Introduction
The incidence of endometrial cancer is increasing;
it is the second most common gynecologic malignancy in Japan.
Increased estrogen exposure, particularly unopposed estrogen, is a
major risk factor for endometrial cancer. Early menarche,
infertility, obesity and late menopause are also associated with
increased risk of the cancer. Endometrial cancer is broadly
classified into one of two clinicopathological types: I and II
(1). The former is estrogen-related
and occurs in both premenopausal and postmenopausal women.
Histologically, it is of the endometrioid type, generally of low
cellular grade and has a favorable prognosis. It is frequently
preceded by endometrial hyperplasia. These tumor cells frequently
express the estrogen receptor (ER), particularly ERα. Type I
endometrial cancer development is associated with a variety of
genetic alterations, including PTEN inactivation, K-ras
mutation, β-catenin mutation and microsatellite instability. Type
II endometrial cancer is non-estrogen-related and occurs primarily
in postmenopausal women. Type II encompasses non-endometrioid
histologies and is represented by serous or clear-cell
adenocarcinoma. It is commonly associated with an atrophic rather
than a hyperplastic endometrium. The cells are negative for ER and
the progesterone receptor (PR); type II endometrial cancer exhibits
a high cellular grade and is associated with poor prognosis.
Genetic alterations in type II carcinomas include p53 mutation, p16
inactivation, HER-2/neu overexpression and reduced E-cadherin
expression (1,2.)
A human homologue of the murine double minute 2
(MDM2) gene (also known as HDM2 in humans) is frequently
overexpressed in several types of human cancer, particularly in
breast carcinomas and soft tissue sarcomas (3). The major contribution of MDM2 to
cancer development is through tight inhibition of tumor suppressor
p53 activity and stability. Biochemically, MDM2 functions as an E3
ubiquitin ligase responsible for p53 ubiquitination and degradation
(4–6). Recent studies have identified numerous
additional MDM2-interacting proteins and p53-independent functions
of MDM2 in the regulation of multiple signaling pathways, including
the pRb/E2F complex and the PI3K/Akt pathway (7). MDM2 overexpression is also strongly
related to the presence of ER (8,9.) Elevated ER expression in
cells lacking ER induces MDM2 overexpression (3). We have shown that overexpression of
wild-type ERα in NIH3T3 cells results in a significant increase in
MDM2 protein levels. MDM2 gene expression is also regulated
by the Ras-driven Raf/MEK/MAP kinase pathway in a p53-independent
manner (10). We have also
demonstrated that the Ras/ER/MDM2 pathway is critical for NIH3T3
cell transformation (11,12) and that blockage of this pathway by
inhibitors or MDM2 siRNA induces p53 and p21 expression and
suppresses cell proliferation in estrogen-dependent cancer such as
endometrial and ovarian cancer (13). These results suggest that the
ER/MDM2/p53/p21 pathway plays an important role in the development
of endometrial cancer.
A single nucleotide polymorphism (SNP309 T>G;
rs2279744) has been identified in the MDM2 promoter. The
SNP309 G allele has high affinity for the transcriptional activator
SP1, which results in a higher level of MDM2 mRNA and MDM2
protein and subsequent attenuation of the p53 pathway. In humans,
the SNP309 G allele is associated with accelerated tumor formation
in hereditary cancer associated with Li-Fraumeni syndrome and
sporadic soft tissue sarcomas (14). SP1 is a well-characterized
co-transcriptional activator of multiple hormone receptors,
including ER. In an analysis of 3 different types of sporadic
cancer (diffuse large B-cell lymphoma, soft-tissue sarcoma and
invasive ductal breast carcinoma), Bond et al(15) showed that the SNP309 G allele is
associated with gender-specific and hormone-dependent acceleration
of tumorigenesis. Several studies, however, found no evidence of an
association between SNP309 and cancer risk (16–18).
The results remain controversial.
The p53 signaling pathway is activated by a wide
variety of stress signals. Activation of p53 induces growth arrest,
cellular senescence and apoptosis (19). Numerous polymorphisms are present in
the TP53 locus. A well-known G to C SNP at TP53 codon
72 results in an arginine (Arg; CGC) or proline (Pro; CCC)
polymorphism (rs1042522). This polymorphism is of particular
interest owing to its functionality, although its biological
function is controversial.
ESR1 is the principal ER expressed in the
endometrium and is thought to be important in the development of
endometrial carcinoma. The ESR1 gene contains several SNPs
whose functional significance remains unknown. The two most
frequently studied polymorphisms located in ESR1 gene intron
1 are often identified by their restriction endonucleases,
PvuII T/C (rs2234693) and XbaI A/G (rs9340799).
p21, a cyclin-dependent kinase inhibitor, is the
major downstream component of the TP53 tumor suppressor pathway.
This protein binds to and inhibits all cyclin-dependent kinase
complexes, causing cell cycle arrest in G1. The p21 codon 31 C/A
(rs1801270) in exon 2 leads to a serine (Ser)/Arg amino acid
substitution and is located in the DNA-binding zinc-finger motif of
the gene (20). This polymorphism
has been implicated in cervical adenocarcinoma (21) and endometrial cancer (22,23).
The MDM2 SNP309 G/G genotype increases the
risk of endometrial cancer in Caucasians (24,25)
and in Japanese women (26).
Furthermore, a combination of the homozygous Arg/Arg genotype of
TP53 codon 72 and the homozygous GG genotype of MDM2
SNP309 is significantly associated with the risk of endometrial
cancer in Japanese women.
We performed the present case control study to
investigate the relationship between endometrial cancer risk and
multiple genetic polymorphisms, including MDM2 SNP309,
TP53 Arg72Pro, ESR1 PvuII and XbaI, and
p21 codon 31, in Japanese women.
Materials and methods
Study subjects
Subjects included 125 endometrial cancer patients
who were diagnosed at the Department of Obstetrics and Gynecology
of Kyushu University Hospital and Kyushu Cancer Center Hospital
between 1993 and 2010. All patients provided informed consent.
There was no family history of endometrial cancer in any of these
cases. Control subjects were selected from 7,132 women aged 49–76
years, living in the East Ward of Fukuoka City, who had completed a
baseline survey between February 2004 and August 2007 of an
on-going cohort study regarding lifestyle-related diseases. Out of
2,055 women aged 49–60 years, 2,783 women aged 60–69 years, and
1,147 women aged ≥70 years who had no history of cancer and who had
donated a blood sample for a genetic study under signed informed
consent, 106, 66 and 28 women, respectively, were randomly selected
in proportion to the age distribution of the 125 cases of
endometrial cancer accrued as of the end of March 2010.
Genotyping
Genomic DNA was extracted from 10-ml blood samples
using the QIAamp® DNA Blood Maxi kit (Qiagen, Hilden,
Germany). DNA of the controls was extracted using an automated DNA
isolation system (NA-300; Kurabo, Osaka, Japan). We performed RFLP
using the digested PCR products, which were electrophoresed in
agarose gels and visualized using ethidium bromide. MDM2
genotyping was performed as previously described (24) using the primers
5′-CGGGAGTTCAGGGTAAAGGT-3′ (sense) and 5′-AGCAAGTCGGTGCTTACCTG-3′
(antisense). The PCR product of 352 bp was digested with MspA1 (New
England Biolabs, Ipswich, MA, USA). A TP53 codon 72
genotyping assay was performed as previously described (27) using primers
5′-TTGCCGTCCCAAGCAATGGATGA-3′ (sense) and
5′-TCTGGGAAGGGACAGAAGATGA-3′ (antisense). The restriction enzyme
Bstu1 (New England Biolabs) was used to digest the 199-bp
PCR product. The ESR1 gene polymorphisms were investigated
using the primers 5′-CTGCCACCCTATCTGTATCTTTTCCTATTC TCC-3′ (sense)
and 5′-TCTTTCTCTGCCACCCTGGCGTC GATTATCTGA-3′ (antisense). The
1374-bp PCR product was digested using the restriction endonuclease
XbaI (Takara; Shiga, Japan), or PvuII (Takara)
(28,29). Genotyping for the p21
polymorphism was performed according to a previously published
method (30,31). A 245-bp fragment was amplified using
the primer sets 5′-ATAGTGTCTAATCTCCGCCG-3′ (sense) and
5′-AAGTCACCCTCCAGTGGTGT-3′ (antisense). The 245-bp PCR product was
digested using Blp 1 (New England Biolabs). To test the
reliability of these assays, genotypes of the MDM2,
TP53, ESR1 and p21 polymorphisms were
validated using direct sequencing for 30 randomly selected samples
of cases and controls (10 of each different genotype). The SNP309
sequencing primers used to amplify a 405-bp sequence were
5′-GATTTCGGACGGCTCTCGCGGC-3′ (sense) and 5′-AGCAAGTCGGTGCTTACCTG-3′
(antisense). The ESR1 PvuII and XbaI sequencing
primers used to amplify a 584-bp sequence were
5′-AGGTTTATGCAATGACG-3′ (sense) and 5′-TCCTTGGCAGATTCCATGGC-3′
(antisense). Sequencing reactions were conducted using BigDye
Terminator version 3.1 (Applied Biosystems, Foster City, CA,
USA).
Cell culture
Endometrial cancer cell lines were maintained in
DMEM supplemented with 10% FBS for HHUA and 15% FBS for Hec6 and
Sawano cells.
Cell growth assay
Cells were plated in a 6-cm dish at 1×105
cells/dish and incubated with a medium supplemented for 24 h.
Subsequently, to reactivate p53 and induce tumor cell apoptosis,
the compound RITA (Tocris, Ellisville, MO, USA), was added, and the
cells were further incubated for 48 and 96 h. Following incubation,
floating cells were washed away and adherent cells were detached
from the dishes using 0.25% trypsin. Detached cells were then
counted using a hemocytometer.
Statistical analysis
Comparisons of means and proportions between cases
and controls were performed using the t-test and Chi-square test,
respectively. Deviation from the Hardy-Weinberg equilibrium was
evaluated using the Chi-square test with 1 degree of freedom. The
association of each genetic polymorphism with endometrial cancer
was examined by the odds ratio (OR) and the 95% confidence interval
(95% CI), which were obtained using logistic regression analysis.
The 95% CI was derived from the standard error of the logistic
regression coefficient. Statistical adjustment was made for age and
body mass index (BMI) with continuous variables for each included
as covariates. Trends in ORs according to the number of minor
alleles were evaluated using the Wald test. For analyzing the
interaction between two polymorphisms, heterozygous and homozygous
genotypes of the minor alleles were combined and statistical
evaluation was conducted using the Wald test for the product term
of the two variables representing the genotypes containing the
minor allele. Statistical significance was declared if a two-sided
P-value <0.05 or if the 95% CI did not include unity.
Statistical analyses were carried out using SAS version 8.2 (SAS
Institute Inc., Cary, NC, USA).
Results
Characteristics of endometrial cancer
cases and controls
Selected characteristics of endometrial cancer cases
and controls are shown in Table I.
The mean age in the cases group was lower than that of the control
group as the latter were selected from women aged 49–76 years,
while the former were recruited regardless of age. BMI was
significantly greater in cases than in controls. A total of 49
(39.2%) overweight women (BMI ≥25 kg/m2) were included
in the cases and 38 (19.0%) in the controls (P<0.001). Most
tumors (112, 89.6%) were of endometrioid histology. Most cases were
of The International Federation of Gynecology and Obstetrics (FIGO)
stage I (95, 76.0%).
 | Table ICharacteristics of cases and
controls. |
Table I
Characteristics of cases and
controls.
|
Characteristics | Cases, n=125 | Controls,
n=200 | P-value |
|---|
| Age (years,
median) | 27–88 (56) | 49–75 (59) | <0.0001 |
| Menopausal status
(%) |
| Premenopausal | 47 (37.6) | 17 (8.5) | <0.05 |
|
Postmenopausal | 78 (62.4) | 183 (91.5) | <0.05 |
| Parity (%) |
| Never | 37 (29.6) | ..20 (10.0) | <0.05 |
| Ever | 88 (70.4) | 180 (90.0) | <0.05 |
| BMI
(kg/m2) mean (SD) | 24.2 (5.0) | 22.9 (3.0) | <0.0001 |
| <25 (%) | 76 (60.8) | 162 (81.0) | |
| ≥25 (%) | 49 (39.2) | ..38 (19.0) | |
| FIGO stage (%) |
| I | 95 (76.0) | | |
| II | 7 (5.6) | | |
| III | 19 (15.2) | | |
| IV | 3 (2.4) | | |
| Unknowna | 1 (0.8) | | |
| Histology |
| Endometrioid
adenocarcinoma (%) | 112 (89.6) | | |
| Grade 1 | 74 | | |
| Grade 2 | 28 | | |
| Grade 3 | 10 | | |
| Non-endometrioid
adenocarcinoma (%) | 13 (10.4) | | |
| Serous papillary
adenocarcinoma | 4 | | |
| Clear cell
adenocarcinoma | 3 | | |
| Mixedb | 3 | | |
|
Undifferentiated | 1 | | |
| Neuroendocrine
carcinoma | 1 | | |
| Squamous cell
carcinoma | 1 | | |
Frequencies of variant alleles between cases and
controls respectively were as follows: SNP309 G allele (0.516 and
0.445), TP53 codon 72 Pro allele (0.364 and 0.370), ESR1
PvuII C allele (0.456, 0.445), ESR1 XbaI G allele
(0.216, 0.205), and p21 codon 31 Ser allele (0.460 and
0.463). Genotype distributions of the SNP309, TP53 Arg72Pro,
ESR1 PvuII and XbaI, and p21 codon 31
polymorphisms in the controls did not deviate from Hardy-Weinberg
equilibrium.
The MDM2 SNP309, TP53, ESR1 and p21
polymorphisms do not individually increase the risk of endometrial
cancer development
Table II shows the
association between endometrial cancer risk and SNP309,
TP53, ESR1 and p21 polymorphisms. The OR for
the SNP309 GG genotype when compared with the SNP309 TT genotype
non-significantly increased risk. The crude OR was 1.76 (95% CI,
0.93–3.30), and the age- and BMI-adjusted OR was 1.64 (95% CI,
0.81–3.28). There was also no measurable association between
endometrial cancer and the TP53 Arg72Pro, ESR1 PvuII
and XbaI, or the p21 codon 31 polymorphisms.
Adjustment for age and BMI did not significantly alter the results.
The analysis was repeated with stratification by menopausal status,
histological status (Table III)
and overweight status. There were 261 postmenopausal women (78
cases and 183 controls) and 102 women with type I endometrial
cancer. For SNP309, the OR associated with the G allele, a slight
increase was seen with postmenopausal status and type I status in
cases that were not statistically significant. When the analysis
was confined to overweight women (49 cases and 38 controls), the
adjusted SNP309 OR for GG vs. TT was 2.39 (95% CI, 0.59–9.66).
 | Table IIMDM2, TP53, ESR1
and p21 genotypes and endometrial cancer risk. |
Table II
MDM2, TP53, ESR1
and p21 genotypes and endometrial cancer risk.
| Genotype | Cases, n=125
(%) | Controls, n=200
(%) | Crude OR (95%
CI) | Adjusteda OR (95% CI) |
|---|
| MDM2
SNP309 |
| TT | 30 (24.0) | 62 (31.0) | 1.00
(reference) | 1.00
(reference) |
| TG | 61 (48.8) | 98 (49.0) | 1.29
(0.75–2.21) | 1.09
(0.60–1.99) |
| GG | 34 (27.2) | 40 (20.0) | 1.76
(0.93–3.30) | 1.64
(0.81–3.28) |
| | | P-trend=0.08 | P-trend=0.45 |
| TG+GG | 95 (76.0) | 138 (69.0) | 1.42
(0.86–2.37) | 1.24
(0.70–2.18) |
| TP53 codon
72 |
| Arg/Arg | 52 (41.6) | 75 (37.5) | 1.00
(reference) | 1.00
(reference) |
| Arg/Pro | 55 (44.0) | 102 (51.0) | 0.78
(0.48–1.26) | 0.66
(0.38–1.15) |
| Pro/Pro | 18 (14.4) | 23 (11.5) | 1.13
(0.55–2.30) | 1.17
(0.54–2.55) |
| | | P-trend=0.08 | P-trend=0.79 |
| Arg/Pro +
Pro/Pro | 73 (58.4) | 125 (62.5) | 0.84
(0.53–1.33) | 0.76
(0.46–1.27) |
| ESR1
PvuII |
| TT | 38 (30.4) | 60 (30.0) | 1.00
(reference) | 1.00
(reference) |
| TC | 60 (48.0) | 102 (51.0) | 0.93
(0.55–1.56) | 0.94
(0.55–1.56) |
| CC | 27 (21.5) | 38 (19.0) | 1.12
(0.59–2.13) | 0.89
(0.43–1.86) |
| | | P-trend=0.78 | P-trend=0.76 |
| TC+CC | 87 (69.6) | 140 (70.0) | 0.98
(0.60–1.60) | 0.93
(0.53–1.60) |
| ESR1
XbaI |
| AA | 77 (61.6) | 127 (63.5) | 1.00
(reference) | 1.00
(reference) |
| AG | 42 (33.6) | 64 (32.0) | 1.08
(0.67–1.75) | 0.88
(0.51–1.53) |
| GG | 6 (4.8) | 9 (4.5) | 1.10
(0.38–3.21) | 0.60
(0.16–2.24) |
| | | P-trend=0.74 | P-trend=0.43 |
| AG+GG | 48 (38.4) | 73 (36.5) | 1.09
(0.68–1.72) | 0.84
(0.50–1.43) |
| p21 codon
31 |
| Ser/Ser | 21 (16.8) | 38 (19.0) | 1.00
(reference) | 1.00
(reference) |
| Ser/Arg | 73 (58.4) | 109 (54.5) | 1.21
(0.66–2.23) | 1.13
(0.57–2.22) |
| Arg/Arg | 31 (24.8) | 53 (26.5) | 1.06
(0.53–2.12) | 1.01
(0.47–2.19) |
| | | P-trend=0.95 | P-trend=0.98 |
| Ser/Arg +
Arg/Arg | 104 (83.2) | 162 (81.0) | 1.16
(0.65–2.09) | 1.09
(0.57–2.09) |
 | Table IIIMDM2 and TP53 genotypes
and endometrial cancer risk. |
Table III
MDM2 and TP53 genotypes
and endometrial cancer risk.
| Genotype | No. of cases
(%) | No. of controls
(%) | Crude OR (95%
CI) | Adjusteda OR (95% CI) |
|---|
| Postmenopause | n=78 | n=183 | | |
| MDM2
SNP309 |
| TT | 22 (28.2) | 57 (31.1) | 1.00
(reference) | 1.00
(reference) |
| TG | 36 (46.2) | 90 (49.2) | 1.04
(0.55–1.94) | 1.12
(0.59–2.15) |
| GG | 20 (25.6) | 36 (19.7) | 1.44
(0.69–3.00) | 1.60
(0.75–3.43) |
| | | P-trend=0.36 | P-trend=0.24 |
| TG + GG | 56 (71.8) | 126 (68.9) | 1.15
(0.64–2.07) | 1.26
(0.69–2.31) |
| TP53 codon
72 |
| Arg/Arg | 33 (42.3) | 69 (37.7) | 1.00
(reference) | 1.00
(reference) |
| Arg/Pro | 32 (41.0) | 93 (50.8) | 0.72
(0.40–1.28) | 0.79
(0.43–1.43) |
| Pro/Pro | 13 (16.7) | 21 (11.5) | 1.29
(0.58–2.90) | 1.44
(0.63–3.31) |
| | | P-trend= 0.95 | P-trend= 0.70 |
| Arg/Pro +
Pro/Pro | 45 (57.7) | 114 (62.3) | 0.83
(0.48–1.42) | 0.91
(0.52–1.58) |
| Type1b |
| MDM2
SNP309 | n=102 | n=200 | | |
| TT | 26 (25.5) | 62 (31.0) | 1.00
(reference) | 1.00
(reference) |
| TG | 47 (46.1) | 98 (49.0) | 1.14
(0.64–2.03) | 0.88
(0.46–1.72) |
| GG | 29 (28.4) | 40 (20.0) | 1.73
(0.89–3.35) | 1.56
(0.73–3.33) |
| | | P-trend=0.11 | P-trend=0.30 |
| TG + GG | 76 (74.5) | 138 (69.0) | 1.31
(0.76–2.25) | 1.07
(0.58–1.97) |
| TP53 codon
72 |
| Arg/Arg | 45 (44.1) | 75 (37.5) | 1.00
(reference) | 1.00
(reference) |
| Arg/Pro | 44 (43.1) | 102 (51.0) | 0.79
(0.43–1.20) | 0.55
(0.30–1.02) |
| Pro/Pro | 13 (12.8) | 23 (11.5) | 0.94
(0.43–2.04) | 1.00
(0.42–2.38) |
| | | P-trend=0.51 | P-trend=0.43 |
| Arg/Pro +
Pro/Pro | 57 (55.9) | 125 (62.5) | 0.76
(0.47–1.23) | 0.64
(0.36–1.12) |
| Postmenopause +
type 1 | n=59 | n=183 | | |
| MDM2
SNP309 |
| TT | 19 (32.2) | 57 (31.1) | 1.00
(reference) | 1.00
(reference) |
| TG | 25 (42.4) | 90 (49.2) | 0.83
(0.42–1.65) | 0.94
(0.46–1.93) |
| GG | 15 (25.4) | 36 (19.7) | 1.25
(0.56–2.77) | 1.50
(0.64–3.47) |
| | | P-trend= 0.66 | P-trend=0.40 |
| TG + GG | 40 (67.8) | 126 (69.9) | 0.95
(0.51–1.79) | 1.09
(0.56–2.13) |
| TP53 codon
72 |
| Arg Arg | 28 (47.5) | 69 (37.7) | 1.00
(reference) | 1.00
(reference) |
| Arg/Pro | 22 (37.3) | 93 (50.8) | 0.58
(0.31–1.11) | 0.66
(0.34–1.30) |
| Pro/Pro | 9 (15.2) | 21 (11.5) | 1.06
(0.43–2.59) | 1.24
(0.49–3.16) |
| | | P-trend=0.55 | P-trend=0.89 |
| Arg/Pro +
Pro/Pro | 31 (52.5) | 114 (62.3) | 0.67
(0.37–1.21) | 0.77
(0.41–1.43) |
Combination of the MDM2 SNP309 G allele
and the Arg/Arg genotype of TP53 codon 72 interacts significantly
to affect the risk of endometrial cancer
In the analysis of the combination of the two
polymorphisms (Table IV), the
SNP309 TG and GG genotypes as well as the TP53 Arg/Pro and
TP53 Pro/Pro genotypes were combined. A significant increase
in the adjusted OR associated with the SNP309 G allele was limited
to those homozygous for the TP53 Arg allele (OR, 2.53; 95%
CI, 1.03–6.21). A statistically significant interaction was
observed between the 2 polymorphisms on the risk of endometrial
cancer (P for the interaction=0.04). We repeated the analysis to
include postmenopausal status and type I endometrial cancer status.
Adjusted ORs for postmenopausal women and type I women possessing
both polymorphisms when compared with being homozygous for both
wild-type alleles were 2.96 (95% CI, 1.04–8.44) and 2.51 (95% CI,
0.97–6.53), respectively. There was a statistically significant
interaction between the two polymorphisms regarding the risk of
endometrial cancer (P for the interaction=0.03 and 0.01,
respectively). Moreover, the corresponding value for type I among
postmenopausal women (59 cases) was 3.24 (95% CI, 1.03–10.16),
which reflected a statistically significant interaction between the
SNP309 and TP53 Arg72Pro polymorphisms (P for the
interaction=0.009).
 | Table IVEndometrial cancer risk for combined
effect of MDM2 and TP53 genotypes. |
Table IV
Endometrial cancer risk for combined
effect of MDM2 and TP53 genotypes.
| Genotype | | No. of cases
(%) | No. of controls
(%) | Crude OR (95%
CI) | Adjusteda OR (95% CI) |
|---|
| All | | n=125 | n=200 | | |
| MDM2
SNP309 | TP53 codon
72 | | | | |
| TT | Arg/Arg | 10 (8.0) | 28 (14.0) | 1.00
(reference) | 1.00
(reference) |
| TT | Arg/Pro + Pro
Pro | 20 (16.0) | 34 (17.0) | 1.65
(0.66–4.09) | 1.79
(0.68–4.73) |
| TG + GG | Arg/Arg | 42 (33.6) | 47 (23.5) | 2.50
(1.09–5.75) | 2.53
(1.03–6.21) |
| TG + GG | Arg/Pro +
Pro/Pro | 53 (42.4) | 91 (45.5) | 1.63
(0.73–3.62) | 1.35
(0.56–3.24) |
| | | |
P-interaction=0.09 |
P-interaction=0.04 |
| Postmenopause | | n=78 | n=183 | | |
| MDM2
SNP309 | TP53 codon
72 | | | | |
| TT | Arg/Arg | 6 (7.7) | 25 (13.7) | 1.00
(reference) | 1.00
(reference) |
| TT | Arg/Pro +
Pro/Pro | 16 (20.5) | 32 (17.5) | 2.08
(0.71–6.10) | 2.46
(0.81–7.44) |
| TG + GG | Arg/Arg | 27 (34.6) | 44 (24.0) | 2.56
(0.93–7.03) | 2.96
(1.04–8.44) |
| TG + GG | Arg/Pro +
Pro/Pro | 29 (37.2) | 82 (44.8) | 1.47
(0.55–3.95) | 1.84
(0.66–5.16) |
| | | |
P-interaction=0.04 |
P-interaction=0.03 |
| Type 1b | | n=102 | n=200 | | |
| MDM2
SNP309 | TP53 codon
72 | | | | |
| TT | Arg/Arg | 9 (8.8) | 28 (14.0) | 1.00
(reference) | 1.00
(reference) |
| TT | Arg/Pro +
Pro/Pro | 17 (16.7) | 34 (17.0) | 1.55
(0.60–4.02) | 1.82
(0.65–5.11) |
| TG + GG | Arg/Arg | 36 (35.3) | 47 (23.5) | 2.38
(1.00–5.67) | 2.51
(0.97–6.53) |
| TG + GG | Arg/Pro +
Pro/Pro | 40 (39.2) | 91 (45.5) | 1.37
(0.59–3.16) | 0.98
(0.37–2.58) |
| | | |
P-interaction=0.07 | P
interaction=0.01 |
| Postmenopause +
Type 1 | | n=59 | n=183 | | |
| MDM2
SNP309 | TP53 codon
72 | | | | |
| TT | Arg/Arg | 5 (8.5) | 25 (13.7) | 1.00
(reference) | 1.00
(reference) |
| TT | Arg/Pro +
Pro/Pro | 14 (23.7) | 32 (17.5) | 2.19
(0.70–6.89) | 2.76
(0.83–9.19) |
| TG + GG | Arg/Arg | 23 (39.0) | 44 (24.0) | 2.61
(0.88–7.73) | 3.24
(1.03–10.16) |
| TG + GG | Arg/Pro +
Pro/Pro | 17 (28.8) | 82 (44.8) | 1.04
(0.35–3.09) | 1.43
(0.45–4.57) |
| | | |
P-interaction=0.01 | P
interaction=0.009 |
Combination of the TP53 72Pro allele and
homozygosity for the p21 codon 31 Ser allele is associated with a
decreased risk of endometrial cancer
We further examined the effect of SNP309 combined
with ESR1 or p21 and SNP309 with both TP53 and
p21 polymorphisms on the risk of endometrial cancer
(Table V). No significant
differences were observed between the combination of the SNP309 and
the PvuII or XbaI or p21 polymorphism. The
combination of having the TP53 72Pro allele and homozygosity
for the p21 codon 31 Ser allele, however, was associated
with a decreased risk of endometrial cancer (crude OR, 0.28; 95%
CI, 0.09–0.92). A statistically significant interaction was
observed between the 2 polymorphisms for a decreased risk of
endometrial cancer (P for the interaction=0.04), although there was
no significant difference once adjustment was made for age and
BMI.
 | Table VEndometrial cancer risk for combined
effect of MDM2, TP53, ESR1 and p21
genotypes. |
Table V
Endometrial cancer risk for combined
effect of MDM2, TP53, ESR1 and p21
genotypes.
| Genotype | | Cases, n=125
(%) | Controls, n=200
(%) | Crude OR (95%
CI) | Adjusteda OR (95% CI) |
|---|
| MDM2
SNP309 | ESR1
PvuII | | | | |
| TT | TT | 8 (6.4) | 20 (10.0) | 1.00
(reference) | 1.00
(reference) |
| TT | TC + CC | 22 (17.6) | 42 (21.0) | 1.31
(0.50–3.45) | 0.96
(0.34–2.69) |
| TG + GG | TT | 30 (24.0) | 40 (20.0) | 1.86
(0.73–4.83) | 1.29
(0.47–3.57) |
| TG + GG | TC + CC | 65 (52.0) | 98 (49.0) | 1.66
(0.69–3.99) | 1.18
(0.46–2.99) |
| | | |
P-interaction=0.49 |
P-interaction=0.93 |
| MDM2
SNP309 | ESR1
XbaI | | | | |
| TT | AA | 20 (16.0) | 42 (21.0) | 1.00
(reference) | 1.00
(reference) |
| TT | AG + GG | 10 (8.0) | 20 (10.0) | 1.05
(0.42–2.65) | 0.83
(0.30–2.23) |
| TG + GG | AA | 57 (45.6) | 85 (42.5) | 1.41
(0.75–2.64) | 1.25
(0.63–2.47) |
| TG + GG | AG + GG | 38 (30.4) | 53 (26.5) | 1.51
(0.77–2.96) | 1.05
(0.49–2.23) |
| | | | P
interaction=0.97 |
P-interaction=0.96 |
| MDM2
SNP309 | p21 codon
31 | | | | |
| TT | Ser/Ser | 8 (6.4) | 11 (5.5) | 1.00
(reference) | 1.00
(reference) |
| TT | Ser/Arg +
Arg/Arg | 22 (17.6) | 51 (25.5) | 0.59
(0.21–1.68) | 0.58
(0.19–1.75) |
| TG + GG | Ser/Ser | 13 (10.4) | 27 (13.5) | 0.66
(0.22–2.04) | 0.58
(0.17–2.00) |
| TG + GG | Ser/Arg +
Arg/Arg | 82 (65.6) | 111 (55.5) | 1.02
(0.39–2.64) | 0.87
(0.31–2.41) |
| | | |
P-interaction=0.14 |
P-interaction=0.18 |
| TP53 codon
72 | p21 codon
31 | | | | |
| Arg/Arg | Ser/Ser | 16 (12.8) | 18 (9.0) | 1.00
(reference) | 1.00
(reference) |
| Arg/Arg | Ser/Arg +
Arg/Arg | 36 (28.8) | 57 (28.5) | 0.71
(0.32–1.57) | 0.85
(0.35–2.02) |
| Arg/Pro +
Pro/Pro | Ser/Ser | 5 (4.0) | 20 (10.0) | 0.28
(0.09–0.92) | 0.39
(0.11–1.43) |
| Arg/Pro +
Pro/Pro | Ser/Arg +
Arg/Arg | 68 (54.4) | 105 (52.5) | 0.73
(0.35–1.53) | 0.72
(0.32–1.64) |
| | | |
P-interaction=0.04 |
P-interaction=0.27 |
The SNP309 GG genotype abrogates the
cytostatic effect of RITA on tumor cells
MDM2 overexpression is observed in multiple
malignancies. Due to the importance of the p53-MDM2 interaction,
restoration of p53 activity by inhibiting MDM2 binding represents a
novel antineoplastic strategy. RITA is a low-molecular-weight
compound previously identified in a cell-based screen for wild-type
p53-reactivating compounds. RITA binds to the amino terminus of
p53, inhibiting p53 binding to MDM2 in cultured cells and in human
tumor xenografts in vivo. This results in derepression of
p53 and highly efficient induction of apoptosis (32).
We demonstrated that a combination of the SNP309 G
allele and the homozygous Arg/Arg genotype of TP53 codon 72
was associated with an increased risk of endometrial cancer
(Table IV). These results suggest
that polymorphisms of MDM2 and TP53 may influence the
effect of RITA. We therefore, assessed the growth-suppressive
effect of RITA on three endometrial cancer cell lines, Hec6, HHUA
and Sawano, in vitro. All three lines express wild-type p53.
SNP309 and TP53 Arg72Pro polymorphisms were analyzed in
these lines with PCR-RFLP and direct sequencing. Hec6 cells were
SNP309 TT homozygous and TP53 Arg72 homozygous, HHUA cells
were SNP309 TG heterozygous and TP53 Arg72 homozygous, and
Sawano cells were SNP309 GG homozygous and TP53 Arg72Pro
heterozygous (Table VI). Treatment
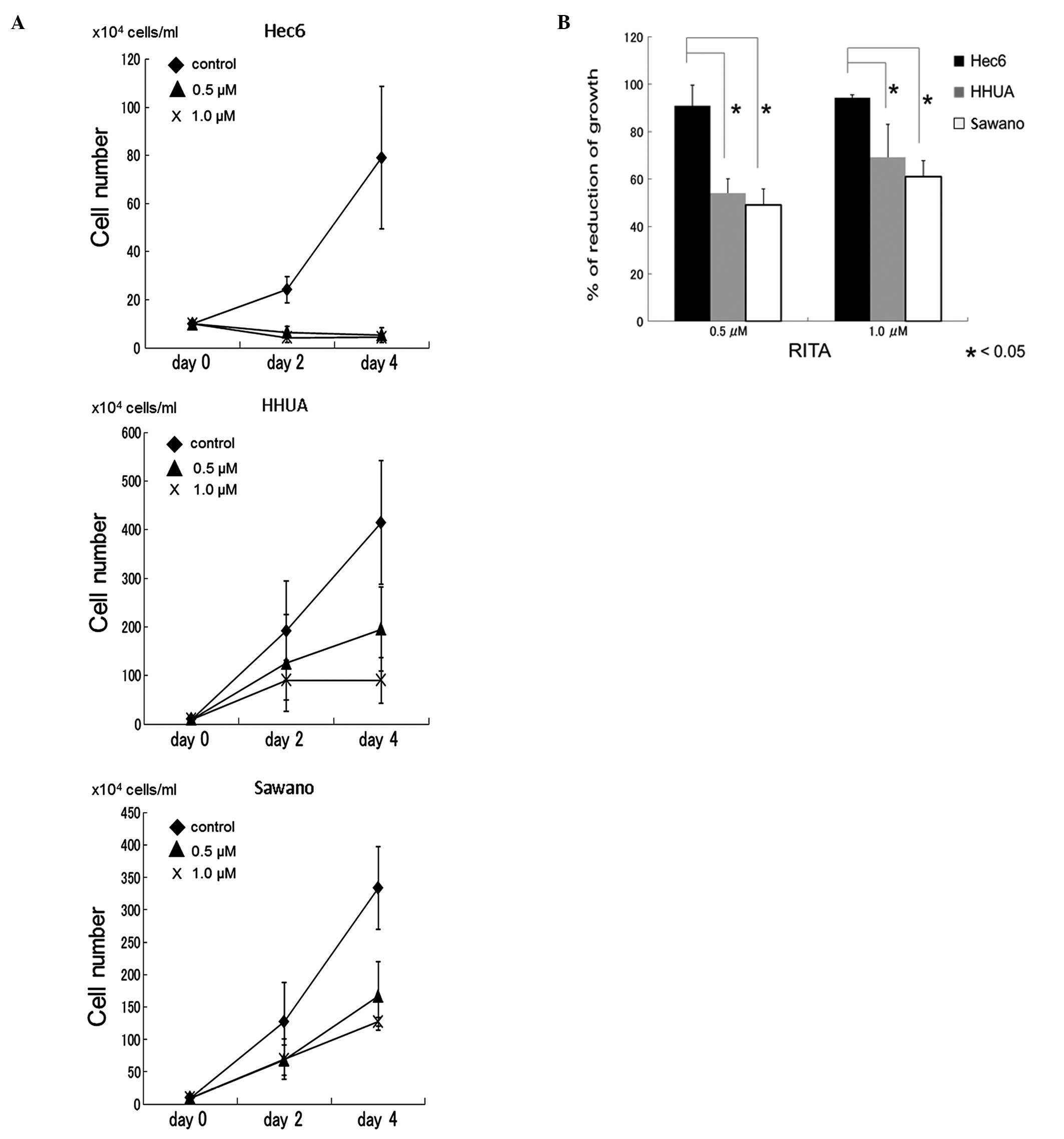
with RITA (0.5 and 1.0 μM) suppressed cell growth in all cell lines
in a dose-dependent manner (Fig.
1A). Following treatment with 0.5 μM RITA for four days, the
rates of inhibition were: Hec6 cells, 90.9±8.7%; HHUA cells,
54.1±6.0%; and Sawano cells, 49.0±15.8%. Following treatment with
1.0 μM RITA, these rates were 94.3±1.2%, 79.0±4.6% and 61.0±6.7%,
respectively. The inhibitory effect was significantly less
pronounced in HHUA and Sawano cells with the SNP309 G allele
compared to that in Hec 6 cells carrying the SNP309 TT genotype
(P<0.05) (Fig. 1B).
 | Table VIGenotype of endometrial cancer cell
lines. |
Table VI
Genotype of endometrial cancer cell
lines.
| Cell line | MDM2
SNP309 | TP53 codon
72 | TP53
status |
|---|
| Hec6 | TT | Arg/Arg | Wild-type |
| HHUA | TG | Arg/Arg | Wild-type |
| Sawano | GG | Arg/Pro | Wild-type |
Discussion
The present study investigated the associations of
endometrial cancer risk with the MDM2 SNP309, TP53
Arg72Pro, ESR1 PvuII or XbaI and p21 codon 31
polymorphisms in Japanese women. Although each polymorphism
individually was unrelated to endometrial cancer risk, the SNP309 G
allele was associated with an increased risk in women homozygous
for the TP53 codon 72 Arg allele. Furthermore, the
relationship remained significant in a subgroup analysis
(postmenopausal, type I endometrial cancer and overweight status.
These were related to unopposed estrogen).
Few studies have addressed the association of SNP309
with the risk of endometrial cancer. Walsh et al(24) reported an OR of 2.76 for the GG vs.
the TT genotype (95% CI, 1.06–7.20) in a United States case-control
study. In a nested case-control study of Caucasian women, an OR of
1.87 (95% CI, 1.29–2.73) was reported for the GG genotype compared
with the TT genotype (Nurses' Health Study, 454 cases and 1,132
controls; Women's Health Study, 137 cases and 411 controls)
(25). Ashton et al(33), however, failed to document a similar
association in an Australian study.
The association between the TP53 Arg72Pro
polymorphism and endometrial cancer risk has been investigated in
several studies in Caucasians with inconsistent findings. The
following findings have been reported for women of Asian descent:
Ueda et al(27) reported an
increased risk of endometrial cancer in Japanese patients harboring
the Arg/Arg genotype compared to those with Arg/Pro and Pro/Pro
genotypes combined. Niwa et al(34), however, found no such association.
By contrast, having the Pro allele conferred an increased risk in
Korean women (22).
Two studies have reported a potential interaction
between the SNP309 G allele and the TP53 Arg72Pro
polymorphisms for endometrial cancer. Nunobiki et
al(35) showed that the risk
with the SNP309 GG vs. TT or TG was greater in women with Arg/Arg
(OR, 3.28; 95% CI, 1.13–9.53) than in women with Arg/Pro or Pro/Pro
of the TP53 Arg72Pro (OR, 1.48; 95% CI, 0.62–3.52), but the
interaction between the two SNPs was not evaluated.
Ashton et al(33) showed no effect of the TP53
polymorphism and MDM2 SNP309 alone or in combination on
endometrial cancer risk. They did, however, show that the
combination of SNP309 and TP53 was associated with
high-grade endometrial cancer (G2+G3). These observations are
incompatible with the findings of the present study showing that
the combination of SNP309 and TP53 was associated with type
I endometrial cancer (G1+G2). This discrepancy may have arisen due
to the fact that G2 and G3 are classified as high-grade tumors. G3
tumors are a distinct entity. For example, in Japanese women, p53
mutations are significantly more common in G3 (43%) than in G1
(11%) tumors (36). p53 mutations
are also detected more frequently in type II endometrial cancer.
Tumors harboring p53 mutations are often more chemoresistant and
have lower apoptotic rates. The status of the TP53 gene
(wild-type or mutant type) is critical when determining the
relationship between grade and a TP53 polymorphism. In the
present study, an analysis of 102 cases that were not type II
demonstrated a significant interaction between the MDM2
SNP309 and TP53 Arg72Pro polymorphism with endometrial
cancer risk. We did not, however, ascertain whether the combination
of MDM2 and TP53 Arg72Pro was associated with
high-grade or non-endometrioid tumors as the overall number of
these cases (G3, 10 cases; non-endometrioid, 13 cases) was
small.
A major limitation of the present study was its
small size. As most of the women with endometrial cancer are early
stage and have positive outcomes, it was difficult to analyze the
association between studied polymorphisms and disease outcome in
the present study.
Five meta-analyses have been published addressing
the MDM2 SNP309 polymorphism and the risk of various types
of tumor. Wilkening et al(37) combined the available genotype data
for breast, colorectal and lung cancer. These results suggest that
the SNP309 variant does not have an impact on the risk of breast or
colorectal cancer. The OR for lung cancer, however, revealed an
increased risk for GG vs. TT (OR, 1.27; 95% CI, 1.12–1.44). Hu
et al(38) conducted a
meta-analysis with different tumor types, including endometrial
cancer. The OR revealed an increased risk for GG vs. TT (OR, 1.17;
95% CI, 1.04–1.33), and (OR, 1.37; 95% CI, 1.23–1.53) for the Asian
population. Wan et al(39)
performed a risk estimate with various tumor types. The OR for
uterine cancer (eight studies) revealed an increased risk for GG
vs. TT (OR, 1.34; 95% CI, 1.07–1.69) and (OR, 1.36; 95%CI,
1.18–1.56) for the Asian population (32 studies). This study
included six studies (two for breast cancer, and one each for acute
myeloid leukemia, hepatocellular carcinoma, lung cancer and gastric
cancer) that explored interaction effects between TP53
Arg72Pro and MDM2 SNP309. They found that the combination of
GG and Pro/Pro, TG and Pro/Pro and GG and Arg/Arg in comparison to
the reference MDM2 SNP309 TT and TP53Arg/Arg
genotype, significantly increased the risk of cancer (OR, 3.38; 95%
CI, 1.77–6.47), (OR, 1.88; 95% CI, 1.26–2.81) and (OR, 1.96; 95%
CI, 1.01–3.78), respectively. Wo et al(40) completed a meta-analysis with
multiple types of tumors, including four endometrial cancer
studies. The OR reflected an increased risk of cancer for GG vs. TT
(OR, 1.123; 95% CI, 1.056–1.193). Li et al(41) conducted a meta-analysis of 1,001
cases and 1,889 controls from six published case-control studies
(24–26,33,35) to
estimate the effect of SNP309 on endometrial cancer risk for GG vs.
TT (OR, 1.54; 95% CI, 1.21–1.95). These analyses indicate that
MDM2 SNP309 serves as a tumor susceptibility marker, and
that there is an association between MDM2 SNP309 and
TP53 Arg/Pro regarding tumor susceptibility.
The other notable finding of the present study was
that the combination of the TP53 72Pro allele and
homozygosity for the p21 codon 31 Ser allele was associated
with a decreased risk of endometrial cancer. This is in contrast to
a previous study showing that the combination of the TP53
Pro allele and p21 Ser/Ser genotype significantly increased
endometrial cancer risk (OR, 9.55; 95% CI, 4.40–21.24) (22). The result requires further
investigation.
Finally, we investigated whether the SNP309 subtype
modified the effect of RITA, an agent that blocks the p53-MDM2
interaction. RITA suppresses the proliferation of endometrial
cancer cell lines. The effect was less pronounced in HHUA and
Sawano cells containing the SNP309 G allele compared with that in
Hec6 cells, which harbor the TT genotype. Arva et
al(42) showed that cells
carrying the SNP309 GG genotype exhibited a compromised TP53
response pathway and formed transcriptionally inactive p53-MDM2
complexes in response to stress. These results support our
findings. Additional functional analyses are required to fully
elucidate the effects of these polymorphisms on the effect of
agents such as RITA on endometrial cancer cell lines.
In conclusion, endometrial cancer is characterized
by numerous genetic alterations, including those in p53, K-ras,
PTEN and β-catenin. We previously demonstrated that the
Ras/ER/MDM2 pathway is important for proliferation of endometrial
cancer cells in vitro. We demonstrated in this case-control
study that polymorphisms of TP53 and MDM2 modify the
effect of this signaling pathway and thus increase the risk of
endometrial cancer.
The increased incidence of endometrial cancer in
Japan may reflect changes in lifestyle. The interplay between
genetic and environmental factors is now being investigated in the
context of gynecologic cancer. It is well known that endometrial
cancer risk includes an environmental component. Further studies
are necessary to elucidate the genetic components of risk.
Understanding this relationship is the first step towards
developing new methods of endometrial cancer prevention and
treatment.
Acknowledgements
The authors acknowledge the generous support of
Professor Suminori Kono (Preventive Medicine, School of Medicine,
Kyushu University) for conducting the survey of controls and for
valuable discussion. We are grateful to Drs Masao Okadome, Takako
Eto, Kazuya Ariyoshi, Tomoko Hagiwara, Shintaro Yanadume, Masahumi
Yasunaga, Hiroko Imamura, Ryosuke Tsunematsu and Kenzo Sonoda for
their help in the survey of cases. We appreciate the technical
support provided by the Research Support Center, Graduate School of
Medical Sciences, Kyushu University. This study was supported by a
grant-in-aid for Cancer Research (17–9) from the Ministry of
Health, Labour and Welfare, Japan.
References
|
1
|
Bansal N, Yendluri V and Wenham RM: The
molecular biology of endometrial cancers and the implications for
pathogenesis, classification, and targeted therapies. Cancer
Control. 16:8–13. 2009.PubMed/NCBI
|
|
2
|
Prat J, Gallardo A, Cuatrecasas M and
Catasus L: Endometrial carcinoma: pathology and genetics.
Pathology. 39:72–87. 2007. View Article : Google Scholar
|
|
3
|
Deb SP: Cell cycle regulatory functions of
the human oncoprotein MDM2. Mol Cancer Res. 1:1009–1016.
2003.PubMed/NCBI
|
|
4
|
Haupt Y, Maya R, Kazaz A and Oren M: Mdm2
promotes the rapid degradation of p53. Nature. 387:296–299. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Honda R, Tanaka H and Yasuda H:
Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53.
FEBS Lett. 420:25–27. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kubbutat MH, Jones SN and Vousden KH:
Regulation of p53 stability by Mdm2. Nature. 387:299–303. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Levav-Cohen Y, Haupt S and Haupt Y: Mdm2
in growth signaling and cancer. Growth Factors. 23:183–192. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gudas JM, Nguyen H, Klein RC, Katayose D,
Seth P and Cowan KH: Differential expression of multiple MDM2
messenger RNAs and proteins in normal and tumorigenic breast
epithelial cells. Clin Cancer Res. 1:71–80. 1995.PubMed/NCBI
|
|
9
|
Sheikh MS, Shao ZM, Hussain A and Fontana
JA: The p53-binding protein MDM2 gene is differentially expressed
in human breast carcinoma. Cancer Res. 53:3226–3228.
1993.PubMed/NCBI
|
|
10
|
Ries S, Biederer C, Woods D, et al:
Opposing effects of Ras on p53: transcriptional activation of mdm2
and induction of p19ARF. Cell. 103:321–330. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kato K, Ueoka Y, Hachiya T, Nishida J and
Wake N: Contribution of enhanced transcriptional activation by ER
to [12Val] K-Ras mediated NIH3T3 cell transformation. Oncogene.
15:3037–3046. 1997.
|
|
12
|
Kato K, Horiuchi S, Takahashi A, et al:
Contribution of estrogen receptor α to oncogenic K-Ras-mediated
NIH3T3 cell transformation and its implication for escape from
senescence by modulating the p53 pathway. J Biol Chem.
277:11217–11224. 2002.
|
|
13
|
Suga S, Kato K, Ohgami T, et al: An
inhibitory effect on cell proliferation by blockage of the
MAPK/estrogen receptor/MDM2 signal pathway in gynecologic cancer.
Gynecol Oncol. 105:341–350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bond GL, Hu W, Bond EE, et al: A single
nucleotide polymorphism in the MDM2 promoter attenuates the p53
tumor suppressor pathway and accelerates tumor formation in humans.
Cell. 119:591–602. 2004. View Article : Google Scholar
|
|
15
|
Bond GL, Hirshfield KM, Kirchhoff T, et
al: MDM2 SNP309 accelerates tumor formation in a gender-specific
and hormone-dependent manner. Cancer Res. 66:5104–5110. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Campbell IG, Eccles DM and Choong DY: No
association of the MDM2 SNP309 polymorphism with risk of breast or
ovarian cancer. Cancer Lett. 240:195–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Petenkaya A, Bozkurt B, Akilli-Ozturk O,
Kaya HS, Gur-Dedeoglu B and Yulug IG: Lack of association between
the MDM2-SNP309 polymorphism and breast cancer risk. Anticancer
Res. 26:4975–4977. 2006.PubMed/NCBI
|
|
18
|
Krekac D, Brozkova K, Knoflickova D, et
al: MDM2SNP309 does not associate with elevated MDM2 protein
expression or breast cancer risk. Oncology. 74:84–87. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vogelstein B, Lane D and Levine AJ:
Surfing the p53 network. Nature. 408:307–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
el-Deiry WS, Tokino T, Velculescu VE, et
al: WAF1, a potential mediator of p53 tumor suppression. Cell.
75:817–825. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roh J, Kim M, Kim J, et al: Polymorphisms
in codon 31 of p21 and cervical cancer susceptibility in Korean
women. Cancer Lett. 165:59–62. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roh JW, Kim JW, Park NH, et al: p53 and
p21 genetic polymorphisms and susceptibility to endometrial cancer.
Gynecol Oncol. 93:499–505. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hachiya T, Kuriaki Y, Ueoka Y, Nishida J,
Kato K and Wake N: WAF1 genotype and endometrial cancer
susceptibility. Gynecol Oncol. 72:187–192. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Walsh CS, Miller CW, Karlan BY and
Koeffler HP: Association between a functional single nucleotide
polymorphism in the MDM2 gene and sporadic endometrial cancer risk.
Gynecol Oncol. 104:660–664. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Terry K, McGrath M, Lee IM, Buring J and
De Vivo I: MDM2 SNP309 is associated with endometrial cancer risk.
Cancer Epidemiol Biomarkers Prev. 17:983–986. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ueda M, Yamamoto M, Nunobiki O, et al:
Murine double-minute 2 homolog single nucleotide polymorphism 309
and the risk of gynecologic cancer. Hum Cell. 22:49–54. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ueda M, Terai Y, Kanda K, et al: Germline
polymorphism of p53 codon 72 in gynecological cancer. Gynecol
Oncol. 100:173–178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yaich L, Dupont WD, Cavener DR and Parl
FF: Analysis of the Pvu II restriction fragment-length
polymorphism and exon structure of the estrogen receptor gene in
breast cancer and peripheral blood. Cancer Res. 52:77–83. 1992.
|
|
29
|
Mizunuma H, Hosoi T, Okano H, et al:
Estrogen receptor gene polymorphism and bone mineral density at the
lumbar spine of pre- and postmenopausal women. Bone. 21:379–383.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun Y, Hildesheim A, Li H, et al: No point
mutation but a codon 31ser→arg polymorphism of the WAF-1/CIP-1/p21
tumor suppressor gene in nasopharyngeal carcinoma (NPC): the
polymorphism distinguishes Caucasians from Chinese. Cancer
Epidemiol Biomarkers Prev. 4:261–267. 1995.PubMed/NCBI
|
|
31
|
Shih CM, Lin PT, Wang HC, Huang WC and
Wang YC: Lack of evidence of association of p21WAF1/CIP1
polymorphism with lung cancer susceptibility and prognosis in
Taiwan. Jpn J Cancer Res. 91:9–15. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Issaeva N, Bozko P, Enge M, et al: Small
molecule RITA binds to p53, blocks p53-HDM-2 interaction and
activates p53 function in tumors. Nat Med. 10:1321–1328. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ashton KA, Proietto A, Otton G, et al:
Polymorphisms in TP53 and MDM2 combined are associated with high
grade endometrial cancer. Gynecol Oncol. 113:109–114. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Niwa Y, Hirose K, Matsuo K, et al:
Association of p73 G4C14-to-A4T14 polymorphism at exon 2 and p53
Arg72Pro polymorphism with the risk of endometrial cancer in
Japanese subjects. Cancer Lett. 219:183–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nunobiki O, Ueda M, Yamamoto M, et al:
Polymorphisms of p53 codon 72 and MDM2 promoter 309 and the risk of
endometrial cancer. Hum Cell. 22:101–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Enomoto T, Fujita M, Inoue M, et al:
Alterations of the p53 tumor suppressor gene and its association
with activation of the c-K-ras-2 protooncogene in premalignant and
malignant lesions of the human uterine endometrium. Cancer Res.
53:1883–1888. 1993.PubMed/NCBI
|
|
37
|
Wilkening S, Bermejo JL and Hemminki K:
MDM2 SNP309 and cancer risk: a combined analysis. Carcinogenesis.
28:2262–2267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hu Z, Jin G, Wang L, Chen F, Wang X and
Shen H: MDM2 promoter polymorphism SNP309 contributes to tumor
susceptibility: evidence from 21 case-control studies. Cancer
Epidemiol Biomarkers Prev. 16:2717–2723. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wan Y, Wu W, Yin Z, Guan P and Zhou B:
MDM2 SNP309, gene-gene interaction, and tumor susceptibility: an
updated meta-analysis. BMC Cancer. 11:2082011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wo X, Han D, Sun H, et al: MDM2 SNP309
contributes to tumor susceptibility: a meta-analysis. J Genet
Genomics. 38:341–350. 2011.
|
|
41
|
Li Y, Zhao H, Sun L, Huang L, Yang Q and
Kong B: MDM2 SNP309 is associated with endometrial cancer
susceptibility: a meta-analysis. Hum Cell. 24:57–64. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Arva NC, Gopen TR, Talbott KE, et al: A
chromatin-associated and transcriptionally inactive p53-Mdm2
complex occurs in mdm2 SNP309 homozygous cells. J Biol Chem.
280:26776–26787. 2005. View Article : Google Scholar : PubMed/NCBI
|