Introduction
Cervical cancer is a malignant neoplasm arising from
cells originating in the cervix uteri. The development of cervical
cancer are closely associated with constitutive infection of HPV
viruses, of which HPV16 is the most common high-risk type,
accounting for more than half (56%) of all cervical cancers
(1). It is well known that Harald
zur Hausen, the German researcher who discovered the human
papillomavirus, was awarded Noble Prize in Medicine on 2008. The
finding of HPV makes prevention and treatment of cervical cancer
possible. In addition to preventive vaccines, such as Gardasil and
Cervarix (2,3), laboratory researches are paying
attention to the treatment of cervical cancer that has been
developed. Preventive vaccines do not cause therapeutic effects on
existing lesions, thus, it is urgent to focus on the treatment of
pre-existing lesions. In addition to traditional surgery,
chemotherapy and radiotherapy, therapeutic HPV vaccines are
attracting our attention. HPV E7 oncoprotein represent an ideal
target for therapeutic intervention because of its constitutive
expression in HPV-associated tumors and their crucial role in the
induction and maintenance of HPV-associated diseases (4,5).
Versatile therapeutic strategies have been developed
(6–10). Various therapeutic HPV vaccines are
aiming to interact with professional antigen-presenting cell (APC)
such as dendritic cell (DC) to generate specific cell-mediated
immunity for clearance of infection and control of HPV-associated
cancers (11,12). Protein-based therapeutic HPV
vaccines are vaccine candidates commonly tested in clinical trials,
compared to other forms of therapeutic HPV vaccines (13). Protein vaccines have some advantages
in vaccine development. For example, compared to live-vector based
vaccines they are safe. Protein vaccines contain all possible
peptide epitopes interacting with the MHC I and II epitopes, so
they are not limited by the specificity of MHC. However, protein
vaccines are troubled by their low immunogenicity. To overcome this
problem and to enhance their potency, we design two novel new
strategies, one is optimization of E7 protein by adding MHC-I
epitope at C terminal and MHC-II epitope at N terminal of fusion
protein, and another is the addition of human hsp70 (huhsp70)
adjuvant.
Effective antigen process and presentation is the
indispensable condition to induce a strong and specific
CD8+ T cell response. Because the normal capacity of APC
to cross-present antigen is generally low, there is significant
interest to develop strategies that enhance the targeting of
exogenous antigens to the cross-presentation pathway (14,15).
Critical factors that determine the efficacy of a vaccine are the
amount of delivered antigen and the surroundings in which the
antigen is presented to the T cells. The addition of MHC I epitope
at C terminal and MHC II epitope at N terminal of fusion protein,
makes more specific antigen peptides to act with MHC molecules, so
that it enlarges the rate of recognition for MHC molecules. Thus,
the delivery of more antigens to the MHC class I cross-presentation
pathway is one of the keys to the improved capacity of APCs to
activate antigen-specific T cells.
Another strategy is the use of hsp70 to enhance the
antigen cross presentation. As an intracellular protein, hsp70 has
been shown to play multiple roles in protein folding, transport,
and degradation act as molecular chaperones (16). However, it is reported that hsps
also are involved in the immune activation in that they transfer
their chaperoned protein-cargo to APC for cross-presentation as an
extracellular protein released when necrotic cells or secretion in
response to cellular stress. Such antigenic cross-presentation is
now considered to be a very important process in the action of
hsp70 (17–20). The extracellular hsp70 is considered
to mediate stimulation of DCs to secrete proinflammatory cytokines
and express costimulatory molecules, thus creating the immunogenic
environment required for the induction of adaptive CD8+
T cell responses. Several endocytic receptors, such as CD91 and
LOX1 have been identified to take part in hsp70-mediated
cross-presentation (21,22). When hsp70 binds to its corresponding
receptors of CD40, TLR-2 and TLR-4 on the DC, enters the cell
plasma and induces activation and maturation DC (23–25).
Hsp70 not only activates and regulates innate immunity but also the
adaptive immunity. The above attributes of hsp70 suggest its
rational use in immunotherapeutic strategies for the treatment of
cancer. Thus, this activity of hsp70 is desirable for therapeutic
vaccine development.
Our previous study on HPV16 E7/hsp70 DNA vaccine has
shown that huhsp70 enhanced more effective antitumor efficacy than
mycobacterium tuberculosis hsp70 (26). In this protein vaccine study, we
optimized the E7 protein by site-directed mutagenesis to eradiate
the transformation activity, MHC epitope and human hsp70 adjuvant
were added to strengthen the cross-presentation potential, and
developed the fusion protein of oE7/huhsp70, expecting to produce
enhanced antitumor efficacy.
Materials and methods
Mice and tumor cell line
Female C57BL/6 mice of 6–8-week-old were maintained
in the Animal Facility of the Laboratory Animal Center, the
Affiliated Hospital of Medical College of Qingdao University. All
animal procedures were performed according to the approved
protocols and in accordance with recommendations for the proper use
and care of laboratory animals. Care was taken to minimize pain and
discomfort to all animals during the procedures in this study. TC-1
cells purchased from Shanghai Meilian Biotechnology Co., Ltd.,
(Shanghai, China) were primary pulmonary epithelial cells of
C57BL/6 mice co-transformed with HPV16 E6, E7 and activated
c-Ha-ras oncogenes. The cells were grown in RPMI-1640 supplemented
with 10% (v/v) fetal bovine serum, 50 U/ml penicillin/streptomycin,
2 mM L-glutamine, 1 mM sodium pyruvate, 2 mM non-essential amino
acids and 0.4 mg/ml G418 at 37°C with 5% CO2.
Plasmids construction
HPV16 oE7 gene was synthesized by Shanghai Generay
Biotech Co., Ltd. (Shanghai, China) and cloned into pMD18T vector
by using two primers, forward primer
5′-GCCATATGATGGACAGCTCAGAGGAGG-3′ (NdeI) and reverse primer
5′-CGGGATCCGGTTACAATA TTGTAATG-3′ (BamHI), then was
subcloned into pET-30a (+) (Novagen, Darmstadt, Germany) and
generated the pET-30a (+)-oE7. The huhsp70 gene (GenBank NM_005345)
was amplified by using forward primer 5′-GCGGATCCCATGGC
CAAAGCCGCGGC-3′ (BamHI) and reverse primer 5′-CGCT
CGAGCTAATCTACCTCCTCAATGGTG-3′ (XhoI) from pMSHsp70, a gift
of Prof. R.I. Morimoto of the Northwestern University (Evanston,
IL, USA), then subcloned into pET-30a (+) and generate pET-30a
(+)-huhsp70. To generate pET-30a (+)-oE7/ huhsp70, huHSP70 digested
with BamHI and XhoI from pET-30a (+)-huhsp70 was
ligated into BamHI/XhoI-digested pET-30a (+)-oE7
without the stop code of oE7. All constructs were validated by
restriction enzyme digestion and DNA sequencing.
Expression, purification and analysis of
oE7, huhsp70 and oE7/huhsp70 proteins
E. coli strain BL21 (DE3) (Novagen) was used
as the host bacterial for all recombinant protein production. All
transformants were grown in Luria Broth (LB) medium containing 50
μg/ml kanamycin. When OD600 reach about 0.5, engineered bacterial
was induced with 1 mM isopropyl-b-D-thiogalactopyranoside (IPTG)
for 4 h at 37°C. Cells were harvested by centrifugation at 10,000 ×
g for 10 min at 4°C, the supernatant was discarded and the cells
resuspended in 4 ml cold bingding buffer with 0.1% NP-40. Using the
His•Bind® Purification kit (Novagen, No. 70239) to
purify these recombinant proteins. The identity and the purity of
the recombinant proteins were determined by SDS-PAGE.
Concentrations of proteins were measured by the Bradford assay. To
confirm the identity of the recombinant protein, all purified
recombinant proteins were verified by western blot analysis against
the human hsp70 antibody (sc-32239; Santa Cruz Biotechnology, Santa
Cruz, CA, USA) and/or HPV16 E7 antibody (sc-51951, Santa Cruz
Biotechnology).
ELISPOT assay
BD ELISPOT plates (BD BioSciences) were coated with
5 μg/ml rat anti-mouse IFN-γ antibody in 100 μl of PBS. After
overnight incubation at 4°C, the wells were washed and blocked with
RPMI-1640 culture medium containing 10% fetal bovine serum.
Different concentrations of freshly isolated spleen cells from each
vaccinated mouse group, from 1×106 to
1.25×105/well, were added to the wells along with 50
IU/ml IL-2 and 1 μg/ml E7-specific MHC class I CD8+ T
cell epitope (H-2 Db, amino acids 49–57, RAHYNIVTF). After culture
at 37°C for 24 h, the plate was washed and then followed by
incubation with 2.5 μg/ml biotinylated IFN-γ antibodies in 100 μl
in PBS containing 10% FCS at 4°C overnight. After washing,
avidin-HRP in 100 μl of PBS was added and incubated for 1 h at room
temperature. After washing five times, spots were developed by
addition of 100 μl AEC solution. The spots were counted using an
ELISPOT Reader system.
Flow cytometry analysis to detect IFN-γ
secretion by E7-specific CD8+ T cells
To detect E7-specific CD8+ T-cell
responses, splenocytes from vaccinated groups of mice were
incubated with the MHC class I E7 peptide. Golgistop (BD
BioSciences) was added 6 h before harvesting the cells from the
culture. The cells were then washed once in staining buffer and
labeled with FITC-conjugated rat anti-mouse CD8 antibodies. The
cells were fixed and permeabilized using a Cytofix/Cytoperm kit (BD
BioSciences) according to the manufacturer’s instructions and then
were stained for PE-conjugated anti-IFN-γ. FITC-conjugated rat
IgG2a, k or PE-conjugated rat IgG1 isotype
control antibody were all purchased from BD BioSciences. Analyses
were performed on a Beckman Coulter Epics XL (Beckman Coulter Inc.,
Brea, CA, USA).
Cytotoxicity assays
C57BL/6 mice were immunized intramuscularly (i.m.)
with PBS, 2 nmol oE7, huhsp70 and oE7/hsp70 at the M. quadriceps,
respectively. These injections were repeated after 1 week. Two
weeks after the last booster, 1×106 splenocytes were
cocultured with 5×104 irradiated TC-1 helper cells in
RPMI-1640 supplemented with 10% FCS and 20 U/ml IL-2 at 37°C in 5%
CO2. After 3 days of stimulation, TC-1 target cells were
plated at 1×104 cells/well on 96-well U-bottomed plates
(Costar), then the prepared splenocytes (effector cells) were added
in a final volume of 100 μl at 40:1, 20:1, 10:1 and 5:1 ratio,
respectively. The CytoTox 96 NonRadioacive Cytotoxicity Assay kit
(Promega Inc.) was used to determine the cytotoxic activity of the
effector cells against TC-1 tumor cells according to the
manufacturer’s protocol. The percentage of cytotoxicity was
calculated by the formula: [A (Experimental) - A (Effector
spontaneous) - A (Target spontaneous)] × 100/[A (Target maximum -
Target spontaneous)].
Anti-E7 ELISA
The anti-HPV16 E7 antibodies in the sera of
vaccinated mice were identified by ELISA. Each well of a
96-microwell plate was coated with 100 μl of 1 μg/ml purified HPV16
oE7 proteins and incubated at 4°C overnight. The wells were then
blocked with PBS containing 5% BSA. Two weeks after the last
booster, sera were prepared from the mice, serially diluted in PBS,
added to the ELISA wells and incubated for 2 h. After washing with
PBS-T containing 0.05% Tween-20, the plate was incubated with
1:3,000 dilution of an HRP-conjugated goat anti-mouse IgG antibody
(sc-2005; Santa Cruz Biotechnology) at room temperature for 1 h.
The plate was washed three times, tetramethyl-benzidine substrate
was added and incubated away from light at 37°C for 15 min. The
reaction was stopped with 50 μl of 2 M H2SO4.
The ELISA plate was read with a standard ELISA reader at 450
nm.
In vivo tumor treatment experiments
To test the ability of protein vaccination to
inhibit the growth of established tumors, C57BL/6 mice (five/group)
were subcutaneously (s.c.) challenged with 7.5xl04 TC-1
cells per mouse in the right flank. Three days after the challenge
with TC-1 cells, mice were given 200 μl of PBS, 2 nmol oE7, huhsp70
or oE7/huhsp70. One week later, these mice were strengthened with
the same regimen as the first vaccination. Mice were monitored
twice a week for tumor growth.
Statistical analysis
The mean of two sample comparison of Poisson
distribution was used to analyze ELISPOT and FACS data. ELISA with
the Student’s t-test, cytotoxicity assays and tumor treatment data
were analyzed by the Fisher’s exact probabilities in a 2×2 table.
Differences were considered statistically significant at
P<0.01.
Results
Production and analysis of recombinant
proteins
oE7, huhsp70 and oE7/huhsp70 recombinant proteins
were expressed effectively in E. coli BL21 in a form of
inclusion body. All proteins with his-tag were purified by the
His•Bind® Purification kit. All recombinant proteins
were purified to >90%. The recombinant proteins used in this
study contained <0.05 endotoxin units (EU)/μg as measured by the
chromogenic Limulus amebocyte lysate assay. Concentrations of
proteins were measured by the Bradford assay. The expression and
analysis of recombinant proteins were confirmed by western blot
analysis (Fig. 1).
oE7/huhsp70 fusion protein significantly
induces strong E7-specific CD8+ T cell responses
CD8+ T cells are the most important cells
involved in antitumor effect, while the ELISPOT assays and flow
cytometry analysis are the two representative methods. To evaluate
whether oE7/huhsp70 is able to prime strong E7-specific
CD8+ T cells, oE7 or huhsp70, the mice were immunized
i.m. with 200 μl of PBS, 2 nmol oE7, huhsp70 or oE7/huhsp70, which
was repeated after 1 week. Two weeks after the second immunization,
splenocytes were harvested and stimulated with 1 μg/ml
H-2Db-restricted E7 peptide. In ELISPOT assays, as shown in
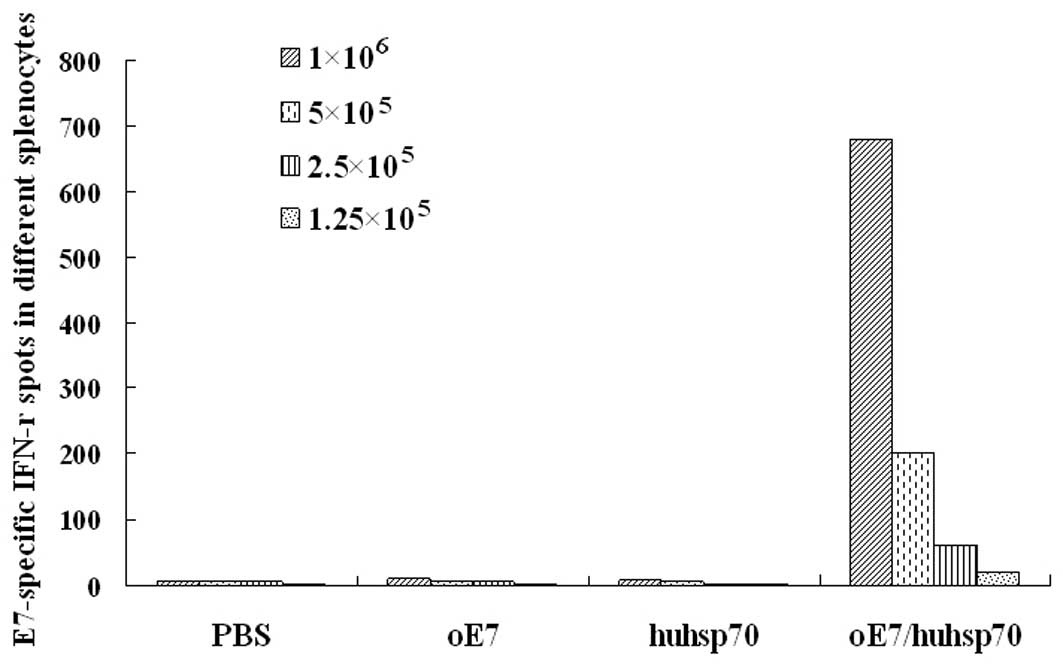
Fig. 2, vaccination with
oE7/huhsp70 generated significantly higher percentage of
E7-specific IFN-γ-secreting CD8+ T cell precursors than
PBS, oE7 or huhsp70. Similar results were also obtained by
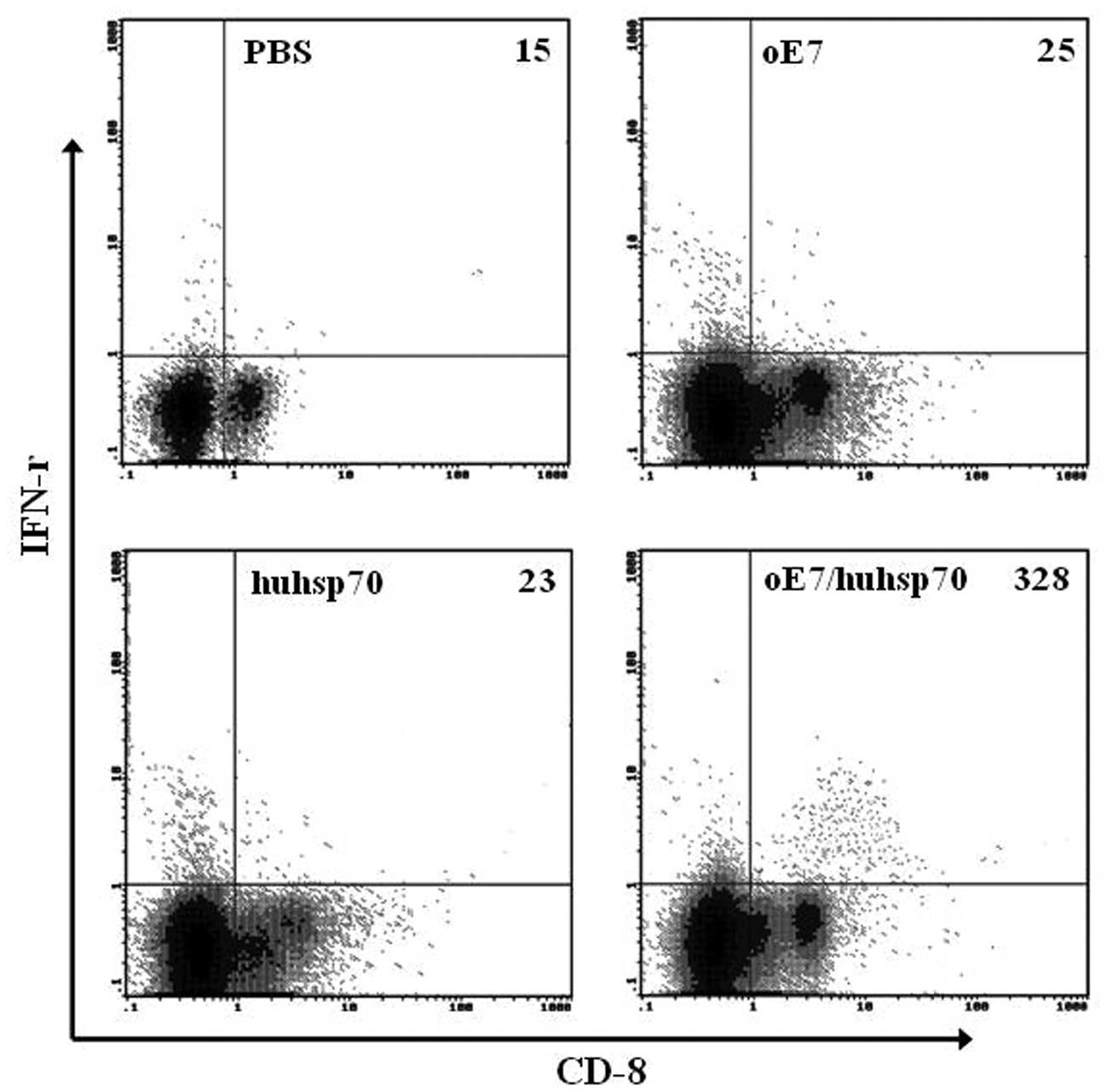
intracellular IFN-γ staining with flow cytometry analysis. As shown
in Fig. 3, vaccination with
oE7/huhsp70 also generated the highest percentage of E7-specific
IFN-γ-secreting CD8+ T cell precursors compared to the
others. In accordance with the ELISPOT and flow cytometry analysis,
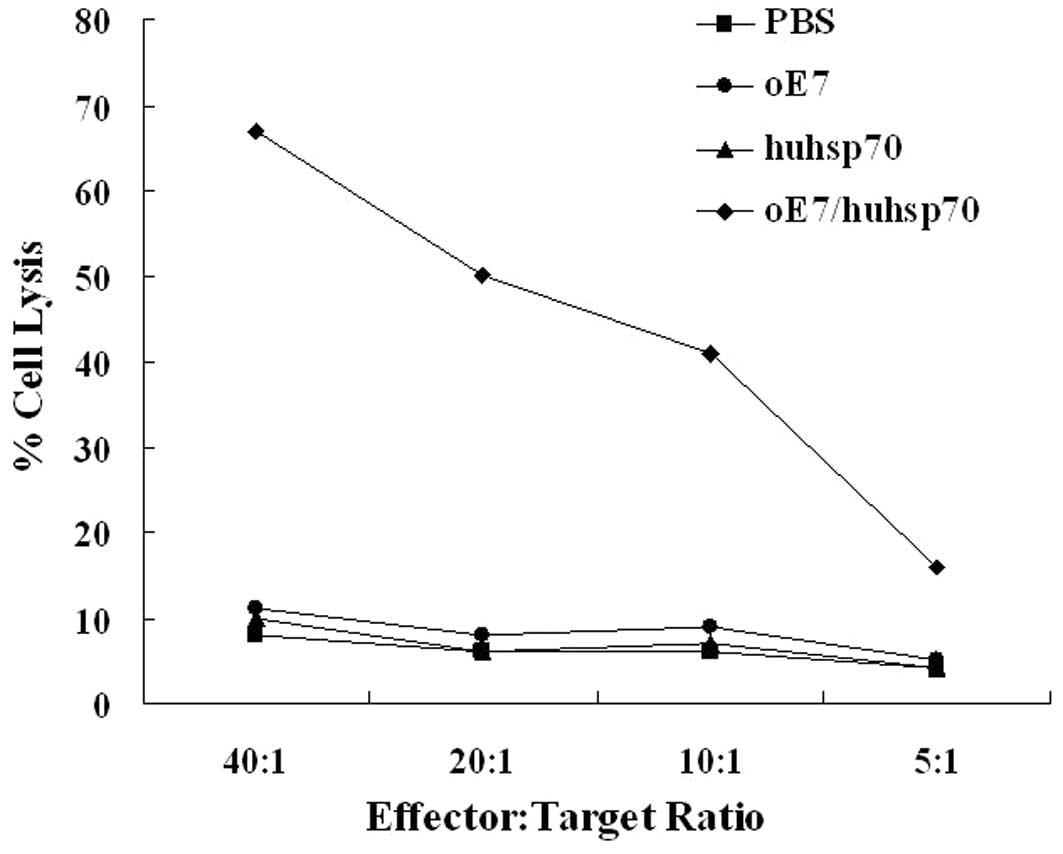
cytotoxicity assays showed that splenocytes of the mice immunized
with oE7/huhsp70 were more cytotoxic to TC-1 target cells
expressing E7 than those in the mice immunized with PBS, oE7 or
huhsp70 (P<0.01) (Fig. 4). Taken
together, our results suggest that oE7/huhsp70 fusion protein
vaccine generates the highest E7-specific CD8+ T cell
response compared with the other vaccinated groups. Results shown
here are all representative of three repeated experiments.
Vaccination with oE7/huhsp70 induces an
E7-specific antibody response
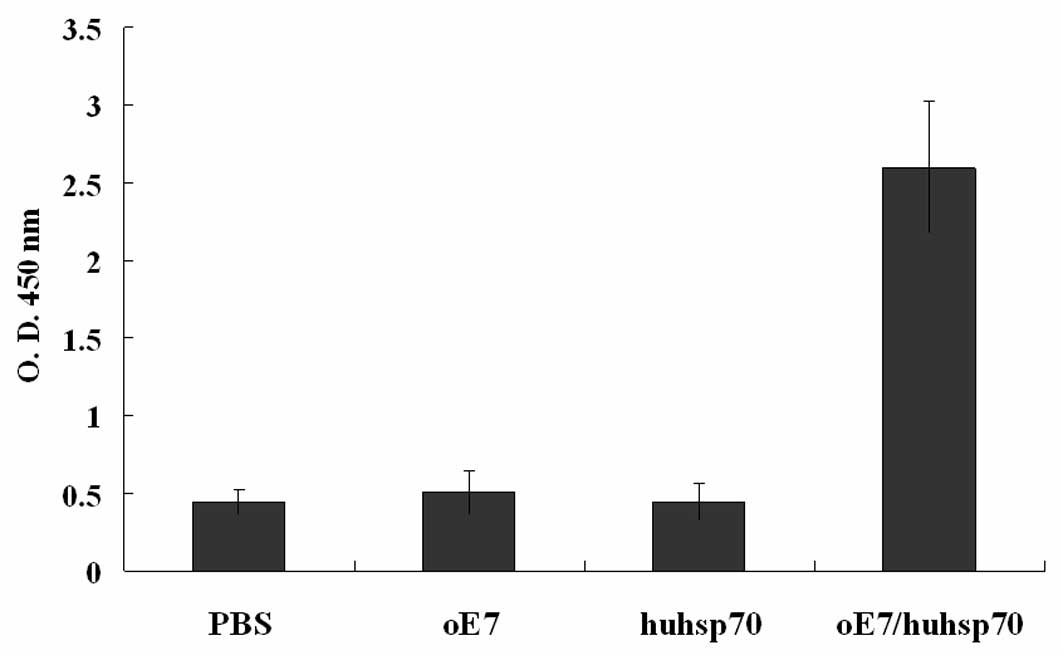
The quantity of anti-HPV16 E7 antibodies in the sera
of the vaccinated mice was determined by ELISA 2 weeks after the
last vaccination. As shown in Fig.
5, only mice vaccinated with oE7/huhsp70 fusion protein produce
a robust antibody response (P<0.01), indicating that secreted
oE7/huhsp70 stimulates B-cell responses.
Tumor treatment experiments
In order to examine the E7-specific CD8+
T response elicited by oE7/huhsp70 fusion protein further, we
investigated the efficacy of each vaccine to induce the eradication
of pre-existing TC-1 tumor expressing E7 in vivo. Female
C57BL/6 mice were challenged with 7.5xl04 TC-1 cells per
mouse s.c. in the right flank. Three days after the challenge with
TC-1 cells, mice were given 200 μl of PBS, 2 nmol oE7, huhsp70 or
oE7/huhsp70. One week later, these mice were boosted with the same
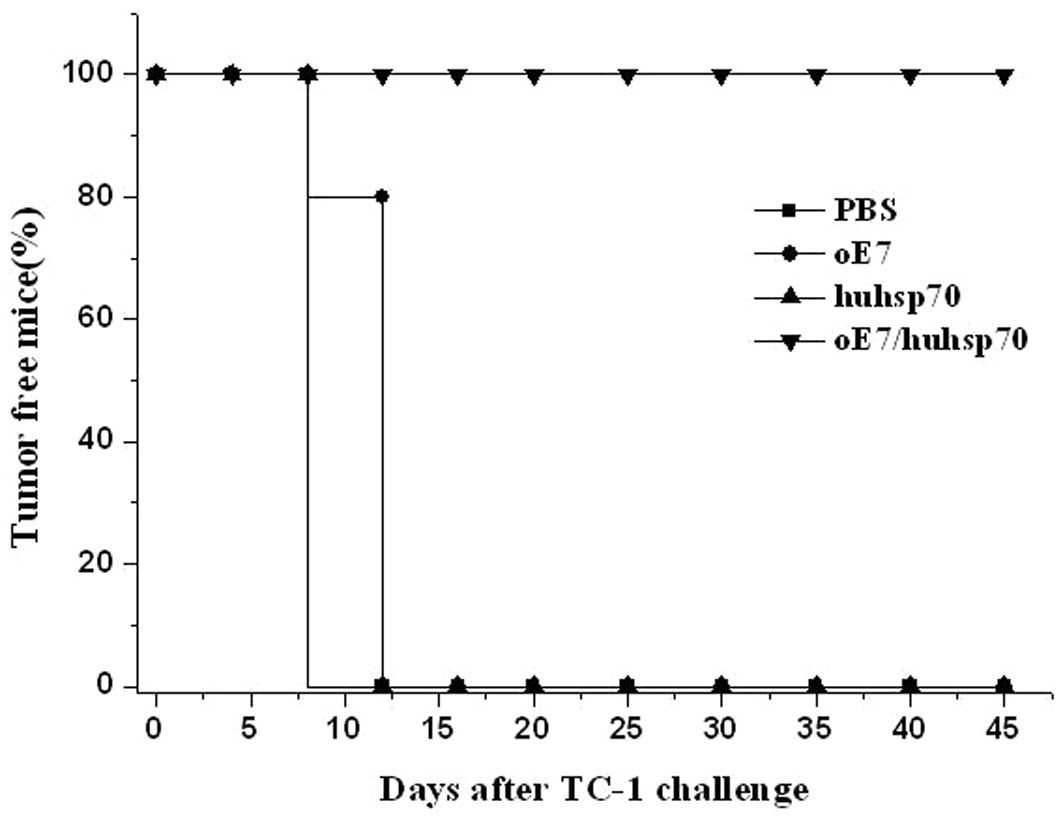
procedure as the first vaccination. As shown in Fig. 6, 100% of the mice immunized with
oE7/huhsp70 were tumor-free until day 45. In contrast, mice
receiving PBS or huhsp70 developed tumor growth on the 12th day
until the 45th day, while 4 mice receiving oE7 developed tumor
growth on the 12th day and 1 mouse developed tumor growth on the
16th day until the 45th day. There was a statistically significant
difference in the therapeutic effect of TC-1 tumor between
oE7/huhsp70 and other vaccinated group (P<0.01). These results
show that oE7/huhsp70 fusion protein vaccine is the most potent and
promising therapeutic vaccine against E7-expressing tumors among
the various protein vaccines which we have tested.
Discussion
Results of the present study revealed that fusion
protein of oE7/huhsp70 not only stimulated very strong
CD8+ T-cell responses but also induced an effective E7
antibody response, indicating that E7-specific CD8+
T-cell responses and B cell mediated humoral immunity play
important roles in anti-tumor effect with this protein vaccine, T
helper cell responses might be simultaneously produced. The
previously reported increased HPV-16 E7-specific IgG levels
appeared to be correlated with a positive therapeutic effect
(27). An important condition of
tumor adaptive immunity is MHC I and II presentation of self and
nonself peptides to CD8+ T and CD4+ T cells
respectively. Endogenous antigens are generally presented by MHC I,
and exogenous antigens by MHC II. In the present study, the
addition of MHC-I epitope at C terminal and MHC-II epitope at N
terminal of fusion protein, aimed to enhance the quantity of
epitope, increased the chance of involvement of antigen
presentation, and strengthened the E7-specific CD8+
T-cell responses and E7 antibody response.
It is well known that exogenous antigens can also be
internalized and displayed by MHC I molecules through a widely
accepted mechanism, called as cross-priming or cross-presentation.
Many studies have recognized and confirmed that hsp70 is involved
in the antigen cross-priming and induction of effective tumor
specific CD8+ T-cell responses (19,20,28).
It has been shown that pathogen-derived molecules are danger
signals and are able to activate innate immunity that in turn
regulates and influences development of adaptive immunity (29,30).
For example, mycobacterium tuberculosis heat shock protein 70 has
been shown to exert a potent adjuvant effect in therapeutic
vaccination against tumors (31).
These studies suggest that mycobacterium tuberculosis also act as
danger signal aside from the molecular chaperon and
cross-presentation function. However, it is clear that there is no
shortage of controversy on the action mechanism of the different
species originated hsp70 in tumor therapeutic vaccines. Bendz et
al(15) have shown that huhsp70
enhances tumor antigen presentation through complex formation and
intracellular antigen delivery without innate immune signaling, in
addition, the fusion DNA vaccine of mouse hsp70 with HPV16 E7
showed that autologous hsp70 was highly potent in enhancing
antigen-specific immune responses (32). These previous therapeutic HPV
vaccine reports showed that whether mouse or human hsp70,
especially huhsp70 induced very strong effective CD8+
T-cell responses and antitumor effect, even 100% of mice were
tumor-free until day 60 after in vivo TC-1 challenge in
tumor protection and treatment experiments (26), which indicated that hsp70 plays an
important role in tumor therapeutic vaccine not for its danger
signal alerting ability but for its molecular chaperon and
cross-presentation function. Thus, huhsp70 fulfills these central
requirements of a tumor vaccination tool and a strong immune
adjuvant.
Safety also is a critical factor to be considered
during development of effective vaccines. The safety problem of
oE7/huhsp70 fusion protein vaccine originats from two areas, one is
the potentially malignant transformation activity of E7 protein,
and another is the homology of hsp70 adjuvant itself. E7 protein
has a tendency to bind with the tumor suppressor protein pRB of the
host, resulting in chromosomal aberration and finally development
of malignant transformation of the host cells. In order to eradiate
the transformation activity of E7 and enhance its immunogenicity,
we muted two zinc finger binding areas and the pRB binding area of
E7. Hsp70 are highly conserved from prokaryotic to eukaryotic
organisms not only functionally but also structurally. However,
allogenous hsp might induce immune reaction to interrupt the
recognition of antigen and antigen presenting cells in mouse or
human (33), or trigger the
induction and expansion of regulatory T-cells with
immunosuppressive functions (34,35). A
potential safety concern arises from the existence of T-lymphocytes
cross-reactive with mycobacterial and human hsp (36). Although we cannot discount this risk
entirely, we believe that in a clinical setting the use of a human
protein will reduce the risk of autoimmunity due to cross-reactive
T-lymphocytes primed by the hsp70 vaccines. Mycobacterium
tuberculosis, mouse and human hsp70 can induce effective antitumor
effects, being a self-protein, toxicity issues are unlikely to
occur, taking into account the future clinical test safety, we
recommend self huhsp70 as a potential and promising candidate
adjuvant to help the therapeutic vaccine to produce enhanced
antitumor effects.
In summary, huhsp70 may be a useful and promising
antigen delivery vehicle for a wide variety of antigens,
cross-presentation is applicable for APC and in various settings of
immune modulation. Our findings not only provide novel insights
into the mechanism by which huhsp70 stimulates T cell responses but
also provide reference for the development of highly effective
molecular tools and immune adjuvant in other tumor biological
treatments.
Acknowledgements
We gratefully acknowledge Professor Richard I.
Morimoto (Northwest University, Evanston, IL, USA) for kindly
providing the pMSHsp70 plasmid and Professor Xuemei Xu (Chinese
Academy of Medical Sciences and Peking Union Medical College,
Beijing, China) for providing the PVR1012mE7/HuHSP70 plasmid. The
present study was supported by a grant from the Nature Science
Foundation for Young Scholars of Shandong Province
(ZR2010HQ009).
Abbreviations:
|
HPV
|
human papilloma virus
|
|
MHC
|
major histocompatibility complex
|
|
hsp70
|
heat shock protein 70
|
|
huhsp70
|
human hsp70
|
|
APC
|
antigen-presenting cell
|
|
DC
|
dendritic cell
|
|
oE7
|
optimized HPV16 E7
|
|
TLR
|
toll-like receptor
|
|
LOX-1
|
Lectin-like, oxidized low-density
lipoprotein receptor-1
|
|
pRB
|
retinoblastoma protein
|
References
|
1
|
Clifford GM, Smith JS, Plummer M, et al:
Human papillomavirus types in invasive cervical cancer worldwide: a
meta-analysis. Br J Cancer. 88:63–73. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siddiqui MA and Perry CM: Human
papillomavirus quadrivalent (types 6, 11, 16, 18) recombinant
vaccine (Gardasil). Drugs. 66:1263–1273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crosbie EJ and Kitchener HC: Cervarix™-a
bivalent L1 virus-like particle vaccine for prevention of human
papillomavirus type 16- and 18-associated cervical cancer. Expert
Opin Biol Ther. 7:391–396. 2007.
|
|
4
|
Dell G and Gaston K: Human
papillomaviruses and their role in cervical cancer. Cell Mol Life
Sci. 58:1923–1942. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
zur Hausen H: Papillomaviruses and cancer:
from basic studies to clinical application. Nat Rev Cancer.
2:342–350. 2002.PubMed/NCBI
|
|
6
|
Li YL, Liu J, Liu JN, et al: Immunization
of protein HPV16 E7 in fusion with mouse HSP70 inhibits the growth
of TC-1 cells in tumor bearing mice. Vaccine. 29:5959–5962. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Vos van Steenwijk PJ, Ramwadhdoebe TH,
Löwik MJ, et al: A placebo-controlled randomized HPV16 synthetic
long-peptide vaccination study in women with high-grade cervical
squamous intraepithelial lesions. Cancer Immunol Immunother.
61:1485–1492. 2012.PubMed/NCBI
|
|
8
|
Zhou L, Zhu T, Ye X, et al: Long-term
protection against human papillomavirus e7-positive tumor by a
single vaccination of adeno-associated virus vectors encoding a
fusion protein of inactivated e7 of human papillomavirus 16/18 and
heat shock protein 70. Hum Gene Ther. 21:109–119. 2010. View Article : Google Scholar
|
|
9
|
Peng S, Monie A, Pang X, et al: Vascular
disrupting agent DMXAA enhances the antitumor effects generated by
therapeutic HPV DNA vaccines. J Biomed Sci. 8:18–21.
2011.PubMed/NCBI
|
|
10
|
Santin AD, Hermonat PL, Ravaggi A, et al:
Induction of human papillomavirus-specific CD4 and CD8 lymphocytes
by E7-pulsed autologous dendritic cells in patients with human
papillomavirus type 16-and 18-positive cervical cancer. J Virol.
73:5402–5410. 1999.
|
|
11
|
Kim D, Hoory T, Wu TC, et al: Enhancing
DNA vaccine potency by combining a strategy to prolong dendritic
cell life and intracellular targeting strategies with a strategy to
boost CD4+ T cell. Hum Gene Ther. 18:1129–1139. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hauser H and Chen SY: Augmentation of DNA
vaccine potency through secretory heat shock protein-mediated
antigen targeting. Methods. 31:225–231. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma B, Xu YJ, Hung CF, et al: HPV and
therapeutic vaccines: Where are we in 2010? Curr Cancer Ther Rev.
6:81–103. 2010. View Article : Google Scholar
|
|
14
|
Maecker HT, Ghanekar SA, Suni MA, et al:
Factors affecting the efficiency of CD8+ T cell
cross-priming with exogenous antigens. J Immunol. 166:7268–7275.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bendz H, Ruhland SC, Pandya MJ, et al:
Human heat shock protein 70 enhances tumor antigen presentation
through complex formation and intracellular antigen delivery
without innate immune signaling. J Biol Chem. 282:31688–31702.
2007. View Article : Google Scholar
|
|
16
|
Brodsky JL and Chiosis G: Hsp70 molecular
chaperones: emerging roles in human disease and identification of
small molecule modulators. Curr Top Med Chem. 6:1215–1225. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Calderwood SK, Theriault JR and Gong J:
Message in a bottle: role of the 70-kDa heat shock protein family
in anti-tumor immunity. Eur J Immunol. 35:2518–2527. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lancaster GI and Febbraio MA:
Exosome-dependent trafficking of HSP70: a novel secretory pathway
for cellular stress proteins. J Biol Chem. 280:23349–23355. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Binder RJ and Srivastava PK: Peptides
chaperoned by heat-shock proteins are a necessary and sufficient
source of antigen in the cross-priming of CD8+ T cells.
Nat Immunol. 6:593–599. 2005.PubMed/NCBI
|
|
20
|
Binder RJ, Blachere NE and Srivastava PK:
Heat shock protein-chaperoned peptides but not free peptides
introduced into the cytosol are presented efficiently by major
histocompatibility complex I molecules. J Biol Chem.
276:17163–17171. 2001. View Article : Google Scholar
|
|
21
|
Tobian AA, Canaday DH, Boom WH, et al:
Bacterial heat shock proteins promote CD91-dependent class I MHC
cross-presentation of chaperoned peptide to CD8+ T cells
by cytosolic mechanisms in dendritic cells versus vacuolar
mechanisms in macrophages. J Immunol. 172:5277–5286. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Delneste Y, Magistrelli G, Gauchat J, et
al: Involvement of LOX-1 in dendritic cell-mediated antigen
cross-presentation. Immunity. 17:353–362. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Asea A, Kraeft SK, Kurt-Jones EA, et al:
HSP70 stimulates cytokine production through a CD14-dependent
pathway, demonstrating its dual role as a chaperone and cytokine.
Nat Med. 6:435–442. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singh-Jasuja H, Scherer HU, Hilf N, et al:
The heat shock protein gp96 induces maturation of dendritic cells
and down-regulation of its receptor. Eur J Immunol. 30:2211–2215.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuppner MC, Gastpar R, Gelwer S, et al:
The role of heat shock protein (hsp70) in dendritic cell
maturation: hsp70 induces the maturation of immature dendritic
cells but reduces DC differentiation from monocyte precursors. Eur
J Immunol. 31:1602–1609. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zong JB, Peng QL, Wang QY, et al: Human
HSP70 and modified HPV16 E7 fusion DNA vaccine induces enhanced
specific CD8+ T cell responses and anti-tumor effects.
Oncol Rep. 22:953–961. 2009.PubMed/NCBI
|
|
27
|
Van Doorslaer K, Reimers LL, Studentsov
YY, et al: Serological response to an HPV16 E7 based therapeutic
vaccine in women with high-grade cervical dysplasia. Gynecol Oncol.
116:208–212. 2010.PubMed/NCBI
|
|
28
|
Srivastava P: Roles of heat-shock proteins
in innate and adaptive immunity. Nat Rev Immunol. 2:185–194. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stewart GR and Young DB: Heat-shock
proteins and the host-pathogen interaction during bacterial
infection. Curr Opin Immunol. 16:506–510. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Segal BH, Wang XY, Dennis CG, et al: Heat
shock proteins as vaccine adjuvants in infections and cancer. Drug
Discov Today. 11:534–540. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Harmala LA, Ingulli EG, Curtsinger JM, et
al: The adjuvant effects of Mycobacterium tuberculosis heat
shock protein 70 result from the rapid and prolonged activation of
antigen-specific CD8+ T cells in vivo. J Immunol.
169:5622–5629. 2002.
|
|
32
|
Li Y, Subjeck J, Yang G, et al: Generation
of anti-tumor immunity using mammalian heat shock protein 70 DNA
vaccines for cancer immunotherapy. Vaccine. 24:5360–5370. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma JH, Sui YF, Ye J, et al: Heat shock
protein 70/MAGE-3 fusion protein vaccine can enhance cellular and
humoral immune responses to MAGE-3 in vivo. Cancer Immunol
Immunother. 54:907–914. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
van Eden W, Koets A, van Kooten P, et al:
Immunopotentiating heat shock proteins: negotiators between innate
danger and control of autoimmunity. Vaccine. 21:897–901.
2003.PubMed/NCBI
|
|
35
|
Rook GA, Martinelli R and Brunet LR:
Innate immune responses to mycobacteria and the downregulation of
atopic responses. Curr Opin Allergy Clin Immunol. 3:337–342. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Salvetti M, Ristori G, Buttinelli C, et
al: The immune response to mycobacterial 70-kDa heat shock proteins
frequently involves autoreactive T cells and is quantitatively
disregulated in multiple sclerosis. J Neuroimmunol. 65:143–153.
1996. View Article : Google Scholar
|