Introduction
Cancer is one of the leading causes of death
worldwide. In Korea, gastric cancer (GC) is the most common cause
of cancer-related death in women and the second most common in men
(1). Moreover, colorectal cancer
(CRC) is the fourth leading cause of cancer-related mortality
(2). Recently, in Korea, CRC has
shown the most sharply increasing tendency of all malignancies. In
spite of improvements in cancer diagnosis and therapy, many
patients are still diagnosed at the late stages of the disease, and
this often occurs only after curative surgery.
Cancer develops as a result of multiple genetic and
epigenetic alterations (3,4). Better knowledge of the molecular
changes in the gene expression during gastric and colorectal
carcinogenesis could lead to improvements at several levels,
including diagnosis, treatment and prevention. In order to identify
potential molecular markers for GC and CRC carcinogenesis and to
better understand the development of GC and CRC at the molecular
level, comprehensive analyses of gene expression are useful
(5,6).
To date, many researchers have studied the
classification and the diagnostic prediction of cancers using gene
expression. These molecular markers were consequently correlated
with patient prognosis and survival. Thus, if GC and CRC are
diagnosed at an early stage, patients may have a highly favorable
prognosis and avoid extensive surgery.
Many human melanomas express antigens that are
specific targets of the cytotoxic T lymphocytes of tumor-bearing
patients. Melanoma antigen gene family A (MAGEA) is one of them and
it has been studied for cancer diagnosis and immunotherapy
(7). The MAGEA family consists of
12 subtypes, including MAGEA1 to MAGEA12 (8). MAGEA genes are highly expressed in
different types of cancer, such as melanoma, lymphocytic leukemia,
and various cancers of the lung, head and neck, esophagus, bladder,
stomach, colorectum, breast, liver and ovary (7,9).
Furthermore, it is known that MAGEA is activated by demethylation
of the promoter region in most cancer cells (10). Since MAGEA genes are expressed in
many types of cancers, MAGEA has been assessed as an important
marker for cancer diagnosis (11–13).
Although many studies have reported that MAGEA genes function as
oncogenes, evidence of the role played by MAGEA in the
carcinogenesis of GC and CRC is still lacking.
In the present study, we examined the expression of
MAGEA genes in GC and CRC cancer cell lines and related clinical
tissues to evaluate their role in carcinogenesis. In the present
study, we report that the expression of MAGEA plays an important
role in gastric and colorectal carcinogenesis.
Furthermore, our results suggest that MAGEA can be
used as a diagnostic marker to predict gastric and colorectal
carcinogenesis.
Materials and methods
Cell culture
Ten human gastric adenocarcinoma cell lines (SNU-1,
−5, −16, −216, −484, −601, −620, −638, −668 and −719) and 9
colorectal adenocarcinoma cell lines (SNU-C1, -C4, -C5, COLO320HSR,
LoVo, DLD-1, HT-29, HCT-8 and HCT-116) were obtained from the
Cancer Research Center at Seoul National University (Korea) and
used in this study. All cells were cultured at 37°C in a 5%
CO2 atmosphere using RPMI-1640 medium (Invitrogen,
Carlsbad, CA, USA) with 10% heat inactivated fetal bovine serum
(Sigma, St. Louis, MO, USA). The cells were maintained either as a
suspension or as a monolayer culture and subcultured until they
reached confluence.
Real-time RT-PCR
The total RNA was extracted using the
MagExtractor® for the MFX-2100 (Toyobo, Osaka, Japan)
auto-nucleic acid purification system, according to the
manufacturer's instructions. The 1 μg RNA extracted from each
sample was then reverse transcribed using 200 units of Moloney
murine leukemia virus reverse transcriptase (Invitrogen) and an
oligo (dT) primer for 1 h at 37°C.
Real-time PCR was performed with the LightCycler 2.0
Instrument (Roche Diagnostics, Mannheim, Germany) using the TaqMan
Master Mix (Roche Diagnostics). Each reaction (20 μl) contained 4
μl of 5-fold diluted cDNA, 10 pmol of each primer and probe, and 4
μl of Master Mix containing buffer, dNTPs, MgCl2 and Taq
polymerase. Primer, probe and cycling conditions, as presented in
Table I, have been used in previous
studies (14,15). Data were analyzed using the
LightCycler software version 4.0 (Roche Diagnostics).
 | Table IPrimers, probes and thermal cycling
conditions of the RT-PCR. |
Table I
Primers, probes and thermal cycling
conditions of the RT-PCR.
| Gene | Sense
(5′→3′)
Antisense (5′→3′) | Probe (5′→3′) | Annealing
extension |
|---|
| MAGEA1 |
GCCGAAGGAACCTGACC
ACTGGGTTGCCTCTGTCG |
TGTGTGCAGGCTGCCACCTCCT | 90 sec, 65°C |
| MAGEA2 |
AAGTAGGACCCGAGGCACTG
GAAGAGGAAGAAGCGGTCTG |
CATTGAAGGAGAAGATCTGCCTGTGGGTCTTC | 1 min, 60°C |
| MAGEA3 |
GTCGTCGGAAATTGGCAGTAT
GCAGGTGGCAAAGATGTACAA |
AAAGCTTCCAGTTCCTT | 1 min, 62°C |
| MAGEA4 |
CCACTACCATCAGCTTCACTTGC
CTTCTCGGAACAAGGACTCTGC |
AGGCAACCCAATGAGGGTTCCAGC | 1 min, 63°C |
| MAGEA6 |
GTCGTCGGAAATTGGCAGT
GCAGGTGGCAAAGATGTACAC |
TGCAAGGAATCGGAAGC | 1 min, 65°C |
| MAGEA10 |
TACTGCACCCCTGAGGAGGTC
TGTGGTGGCAATTCTGTCCTG |
AAATGGGAGTGATCCAAGATCCTTCCCAC | 1 min, 64°C |
| MAGEA12 |
GGTGGAAGTGGTCCGCATCG
GCCCTCCACTGATCTTTAGCAA |
AGGCATCTGATGGGAGG | 1 min, 60°C |
| β-actin |
GGGAATCTGACGGATCGGA
GGAATGGAACGCCTGGAAC |
TGCTCCTGAAGAAGTCGTCATGCCTCC | 1 min, 60°C |
Protein extraction and western blot
analysis
The cells were washed with phosphate-buffered saline
(PBS) and lysed in 50 mM Tris-Cl (pH 7.4), 250 mM NaCl, 0.5% Triton
X-100, 10% glycerol, 1 mM DTT, 1 mM PMSF and protease inhibitor
cocktail (Pierce Biotechnology, Rockford, IL, USA). The cell
lysates were then centrifuged and then fractionated by SDS-PAGE,
and western blotting was performed using a slight modification of
the method as previously described (16). The membrane was incubated with
primary rabbit polyclonal antibodies for MAGEA (detection of
MAGEA1, -A2, -A3, -A4, -A6, -A10 and -A12; 1:1,000; Santa Cruz
Biotechnology, Santa Cruz, CA, USA) and β-actin (1:2,500; Santa
Cruz Biotechnology). The membrane was then washed and incubated
with horseradish peroxidase-conjugated secondary antibody (1:2,000
for MAGEA; 1:5,000 for β-actin) against each IgG for hosts of
primary antibodies for 1 h. The membrane was then stained using the
detection reagent of the ECL detection kit (Amersham Pharmacia
Biotech Inc., Piscataway, NJ, USA).
Case selection and tissue sampling
Among the patients that underwent curative surgery
for gastric adenocarcinoma [20 and 21 cases of early-stage gastric
cancer (EGC) and advanced-stage gastric cancer (AGC),
respectively], and colorectal adenocarcinoma (19 cases) at the
Chosun University Hospital (Gwangju, Korea) from January 2008 to
December 2009, non-consecutive patients were selected for this
study, including relatively well-preserved paraffin-embedded
tissues and complete medical records. Twenty cases of colorectal
adenoma, with all samples obtained endoscopically, were subjected
to analysis for a comparison study. Those patients who underwent
preoperative chemo/radiotherapy and emergency surgery, and those
who had evidence of hereditary non-polyposis colorectal cancer or
familial adenomatous polyposis were excluded from the study.
Informed consent was obtained from each subject according to the
institutional guidelines, and the research protocols were approved
by the IRB of our hospital.
Immunohistochemical staining
All tissues investigated in the study were tested
for MAGEA mouse monoclonal antibody (1:2,000, Santa Cruz
Biotechnology). Immunolocalization for MAGEA was performed using a
Polink-2 HRP Plus Mouse DAB Detection system (Golden Bridge
International, Inc., Mukilteo, WA, USA), according to the
supplier's protocol. Briefly, 4-μm sections obtained after formalin
fixation and paraffin embedding were deparaffinized in xylene and
rehydrated with distilled water through graded concentrations of
ethanol. After quenching the endogenous peroxidase activity in 0.3%
hydrogen peroxide for 10 min, the slides were rinsed with distilled
water. The sections were then placed in a glass jar with 10 mM
citrate buffer (pH 6.0) and irradiated in a microwave oven for 15
min, and cooled down in the jar at room temperature for 20 min. The
slides were then rinsed with Tris-buffered saline (TBS) and a
blocking reagent was added for 10 min. After tapping off the excess
blocking reagent, the specimen was carefully wiped around, and
enough primary antibody to cover the specimen was applied for 1 h
in a moist chamber at 37°C. After washing with TBS, mouse antibody
enhancer was applied for 10 min, followed by washing with TBS, as
before. Then, polymer-HRP (horseradish peroxidase) for mouse was
applied for 10 min to cover each section. After washing again with
TBS, the localization of antibodies was visualized by incubating
the sections for 5 min in DAB and counterstaining with Mayer's
hematoxylin for 10 sec. An isotype matched control antibody was
also used. The positive control for MAGEA used in the present study
was early placental tissue. In contrast, instead of the primary
antibody, normal goat serum was used as the negative control.
Analysis and interpretation of the
staining
Staining for MAGEA was deemed positive when nuclear
staining was identified under an optical microscope in >1% of
the tumor cells in each tissue section. Positive expression of
MAGEA was then classified into level 1 (weakly positive), when
1–25% of tumor cells were stained; level 2 (moderately positive),
when 26–50% of tumor cells were stained; and level 3 (strongly
positive), when >50% of tumor cells were stained.
Statistical analysis
Statistical analysis was performed using the
Student's t-test. P-values <0.05 were considered to indicate
statistically significant differences.
Results
Comparison of the MAGEA expression in
gastric and colorectal cancer cell lines
mRNA and protein expression of the MAGEA genes,
including MAGEA-1, -A2, -A3, -A4, -A6, -A10 and -A12, was analyzed
using real-time PCR (RT-PCR) and western blot methods in 10 gastric
and 9 colorectal cancer cell lines, respectively.
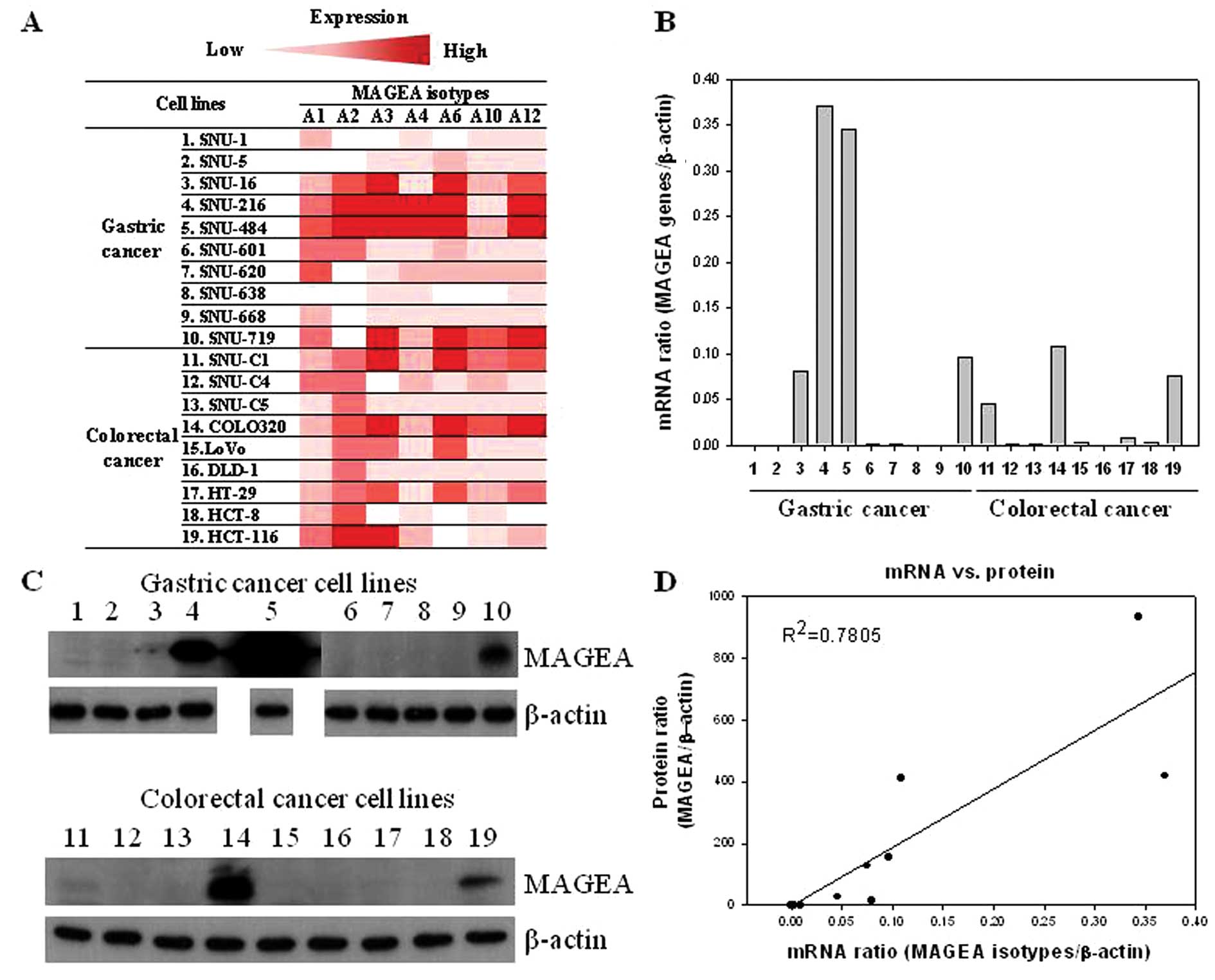
The color gradient from dark red to light red
indicates the mRNA expression level of the MAGEA genes (Fig. 1A). Furthermore, the mRNA level of
total MAGEA genes was determined as the sum of the mRNA levels
found in each MAGEA gene. According to the MAGEA gene/β-actin ratio
in the gastric cancer cell lines, the rank order was as follows:
SNU-216 (0.37) > SNU-484 (0.34) > SNU-719 (0.1) > SNU-16
(0.08) > SNU-620 (0.002) > SNU-601 (0.0007) > SNU-1
(0.0001) > SNU-5 (0.00008) > SNU-668 (0.00005) > SNU-638
(0.00001) (Fig. 1B). Similarly, in
the colorectal cancer cell lines, according to the MAGEA
gene/β-actin ratio, the rank order was as follows: COLO320HSR
(0.11) > HCT-116 (0.08) > SNU-C1 (0.04) > HT-29 (0.008)
> LoVo = HCT-8 (0.003) > SNU-C4 = SNU-C5 = DLD-1 (0.001)
(Fig. 1B).
We examined the protein level of MAGEA using western
blot analysis to compare the mRNA levels obtained from real-time
RT-PCR. Among the 10 investigated gastric cancer cell lines, the
protein expression of MAGEA was be detected in 3 (30%) (SNU-484,
SNU-216 and SNU-719) (Table II).
Of the 9 investigated colorectal cancer cell lines, MAGEA was also
detected in 2 (22.2%) (COLO320HSR and HCT-116) (Table II). MAGEA gene expression at the
mRNA level was generally correlated with that at the protein level
(Fig. 1D).
 | Table IIComparison of protein expression of
MAGEA in gastric and colorectal cancer cell lines. |
Table II
Comparison of protein expression of
MAGEA in gastric and colorectal cancer cell lines.
| Cell lines | Histopathology | MAGEA expression | Positive n (%) |
|---|
| Gastric |
| SNU-1 | Adenocarcinoma | − | 3 (30) |
| SNU-5 | Adenocarcinoma | − | |
| SNU-16 | Adenocarcinoma | − | |
| SNU-216 | Adenocarcinoma | +++ | |
| SNU-484 | Adenocarcinoma | ++++++ | |
| SNU-601 | Adenocarcinoma | − | |
| SNU-620 | Adenocarcinoma | − | |
| SNU-638 | Adenocarcinoma | − | |
| SNU-668 | Carcinoma | − | |
| SNU-719 | Adenocarcinoma | ++ | |
| Colorectal |
| SNU-C1 | Adenocarcinoma | − | 2 (22.2) |
| SNU-C4 | Adenocarcinoma | − | |
| SNU-C5 | Adenocarcinoma | − | |
| COLO320 | Adenocarcinoma | +++ | |
| LoVo | Adenocarcinoma | − | |
| DLD-1 | Adenocarcinoma | − | |
| HT-29 | Adenocarcinoma | − | |
| HCT-8 | Adenocarcinoma | − | |
| HCT-116 | Carcinoma | + | |
Clinicopathological significance of MAGEA
in gastric and colorectal cancer tissues
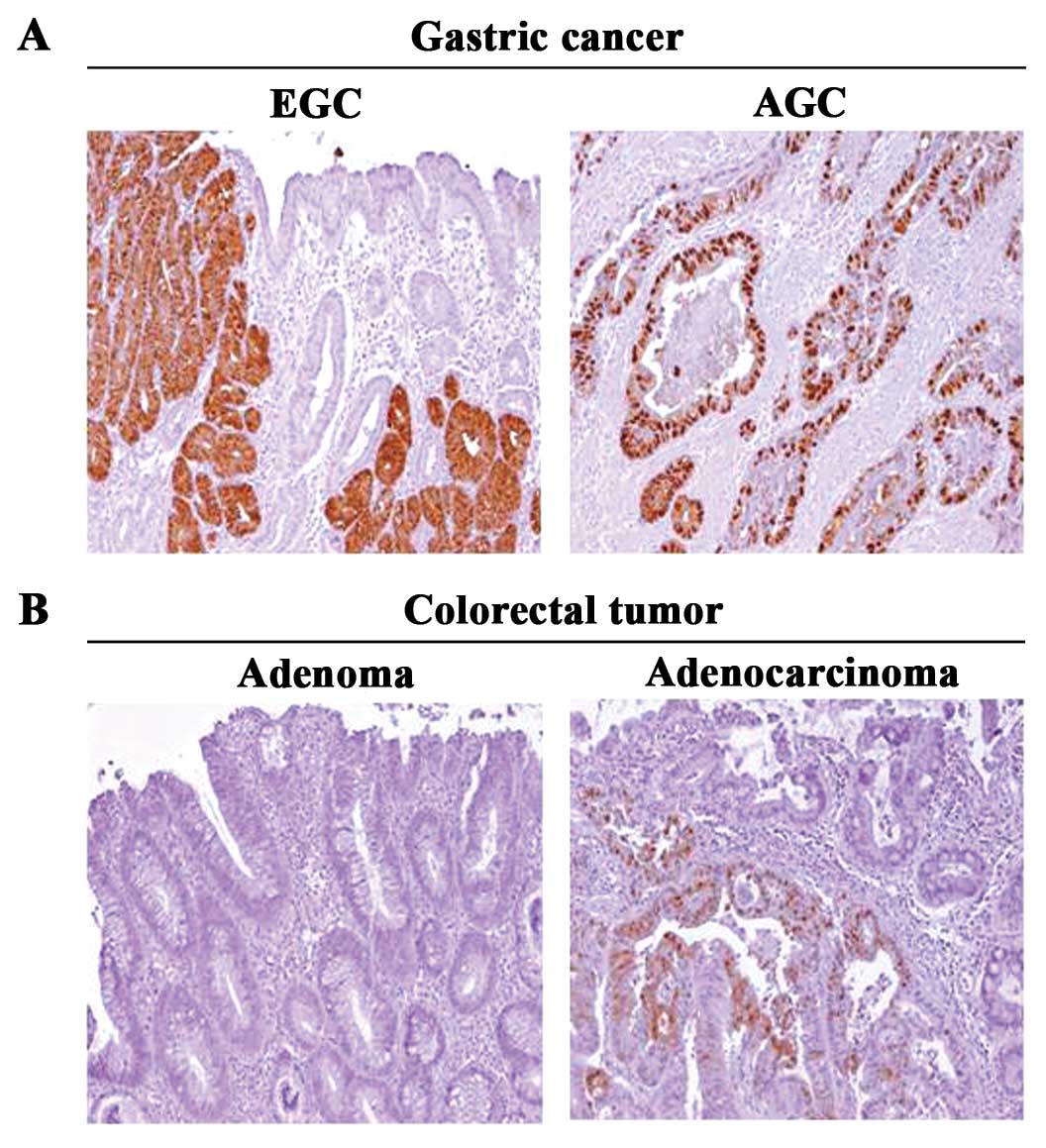
Immunoreactivity of MAGEA by immunohistochemical
staining was examined comparatively between EGC and AGC in the
gastric adenocarcinoma, and between adenoma and adenocarcinoma in
the colorectal cancers.
Among the 41 investigated gastric cancer cases,
MAGEA expression was positive in 25% (5 cases) of EGC and 28.6% (6
cases) of AGC (Table III).
However, among the 39 investigated colorectal cancer cases, MAGEA
was detected only in adenocarcinoma, i.e. 3 1.6% (6 cases)
(Table III).
 | Table IIIMAGEA expression between EGC and AGC
in gastric adenocarcinoma, and colorectal adenoma and
adenocarcinoma. |
Table III
MAGEA expression between EGC and AGC
in gastric adenocarcinoma, and colorectal adenoma and
adenocarcinoma.
| Tissue | Classification | No. of cases | Positive n (%) | P-value |
|---|
| Gastric | EGC | 20 | 5 (25.0) | 0.5704 |
| AGC | 21 | 6 (28.6) | |
| Colorectal | Adenoma | 20 | 0 (0) | 0.0157 |
| Adenocarcinoma | 19 | 6 (31.6) | |
Moreover, MAGEA was not detected in any adjacent
normal tissues in either gastric or colorectal cases.
Representative examples of the MAGEA immunohistochemical staining
in both gastric and colorectal cancer tissues are shown in Fig. 2.
Discussion
In the present study, we examined the expression of
MAGEA genes in gastric and colorectal cancer cell lines and in
related clinical tissues to evaluate their role in
carcinogenesis.
The inherited and acquired genetic and molecular
alterations, leading to gastric and colorectal carcinogenesis, have
been extensively studied over the past 20 years (17,18).
However, the precise molecular alterations that might
differentiate, for example EGC from AGC in gastric cancer, are not
yet clear (19). In addition, the
determinants of malignancy in colorectal cancer are lacking to date
(20). Therefore, it is very
critical to predict the carcinogenesis of gastric and colorectal
cancers, allowing early diagnosis using biopsy samples, and to
select the appropriate therapeutic regimens (19). It has been demonstrated that
prognosis largely depends on whether gastric and colorectal cancers
are diagnosed as EGC or AGC, and colorectal adenoma or
adenocarcinoma, respectively (19,21).
Since the MAGEA genes are expressed exclusively in
tumor cells, except for placental and normal testis tissues, they
may be used as diagnostic markers for detecting malignancy, as
previously suggested (9). However,
the expression profile of the MAGEA genes as applied to gastric and
colorectal carcinogenesis has been insufficiently studied. There
was no significant difference in the MAGEA expression when
comparing EGC and AGC. However, MAGEA expression was not detected
in colorectal adenoma, nor in any adjacent normal colorectal
tissues. In contrast, it was detected in several of the colorectal
adenocarcinoma cases, thus suggesting the potential role of the
MAGEA genes in the colorectal adenoma-adenocarcinoma sequence.
However, the correlation between MAGEA expression and
clinicopathological parameters, such as tumor stage and
differentiation, was not statistically significant (data not
shown).
In conclusion, expression of the MAGEA genes may
play an important role in both gastric and colorectal
carcinogenesis. Furthermore, MAGEA genes can be used as a
diagnostic marker to predict gastric and colorectal
carcinogenesis.
Acknowledgements
This study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Ministry of
Education, Science and Technology (MEST) through the Research
Center for Resistant Cells (R13-2003-009).
References
|
1
|
Lee WC: Breast, stomach and colorectal
cancer screening in Korea. J Med Screen. 13(Suppl 1): S20–S22.
2006.PubMed/NCBI
|
|
2
|
Kim MY, Yim SH, Kwon MS, et al: Recurrent
genomic alterations with impact on survival in colorectal cancer
identified by genome-wide array comparative genomic hybridization.
Gastroenterology. 131:1913–1924. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ushijima T and Sasako M: Focus on gastric
cancer. Cancer Cell. 5:121–125. 2004. View Article : Google Scholar
|
|
4
|
Yasui W, Sentani K, Motoshita J, et al:
Molecular pathobiology of gastric cancer. Scand J Surg. 95:225–231.
2006.
|
|
5
|
Yasui W, Oue N, Ito R, et al: Search for
new biomarkers of gastric cancer through serial analysis of gene
expression and its clinical implications. Cancer Sci. 95:385–392.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suk KT and Kim HS: Biomarkers for
colorectal cancer treatment. Korean J Gastroenterol. 53:68–75.
2009.(In Korean).
|
|
7
|
Park JW, Kwon TK, Kim IH, et al: A new
strategy for the diagnosis of MAGE-expressing cancers. J Immunol
Methods. 266:79–86. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zammatteo N, Lockman L, Brasseur F, et al:
DNA microarray to monitor the expression of MAGE-A genes. Clin
Chem. 48:25–34. 2002.PubMed/NCBI
|
|
9
|
De Plaen E, Arden K, Traversari C, et al:
Structure, chromosomal localization, and expression of 12 genes of
the MAGE family. Immunogenetics. 40:360–369. 1994.PubMed/NCBI
|
|
10
|
Yang B, Wu J, Maddodi N, et al: Epigenetic
control of MAGE gene expression by the KIT tyrosine kinase. J
Invest Dermatol. 127:2123–2128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kufer P, Zippelius A, Lutterbuse R, et al:
Heterogeneous expression of MAGE-A genes in occult disseminated
tumor cells: a novel multimarker reverse transcription-polymerase
chain reaction for diagnosis of micrometastatic disease. Cancer
Res. 62:251–261. 2002.
|
|
12
|
Dhodapkar MV, Osman K, Teruya-Feldstein J,
et al: Expression of cancer/testis (CT) antigens MAGE-A1, MAGE-A3,
MAGE-A4, CT-7, and NY-ESO-1 in malignant gammopathies is
heterogeneous and correlates with site, stage and risk status of
disease. Cancer Immun. 3:92003.PubMed/NCBI
|
|
13
|
Qiu G, Fang J and He Y: 5′ CpG island
methylation analysis identifies the MAGE-A1 and MAGE-A3 genes as
potential markers of HCC. Clin Biochem. 39:259–266. 2006.
|
|
14
|
Jacobs JF, Grauer OM, Brasseur F, et al:
Selective cancer-germline gene expression in pediatric brain
tumors. J Neurooncol. 88:273–280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sigalotti L, Covre A, Zabierowski S, et
al: Cancer testis antigens in human melanoma stem cells:
expression, distribution, and methylation status. J Cell Physiol.
215:287–291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Towbin H, Staehelin T and Gordon J:
Electrophoretic transfer of proteins from polyacrylamide gels to
nitrocellulose sheets: procedure and some applications. Proc Natl
Acad Sci USA. 76:4350–4354. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Panani AD: Cytogenetic and molecular
aspects of gastric cancer: clinical implications. Cancer Lett.
266:99–115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soreide K, Nedrebo BS, Reite A, et al:
Endoscopy, morphology, morphometry and molecular markers:
predicting cancer risk in colorectal adenoma. Expert Rev Mol Diagn.
9:125–137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vecchi M, Nuciforo P, Romagnoli S, et al:
Gene expression analysis of early and advanced gastric cancers.
Oncogene. 26:4284–4294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kidd M, Modlin IM, Mane SM, et al: The
role of genetic markers, NAP1L1, MAGE-D2, and MTA1, in defining
small-intestinal carcinoid neoplasia. Ann Surg Oncol. 13:253–262.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pasche B, Mulcahy M and Benson AB III:
Molecular markers in prognosis of colorectal cancer and prediction
of response to treatment. Best Pract Res Clin Gastroenterol.
16:331–345. 2002. View Article : Google Scholar : PubMed/NCBI
|