Introduction
Multiple myeloma (MM), which mainly occurs in older
patients and particularly in elderly men, is a clonal B-cell
malignancy characterized by the abnormal proliferation of plasma
cells secreting high levels of abnormal monoclonal immunoglobulin.
MM accounts for 10% of all hematological malignancies (1) and for 20% of deaths caused by
hematological malignancies (2). In
the past, alkylating agents such as melphalan, dexamethasone,
interferon and vincristine have traditionally been used against MM.
In recent years, new agents, including lenalidomide, thalidomide,
proteasome inhibitor (bortezomib) and bendamustine, have been
introduced into the treatment of MM (3,4).
Immunotherapy and hematopoietic stem cell transplantation are also
being applied therapeutically to treat MM patients. At present, the
estimated overall survival is higher than 80% at 3 years. However,
despite all the therapeutic advances, MM patients still have a poor
prognosis, with a median survival of approximately 3–5 years
(5). Consequently, novel agents for
the treatment of MM are urgently needed.
Many cytokines are involved in the proliferation of
MM cells and the progression of the disease, including tumor
necrosis factor-α (TNF-α), insulin-like growth factor (IGF),
interleukin (IL)-6, vascular endothelial growth factor (VEGF),
basic fibroblast growth factor (b-FGF) and hepatocyte growth factor
(HGF). Among them, IL-6 is a pivotal cytokine in mediating cell
proliferation, survival and drug resistance in MM (6). In addition, an increased level of
serum IL-6 has been widely recognized as a prognostic factor,
associated with a highly malignant phenotype of MM (7). Bone marrow stromal cells constitute
one producer of IL-6, while an additional producer of IL-6 are MM
cells themselves which show an autocrine response to IL-6, leading
to an autocrine proliferation loop (7). IL-6 binds to the IL-6 receptor
(IL-6R), a cell surface membrane protein composed of two subunits:
an IL-6-specific ligand-binding gp80 subunit and a signal
transducing gp130 subunit. Binding of IL-6 to IL-6R activates
downstream targets, including STAT3, MAPK and AKT (8), followed by a variety of downstream
transcription factors, thus promoting the proliferation of myeloma
cells.
Berbamine is a herbal compound derived from
Berberis amurensis, which is used in Traditional Chinese
Medicine. It has been shown to have immunosuppressive (9), anti-inflammatory, antinociceptive and
antipyretic activities (10).
Berbamine has been previously found to have an antitumor activity
in cancer cells including HeLa, SMMC7721 (a hepatoma cell line),
K562 and HL-60 cell lines (11–14).
Recently, many berbamine derivatives have been synthesized and have
been shown to possess potent antitumor activities (15,16).
The novel berbamine derivative, 4-chlorobenzoyl berbamine (BBD9),
has also been designed for its potent antitumor activity.
In the present study, we investigated the effect of
BBD9 on the proliferation of myeloma cells and, for the first time,
on the IL-6 signaling pathway. In order to further clarify the
mechanism of action of BBD9, we also investigated the downstream
targets of IL-6, including the expression of FOXO3a as well as the
transcription and expression of Bim, the target gene of
FOXO3a, in BBD9-treated MM cells.
Materials and methods
Cells, culture conditions and
reagents
The human myeloma cell lines, U266 and RPMI 8226,
were obtained from the Institute of Cell Biology (Shanghai, China).
MM1.R and MM1.S cells were kindly provided by Professor Steven
Rosen (Northwestern University, Chicago, IL, USA). The cell lines
were cultured in RPMI 1640 medium (Gibco) and supplemented with 10%
heat-inactivated fetal bovine serum (FBS) (Gibco), 100 U/ml
penicillin/streptomycin and 2 mmol/l L-glutamine (Gibco) in a
humidified atmosphere with 5% CO2 at 37°C. Primary MM
cells were purified from bone marrow aspirates obtained from three
patients with de novo MM. BBD9 was kindly provided by
Professor Rong-Zhen Xu (The Second Affiliated Hospital, College of
Medicine, Zhejiang University, Hangzhou, China). The chemical
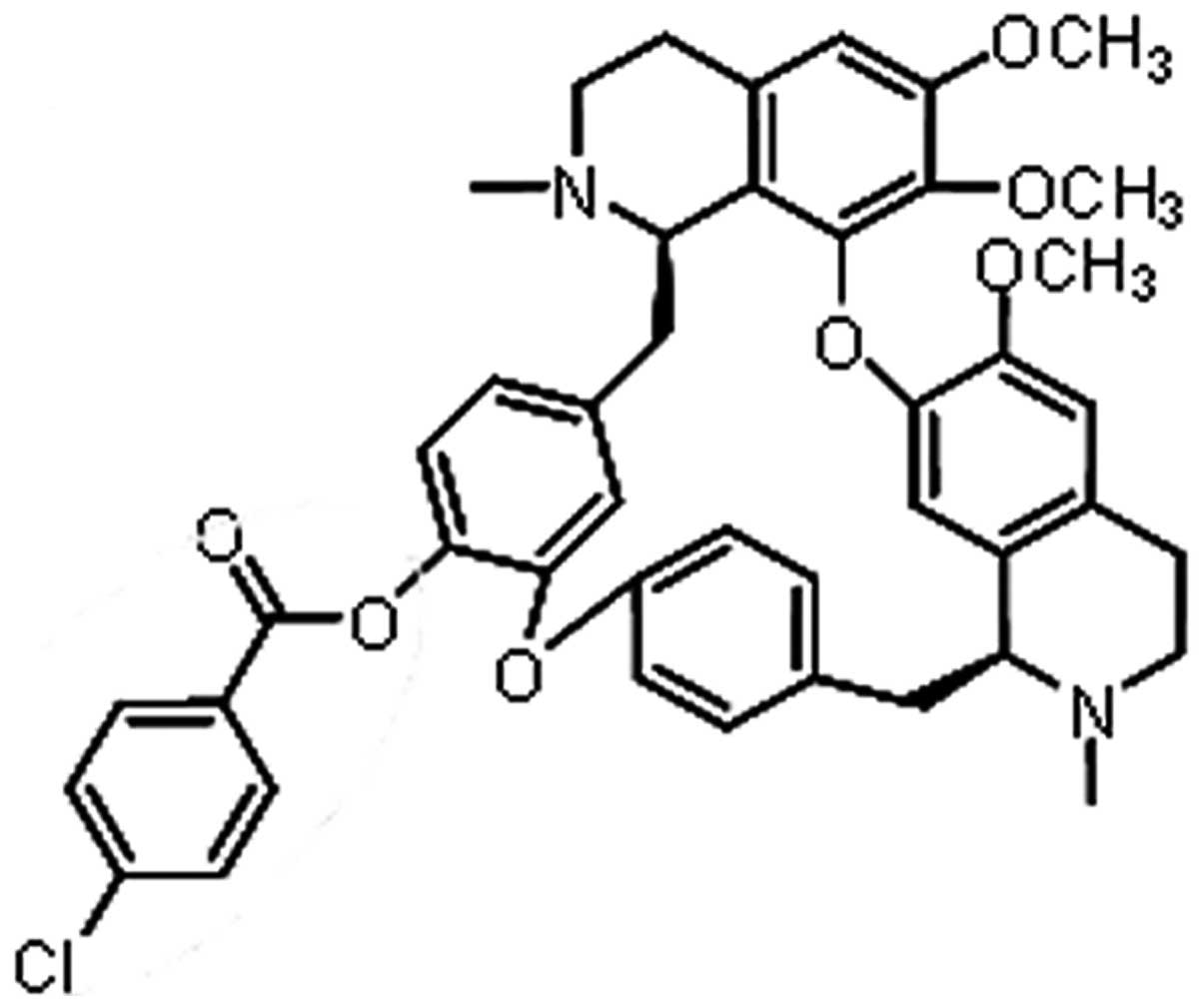
structure of BBD9 is shown in Fig.
1. BBD9 (molecular weight, 747.25) was dissolved in DMSO and
stored at −20°C.
MTT assays for cell viability
The in vitro cytotoxic effect of BBD9 on MM
cells was determined using an MTT assay. Briefly, exponentially
growing cells were plated on 96-well microtiter plates in a total
volume of 200 μl at densities of 2×105/ml for cell lines
and 5×105/ml for primary myeloma cells. Following
incubation with various concentrations of BBD9 (0.5–4.0 μg/ml) for
the indicated times, 20 μl of MTT solution (5 mg/ml) was added to
each well. The plates were incubated for an additional 4 h at 37°C.
The supernatant was aspirated off and 200 μl of DMSO was added to
each well to dissolve the purple formazan dye produced by the
reduction of MTT in viable cells. The absorbance was measured at
570 nm using an ELISA plate reader (Bio-Rad).
Flow cytometry assays for determination
of apoptosis, cell cycle distribution and IL-6R
Following incubation with the indicated
concentrations of agents, the cells were harvested and washed with
phosphate-buffered saline (PBS) before the next step. In order to
investigate apoptosis, the cells were stained with Annexin V (AV)
and propidium iodide (PI) according to the manufacturer’s
instructions (BioVision). For cell cycle analysis,
~1×106 cells were incubated with PI staining solution
(containing 8 μg/ml PI, 0.5 mg/ml RNase A, 1% Triton X-100), after
fixation with cold 70% ethanol for 30 min. To investigate the
expression of IL-6R, the cells were incubated with FITC-conjugated
antibody against IL-6Rα (Chemicon International). After staining
with AV-PI, PI or IL-6Rα-FITC, the cells were run on a FACSCalibur™
flow cytometer and analyzed using CellQuest™ software
(Becton-Dickinson). The mean fluorescence intensity was used as a
parameter to measure the expression of membrane IL-6R. The
immunofluorescent intensity of untreated samples was considered to
be 100%. The values for the expression of IL-6R on treated cell
membranes were expressed as a percentage of the value for untreated
samples.
Western blot analysis
Whole cell lysates were prepared as follows. The
cells were suspended in cell lysis buffer (Cell Signaling
Technology, Inc.) for 30 min, and the cell suspension was
centrifuged at 13,000 × g for 10 min. The resulting supernatant was
collected as a whole cell lysate.
Nuclear and cytosolic extracts were isolated using a
nuclear extraction kit (Chemicon International). Briefly, after
treating with the indicated agents, the cells were collected and
suspended in cytoplasmic lysis buffer (no. 90497; Chemicon
International) containing 0.5 mM DTT and 1/1,000 protease inhibitor
cocktail (no. 90492; Chemicon International). The cell suspension
was centrifuged at 8,000 × g for 20 min at 4°C. The supernatant
containing the cytosolic portion of the cell lysate was collected.
The remaining pellet containing the nuclear portion of the cell
lysate was resuspended in nuclear extraction buffer (no. 90498;
Chemicon International) containing 0.5 mM DTT and 1/1,000 protease
inhibitor cocktail (part no. 90492; Chemicon International). The
nuclear suspension was centrifuged at 16,000 × g for 5 min at 4°C.
The supernatant containing the nuclear portion of the cell lysate
was collected and stored at −20°C.
Equivalent amounts of protein from each lysate were
resolved by 12% SDS-PAGE gel and electrotransferred onto PVDF
membranes (Millipore). The membranes were incubated overnight at
4°C with primary antibodies against the following proteins:
caspase-3, caspase-8, caspase-9, PARP, AKT, pAKT, STAT3, pSTAT3,
cyclin B1, cyclin A, FOXO3a, Bim, β-actin (Santa Cruz
Biotechnology, Inc.) and lamin B. After incubation with the
appropriate horsadish peroxidase (HRP)-conjugated secondary
antibodies, the proteins were detected using an EZ-ECL kit
(Biological Industries).
All antibodies were purchased from Cell Signaling
Biotechnology, Inc. (USA) except where other suppliers were
indicated.
RNA extraction and real-time PCR
Total RNA was extracted from cells using TRIzol™
(Invitrogen). Following treatment with BBD9 for 8 h, the cells were
harvested and washed twice with PBS. TRIzol was added to lyse the
cells. Following the addition of chloroform, the mixture was
centrifuged at 12,000 × g for 15 min. The supernatant containing
RNA was collected and isopropanol was added to precipitate the RNA.
After further centrifugation, the supernatant was discarded and the
pellet containing RNA was washed in 75% ethanol. The RNA was
resuspended in 0.1% (v/v) DEPC-treated water, and 1 μg of total RNA
was reverse-transcribed into cDNA using the Invitrogen RT kit and
an oligo(dT) primer. Real-time PCR was conducted in a volume of 25
μl containing 1 μl of primers, 12.5 μl of 2X SYBR Premix EX Taq™
(Takara), 2 μl of sample and 9.5 μl of double-distilled water. The
samples were amplified in the IQ5™ Real-Time PCR system (Bio-Rad)
for 40 cycles under the following conditions: denaturation for 15
sec at 95°C, annealing and extension for 60 sec at 60°C. The
following primers were used for real-time PCR: Bim forward, 5′-CAC
CCA TGA GTT GTG ACA AAT C-3′ and reverse, 5′-CGT TAA ACT CGT CTC
CAA TAC GC-3′; c-abl forward, 5′-CCG CTG ACC ATC AAT AAG GAA-3′ and
reverse, 5′-GAT GTA GTT GCT TGG GAC CCA-3′. All the primers were
purchased from Sangon Biotech Co., Ltd. (Shanghai).
Enzyme-linked immunosorbent assay (ELISA)
for IL-6
Following incubation of U266 cells with BBD9 for 24
h, the level of IL-6 in the culture supernatant was determined by
ELISA according to the manufacturer’s instructions (R&D
Systems).
Statistical analysis
Data are shown as the means ± standard deviation
(SD). Data were analyzed using the unpaired Student’s t-test.
P<0.05 was considered to indicate a statistically significant
result.
Results
BBD9 inhibits MM cell growth and triggers
apoptosis
In order to investigate the effects of BBD9 on the
viability of MM cells, 4 MM cell lines, RPMI 8226, U266, MM1.R and
MM1.S, were exposed to various concentrations of BBD9 for 24, 48
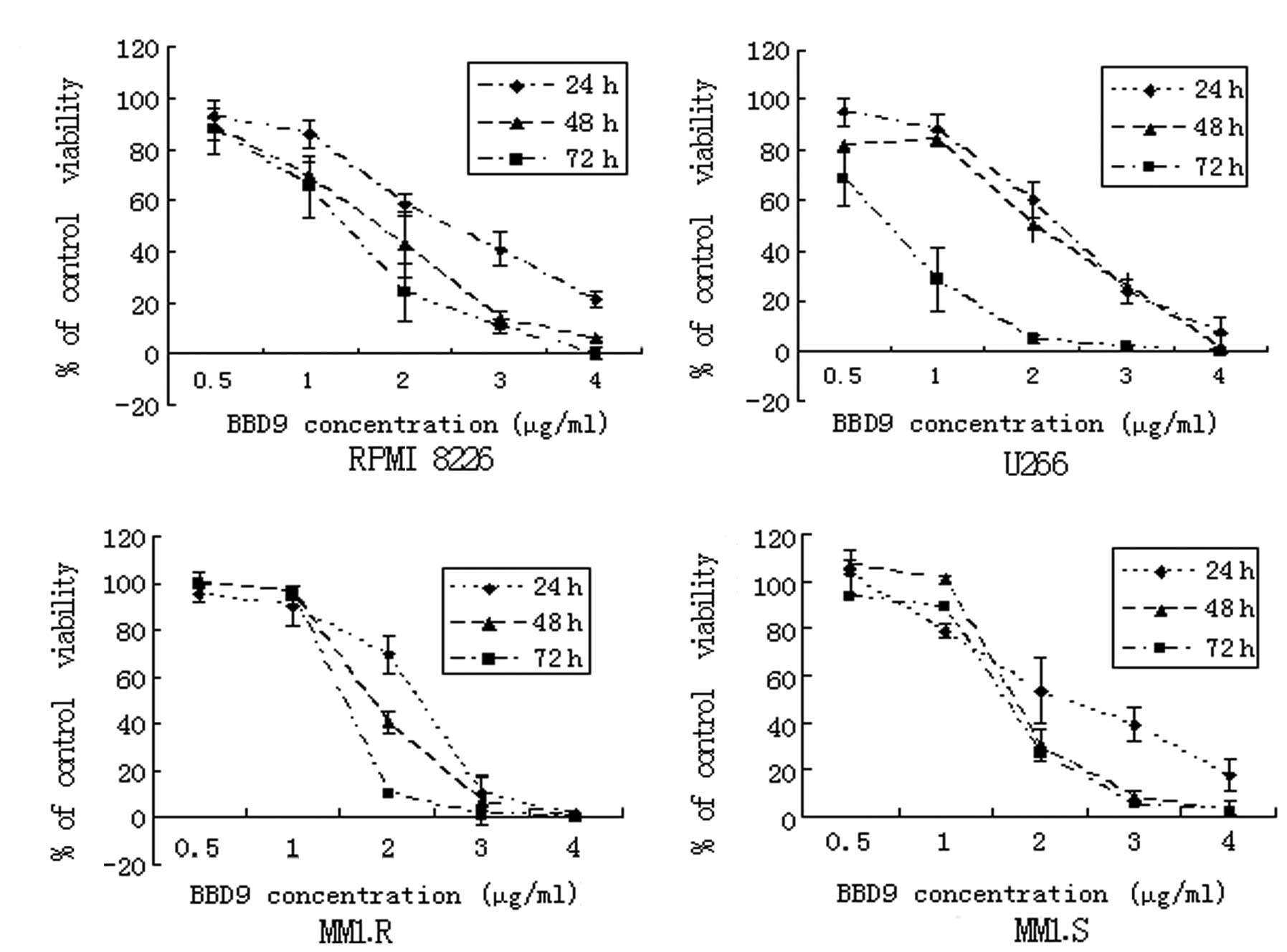
and 72 h, and then analyzed using MTT assays. As shown in Fig. 2, the cell growth of RPMI 8226, U266,
MM1.R and MM1.S was inhibited by BBD9 in a time- and dose-dependent
manner. The IC50 values, determined after 24 h of
incubation, were 2.3 μg/ml for RPMI 8226, 1.8 μg/ml for U266, 1.5
μg/ml for MM1.R and 2.4 μg/ml for MM1.S. These results suggest that
BBD9 is a potent inhibitor of the growth of MM cells in
vitro.
Apoptosis is an important part of the mechanism of
cell death induced by anticancer agents. We next investigated the
role of apoptosis in the inhibition of cell growth in these MM cell
lines. One of the early events of apoptosis is the translocation of
phosphatidylserine (PS) from the internal to the external cell
surface (17); this translocated PS
is detected by AV staining. Hence, we used AV staining combined
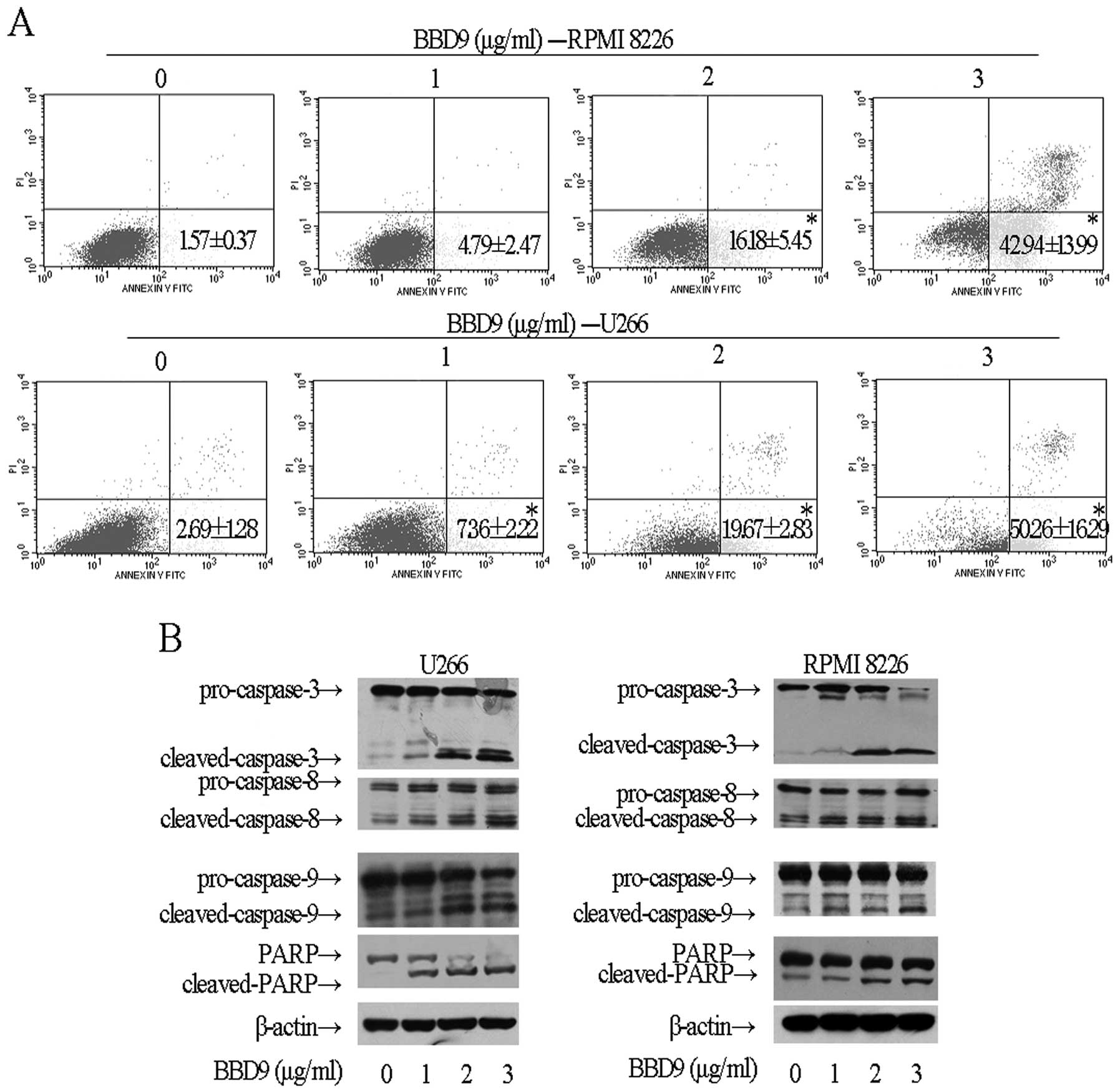
with PI staining and flow cytometric analysis. With increasing
concentrations of BBD9, the number of U266 and RPMI 8226 cells that
were AV+PI− (indicating the cells in early
stages of apoptosis), was significantly increased for 24 h after
treatment (Fig. 3A). Additional
characteristic signs of apoptosis include the cleavage of PARP and
the activation of caspase-3. Western blot analysis (Fig. 3B) demonstrated that BBD9 induced
PARP cleavage and activated caspase-3 in U266 and RPMI 8226 cells
in a dose-dependent manner, thus confirming the induction of
apoptosis. Furthermore, caspase-8 and -9 were also cleaved in U266
and RPMI 8226 cells (Fig. 3B),
indicating that BBD9 induced apoptosis via both
mitochondrial-dependent and -independent apoptotic pathways. These
data indicate that BBD9 inhibits cell growth and leads to apoptosis
in MM cells.
BBD9 induces G2/M phase arrest and
regulates G2/M transition-related proteins in MM cells
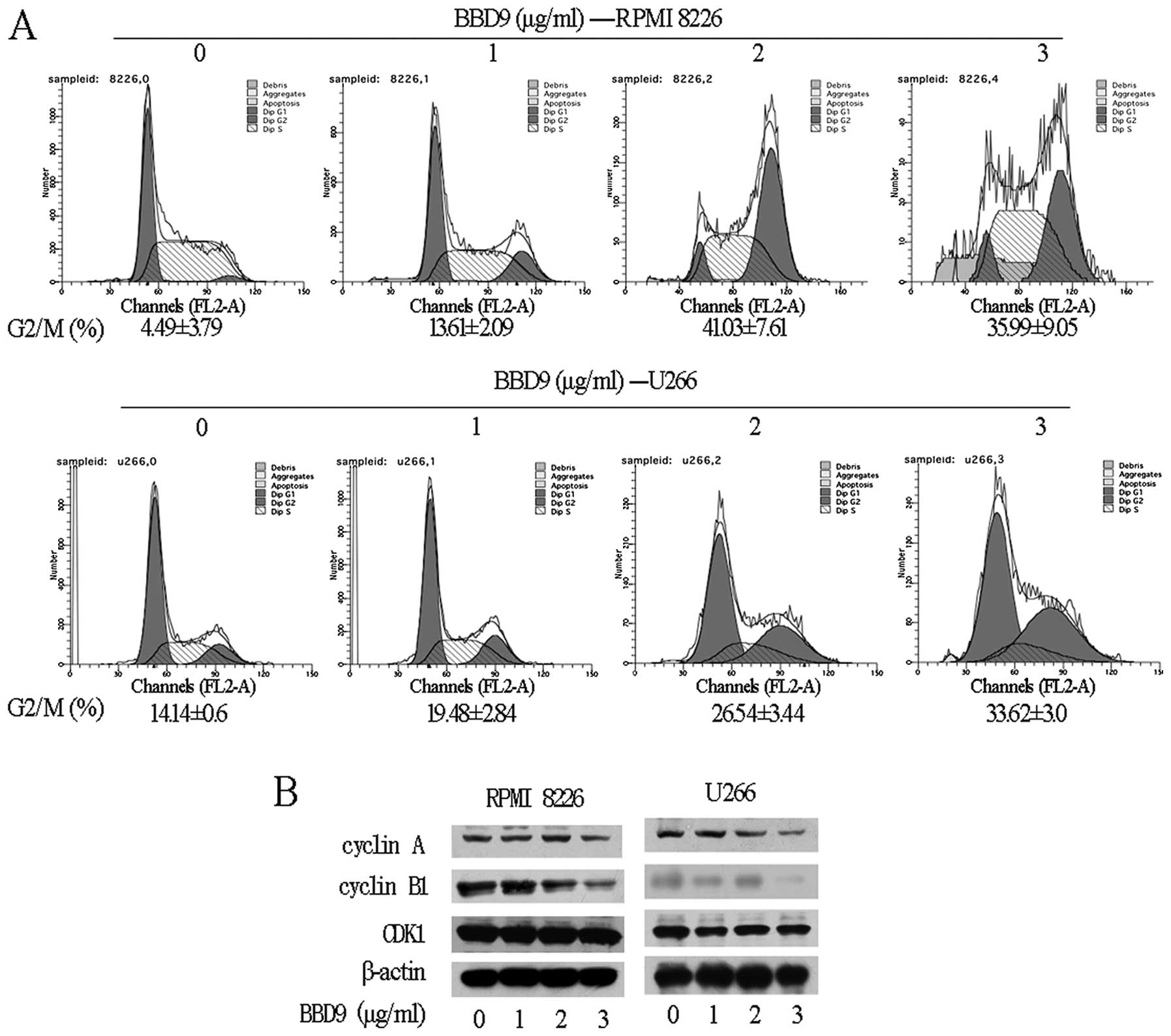
To determine whether MM cell growth inhibition
induced by BBD9 involves cell cycle changes, we examined the cell
cycle phase distribution by flow cytometry. As shown in Fig. 4A, treating U266 and RPMI 8226 cells
with increasing concentrations of BBD9 (1–3 μg/ml) for 24 h
resulted in a dose-dependent cell cycle arrest of U266 and RPMI
8226 cells in the G2/M phase. Following incubation with 1, 2 or 3
μg/ml of BBD9 for 24 h, the percentage of cells in the G2/M phase
was markedly increased compared with the controls (Fig. 4A). These results suggest that BBD9
arrests the cell cycle in the G2/M phase.
The cell cycle is precisely regulated by cyclin,
cyclin-dependent kinase (CDK) and CDK inhibitor (CKI). We
investigated the levels of proteins involved in G2 to M transition
by western blot analysis. The results showed that cyclin B1 and
cyclin A, proteins known to increase during the G2/M transition
phase, were markedly decreased in both U266 and RPMI 8226 cells
treated with 1, 2 or 3 μg/ml of BBD9 for 24 h, while CDK1 remain
unchanged (Fig. 4B).
Cell growth inhibition induced by BBD9 is
abrogated by exogenous IL-6
In consideration of the important role of IL-6 in
the survival and proliferation of MM cells, we next investigated
the impact of exogenous IL-6 on BBD9-induced cell growth inhibition
by MTT assay. Compared with the cell survival rate of MM cells
treated with BBD9 alone, the cell survival rate of cells treated
with 1 or 2 μg/ml of BBD9 combined with 150 ng/ml of IL-6 for 24 h
was significantly increased from 88.83±5.49 to 104.83±4.92% and
from 43.035±12.77 to 55.93±12.24% (mean ± SD), respectively
(Fig. 5A). These results
demonstrate that the cell growth inhibition induced by BBD9 is
abrogated by exogenous IL-6, indicating that the IL-6 signaling
pathway is involved in BBD9-induced apoptosis. We, therefore,
proceeded to investigate changes in proteins related to IL-6
signaling in MM cells exposed to BBD9.
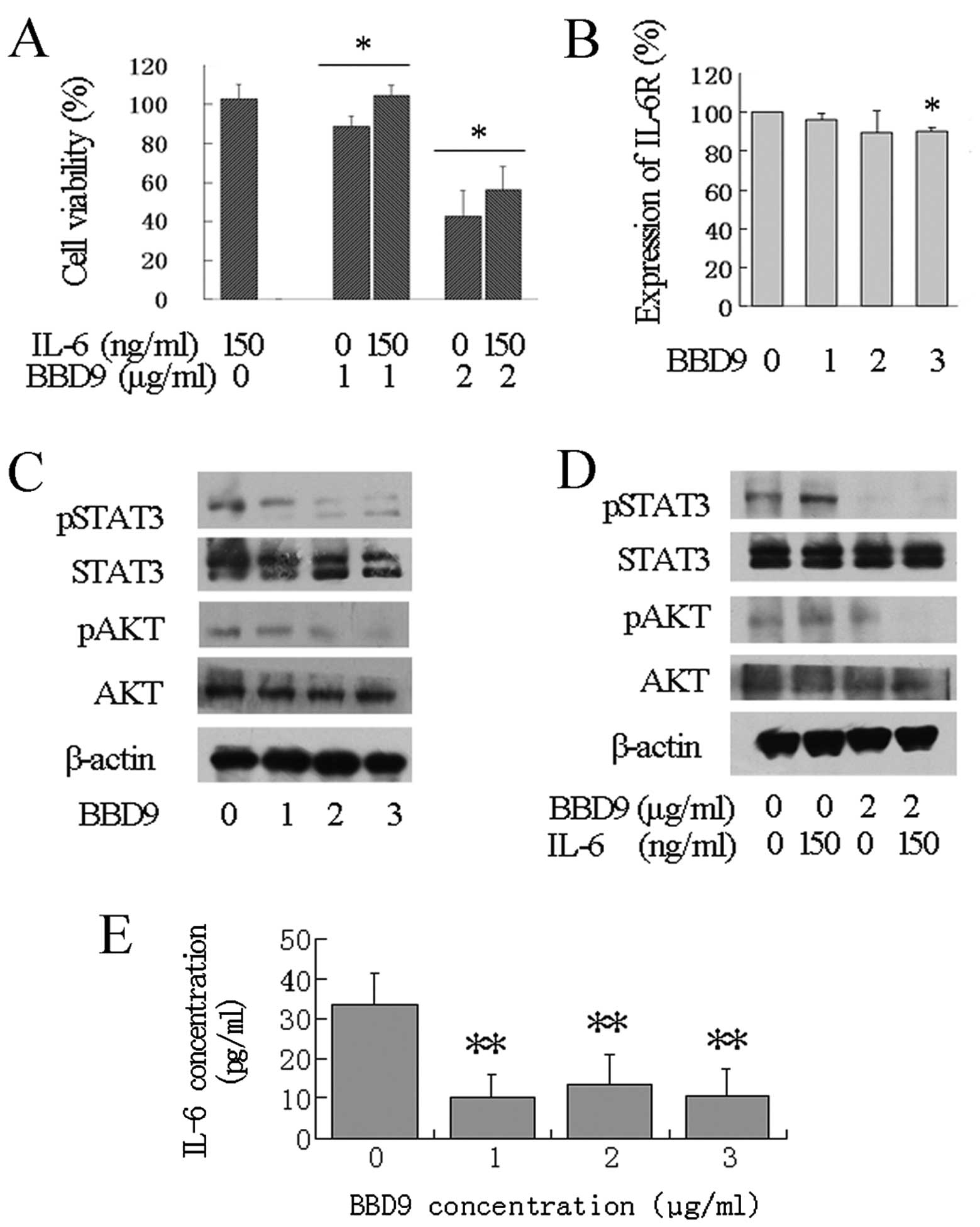 | Figure 5Effects of BBD9 on the IL-6 signaling
pathway in U266 cells. (A) Effects of IL-6 on cell growth
inhibition induced by BBD9. U266 cells were treated with 0, 1 or 2
μg/ml of BBD9 combined with 0 or 150 ng/ml of IL-6 for 24 h, and
the cell viability was determined using MTT assay.
*P<0.05 between the two groups indicated. (B)
Expression of membrane IL-6R in U266 cells treated with BBD9 (0, 1,
2 or 3 μg/ml for 24 h), as determined using the FITC-conjugated
anti-IL-6R antibody and FACS analysis, as described in Materials
and methods. *P<0.05 vs. control cells. (C) Western
blot analysis of AKT and STAT3 in U266 cells treated with 0, 1, 2
or 3 μg/ml of BBD9 for 24 h. (D) Western blot analysis of AKT and
STAT3 in U266 cells treated with 0 or 2 μg/ml of BBD9 combined with
0 or 150 ng/ml of IL-6 for 24 h. The blots are representative of
three independent experiments with similar results. (E) Effects of
BBD9 on IL-6 secretion in U266 cells. The cells were treated with
0, 1, 2 or 3 μg/ml of BBD9 for 24 h. The supernatants were
collected and the concentration of IL-6 was determined using ELISA
assay. Data are the means ± standard error of three independent
experiments. **P<0.01 vs. untreated control
cells. |
BBD9 inhibits IL-6 production and IL-6R
expression
One of the characteristics of U266 cells is that
they exhibit autocrine IL-6 production and constitutive IL-6R
expression; hence, U266 cells constitute a suitable model with
which to examine the effects of BBD9 on the IL-6 signaling pathway.
We, therefore, used U266 cells to investigate the effects of BBD9
on the IL-6 signaling pathway in the following experiments. We
found a significant decrease in IL-6 levels in U266 culture
supernatants following treatment with BBD9 for 24 h using an ELISA
assay (Fig. 5E). The expression of
IL-6R on the cell surface, as determined by FACS analysis, also
decreased significantly in BBD9-treated U266 cells (Fig. 5B). These results suggest that the
expression of both IL-6 and IL-6R was inhibited by BBD9 in U266
cells.
BBD9 inhibits STAT3 and AKT activation by
exogenous IL-6
STAT3 and AKT are two important downstream targets
of IL-6 signaling. Activation of STAT3 and AKT by IL-6 leads to
cell survival and proliferation in myeloma cells, while inhibition
of the phosphorylation of STAT3 and AKT results in cell death. We
first analyzed the effects of BBD9 on STAT3 and AKT in U266 cells.
We found that BBD9 markedly inhibited the phosphorylation of STAT3
and AKT in a dose-dependent manner. However, the total amounts of
STAT3 and total AKT remained unchanged (Fig. 5C). We then investigated whether BBD9
inhibits the activation of STAT3 and AKT by exogenous IL-6 using
western blot analysis. As shown in Fig.
5D, incubation of U266 cells for 24 h with 150 ng/ml of IL-6
led to an increase in the phosphorylation of STAT3 and AKT, while
this effect was abrogated by treatment with 2 μg/ml of BBD9. These
results suggest that both the constitutive and exogenous activation
of STAT3 and AKT are blocked by BBD9 in myeloma cells.
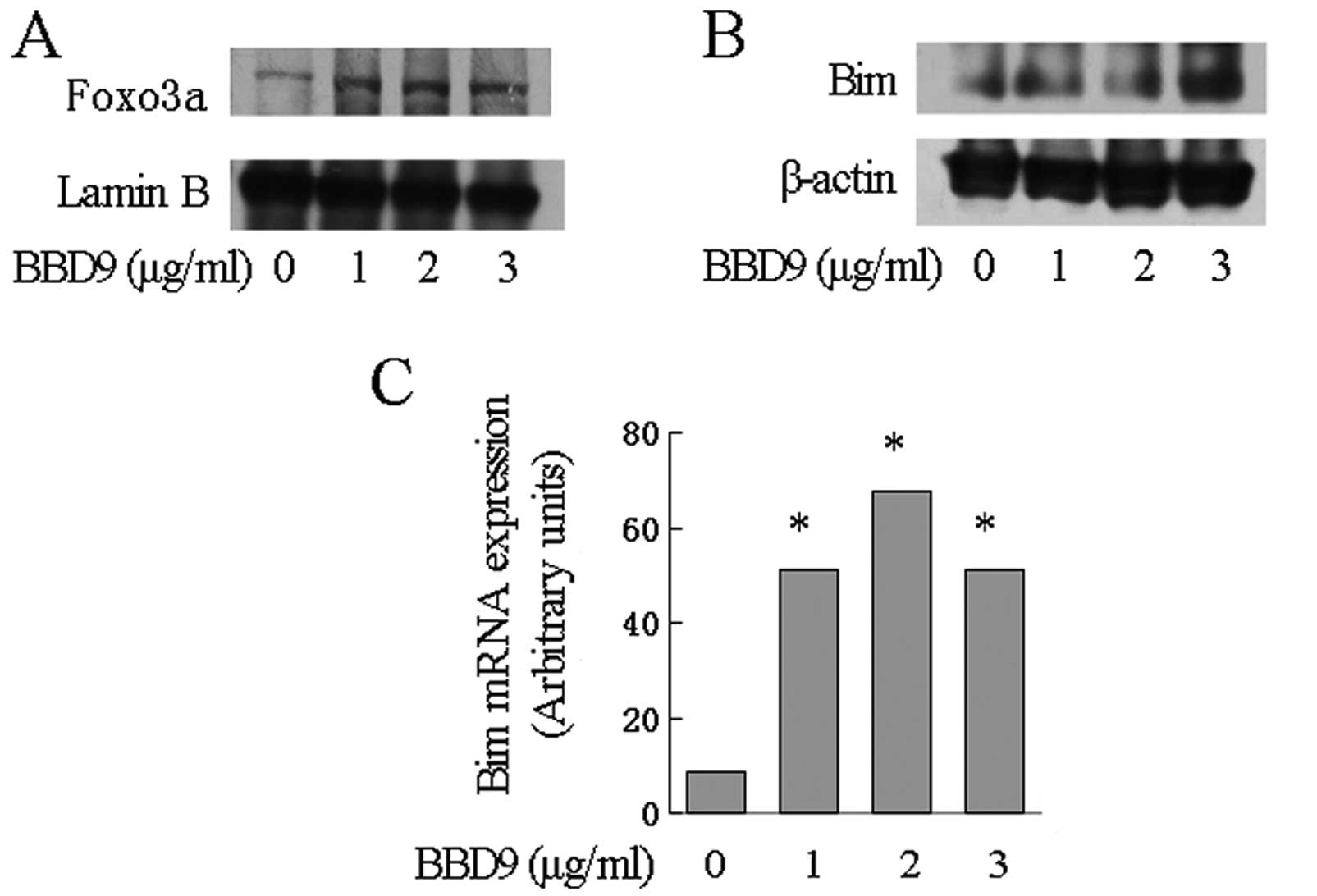
BBD9 activates FOXO3a and increases the
transcription of the Bim gene
FOXO3 is a transcription factor that regulates the
transcription of various genes including Bim. Notably, FOXO3
is a target of AKT. The activation of AKT downregulates the
expression of FOXO3a. Since AKT phosphorylation was inhibited by
BBD9, we hypothesized that FOXO3 is upregulated by BBD9 in MM
cells. To test this hypothesis, we first assessed the expression of
FOXO3a in nuclear extraction lysate from U266 cells treated with
BBD9. We found that FOXO3 was markedly increased as detected by
western blot analysis (Fig. 6A).
The expression of Bim, one of the targets of FOXO3, was then
examined. qRT-PCR assays demonstrated a significant increase in the
levels of Bim transcripts in U266 cells treated with increasing
concentrations of BBD9 (Fig. 6C).
Notably, the upregulation of Bim transcripts was associated with an
increase in the expression of Bim protein as demonstrated by
western blot analysis (Fig.
6B).
Discussion
Apoptosis is a universal and efficient mechanism of
programmed cell death, and is an ideal way of removing unwanted,
diseased or damaged cells. The induction of apoptosis has been
shown to be a promising strategy for the development of novel
anticancer agents. In the present study, we showed that BBD9
inhibits the growth of MM cells by triggering apoptosis, as well as
inducing cell cycle arrest. BBD9 inhibited cell proliferation in 4
MM cell lines in a dose- and time-dependent manner. Notably, there
was no marked difference in susceptibility to BBD9 among the 4 cell
lines, where IC50 at 24 h was ~2 μg/ml. These results
suggest that BBD9 may be clinically useful in the treatment of
patients with MM.
Apoptosis is closely associated with cell cycle
arrest in proliferating cells. A large number of anticancer agents
have been shown to induce apoptosis by arresting cell cycle
progression in the G2/M phase in various types of cancer. This was
confirmed by our study which showed that G2/M phase cell cycle
arrest was associated with cell growth inhibition and apoptosis in
BBD9-treated myeloma cells. G2/M transition is regulated by the
sequential activation and inhibition of cyclins, CDKs and CKIs,
including cyclin A, cyclin B1 and CDK1 (also known as cdc2). Among
these, the cyclin B1/CDK1 and cyclin A/CDK1 complexes are of the
utmost importance in regulating the transition from G2 to M phase
of the cell cycle and mitosis (18–20).
In contrast, the inhibition of cyclin B1/CDK1 or cyclin A/CDK1
complex leads to arrest of cell cycle progression in the G2/M
phase. The downregulation of cyclin B1 and cyclin A, as noted in
the present study, was critical in determining the G2/M arrest in
MM cells caused by BBD9, since CDK1 levels were unaffected.
Therefore, the downregulation of cyclin A and cyclin B1 provides a
mechanism for G2/M phase arrest in BBD9-treated myeloma cells.
The IL-6 signaling pathway plays an important role
in transducing signals for survival and proliferation in myeloma
cells (21). Inhibition of the IL-6
signaling pathway leads to cell death and apoptosis. The effects of
BBD9 on the expression of IL-6R and IL-6 have been investigated in
a dose range of 1–3 μg/ml. The present study demonstrated that both
the expression of membrane IL-6R and autocrine IL-6 decreased
significantly. It has been reported that exogenous IL-6
significantly upregulates the expression of membrane IL-6R in
myeloma cells although the mechanisms have remained elusive
(22). We continue to speculate
whether the downregulation of autocrine IL-6, as noted in the
present study, results in the decreased expression of IL-6R in
myeloma cells.
In addition to the downregulation of IL-6 and IL-6R
by BBD9, we also demonstrated the involvement of downstream
elements of the IL-6 signaling pathway in BBD9-induced apoptosis in
MM. Two important signal transducers in the IL-6 signaling pathway,
STAT3 and AKT, were both found to be inactivated in BBD9-treated
myeloma cells in the present study. Even when U266 cells were
incubated with exogenous IL-6, STAT3 and AKT were still
inactivated. This indicates that the IL-6 signaling pathway is an
important target in the apoptosis induced by BBD9 in MM. The
blockade of the IL-6 signaling pathway is a useful strategy for the
development of novel therapeutic agents (23).
Bim is a member of the BCL-2 family, which contains
more than 20 types of structurally similar proteins, which are
divided into two groups: one consisting of pro-apoptotic proteins
including Bax, Bim and Bak; and the other consisting of
anti-apoptotic proteins including BCL-2, BCL-xL and MCL-1 (24). Bim initiates apoptosis by binding to
BCL-2, BCL-xL and MCL-1 (25,26),
directly neutralizing their anti-apoptotic function. In the present
study, the expression of Bim was elevated in MM cells treated with
BBD9 for 24 h, suggesting the involvement of Bim in the apoptosis
induced by BBD9.
It has been demonstrated that FOXO3a is a downstream
target of AKT (27) and is a
critical nuclear factor in regulating transcription of
apoptosis-related genes (28,29).
By binding to DNA, FOXO3a promotes the transcription of target
genes including pro-apoptotic Bim (28,30).
In addition, FOXO3a is inactivated through phosphorylation by
activated AKT (27), resulting in
the release of bound FOXO3a from DNA, and leading to sustained
nuclear exclusion and subsequent inhibition of apoptosis. In
contrast, inhibition of AKT induces apoptosis via the upregulation
of FOXO3 and Bim. The present study showed that BBD9 inactivated
AKT, leading to elevation of the expression of FOXO3a and Bim, at
both the transcription and protein levels. Therefore, we confirm
that BBD9 induces apoptosis through the AKT/FOXO3a/Bim pathway in
MM cells.
In conclusion, BBD9 effectively inhibited cell
growth and induced apoptosis in myeloma cells in vitro. The
apoptosis induced by BBD9 was accompanied by the arrest of cell
cycle progression in the G2/M phase and concomitant downregulation
of cyclin A and cyclin B1. In addition, BBD9 induced apoptosis
through the inactivation of the IL-6 signaling pathway, including
the inhibition of the expression of IL-6 and IL-6R, downregulation
of STAT3 and AKT phosphorylation, upregulation of FOXO3a in the
nucleus and a subsequent increase in the expression of Bim. To
conclude, BBD9 is a potential novel therapeutic agent for the
treatment of MM. It inactivates the IL-6 signaling pathway, which
underlies the molecular mechanisms of apoptosis. These data provide
a rationale for the clinical study of BBD9 in patients with MM.
Acknowledgements
We thank Professor Rong-Zhen Xu (The Second
Affiliated Hospital, College of Medicine, Zhejiang University,
Hangzhou, China) for kindly providing BBD9. We also thank Dr
Wan-Mao Ni for FACS analysis.
Abbreviations:
|
CDK
|
cyclin-dependent kinase
|
|
CDKI
|
cyclin-dependent kinase inhibitor
|
|
IL-6
|
interleukin-6
|
|
IL-6R
|
IL-6 receptor
|
|
MM
|
multiple myeloma
|
References
|
1
|
Barillé-Nion S, Barlogie B, Bataille R, et
al: Advances in biology and therapy of multiple myeloma. Hematology
Am Soc Hematol Educ Program. 2003:248–278. 2003. View Article : Google Scholar
|
|
2
|
Kuehl WM and Bergsagel PL: Multiple
myeloma: evolving genetic events and host interactions. Nat Rev
Cancer. 2:175–187. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Richardson PG, Mitsiades C, Schlossman R,
Munshi N and Anderson K: New drugs for myeloma. Oncologist.
12:664–689. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lentzsch S, O’Sullivan A, Kennedy RC, et
al: Combination of bendamustine, lenalidomide, and dexamethasone
(BLD) in patients with relapsed or refractory multiple myeloma is
feasible and highly effective: results of phase 1/2 open-label,
dose escalation study. Blood. 119:4608–4613. 2012. View Article : Google Scholar
|
|
5
|
Palumbo A, Attal M and Roussel M: Shifts
in the therapeutic paradigm for patients newly diagnosed with
multiple myeloma: maintenance therapy and overall survival. Clin
Cancer Res. 17:1253–1263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lauta VM: A review of the cytokine network
in multiple myeloma: diagnostic, prognostic, and therapeutic
implications. Cancer. 97:2440–2452. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trikha M, Corringham R, Klein B and Rossi
JF: Targeted anti-interleukin-6 monoclonal antibody therapy for
cancer: a review of the rationale and clinical evidence. Clin
Cancer Res. 9:4653–4665. 2003.PubMed/NCBI
|
|
8
|
Hideshima T, Nakamura N, Chauhan D and
Anderson KC: Biologic sequelae of interleukin-6 induced PI3-K/Akt
signaling in multiple myeloma. Oncogene. 20:5991–6000. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ramgolam V, Ang SG, Lai YH, Loh CS and Yap
HK: Traditional Chinese medicines as immunosuppressive agents. Ann
Acad Med Singapore. 29:11–16. 2000.PubMed/NCBI
|
|
10
|
Küpeli E, Koşar M, Yeşilada E, Hüsnü K and
Başer C: A comparative study on the anti-inflammatory,
antinociceptive and antipyretic effects of isoquinoline alkaloids
from the roots of Turkish Berberis species. Life Sci.
72:645–657. 2002.PubMed/NCBI
|
|
11
|
Zhao XY, He ZW, Wu D and Xu RZ: Berbamine
selectively induces apoptosis of human acute promyelocytic leukemia
cells via survivin-mediated pathway. Chin Med J (Engl).
120:802–806. 2007.PubMed/NCBI
|
|
12
|
Wang GY, Zhang JW, Lu QH, Xu RZ and Dong
QH: Berbamine induces apoptosis in human hepatoma cell line
SMMC7721 by loss in mitochondrial transmembrane potential and
caspase activation. J Zhejiang Univ Sci B. 8:248–255. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu R, Dong Q, Yu Y, et al: Berbamine: a
novel inhibitor of bcr/abl fusion gene with potent anti-leukemia
activity. Leuk Res. 30:17–23. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li BY, Fu B, Zhao YL and Li WH: Effects of
berbamine on intracellular calcium concentration in cultured HeLa
cells. Zhongguo Yao Li Xue Bao. 20:1011–1014. 1999.PubMed/NCBI
|
|
15
|
Du HP, Shen JK, Yang M, et al:
4-Chlorobenzoyl berbamine induces apoptosis and G2/M cell cycle
arrest through the PI3K/Akt and NF-κB signal pathway in lymphoma
cells. Oncol Rep. 23:709–716. 2010.PubMed/NCBI
|
|
16
|
Cui Y, Xu M, Mao J, Ouyang J, Xu R and Yu
Y: Synthesis of berbamine acetyl glycosides and evaluation of
antitumor activity. Eur J Med Chem. 54:867–872. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vermes I, Haanen C, Steffens-Nakken H and
Reutelingsperger C: A novel assay for apoptosis. Flow cytometric
detection of phosphatidylserine expression on early apoptotic cells
using fluorescein labelled Annexin V. J Immunol Methods. 184:39–51.
1995. View Article : Google Scholar
|
|
18
|
Dorée M and Hunt T: From Cdc2 to Cdk1:
when did the cell cycle kinase join its cyclin partner? J Cell Sci.
115:2461–2464. 2002.PubMed/NCBI
|
|
19
|
Galea CA, Wang Y, Sivakolundu SG and
Kriwacki RW: Regulation of cell division by intrinsically
unstructured proteins: intrinsic flexibility, modularity, and
signaling conduits. Biochemistry. 47:7598–7609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Toyoshima-Morimoto F, Taniguchi E, Shinya
N, Iwamatsu A and Nishida E: Polo-like kinase 1 phosphorylates
cyclin B1 and targets it to the nucleus during prophase. Nature.
410:215–220. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Klein B, Zhang XG, Lu ZY and Bataille R:
Interleukin-6 in human multiple myeloma. Blood. 85:863–872.
1995.PubMed/NCBI
|
|
22
|
Kovacs E: Multiple myeloma and B cell
lymphoma. Investigation of IL-6, IL-6 receptor antagonist (IL-6RA),
and GP130 antagonist (GP130A) using various parameters in an in
vitro model. Sc World J. 6:888–898. 2006. View Article : Google Scholar
|
|
23
|
Guo Y, Xu F, Lu T, Duan Z and Zhang Z:
Interleukin-6 signaling pathway in targeted therapy for cancer.
Cancer Treat Rev. 38:904–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Petros AM, Olejniczak ET and Fesik SW:
Structural biology of the Bcl-2 family of proteins. Biochim Biophys
Acta. 1644:83–94. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Willis SN, Fletcher JI, Kaufmann T, et al:
Apoptosis initiated when BH3 ligands engage multiple Bcl-2
homologs, not Bax or Bak. Science. 315:856–859. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hübner A, Barrett T, Flavell RA and Davis
RJ: Multisite phosphorylation regulates Bim stability and apoptotic
activity. Mol Cell. 30:415–425. 2008.PubMed/NCBI
|
|
27
|
Brunet A, Bonni A, Zigmond MJ, et al: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barreyro FJ, Kobayashi S, Bronk SF,
Werneburg NW, Malhi H and Gores GJ: Transcriptional regulation of
Bim by FoxO3A mediates hepatocyte lipoapoptosis. J Biol Chem.
282:27141–27154. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Modur V, Nagarajan R, Evers BM and
Milbrandt J: FOXO proteins regulate tumor necrosis factor-related
apoptosis inducing ligand expression. Implications for PTEN
mutation in prostate cancer. J Biol Chem. 277:47928–47937. 2002.
View Article : Google Scholar
|
|
30
|
Burgering BM and Kops GJ: Cell cycle and
death control: long live Forkheads. Trends Biochem Sci. 27:352–360.
2002. View Article : Google Scholar : PubMed/NCBI
|