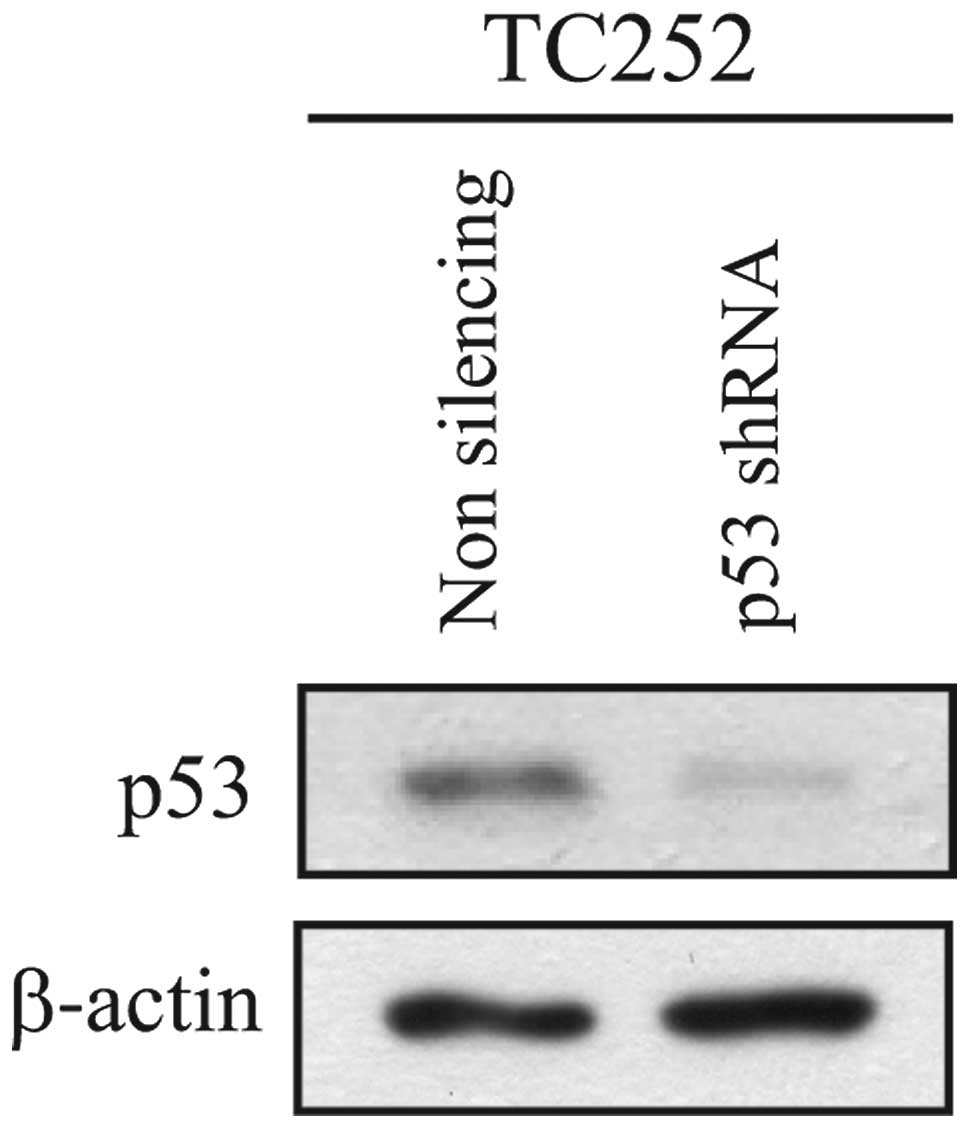
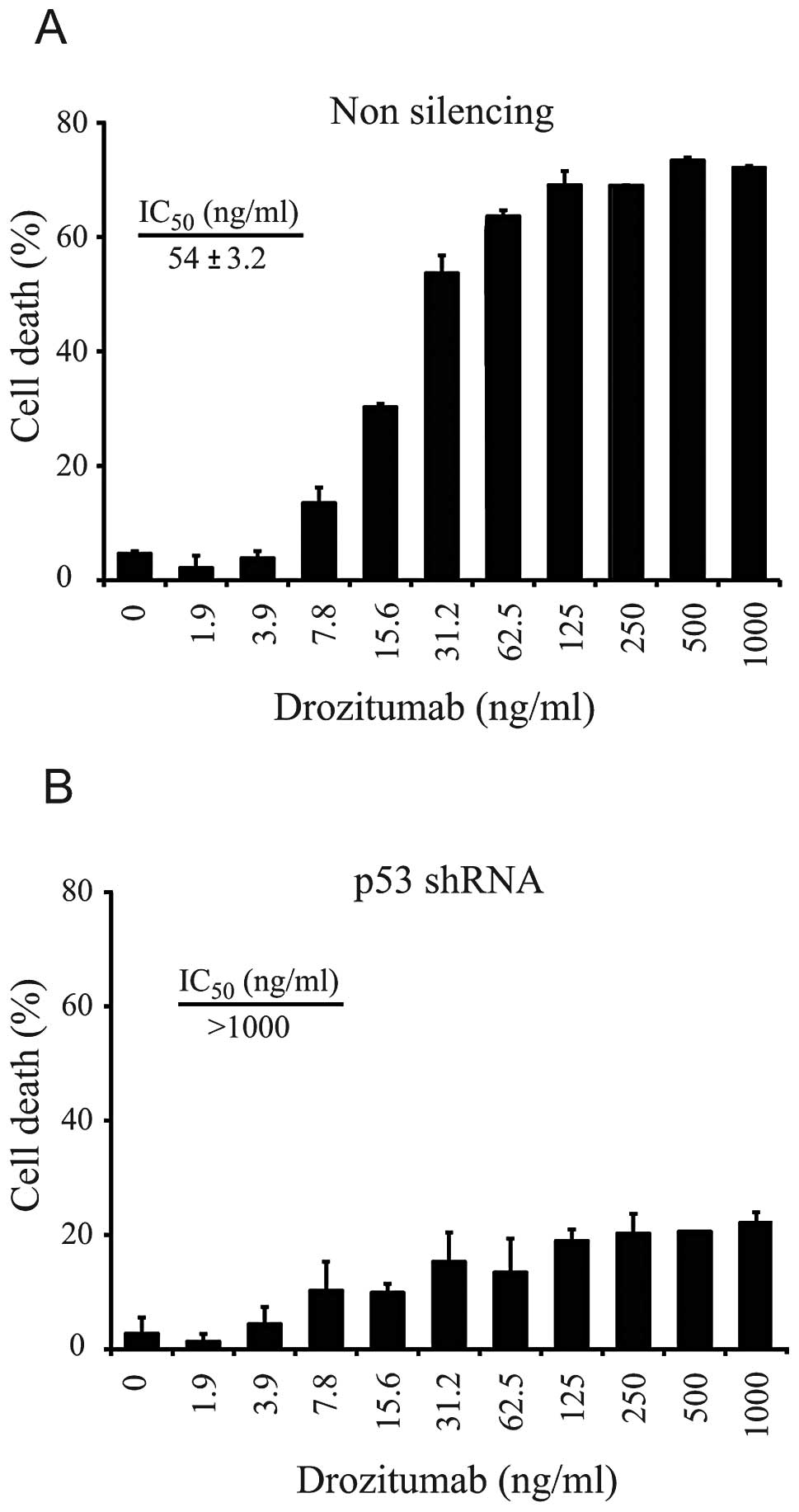
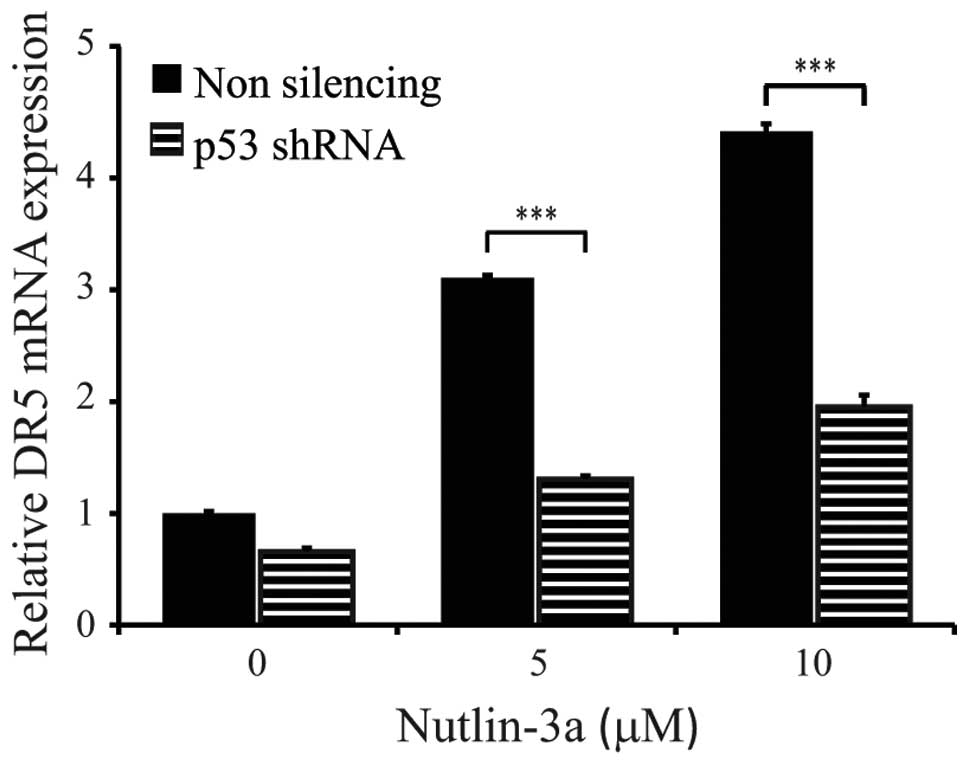
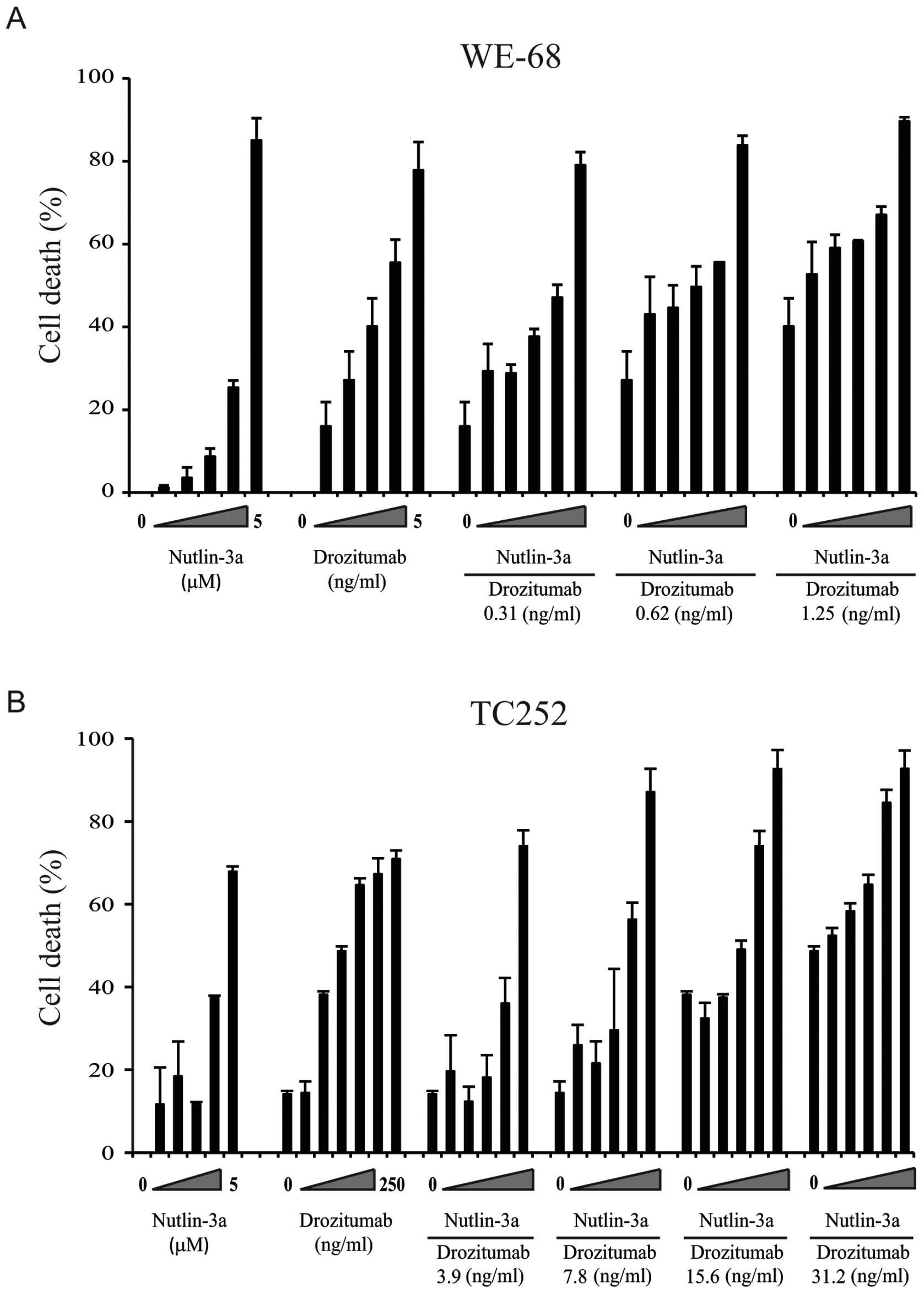
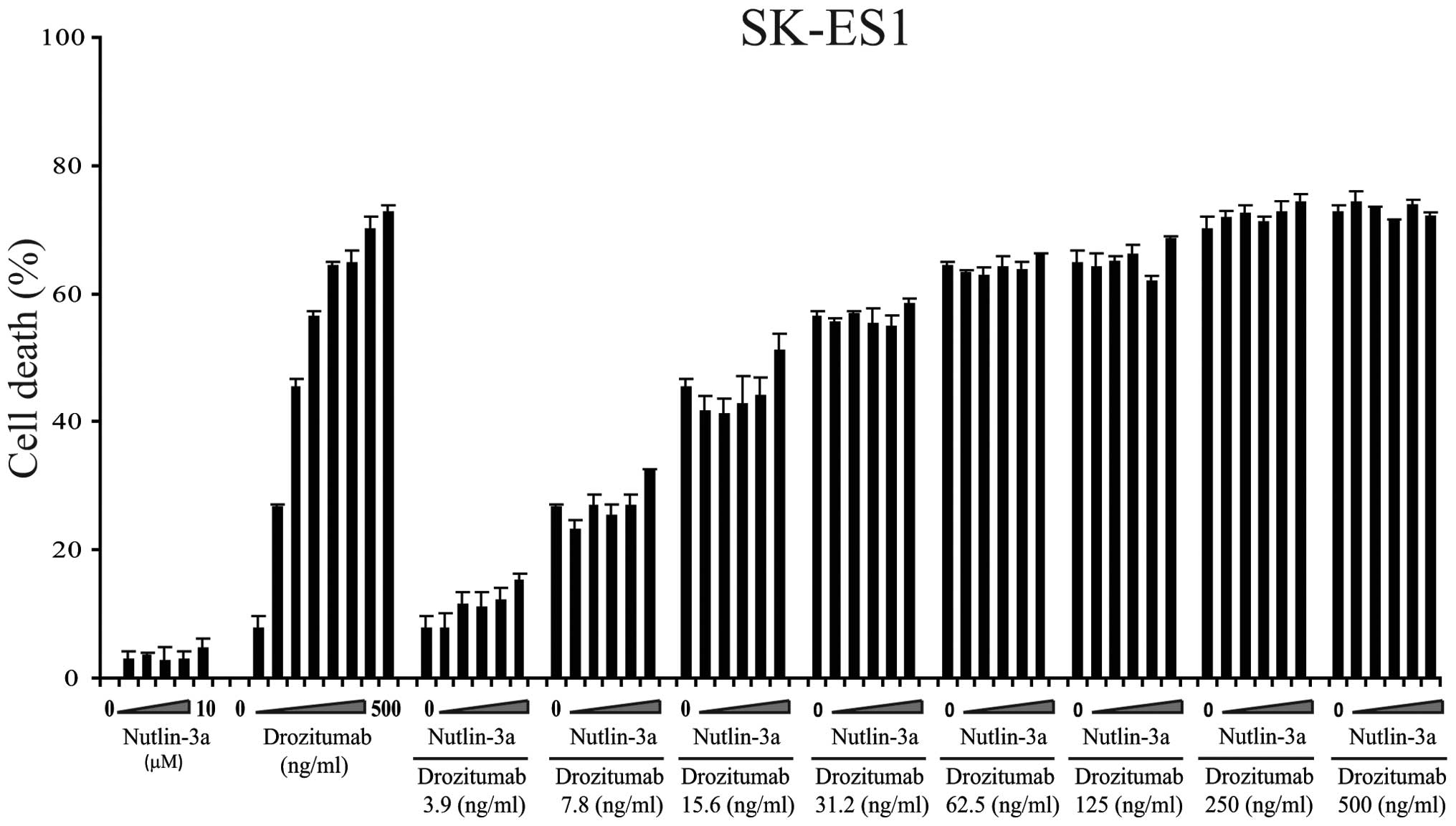
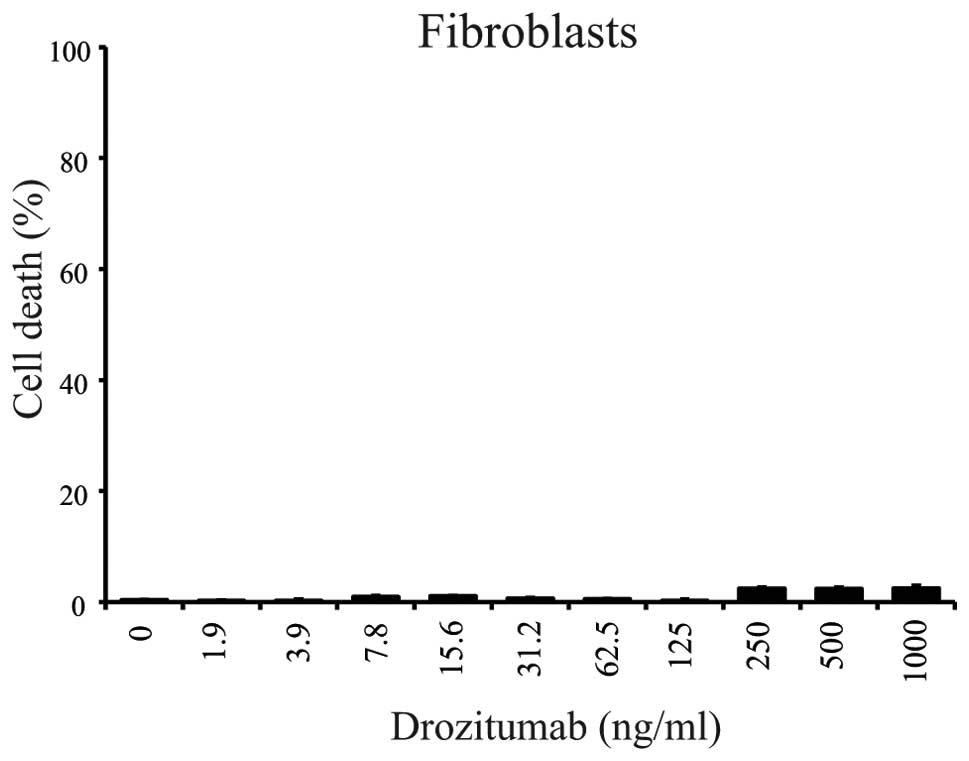
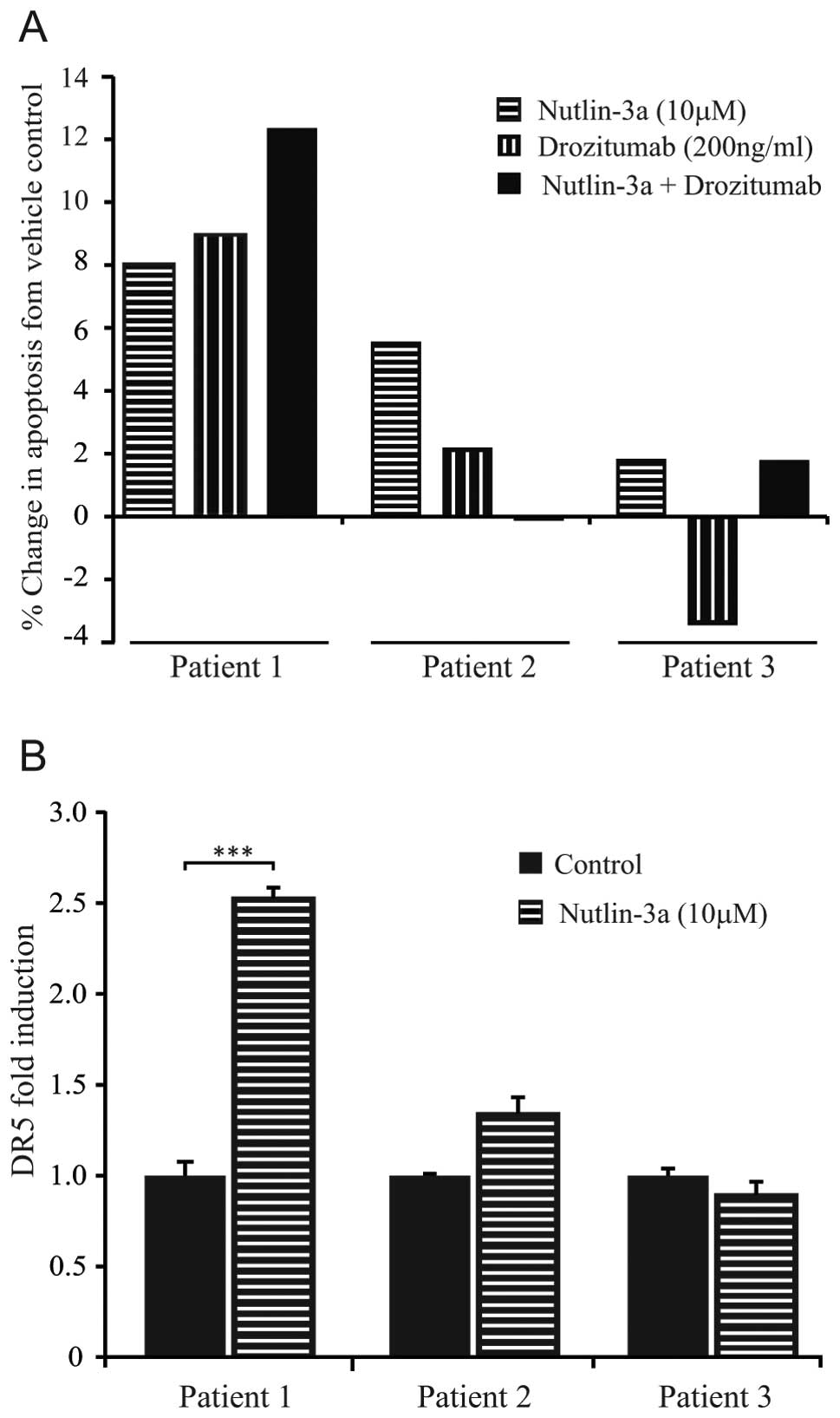
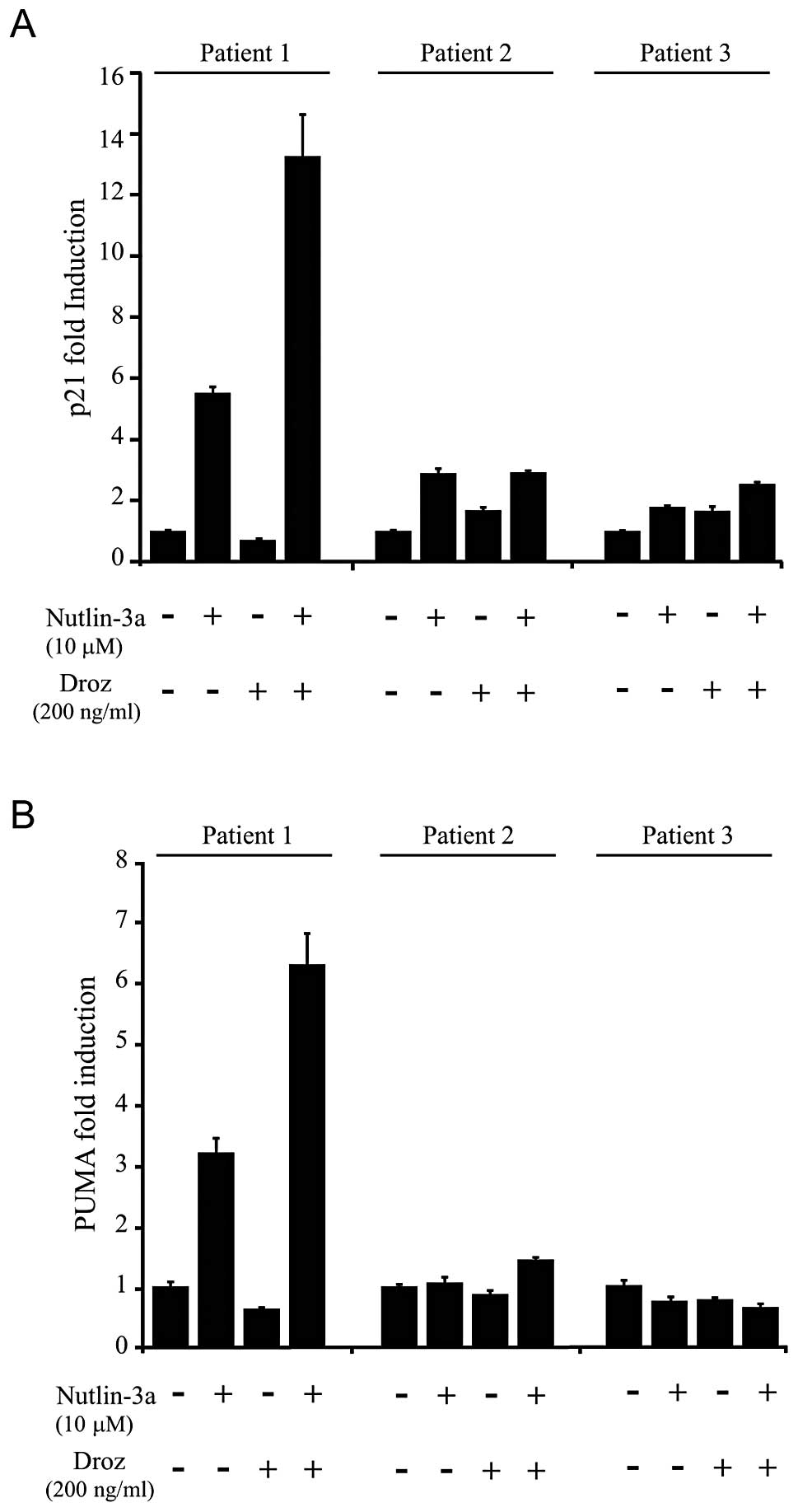
|
1
|
Ashkenazi A, Pai RC, Fong S, et al: Safety
and antitumor activity of recombinant soluble Apo2 ligand. J Clin
Invest. 104:155–162. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Camidge DR, Herbst RS, Gordon MS, et al: A
phase I safety and pharmacokinetic study of the death receptor 5
agonistic antibody PRO95780 in patients with advanced malignancies.
Clin Cancer Res. 16:1256–1263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wakelee HA, Patnaik A, Sikic BI, et al:
Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given
every 2 weeks in patients with advanced solid tumors. Ann Oncol.
21:376–381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Plummer R, Attard G, Pacey S, et al: Phase
1 and pharmacokinetic study of lexatumumab in patients with
advanced cancers. Clin Cancer Res. 13:6187–6194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Merchant MS, Chou AJ, Price A, Geller JI,
Tsokos M, Graham C, Charles A, Meyers PA and Mackall C:
Lexatumumab: results of a phase I trial in pediatric patients with
advanced solid tumors. J Clin Oncol. 18(Suppl: 15): 95002010.
|
|
6
|
Sikic BI, Wakelee HA, von Mehren M, Lewis
N, Calvert AH, Plummer ER, Fox NL, Howard T, Jones SF and Burris
HA: A phase Ib study to assess the safety of lexatumumab, a human
monoclonal antibody that activates TRAIL-R2, in combination with
gemcitabine, pemetrexed, doxorubicin or FOLFIRI. J Clin Oncol.
25(Suppl 18): 140062007.
|
|
7
|
Demetri GD, Le Cesne A, Chawla SP, et al:
First-line treatment of metastatic or locally advanced unresectable
soft tissue sarcomas with conatumumab in combination with
doxorubicin or doxorubicin alone: a phase I/II open-label and
double-blind study. Eur J Cancer. 48:547–563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Doi T, Murakami H, Ohtsu A, et al: Phase 1
study of conatumumab, a pro-apoptotic death receptor 5 agonist
antibody, in Japanese patients with advanced solid tumors. Cancer
Chemother Pharmacol. 68:733–741. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herbst RS, Kurzrock R, Hong DS, et al: A
first-in-human study of conatumumab in adult patients with advanced
solid tumors. Clin Cancer Res. 16:5883–5891. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chawla SP, Tabernero J, Kindler HL,
Chiorean EG, LoRusso P, Hsu M, Haddad V, Bach BA and Baselga J:
Phase I evaluation of the safety of conatumumab (AMG 655) in
combination with AMG 479 in patients (pts) with advanced,
refractory solid tumors. J Clin Oncol. 28:31022010.
|
|
11
|
Sharma S, de Vries EG, Infante JR,
Oldenhuis C, Chiang L, Bilic S, Goldbrunner M, Scott JW and Burris
HA III: Phase I trial of LBY135, a monoclonal antibody agonist to
DR5, alone and in combination with capecitabine in advanced solid
tumors. J Clin Oncol. 26:35382008.
|
|
12
|
Hotte SJ, Hirte HW, Chen EX, et al: A
phase 1 study of mapatumumab (fully human monoclonal antibody to
TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer
Res. 14:3450–3455. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leong S, Cohen RB, Gustafson DL, et al:
Mapatumumab, an antibody targeting TRAIL-R1, in combination with
paclitaxel and carboplatin in patients with advanced solid
malignancies: results of a phase I and pharmacokinetic study. J
Clin Oncol. 27:4413–4421. 2009. View Article : Google Scholar
|
|
14
|
Mom CH, Verweij J, Oldenhuis CN, et al:
Mapatumumab, a fully human agonistic monoclonal antibody that
targets TRAIL-R1, in combination with gemcitabine and cisplatin: a
phase I study. Clin Cancer Res. 15:5584–5590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tolcher AW, Mita M, Meropol NJ, et al:
Phase I pharmacokinetic and biologic correlative study of
mapatumumab, a fully human monoclonal antibody with agonist
activity to tumor necrosis factor-related apoptosis-inducing ligand
receptor-1. J Clin Oncol. 25:1390–1395. 2007. View Article : Google Scholar
|
|
16
|
Trarbach T, Moehler M, Heinemann V, et al:
Phase II trial of mapatumumab, a fully human agonistic monoclonal
antibody that targets and activates the tumour necrosis factor
apoptosis-inducing ligand receptor-1 (TRAIL-R1), in patients with
refractory colorectal cancer. Br J Cancer. 102:506–512. 2010.
View Article : Google Scholar
|
|
17
|
Younes A, Vose JM, Zelenetz AD, et al: A
Phase 1b/2 trial of mapatumumab in patients with
relapsed/refractory non-Hodgkin’s lymphoma. Br J Cancer.
103:1783–1787. 2010.PubMed/NCBI
|
|
18
|
Herbst RS, Eckhardt SG, Kurzrock R, et al:
Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a
dual proapoptotic receptor agonist, in patients with advanced
cancer. J Clin Oncol. 28:2839–2846. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
den Hollander MW, Gietema JA, de Jong S,
et al: Translating TRAIL-receptor targeting agents to the clinic.
Cancer Lett. 332:194–201. 2013.PubMed/NCBI
|
|
20
|
Van Valen F, Fulda S, Truckenbrod B, et
al: Apoptotic responsiveness of the Ewing’s sarcoma family of
tumours to tumour necrosis factor-related apoptosis-inducing ligand
(TRAIL). Int J Cancer. 88:252–259. 2000.
|
|
21
|
Picarda G, Lamoureux F, Geffroy L, et al:
Preclinical evidence that use of TRAIL in Ewing’s sarcoma and
osteosarcoma therapy inhibits tumor growth, prevents osteolysis,
and increases animal survival. Clin Cancer Res. 16:2363–2374.
2010.PubMed/NCBI
|
|
22
|
Tomek S, Koestler W, Horak P, et al:
Trail-induced apoptosis and interaction with cytotoxic agents in
soft tissue sarcoma cell lines. Eur J Cancer. 39:1318–1329. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang Z, Chen JJ, Yu Y, et al: Drozitumab,
a human antibody to death receptor 5, has potent antitumor activity
against rhabdomyosarcoma with the expression of caspase-8
predictive of response. Clin Cancer Res. 17:3181–3192. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kontny HU, Hammerle K, Klein R, Shayan P,
Mackall CL and Niemeyer CM: Sensitivity of Ewing’s sarcoma to
TRAIL-induced apoptosis. Cell Death Differ. 8:506–514. 2001.
|
|
25
|
Mitsiades N, Poulaki V, Mitsiades C and
Tsokos M: Ewing’s sarcoma family tumors are sensitive to tumor
necrosis factor-related apoptosis-inducing ligand and express death
receptor 4 and death receptor 5. Cancer Res. 61:2704–2712.
2001.
|
|
26
|
Wu GS, Burns TF, McDonald ER III, et al:
KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor
gene. Nat Genet. 17:141–143. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pishas KI, Al-Ejeh F, Zinonos I, et al:
Nutlin-3a is a potential therapeutic for Ewing sarcoma. Clin Cancer
Res. 17:494–504. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zinonos I, Labrinidis A, Lee M, et al:
Apomab, a fully human agonistic antibody to DR5, exhibits potent
antitumor activity against primary and metastatic breast cancer.
Mol Cancer Ther. 8:2969–2980. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Noll JE, Jeffery J, Al-Ejeh F, et al:
Mutant p53 drives multinucleation and invasion through a process
that is suppressed by ANKRD11. Oncogene. 31:2836–2848. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Neilsen PM, Noll JE, Suetani RJ, et al:
Mutant p53 uses p63 as a molecular chaperone to alter gene
expression and induce a pro-invasive secretome. Oncotarget.
12:1203–1217. 2011.PubMed/NCBI
|
|
31
|
Neilsen PM, Cheney KM, Li CW, et al:
Identification of ANKRD11 as a p53 coactivator. J Cell Sci.
121:3541–3552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suetani RJ, Ho K, Jindal S, et al: A
comparison of vitamin D activity in paired non-malignant and
malignant human breast tissues. Mol Cell Endocrinol. 362:202–210.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao L, Wientjes MG and Au JL: Evaluation
of combination chemotherapy: integration of nonlinear regression,
curve shift, isobologram, and combination index analyses. Clin
Cancer Res. 10:7994–8004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fulda S, Kufer MU, Meyer E, van Valen F,
Dockhorn-Dworniczak B and Debatin KM: Sensitization for death
receptor- or drug-induced apoptosis by re-expression of caspase-8
through demethylation or gene transfer. Oncogene. 20:5865–5877.
2001. View Article : Google Scholar : PubMed/NCBI
|