Introduction
Sarcomas represent a diverse group of heterogeneous
mesenchymal neoplasms that affect ~200,000 individuals worldwide
each year. There has been limited improvement in overall 5-year
survival rates for sarcoma patients over the past 30 years,
particularly for patients with metastatic disease. As such, the use
of molecular-targeted therapies has emerged as a promising new
therapeutic approach for the treatment of sarcomas.
Activation of the extrinsic pathway of apoptosis
through the use of recombinant ligand and agonistic monoclonal
antibodies directed against TRAIL receptors DR4 (TRAIL-R1) and/or
DR5 (TRAIL-R2) has been explored as a promising new targeted
therapy for numerous types of malignancies. Based on their
selective ability to induce apoptosis in a variety of human cancer
cell lines and xenografts while sparing normal cells (1), several agents are currently undergoing
phase I and II clinical testing both as single agents and in
combination with traditional chemotherapies. These include
monoclonal agonistic antibodies which specifically target either
DR5, drozitumab (Genetech) (2),
lexatumumab (Human Genome Sciences) (3–6),
conatumumab (Amgen) (7–10), tigatuzumab (humanized IgG1 antibody;
Daiichi-Sankyo) and LBY135 [chimeric (mouse/human) IgG1 antibody;
Novartis] (11) or DR4, mapatumumab
(Human Genome Sciences) (12–17) or
both cognate receptors as well as three decoy receptors of
recombinant human TRAIL (rhApo2L/TRAIL) (dulanermin,
Amgen/Genentech) (18).
Results from early trials have established that DR4
and DR5 agonistic antibodies can be considered safe and are well
tolerated with responses not limited to histological subtype.
However, the clinical efficacy of these agonistic antibodies as
monotherapeutic agents has proven to be quite poor, with only a few
patients showing partial or complete responses (19). Out of the 41 evaluable patients who
participated in dose escalation phase I clinically testing of
drozitumab, partial responses were reported in only 3 patients
(chondrosarcoma, colorectal cancer and ovarian cancer) (2). These findings reflect cell culture
studies which indicate that only a small subset of sarcoma cell
lines are highly sensitive to DR5 agonistic antibodies (20–23).
The poor clinical efficacy of drozitumab as a
monotherapy warrants further investigation into combinational
therapeutic approaches involving drozitumab with other systemic
therapies to synergistically drive tumour regression. Furthermore,
additional mechanistic studies are required to completely delineate
the fundamental regulators of drozitumab. A broad spectrum of
apoptosis regulatory molecules (FLIP, XIAP and Bcl-XL)
and signalling pathways (NF-κB and Akt) are believed to confer
resistance; however, little is known concerning the influence of
proteins which sensitise tumour cells to drozitumab therapy, apart
from DR5 itself. The protein and mRNA expression levels of DR5 and
DR4 in sarcoma cell lines have been extensively documented in
literature. Notably, resistance to TRAIL-mediated apoptosis is not
associated with differential expression of TRAIL-receptors between
sensitive and resistant sarcoma cell lines (24,25).
As DR5 has been shown to be a transcriptional target of p53
(26), this study assessed the role
of p53 in mediating sensitivity to drozitumab in sarcoma cell lines
and human sarcoma patient material. As expected, knockdown of p53
ablated drozitumab-induced apoptosis in vitro. Furthermore,
pre-activation of the p53 pathway through Nutlin-3a (p53-MDM2
antagonist) enhanced the cytotoxic effects of drozitumab both in
vitro and ex vivo. Our study provides the first
pre-clinical evidence that pre-activation of the p53 pathway in
conjunction with drozitumab will potentially provide an effective
therapeutic means to maximise the apoptotic response from both the
extrinsic and intrinsic pathway for the treatment of sarcomas.
Materials and methods
Cell culture
Osteosarcoma (Saos-2, U20S) and Ewing’s sarcoma
(SK-ES1, RD-ES) cell lines were purchased from American Type Tissue
Culture (ATCC, Manassas, VA, USA). Additional Ewing’s sarcoma cell
lines CADO-ES1, STA-ET1, SK-N-MC, TC252, VH-64, WE-68 were kindly
supplied by J. Sonnemann (Department of Pediatric Haematology and
Oncology, University Children’s Hospital, Jena, Germany), P. Ambros
(Children’s Cancer Research Institute, St. Anna Children’s
Hospital, Vienna, Austria), V. Russo (Murdoch Children’s Research
Institute, Royal Children’s Hospital, Victoria, Australia), G.
Hamilton (Department of Surgery, University of Vienna, Austria) and
F. van Valen (Department of Orthopaedic Surgery, Westfälische
Wilhelms University, Germany). Cell lines were cultured as
previously described (27).
Cell viability assays
Cells were seeded in 96-well microtiter plates at a
density of 3×104 cells/well in the presence of
drozitumab + anti-Fcγ at the indicated concentrations for 24 h.
Drozitumab (a kind gift from Dr Avi Ashkenazi, Genentech Inc.,
South San Francisco, CA, USA), was cross-linked with anti-human IgG
Fcγ antibody (Jackson ImmunoResearch Laboratories, Inc., West
Grove, PA, USA) as previously described (28). For synergy experiments, cells were
pre-treated with Nutlin-3a (Cayman Biochemicals, Ann Arbor, MI,
USA) for 24 h prior to the addition of drozitumab + anti-Fcγ. Cells
were harvested and processed as previously described (27). The viability of harvested cells was
determined using 7-amino-actinomycin-D staining and processed on a
FACSCalibur flow cytometer (Becton-Dickinson Immunocytometry
Systems, Franklin Lakes, NJ, USA).
RNA interference
Cell lines with silenced expression of p53 were
generated using the pGIPZ lentiviral shRNAmir system (Open
Biosystems) as previously described (29). Briefly, HEK-293T cells were seeded
at 50% confluency and transfected with either a non-silencing
scramble control (RHS4346) or shRNA directed against human p53
(V2LHS217) using Trans-Lentiviral packaging mix according to the
manufacturer’s protocol (Thermo Fisher Scientific, Waltham, MA,
USA). Forty-eight hours post-transfection, growth medium containing
lentivirus particles was filtered and added to recipient TC252
cells seeded at 50% confluency. Polyclonal populations of
transduced cells were generated through subsequent puromycin
selection
Western blot analysis
Western blot analysis was performed as previously
described (30). Protein extracts
were resolved by SDS polyacrylamide gel electrophoresis on 8%
polyacrylamide gels and incubated with anti-p53 DO-1 (1:1,000,
Santa Cruz Biotechnology, Santa Cruz, CA, USA). Anti-β-actin
(1:2,000; Sigma Aldrich, St. Louis, MO, USA) was used as an
internal loading control.
Real-time PCR
Total RNA was extracted using RNeasy Mini kit
(Qiagen), using On-Column RNase-free DNase digestion according to
the manufacturer’s instructions. cDNAs were generated and real-time
PCR reactions were processed and normalised as previously described
(31). Primer sequences are listed
in Table I.
 | Table IPrimer sequences utilised in this
study. |
Table I
Primer sequences utilised in this
study.
| Primer sequence
5′→3′ |
|---|
|
|
|---|
| Forward | Reverse |
|---|
| PPIG
(housekeeping) |
CAGATGCAGCTAGCAAACCGTTTG |
CTCTTCAGTAGCACTTTCGGAATCAGAGG |
| DR5 (TRAIL-R2) |
CGCTGCACCAGGTGTGATT |
GTGCCTTCTTCGCACTGACA |
| p21 (CDKN1A) |
TGGACCTGGAGACTCTCAGGGTCG |
TTAGGGCTTCCTCTTGGAGAAGATC |
| PUMA (BBC3) |
ACGACCTCAACGCACAGTACG |
TCCCATGATGAGATTGTACAGGAC |
| p53 exons 2–4 |
GTGTCTCATGCTGGATCCCCACT |
GGATACGGCCAGGCATTGAAGT |
| p53 exons 5–6 |
TGCAGGAGGTGCTTACGCATGT |
CCTTAACCCCTCCTCCCAGAGAC |
| p53 exons 7–9 |
ACAGGTCTCCCCAAGGCGCACT |
TTGAGGCATCACTGCCCCCTGAT |
| p53 exon 10 |
GTCAGCTGTATAGGTACTTGAAGTGCAG |
TGGCAGCTGAGCTAGACCTCG |
| p53 exon 11 |
CCTTAGGCCCTTCAAAGCATTGGTCA |
GTGCTTCTGACGCACACCTATTGCAAG |
Explant system
The ex vivo sarcoma tissue explant system was
adapted from methods previously described (32). Briefly sarcoma tissue from patients
previously not exposed to neo-adjuvant therapy was collected
immediately following surgical resection and treated with the
following: vehicle control (DMSO), Nutlin-3a (10 μM), drozitumab as
a monotherapy (200 ng/ml) and in combination with Nutlin-3a (10 μM)
for 48 h. For synergy experiments, explants were pre-treated with
Nutlin-3a for 24 h prior to the addition of drozitumab + anti Fcγ.
Paraffin-embedded sections were subjected to immunohistochemical
(IHC) analysis for activated-caspase 3 (ab4051, 1:100; Abcam,
Cambridge, UK). IHC analysis was adapted from methods previously
described (32). Sequencing of
exons 2-11 of the TP53 gene was conducted to confirm the p53 status
of the sarcoma tissue.
Ethical approval
This study was performed with the approval of the
Royal Adelaide Hospital Human Research Ethics Committee (protocol
#100505). The research conducted throughout is compliant with the
Helsinki Declaration and adheres to the guidelines stated by the
National Health and Medical Research Council (NHMRC) of
Australia.
Statistics
Combination index (CI) values were used to determine
the effects of drozitumab on cell viability in the presence and
absence of Nutlin-3a, as previously described (33). A CI of 1 indicates an additive
effect; >1, an antagonistic effect; and <1, a synergistic
effect.
Results and Discussion
In an effort to define the role of p53 in the
cytotoxic response of sarcoma cells to drozitumab, lentiviral-based
delivery of shRNAs targeting p53 (or non-targeting control shRNAs)
were delivered into the wild-type p53 Ewing’s sarcoma cell line
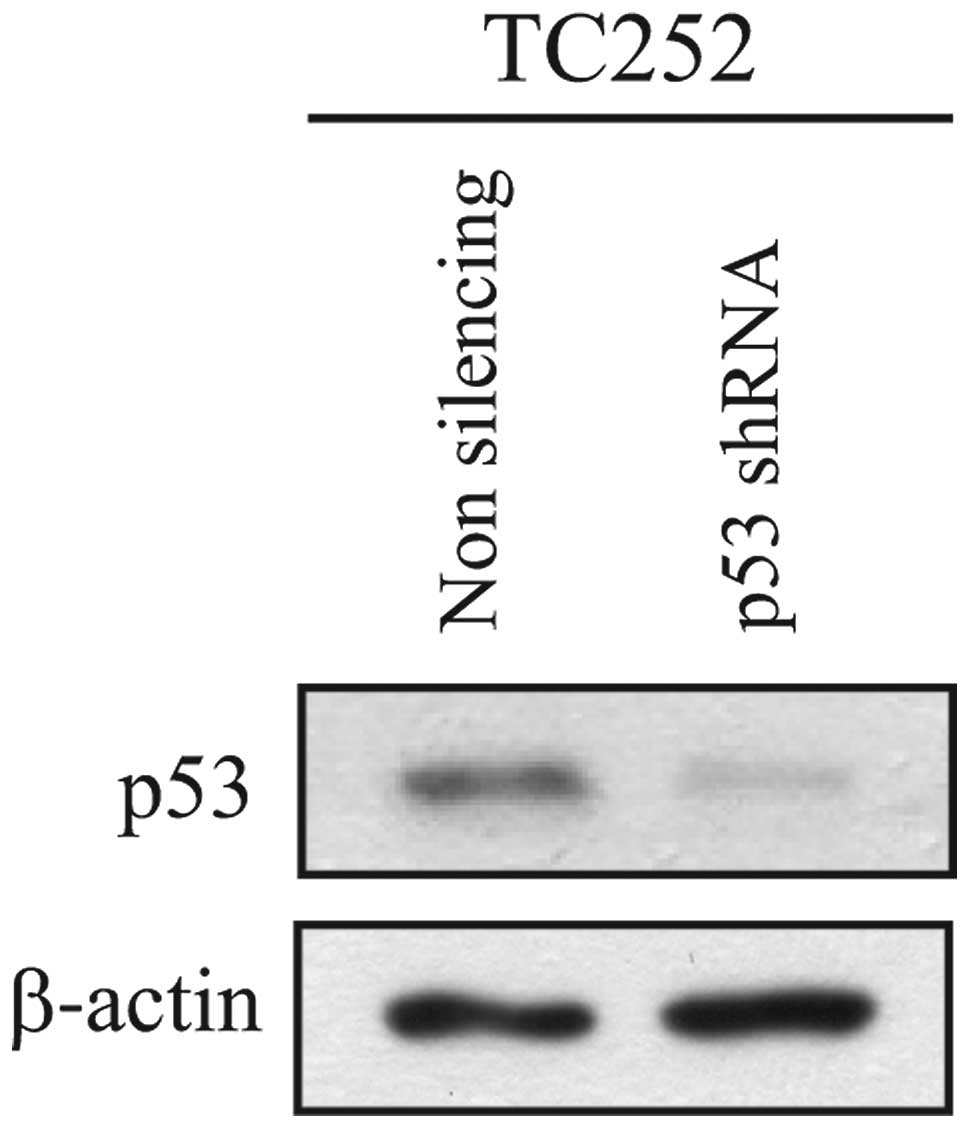
TC252. Knockdown of p53 resulted in effective ablation of p53
protein levels (Fig. 1). The
sensitivity of these TC252 derivatives expressing either p53 shRNA
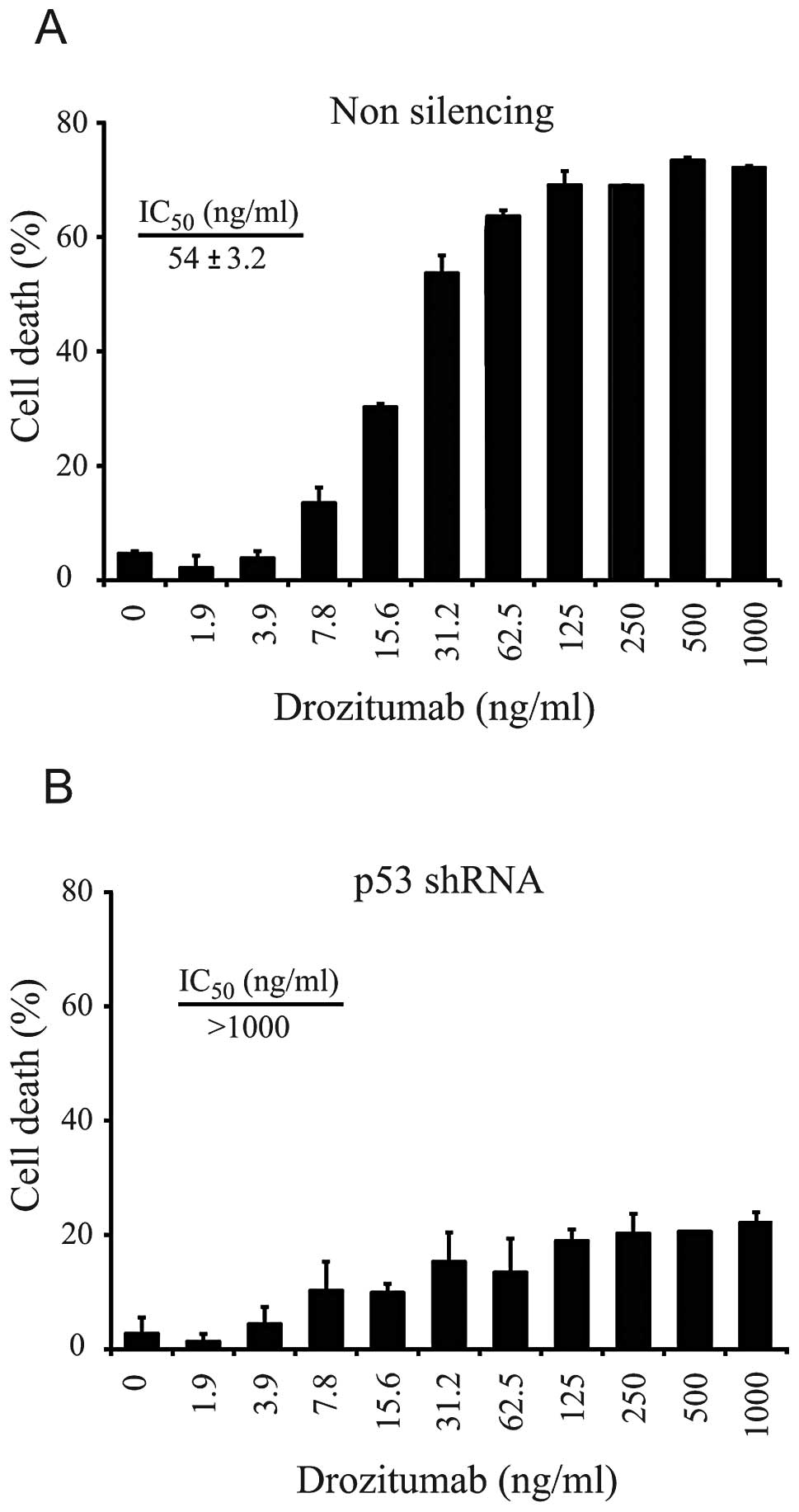
or control shRNA to drozitumab was subsequently determined. In the
control (non-silencing shRNA) cell line derivative, drozitumab
induced a dose-dependent increase in cytotoxicity, with an
IC50 of 54 ng/ml (Fig.
2A). In contrast, silencing of p53 significantly ablated the
ability of drozitumab to induce apoptosis (IC50
>1,000 ng/ml). Even at the maximum concentration tested (1,000
ng/ml), drozitumab was only able to induce 22% cell death (Fig. 2B). As DR5 is a p53-regulated gene
(26), we wished to confirm that
this observed ablation in drozitumab-induced cytotoxicity was
attributed to reduced DR5 expression. Activation of the p53 pathway
was achieved through the use of the non-genotoxic agent Nutlin-3a.
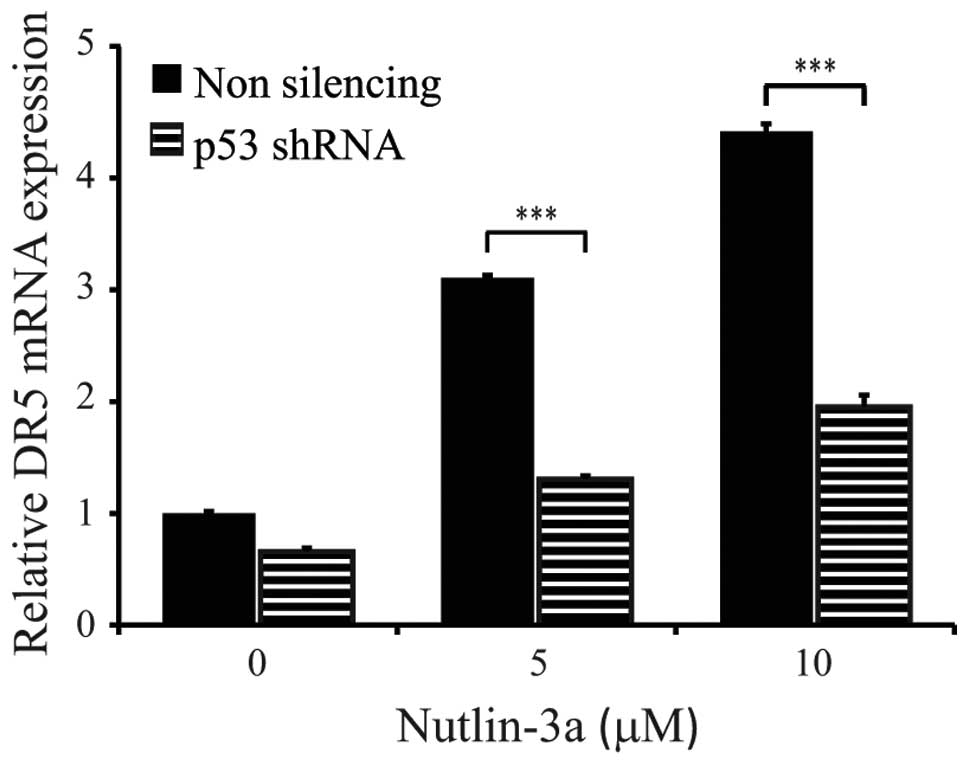
Indeed, significant DR5 upregulation was observed following
Nutlin-3a treatment of control (non-silencing shRNA) TC252 cells.
In contrast, TC252 cells expressing the p53-specific shRNA were
associated with a significantly diminished capacity to upregulate
DR5 upon p53 activation (P<0.0001) (Fig. 3). These results suggest that
p53-induced expression of DR5 is required for conferring drozitumab
sensitivity.
As Nutlin-3a treatment upregulated DR5 mRNA
expression in a p53-dependent manner, we sought to determine
whether pre-activation of the p53 pathway with Nutlin-3a augments
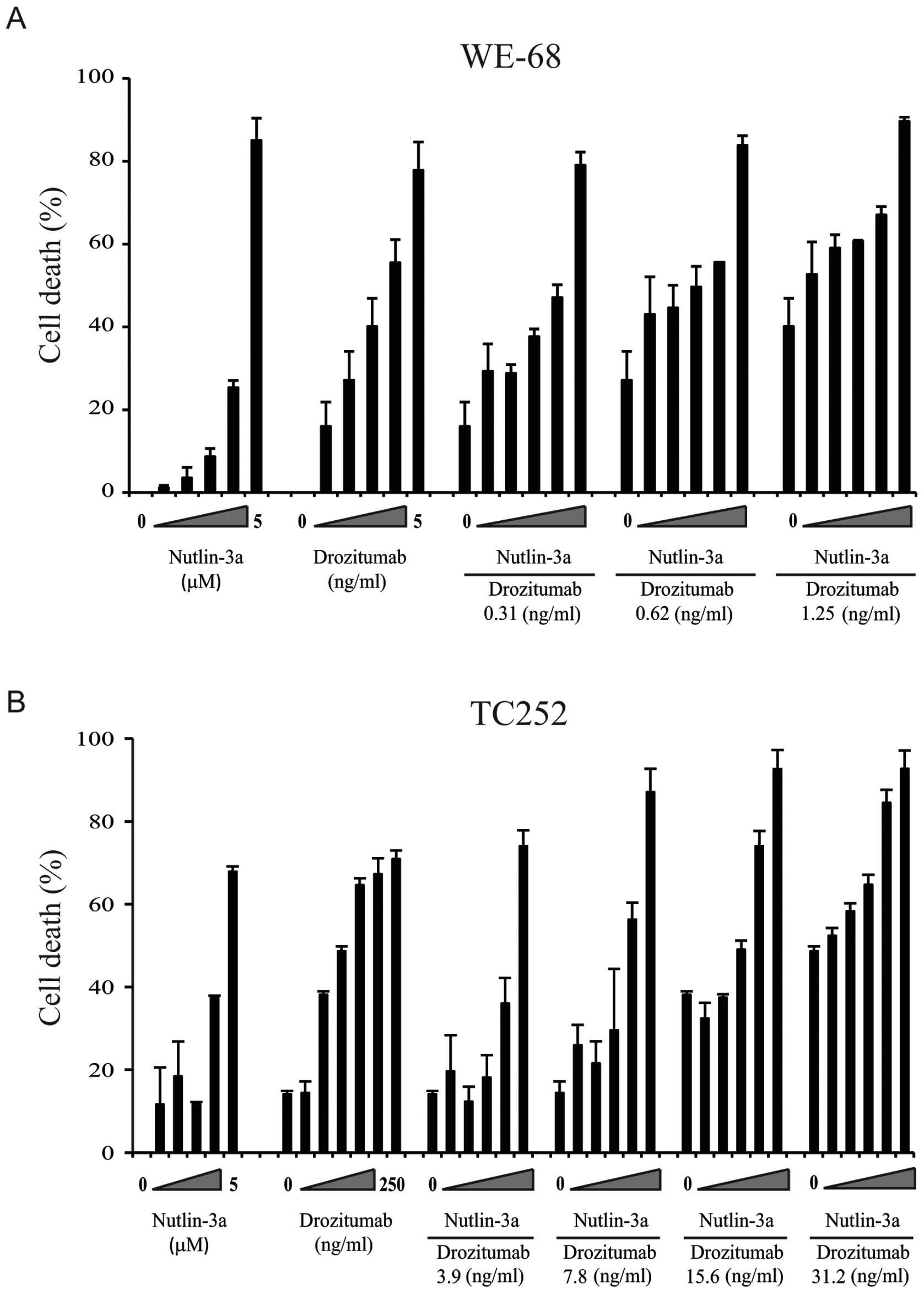
drozitumab induced apoptosis in vitro. WE-68 or TC252
sarcoma cell lines were pre-treated with Nutlin-3a for 24 h before
the addition of drozitumab for an additional 24 h. This combined
dose and schedule of Nutlin-3a and drozitumab resulted in
synergistic cell death, with maximum combination indices of ~0.5 in
both cell lines (Fig. 4, Table II). Furthermore, synergism between
these two agents was only achieved in the wild-type p53 cell lines
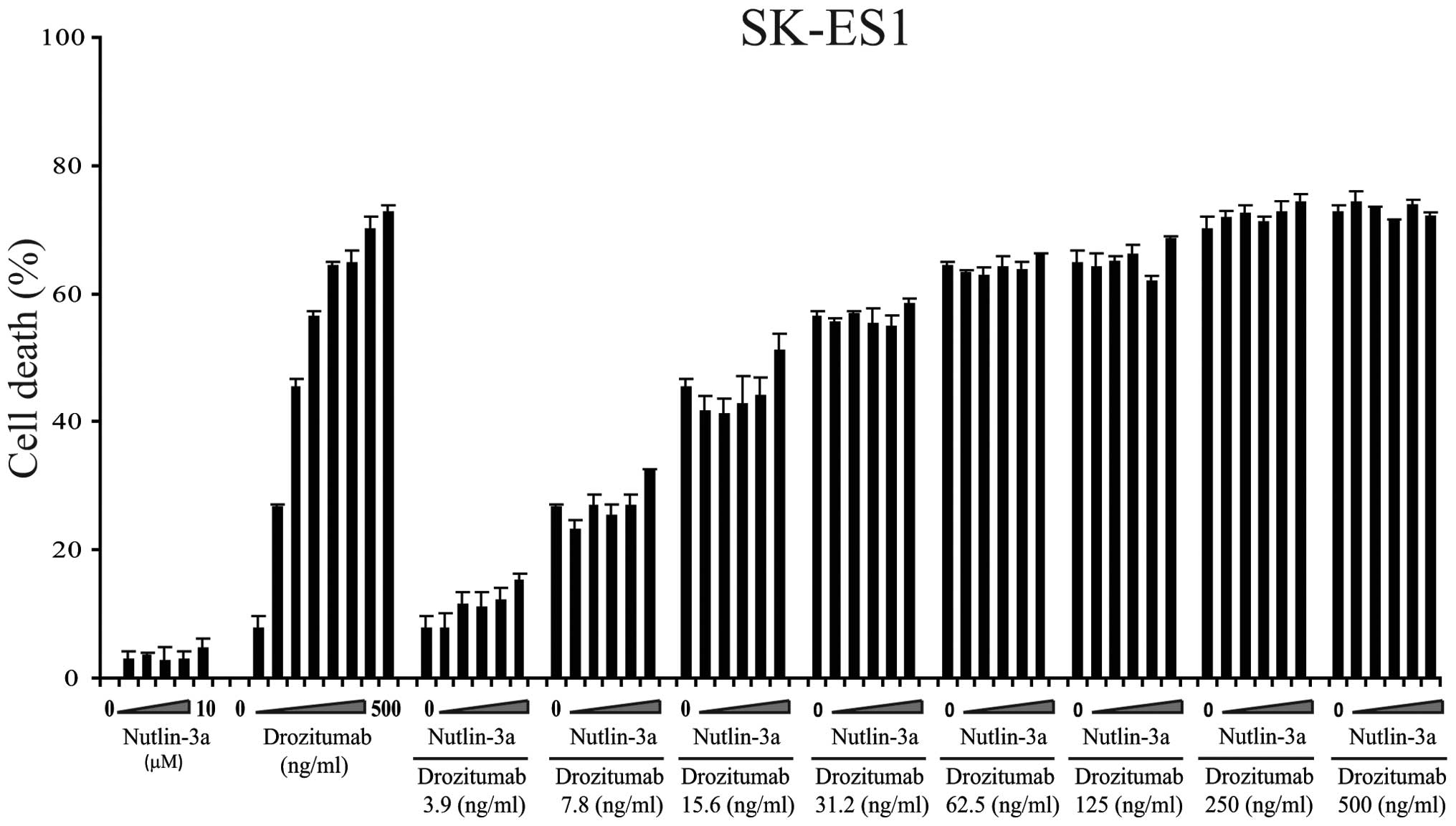
WE-68 and TC-252 but not in the mutant p53-expressing cell line
SK-ES1 (Fig. 5). Importantly, the
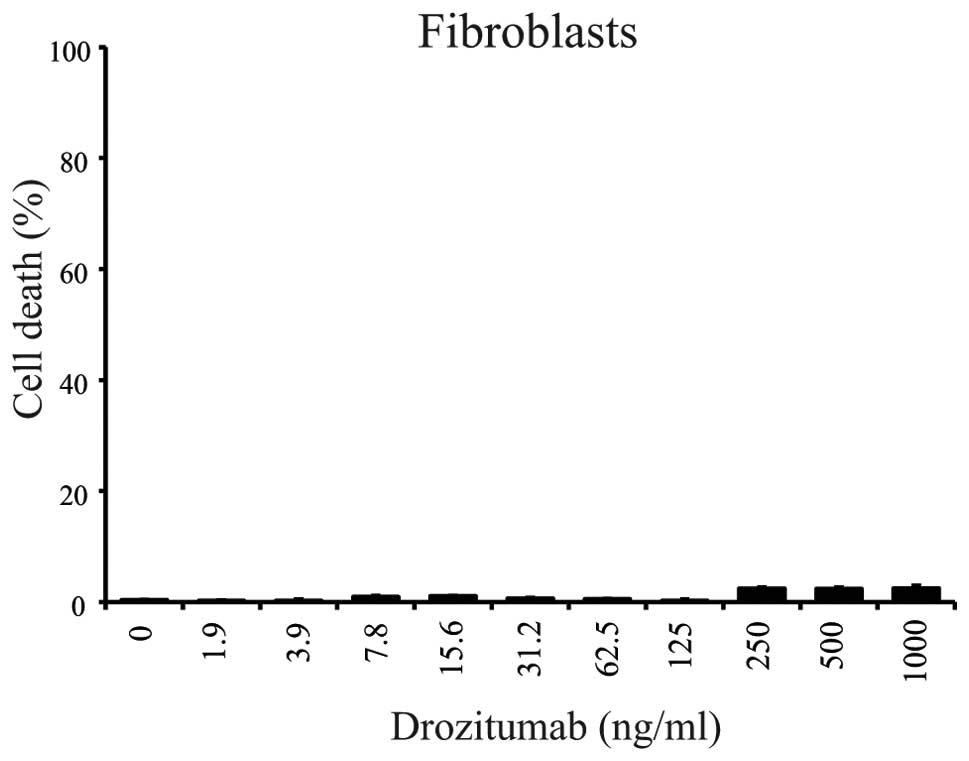
viability of normal human fibroblasts was unaffected following
exposure to drozitumab at these concentrations (Fig. 6).
 | Table IICombination index (CI) values. |
Table II
Combination index (CI) values.
| | In combination with
Nutlin-3a |
|---|
| |
|
|---|
| Cell line | Drozitumab
IC50 (ng/ml) | Drozitumab dose
(ng/ml) | Combination
index |
|---|
| WE-68 | 1.5±0.8 | 0.31 | 0.62 |
| | 0.62 | 0.54 |
| | 1.25 | 0.87 |
| TC252 | 40.7±2.2 | 3.9 | 0.96 |
| | 7.8 | 0.57 |
| | 15.6 | 0.52 |
| | 31.2 | 0.79 |
We subsequently investigated whether Nutlin-3a
augments the apoptotic response of drozitumab in human sarcoma
patient samples ex vivo. Sarcoma tissues from 3 patients
were collected immediately following surgical resection, dissected
in 1-mm3 pieces and pre-treated using an ex vivo
tissue explant system with Nutlin-3a (10 μM) for 24 h prior to the
addition of drozitumab (200 ng/ml) for a subsequent 24 h. An
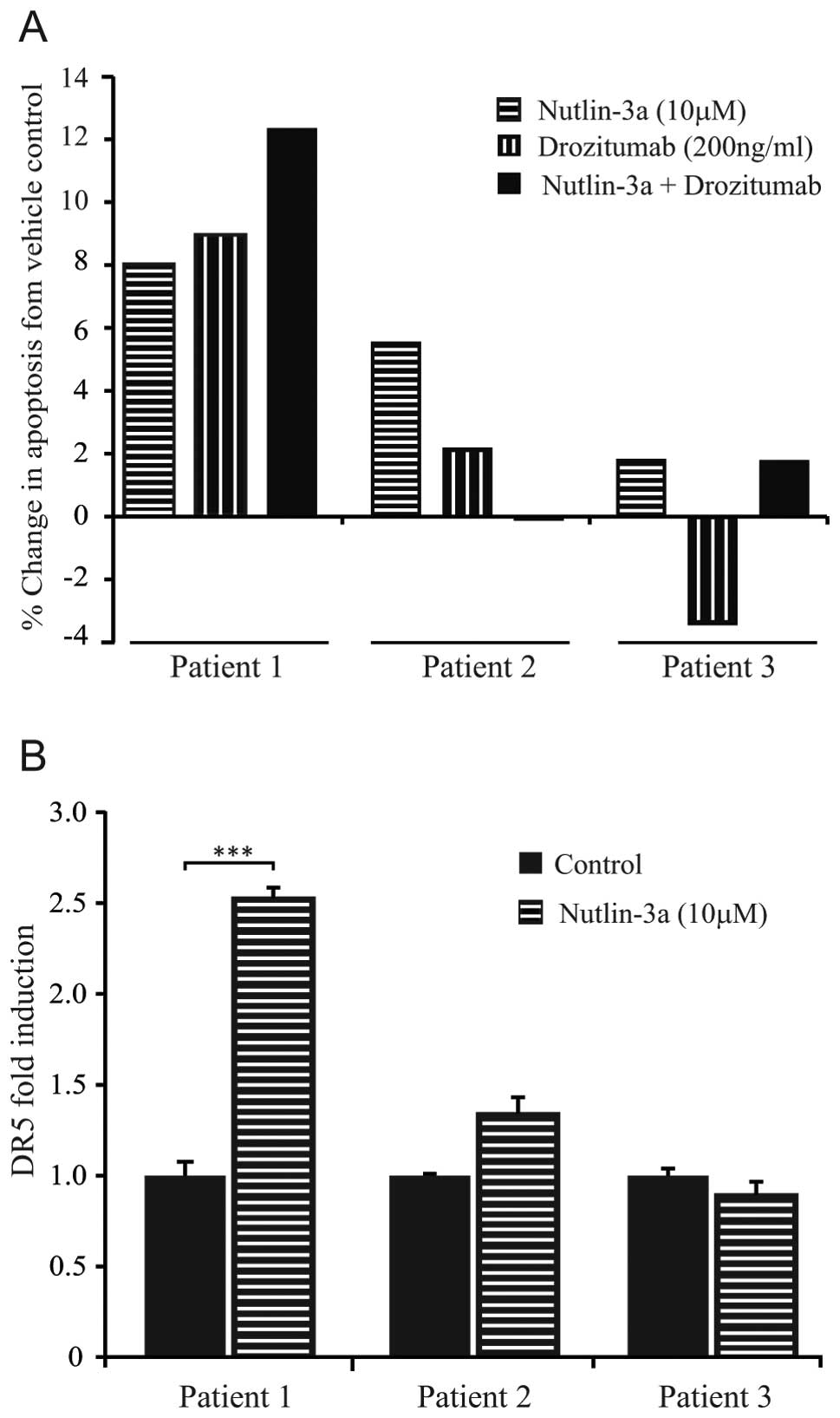
increased level of apoptosis following this combination treatment
was achieved in 1 of the 3 patients (patient 1; malignant fibrous
histiocytoma) (Fig. 7A). This
response was associated with a significant upregulation of DR5
receptor expression (P<0.0001) following Nutlin-3a treatment,
thus, providing a plausible mechanism for this enhanced apoptotic
response (Fig. 7B). In contrast,
sarcomas from patients 2 and 3 showed neither an increase in
drozitumab efficacy upon pre-treatment with Nutlin-3a, nor DR5
upregulation following Nutlin-3a pre-treatment. Furthermore, the
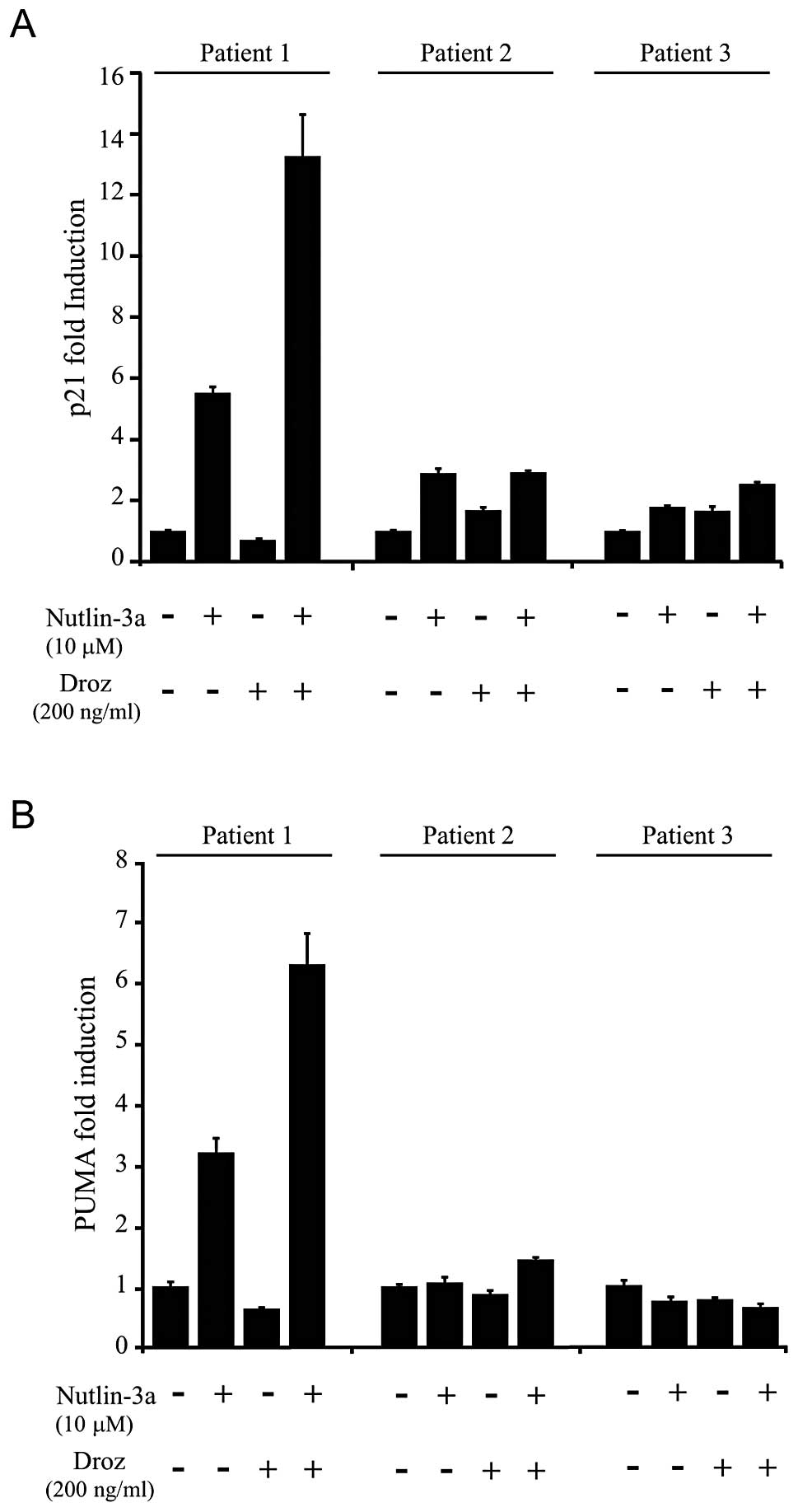
ability of this combination treatment to induce the expression of
other verified p53 target genes (p21 and PUMA) was also only
observed in the responding patient (patient 1) (Fig. 8). Therefore, the ability of p53 to
stimulate DR5 expression in sarcoma tissues is a key factor that
contributes towards the synergistic effects between p53 activators
and drozitumab.
To further investigate the role of p53 in modulating
susceptibility of sarcoma cell lines to the cytotoxic effects of
drozitumab, viability assays were carried out on a panel of 10
sarcoma cell lines with varying p53 statuses in vitro.
Notably, there was no significant correlation between p53 status of
the sarcoma cell lines and sensitivity to drozitumab (Table III), suggesting that p53 status
alone is not an indispensable determinant for driving drozitumab
sensitivity. In particular, two of the wild-type p53 sarcoma cell
lines (CADO-ES1 and U2OS) were completely resistant to drozitumab
(IC50 >1,000 ng/ml). However, it must be noted that
the Ewing’s sarcoma cell line CADO-ES1 is deficient in caspase-8
expression, an essential protein required for the initiation of the
extrinsic pathway of apoptosis (34). In summary, although p53 may play a
critical role in drozitumab sensitivity in sarcomas that have
retained a wild-type p53, our data suggest that the p53 status of
cell lines alone is not enough to predict drozitumab cytotoxicity,
most likely due to secondary genetic alterations in the tumour that
drive fundamental defects in the apoptotic pathway. Thus, further
mechanistic studies are required to define other factors that can
influence the susceptibility of sarcomas to drozitumab-mediated
apoptosis. Collectively, our results justify further pre-clinical
investigations of therapeutic regimes that combine DR5 agonists
with p53 activators as a new means to amplify crosstalk signals
from both the extrinsic and intrinsic pathways of apoptosis for the
targeted treatment of sarcoma.
 | Table IIISensitivity of sarcoma cell lines to
drozitumab. |
Table III
Sensitivity of sarcoma cell lines to
drozitumab.
| Cell line | Histology | TP53
status | Drozitumab
IC50 (ng/ml) |
|---|
| WE-68 | ES | Wild-type | 6.0±0.1 |
| SK-N-MC | ES | Null | 6.8±0.1 |
| STA-ET1 | pPNET | Wild-type | 7.7±2.2 |
| RD-ES | ES | Mutant | 37.2±5.5 |
| TC252 | ES | Wild-type | 54.9±3.2 |
| VH-64 | ES | Wild-type | 65.2±1.7 |
| SK-ES1 | ES | Mutant | 214.1±19.2 |
| CADO-ES1 | ES | Wild-type | >1,000 |
| U2OS | OS | Wild-type | >1,000 |
| Saos-2 | OS | Null | >1,000 |
Acknowledgements
This study was supported in part by the ASSG Sarcoma
Research Award funded through the Rainbows for Kate Foundation in
memory of Tom Wood. We wish to acknowledge financial support from
the Florey Medical Research Foundation, Royal Adelaide Hospital
Clinical Project Grant, Cancer Australia (APP1034715) and the Cure
Cancer Australia Foundation.
References
|
1
|
Ashkenazi A, Pai RC, Fong S, et al: Safety
and antitumor activity of recombinant soluble Apo2 ligand. J Clin
Invest. 104:155–162. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Camidge DR, Herbst RS, Gordon MS, et al: A
phase I safety and pharmacokinetic study of the death receptor 5
agonistic antibody PRO95780 in patients with advanced malignancies.
Clin Cancer Res. 16:1256–1263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wakelee HA, Patnaik A, Sikic BI, et al:
Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given
every 2 weeks in patients with advanced solid tumors. Ann Oncol.
21:376–381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Plummer R, Attard G, Pacey S, et al: Phase
1 and pharmacokinetic study of lexatumumab in patients with
advanced cancers. Clin Cancer Res. 13:6187–6194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Merchant MS, Chou AJ, Price A, Geller JI,
Tsokos M, Graham C, Charles A, Meyers PA and Mackall C:
Lexatumumab: results of a phase I trial in pediatric patients with
advanced solid tumors. J Clin Oncol. 18(Suppl: 15): 95002010.
|
|
6
|
Sikic BI, Wakelee HA, von Mehren M, Lewis
N, Calvert AH, Plummer ER, Fox NL, Howard T, Jones SF and Burris
HA: A phase Ib study to assess the safety of lexatumumab, a human
monoclonal antibody that activates TRAIL-R2, in combination with
gemcitabine, pemetrexed, doxorubicin or FOLFIRI. J Clin Oncol.
25(Suppl 18): 140062007.
|
|
7
|
Demetri GD, Le Cesne A, Chawla SP, et al:
First-line treatment of metastatic or locally advanced unresectable
soft tissue sarcomas with conatumumab in combination with
doxorubicin or doxorubicin alone: a phase I/II open-label and
double-blind study. Eur J Cancer. 48:547–563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Doi T, Murakami H, Ohtsu A, et al: Phase 1
study of conatumumab, a pro-apoptotic death receptor 5 agonist
antibody, in Japanese patients with advanced solid tumors. Cancer
Chemother Pharmacol. 68:733–741. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herbst RS, Kurzrock R, Hong DS, et al: A
first-in-human study of conatumumab in adult patients with advanced
solid tumors. Clin Cancer Res. 16:5883–5891. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chawla SP, Tabernero J, Kindler HL,
Chiorean EG, LoRusso P, Hsu M, Haddad V, Bach BA and Baselga J:
Phase I evaluation of the safety of conatumumab (AMG 655) in
combination with AMG 479 in patients (pts) with advanced,
refractory solid tumors. J Clin Oncol. 28:31022010.
|
|
11
|
Sharma S, de Vries EG, Infante JR,
Oldenhuis C, Chiang L, Bilic S, Goldbrunner M, Scott JW and Burris
HA III: Phase I trial of LBY135, a monoclonal antibody agonist to
DR5, alone and in combination with capecitabine in advanced solid
tumors. J Clin Oncol. 26:35382008.
|
|
12
|
Hotte SJ, Hirte HW, Chen EX, et al: A
phase 1 study of mapatumumab (fully human monoclonal antibody to
TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer
Res. 14:3450–3455. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leong S, Cohen RB, Gustafson DL, et al:
Mapatumumab, an antibody targeting TRAIL-R1, in combination with
paclitaxel and carboplatin in patients with advanced solid
malignancies: results of a phase I and pharmacokinetic study. J
Clin Oncol. 27:4413–4421. 2009. View Article : Google Scholar
|
|
14
|
Mom CH, Verweij J, Oldenhuis CN, et al:
Mapatumumab, a fully human agonistic monoclonal antibody that
targets TRAIL-R1, in combination with gemcitabine and cisplatin: a
phase I study. Clin Cancer Res. 15:5584–5590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tolcher AW, Mita M, Meropol NJ, et al:
Phase I pharmacokinetic and biologic correlative study of
mapatumumab, a fully human monoclonal antibody with agonist
activity to tumor necrosis factor-related apoptosis-inducing ligand
receptor-1. J Clin Oncol. 25:1390–1395. 2007. View Article : Google Scholar
|
|
16
|
Trarbach T, Moehler M, Heinemann V, et al:
Phase II trial of mapatumumab, a fully human agonistic monoclonal
antibody that targets and activates the tumour necrosis factor
apoptosis-inducing ligand receptor-1 (TRAIL-R1), in patients with
refractory colorectal cancer. Br J Cancer. 102:506–512. 2010.
View Article : Google Scholar
|
|
17
|
Younes A, Vose JM, Zelenetz AD, et al: A
Phase 1b/2 trial of mapatumumab in patients with
relapsed/refractory non-Hodgkin’s lymphoma. Br J Cancer.
103:1783–1787. 2010.PubMed/NCBI
|
|
18
|
Herbst RS, Eckhardt SG, Kurzrock R, et al:
Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a
dual proapoptotic receptor agonist, in patients with advanced
cancer. J Clin Oncol. 28:2839–2846. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
den Hollander MW, Gietema JA, de Jong S,
et al: Translating TRAIL-receptor targeting agents to the clinic.
Cancer Lett. 332:194–201. 2013.PubMed/NCBI
|
|
20
|
Van Valen F, Fulda S, Truckenbrod B, et
al: Apoptotic responsiveness of the Ewing’s sarcoma family of
tumours to tumour necrosis factor-related apoptosis-inducing ligand
(TRAIL). Int J Cancer. 88:252–259. 2000.
|
|
21
|
Picarda G, Lamoureux F, Geffroy L, et al:
Preclinical evidence that use of TRAIL in Ewing’s sarcoma and
osteosarcoma therapy inhibits tumor growth, prevents osteolysis,
and increases animal survival. Clin Cancer Res. 16:2363–2374.
2010.PubMed/NCBI
|
|
22
|
Tomek S, Koestler W, Horak P, et al:
Trail-induced apoptosis and interaction with cytotoxic agents in
soft tissue sarcoma cell lines. Eur J Cancer. 39:1318–1329. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang Z, Chen JJ, Yu Y, et al: Drozitumab,
a human antibody to death receptor 5, has potent antitumor activity
against rhabdomyosarcoma with the expression of caspase-8
predictive of response. Clin Cancer Res. 17:3181–3192. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kontny HU, Hammerle K, Klein R, Shayan P,
Mackall CL and Niemeyer CM: Sensitivity of Ewing’s sarcoma to
TRAIL-induced apoptosis. Cell Death Differ. 8:506–514. 2001.
|
|
25
|
Mitsiades N, Poulaki V, Mitsiades C and
Tsokos M: Ewing’s sarcoma family tumors are sensitive to tumor
necrosis factor-related apoptosis-inducing ligand and express death
receptor 4 and death receptor 5. Cancer Res. 61:2704–2712.
2001.
|
|
26
|
Wu GS, Burns TF, McDonald ER III, et al:
KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor
gene. Nat Genet. 17:141–143. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pishas KI, Al-Ejeh F, Zinonos I, et al:
Nutlin-3a is a potential therapeutic for Ewing sarcoma. Clin Cancer
Res. 17:494–504. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zinonos I, Labrinidis A, Lee M, et al:
Apomab, a fully human agonistic antibody to DR5, exhibits potent
antitumor activity against primary and metastatic breast cancer.
Mol Cancer Ther. 8:2969–2980. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Noll JE, Jeffery J, Al-Ejeh F, et al:
Mutant p53 drives multinucleation and invasion through a process
that is suppressed by ANKRD11. Oncogene. 31:2836–2848. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Neilsen PM, Noll JE, Suetani RJ, et al:
Mutant p53 uses p63 as a molecular chaperone to alter gene
expression and induce a pro-invasive secretome. Oncotarget.
12:1203–1217. 2011.PubMed/NCBI
|
|
31
|
Neilsen PM, Cheney KM, Li CW, et al:
Identification of ANKRD11 as a p53 coactivator. J Cell Sci.
121:3541–3552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suetani RJ, Ho K, Jindal S, et al: A
comparison of vitamin D activity in paired non-malignant and
malignant human breast tissues. Mol Cell Endocrinol. 362:202–210.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao L, Wientjes MG and Au JL: Evaluation
of combination chemotherapy: integration of nonlinear regression,
curve shift, isobologram, and combination index analyses. Clin
Cancer Res. 10:7994–8004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fulda S, Kufer MU, Meyer E, van Valen F,
Dockhorn-Dworniczak B and Debatin KM: Sensitization for death
receptor- or drug-induced apoptosis by re-expression of caspase-8
through demethylation or gene transfer. Oncogene. 20:5865–5877.
2001. View Article : Google Scholar : PubMed/NCBI
|