPK p38 in the
lung metastatic tissues from the mice treated with CytD (Fig. 5A and C). In addition, upregulation
and phosphorylation of MAPK p38 was also found in B16 melanoma
cells co-cultured with CytD and recombinant FVIIa but not in B16
melanoma cells cultured with only CytD or other control agents
(Fig. 5B and D).
Based on these findings, the binding of TF to FVIIa
is the major step of the coagulation cascade. Since we found that
the binding of TF to FVIIa could induce upregulation and
phosphorylation of MAPK p38 in B16 melanoma cells cultured ex
vivo and in metastatic tumor tissues in vivo, we
conclude that both coagulation-dependent and -independent pathways
may be involved in lung metastasis in B16 melanoma cells.
We also found that interference against TF can
significantly inhibit lung metastasis (Fig. 4) when compared to the results shown
in Fig. 1. These results are
concordant with those of a previous report (10), and indicate that TF expression can
play a critical role in the metastatic process in B16 melanoma
cells. Collectively, these results suggest that interference
against TF by lentiviruses expressing TF siRNA may become a
clinical approach for the prevention of tumor metastasis.
In summary, our present study suggests that CytD can
stimulate B16 melanoma cell expression of TF. The binding of TF to
FVIIa may cause various signal activations via both
coagulation-dependent and -independent pathways, which promote lung
metastasis in B16 melanoma cells. Thus, it is necessary to
reevaluate the value of CytD and its modified formula when
considering it as an antitumor agent against melanoma.
Acknowledgements
This study was funded in part by grants from the
National Basic Research Program of China (2010CB534909), the
National Natural Science Foundation of China (30960411, 81160288,
81272477 and 81260262), and the Hainan Provincial Natural Science
Foundation (812198 and 061009).
References
|
1
|
Talmadge JE: Clonal selection of
metastasis within the life history of a tumor. Cancer Res.
67:11471–11475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Manavathi B and Kumar R: Metastasis tumor
antigens, an emerging family of multifaceted master coregulators. J
Biol Chem. 282:1529–1533. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Langley RR and Fidler IJ: Tumor cell-organ
microenvironment interactions in the pathogenesis of cancer
metastasis. Endocr Rev. 28:297–321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Denko NC and Giaccia AJ: Tumor hypoxia,
the physiological link between Trousseau’s syndrome
(carcinoma-induced coagulopathy) and metastasis. Cancer Res.
61:795–798. 2001.
|
|
5
|
Orfeo T, Butenas S, Brummel-Ziedins KE and
Mann KG: The tissue factor requirement in blood coagulation. J Biol
Chem. 280:42887–42896. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vestjens JH, Sassen S and Prins MH: Blood
coagulation and cancer: thrombosis and survival, clinical relevance
and impact. An introduction. Pathophysiol Haemost Thromb.
36:113–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boccaccio C and Medico E: Cancer and blood
coagulation. Cell Mol Life Sci. 63:1024–1027. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sawada M, Miyake S, Ohdama S, et al:
Expression of tissue factor in non-small-cell lung cancers and its
relationship to metastasis. Br J Cancer. 79:472–477. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koomagi R and Volm M: Tissue-factor
expression in human non-small-cell lung carcinoma measured by
immunohistochemistry: correlation between tissue factor and
angiogenesis. Int J Cancer. 79:19–22. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amarzguioui M, Peng Q, Wiiger MT, et al:
Ex vivo and in vivo delivery of anti-tissue factor short
interfering RNA inhibits mouse pulmonary metastasis of B16 melanoma
cells. Clin Cancer Res. 12:4055–4061. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amirkhosravi A, Meyer T, Chang JY, et al:
Tissue factor pathway inhibitor reduces experimental lung
metastasis of B16 melanoma. Thromb Haemost. 87:930–936.
2002.PubMed/NCBI
|
|
12
|
Mueller BM, Reisfeld RA, Edgington TS and
Ruf W: Expression of tissue factor by melanoma cells promotes
efficient hematogenous metastasis. Proc Natl Acad Sci USA.
89:11832–11836. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bromberg ME, Konigsberg WH, Madison JF,
Pawashe A and Garen A: Tissue factor promotes melanoma metastasis
by a pathway independent of blood coagulation. Proc Natl Acad Sci
USA. 92:8205–8209. 1995. View Article : Google Scholar
|
|
14
|
Udagawa T, Yuan J, Panigrahy D, Chang YH,
Shah J and D’Amato RJ: Cytochalasin E, an epoxide containing
Aspergillus-derived fungal metabolite, inhibits angiogenesis and
tumor growth. J Pharmacol Exp Ther. 294:421–427. 2000.PubMed/NCBI
|
|
15
|
Bousquet PF, Paulsen LA, Fondy C, Lipski
KM, Loucy KJ and Fondy TP: Effects of cytochalasin B in culture and
in vivo on murine Madison 109 lung carcinoma and on B16 melanoma.
Cancer Res. 50:1431–1439. 1990.PubMed/NCBI
|
|
16
|
Cooper JA: Effects of cytochalasin and
phalloidin on actin. J Cell Biol. 105:1473–1478. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kustermans G, Piette J and Legrand-Poels
S: Actin-targeting natural compounds as tools to study the role of
actin cytoskeleton in signal transduction. Biochem Pharmacol.
76:1310–1322. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miyazaki J, Nishiyama H, Yano I, et al:
The therapeutic effects of R8-liposome-BCG-CWS on BBN-induced rat
urinary bladder carcinoma. Anticancer Res. 31:2065–2071.
2011.PubMed/NCBI
|
|
19
|
Nemeth ZH, Deitch EA, Davidson MT, Szabo
C, Vizi ES and Hasko G: Disruption of the actin cytoskeleton
results in nuclear factor-kappaB activation and inflammatory
mediator production in cultured human intestinal epithelial cells.
J Cell Physiol. 200:71–81. 2004. View Article : Google Scholar
|
|
20
|
Mortensen K and Larsson LI: Effects of
cytochalasin D on the actin cytoskeleton: association of neoformed
actin aggregates with proteins involved in signaling and
endocytosis. Cell Mol Life Sci. 60:1007–1012. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Milsom C and Rak J: Regulation of tissue
factor and angiogenesis-related genes by changes in cell shape.
Biochem Biophys Res Commun. 337:1267–1275. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang JL, Dai HF, Wang H, et al: Cytotoxic
active metabolites from endophytic fungus S15 From Hainan Plum-Yew
(Cephalotaxus hainanensis). J Microbiol (Chinese). 30:10–14.
2010.
|
|
23
|
Tan GH, Wei YQ, Tian L, et al: Active
immunotherapy of tumors with a recombinant xenogeneic endoglin as a
model antigen. Eur J Immunol. 34:2012–2021. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tan GH, Tian L, Wei YQ, et al: Combination
of low-dose cisplatin and recombinant xenogeneic endoglin as a
vaccine induces synergistic antitumor activities. Int J Cancer.
112:701–706. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Apte RS, Niederkorn JY, Mayhew E and
Alizadeh H: Angiostatin produced by certain primary uveal melanoma
cell lines impedes the development of liver metastases. Arch
Ophthalmol. 119:1805–1809. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen SX, Huang FY, Tan GH, et al: RNA
interference against interleukin-5 attenuates airway inflammation
and hyperresponsiveness in an asthma model. J Zhejiang Univ Sci B.
10:22–28. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ailenberg M and Silverman M: Cytochalasin
D disruption of actin filaments in 3T3 cells produces an
anti-apoptotic response by activating gelatinase A extracellularly
and initiating intracellular survival signals. Biochim Biophys
Acta. 1593:249–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pollard TD, Blanchoin L and Mullins RD:
Molecular mechanisms controlling actin filament dynamics in
nonmuscle cells. Annu Rev Biophys Biomol Struct. 29:545–576. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Levee MG, Dabrowska MI, Lelli JL Jr and
Hinshaw DB: Actin polymerization and depolymerization during
apoptosis in HL-60 cells. Am J Physiol. 271:C1981–C1992.
1996.PubMed/NCBI
|
|
30
|
Seufferlein T and Rozengurt E:
Lysophosphatidic acid stimulates tyrosine phosphorylation of focal
adhesion kinase, paxillin, and p130. Signaling pathways and
cross-talk with platelet-derived growth factor. J Biol Chem.
269:9345–9351. 1994.PubMed/NCBI
|
|
31
|
Frisch SM and Francis H: Disruption of
epithelial cell-matrix interactions induces apoptosis. J Cell Biol.
124:619–626. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang C, Zhang F, Tsan R and Fidler IJ:
Transforming growth factor-beta2 is a molecular determinant for
site-specific melanoma metastasis in the brain. Cancer Res.
69:828–835. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ueno T, Toi M, Koike M, Nakamura S and
Tominaga T: Tissue factor expression in breast cancer tissues: its
correlation with prognosis and plasma concentration. Br J Cancer.
83:164–170. 2000.PubMed/NCBI
|
|
34
|
Rickles FR, Patierno S and Fernandez PM:
Tissue factor, thrombin, and cancer. Chest. 124(Suppl 3): 58S–68S.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fernandez PM, Patierno SR and Rickles FR:
Tissue factor and fibrin in tumor angiogenesis. Semin Thromb
Hemost. 30:31–44. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Staton CA, Brown NJ and Lewis CE: The role
of fibrinogen and related fragments in tumour angiogenesis and
metastasis. Expert Opin Biol Ther. 3:1105–1120. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dupuy E, Habib A, Lebret M, Yang R,
Levy-Toledano S and Tobelem G: Thrombin induces angiogenesis and
vascular endothelial growth factor expression in human endothelial
cells: possible relevance to HIF-1alpha. J Thromb Haemost.
1:1096–1102. 2003. View Article : Google Scholar
|
|
38
|
Wang X, Gjernes E and Prydz H: Factor VIIa
induces tissue factor-dependent up-regulation of interleukin-8 in a
human keratinocyte line. J Biol Chem. 277:23620–23626. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nakasaki T, Wada H, Shigemori C, et al:
Expression of tissue factor and vascular endothelial growth factor
is associated with angiogenesis in colorectal cancer. Am J Hematol.
69:247–254. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Y, Deng Y, Luther T, et al: Tissue
factor controls the balance of angiogenic and antiangiogenic
properties of tumor cells in mice. J Clin Invest. 94:1320–1327.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ollivier V, Chabbat J, Herbert JM, Hakim J
and de Prost D: Vascular endothelial growth factor production by
fibroblasts in response to factor VIIa binding to tissue factor
involves thrombin and factor Xa. Arterioscler Thromb Vasc Biol.
20:1374–1381. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bromberg ME, Sundaram R, Homer RJ, Garen A
and Konigsberg WH: Role of tissue factor in metastasis: functions
of the cytoplasmic and extracellular domains of the molecule.
Thromb Haemost. 82:88–92. 1999.PubMed/NCBI
|
|
43
|
Røttingen JA, Enden T, Camerer E, Iversen
JG and Prydz H: Binding of human factor VIIa to tissue factor
induces cytosolic Ca2+ signals in J82 cells, transfected COS-1
cells, Madin-Darby canine kidney cells and in human endothelial
cells induced to synthesize tissue factor. J Biol Chem.
270:4650–4660. 1995.
|
|
44
|
Abe K, Shoji M, Chen J, et al: Regulation
of vascular endothelial growth factor production and angiogenesis
by the cytoplasmic tail of tissue factor. Proc Natl Acad Sci USA.
96:8663–8668. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sorensen BB, Rao LV, Tornehave D,
Gammeltoft S and Petersen LC: Antiapoptotic effect of coagulation
factor VIIa. Blood. 102:1708–1715. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Camerer E, Gjernes E, Wiiger M, Pringle S
and Prydz H: Binding of factor VIIa to tissue factor on
keratinocytes induces gene expression. J Biol Chem. 275:6580–6585.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang X, Wang M, Amarzguioui M, Liu F,
Fodstad O and Prydz H: Downregulation of tissue factor by RNA
interference in human melanoma LOX-L cells reduces pulmonary
metastasis in nude mice. Int J Cancer. 112:994–1002. 2004.
View Article : Google Scholar
|
|
48
|
Poggi A, Rossi C, Casella N, et al:
Inhibition of B16-BL6 melanoma lung colonies by semisynthetic
sulfaminoheparosan sulfates from E. coli K5 polysaccharide.
Semin Thromb Hemost. 28:383–392. 2002. View Article : Google Scholar : PubMed/NCBI
|