Introduction
Endoscopic submucosal dissection (ESD) for the
treatment of large gastric superficial neoplasms is increasing due
to the high en bloc resection rate. However, colorectal ESD
is not widely used to treat large superficial colorectal neoplasms
world-wide presumably because of its technically difficulties and
higher incidence of complications (1). In particular, perforation is the most
severe complication after colorectal ESD and was reported 1.4–10.4%
in previous studies (2,3). The risk of perforation after ESD is
higher than after EMR, although most were small in size and
successfully treated by conventional clips. In contrast, delayed
perforation has been reported as a serious complication after ESD
and it requires emergency surgical treatment. The rate of delayed
perforation is reported to be 0.3–0.7% (4,5). The
reasons for delayed perforation are unknown, but it is reported to
be related to excessive coagulation in the muscularis propria
(2). In addition, one of the
reasons for the high rate of perforations and peritoneal
inflammation is the thinness of the colorectal wall compared with
the gastric wall, resulting in transmural electrocautery injury,
and a large resection size may also contribute to complications,
including delayed bleeding and perforation as well as inflammation
without perforation (6,7). However, patients experienced abdominal
pain, fever, leukocytosis, major signs of peritoneal inflammation
in the absence of frank perforation, which occurs after colorectal
ESD (8,24). These symptoms are similar to
postpolypectomy electrocoagulation syndrome (also known as
postpolypectomy syndrome and transmural burn syndrome). Many
reports have revealed perforations and postoperative hemorrhages,
but peritoneal inflammation without perforation and transmural burn
syndrome after colorectal ESD are not well described. In this
regard, whether prophylactic closure after colorectal ESD prevents
perforation and other complications is not known. Therefore, in the
present study we assessed the effectiveness of prophylactic closure
for a large mucosal defect after colorectal ESD using a
conventional clip and OTSC.
Materials and methods
The present study was designed as a retrospective
cohort study, conducted in the Endoscopy Unit at Kagawa University
Hospital during the period April 2010 to December 2012. A total of
77 patients were referred to our hospital during this period, out
of whom 68 patients with superficial colorectal neoplasm (mean
tumor size of 35.4 mm) were enrolled and assigned to undergo
colorectal ESD. After successful colorectal ESD, closure of
artificial wound were achieved by conventional clips (EZ Clip;
Olympus Co., Tokyo, Japan) for small mucosal defects (tumor size
<30 mm) and over-the-scope clip system (OTSC, Ovesco Endoscopy,
Tübingen, Germany) for: i) a large mucosal defect (tumor size
>30 mm); ii) flexure of the colon; and iii) an inability to
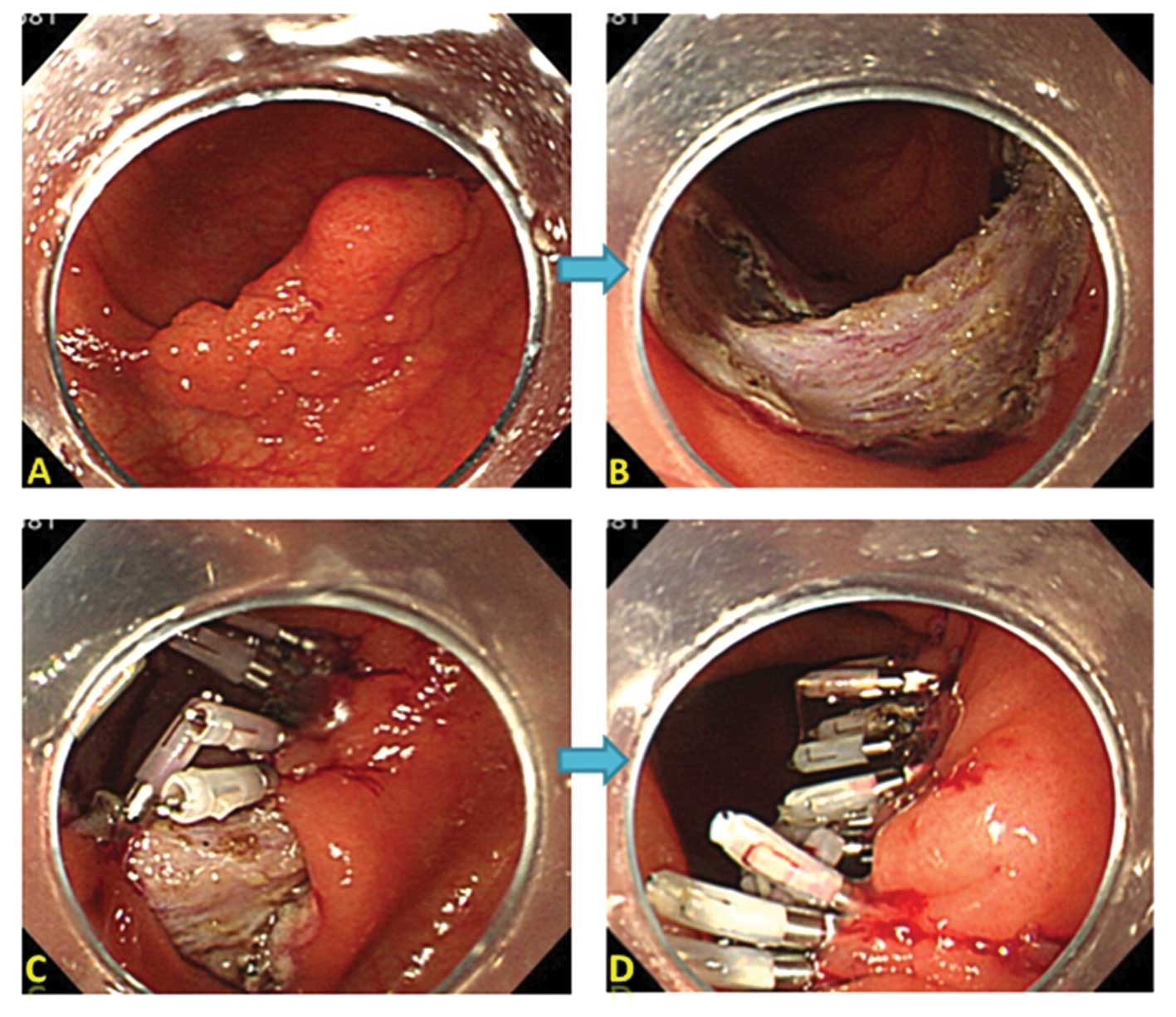
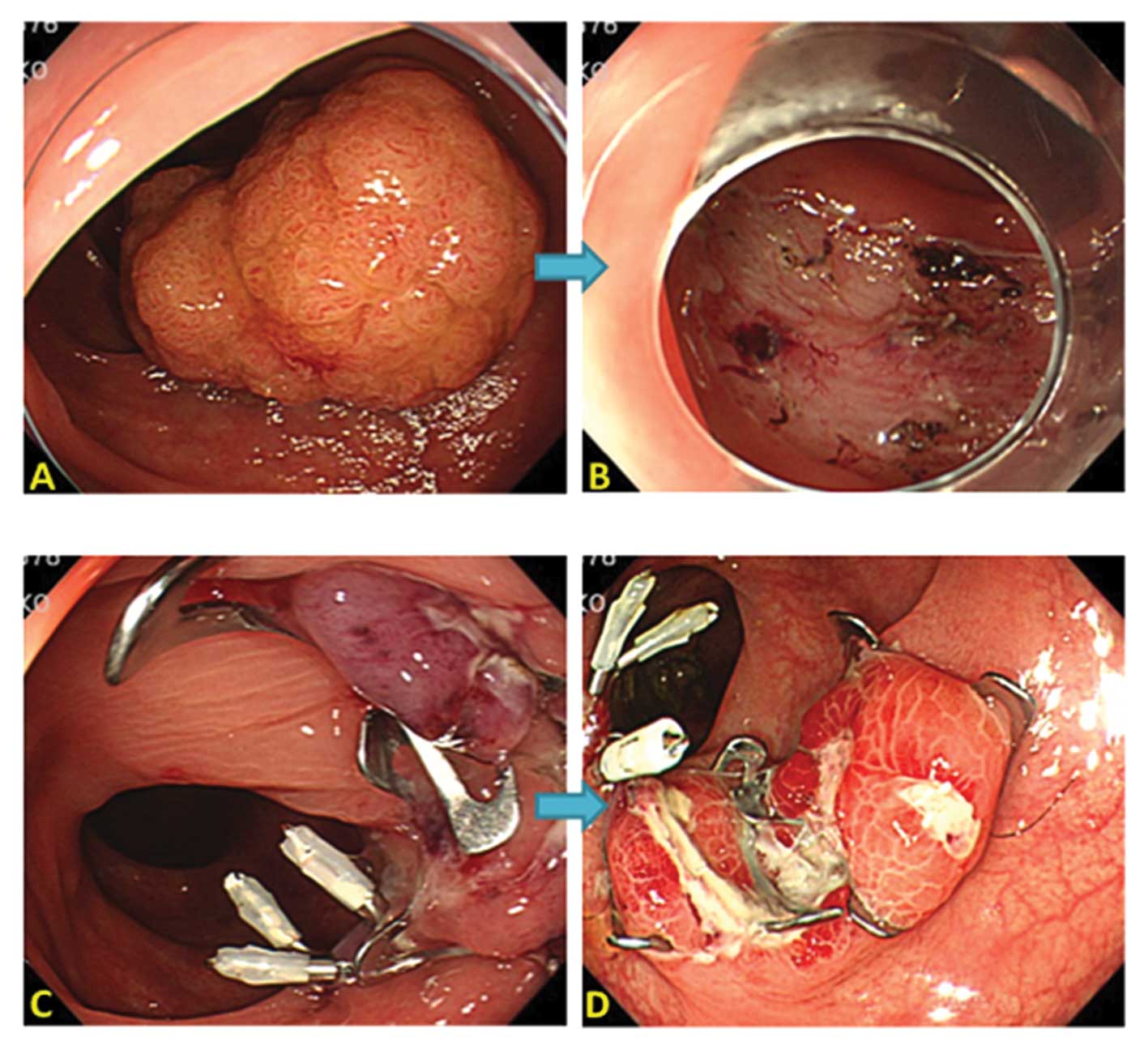
close with conventional clips (Figs.
1 and 2). For OTSC system, we
used 9 mm diameter and 6 mm depth of OTSC caps with atraumatic
blunt teeth.
The patient’s exclusion criteria for the present
study were as follows: i) lesions that could result in en
bloc resection by EMR; ii) lesions located in the anal canal or
at the appendix; iii) tumor size >70 mm; iv) severe organ
failure; v) undergoing anticoagulant therapy; and vi) an inability
to obtain written informed consent. Before colorectal ESD
individual written informed consent were obtained from all
patients.
All ESD procedures were performed by three
experienced endoscopists (H.M, H.K and M.K) in this unit.
The study protocol was approved by the Ethics
Committee of the Kagawa University. The present study has been
registered in the University Hospital Medical Information Network
Clinical Trial Registry (UMIN-CTR) as no. UMIN000007315.
Participants
The depth of invasion was limited to mucosal or SM1,
as estimated endoscopically and by magnification using
chromoendoscopy in most cases (9).
Based on extensive clinicopathological analyses (10–12),
we defined the indications for ESD (13) as non-granular type LSTs >20 mm
and granular type LSTs >30 mm because both have a higher SM
invasion rate and are difficult to treat even by piecemeal EMR
(10,12). Large villous tumors and intramucosal
lesions, recurrent lesions and residual mucosal lesions that showed
a non-lifting sign (14,15) after EMR were also potential
candidates for ESD, with the final decision made by each individual
endoscopist.
Clinicopathological characteristics and
histological assessment
The tumor types were classified according to the
Paris classification (16) and
Kudo’s classification (17) as
follows: type 0-I (protruded) and two subtypes of laterally
spreading tumors (LSTs). The two subtypes of LSTs were either
granular (LST-G) or non-granular (LST-NG). The extent of the tumor
was determined by differences in the color, height, morphological
features and pit patterns between the neoplastic and non-neoplastic
mucosa. The tumor depth was assessed using morphological features.
Tumors that showed evidence of regions of hardness, irregular
nodules, ulceration, or submucosal tumor-like marginal elevation
were suspected to be massive SM >1,000 μm (SM2 or deeper).
The histological classification was performed
according to the Vienna classification of gastrointestinal
epithelial neoplasia (18,19). The extension of tumor cells to the
resected margin was evaluated as following: complete resection
(R0); with the lateral and basal resection margins free of tumor
(where en bloc resection is essential), incomplete resection
(R1); when the tumor extended into the lateral or basal margins, or
not evaluable (Rx); when we were unable to evaluate the
margins.
Premedication before colorectal ESD
The patients were given a low-fiber diet during the
day before ESD and were prescribed 24 mg of sennoside (Pursennid;
Novartis Pharma, Tokyo, Japan) the night before ESD. In the morning
before ESD, Niflec (Ajinomoto Pharmaceuticals, Tokyo, Japan) with
2,000 ml of water was used to clean the bowel. An intravenous
injection was administered immediately before the procedure, which
comprised 20 mg of scopolamine butylbromide (Buscopan; Nippon
Boehringer Ingelheim Co., Ltd., Tokyo, Japan) or 1 mg of glucagon
(Glucagon G Novo; Eisai, Tokyo, Japan) as well as 15 mg of
pentazocine (Pentazin; Daiichi Sankyo, Tokyo, Japan) and 2.5 mg of
midazolam (Dormicum; Astellas Pharma, Tokyo, Japan). Throughout the
procedure, 1.25 mg midazolam was administered as required.
ESD technique
All procedures were performed using a standard
colonoscope (EVIS PCF-Q260AI or GIF H260Z, Olympus Medical Systems
Co., Tokyo, Japan) and carbon dioxide. The disposable distal
attachment (D-201-13404; Olympus) was mounted onto the tip of the
endoscope. A VIO 300D (Erbe Elektromedizin GmbH, Tübingen, Germany)
or ICC200 (Erbe Elektromedizin) was used as a power source for the
electrical cutting and coagulation. During the colorectal ESD
technique in the present study we mainly used the Dual knife
(Olympus Medical Systems) and the insulated tipped (IT) knife
(Olympus Medical Systems). A mixture of 1% hyaluronic acid (MucoUp;
Johnson & Johnson K.K., Tokyo, Japan) and 10% glycerin
(Glyceol; Chugai Pharmaceutical Co., Tokyo, Japan) was used as the
injection liquid.
Treatment protocol
The patients were admitted to our unit the day
before the ESD. After colorectal ESD procedure the patients had a
2-day fasting period, and were discharged from the hospital 7 days
after the colorectal ESD. We checked the laboratory data at
postoperative day 1. If WBC count and CRP were elevated, we checked
the laboratory test at postoperative day 4 as well. All patients
were prescribed cefmetazole (Cefmetazon; Daiichi Sankyo) for 3 days
after colorectal ESD. After dischared from our unit the patients
were followed up for 30 days after the procedure as outpatient
visits to record late adverse events.
Measured outcomes
Postpolypectomy syndrome and transmural burn
syndrome are characterized by a local peritoneal inflammation in
the absence of frank perforation, which occurs after colorectal
ESD. To assess the local peritoneal inflammation, the primary end
point measured for the patient reactions, such as abdominal pain,
recorded initial visual analog scale (VAS) pain score, increases in
WBC count, C-reactive protein levels and body temperature.
We compared the 2 groups with respect to visual
analogue scale (VAS) score 24 h after colorectal ESD. VAS score was
determined based on a scale of 0–10 with 0 equal to no pain and 10
equal to the worse pain imaginable. Abdominal pain was defined as
moderate or greater pain in the region of the ESD site (VAS ≥4).
The body temperature readings were recorded regularly for all
patients during hospitalization.
Adverse events, postoperative bleeding and
perforation after colorectal ESD were evaluated as secondary end
points. Postoperative bleeding was defined as clinical evidence of
bleeding manifested by melena or hematochezia from 0 to 14 days
after the procedure that required endoscopic hemostasis.
Perforation during an ESD procedure was defined as immediate, and
delayed perforation was defined as occurring after the completion
of the ESD procedure.
The number of clips, closure completion rate,
closure procedure time, and closure-related complications,
including patient reactions such as abdominal pain and increases in
white blood cell count, C-reactive protein levels, and body
temperature were counted by recorded video after the procedure and
were also evaluated as secondary end points.
Statistical analysis
The absolute and relative frequencies of qualitative
variables were calculated for each group. Continuous variables were
expressed as the median and range. The continuous variables were
compared using the Student’s t-test if normally distributed or the
Wilcoxon test if not normally distributed. Pearson’s Chi-square
test or Fisher’s exact test was used to analyze the categorical
data to compare the proportions. All P-values were two-tailed, with
P<0.05 being defined as statistically significant. The data
analysis was conducted using JMP version 9.0 (SAS Institute Inc.,
Cary, NC, USA).
Results
Between April 2010 and December 2012, we performed
colorectal ESD for a total of 77 patients with superficial
colorectal neoplasm, out of which 68 patients were referred to our
hospital from other hospital. A total of 68 patients (39 men and 29
women) and mean tumor size were 35.4 mm, 68 patients with
superficial colorectal neoplasms were enrolled in the present study
and were assigned to undergo colorectal ESD with or without
endoscopic closure group. The remaining 9 patients were excluded
from the present study due to following reasons: tumor size >70
mm (n=5); when another endoscopists rather than ours was assigned
to perform the colorectal ESD (n=3); other reasons (inability to
obtain written informed consent) (n=1).
The patient characteristics and tumor
clinicopathological features are summarized in Table I and were not significantly
different among the closure and non-closure group.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Groups | |
|---|
|
| |
|---|
| Characteristics | Closure (n=27 | Non-closure
(n=41) | P-value |
|---|
| Patient |
| Age, mean (range),
years | 67 (37–88) | 71 (51–87) | 0.6513 |
| Gender,
male/female | 18:9 | 21:20 | 0.2076 |
| Tumor |
| Diameter, mean
(range), mm | 32 (16–55) | 35 (18–70) | 0.2188 |
| Location, n | | | 0.2402 |
| Cecum | 8 | 5 | |
| Ascending
colon | 3 | 9 | |
| Transverse
colon | 1 | 4 | |
| Descending
colon | 2 | 0 | |
| Sigmoid colon | 6 | 9 | |
| Upper rectum | 3 | 5 | |
| Lower rectum | 4 | 9 | |
| Macroscopic type | | | 0.7857 |
| Granular type
laterally spreading | 15 | 26 | |
| Non-granular type
laterally spreading | 4 | 6 | |
| 0-Is | 2 | 3 | |
| 0-Isp | 4 | 2 | |
| Post EMR
residual | 1 | 3 | |
| Others | 1 | 1 | |
| Histology | | | 0.1974 |
| Low grade
adenoma | 4 | 0 | |
| Moderately grade
adenoma | 2 | 1 | |
| High grade
adenoma | 7 | 11 | |
| Well
differentiated adenocarcinoma | 10 | 23 | |
| Moderately
differentiated adenocarcinoma | 3 | 4 | |
| Others | 1 | 2 | |
| Depth of
invasion | | | 0.8770 |
| Mucosal | 9 | 18 | |
| SM1 | 1 | 3 | |
| SM2 | 3 | 5 | |
| Vessel
infiltration, n | 3 | 3 | 0.3754 |
| Ulcer presentation,
n | 1 | 2 | 0.8157 |
Tables II and
III summarize the closure
technique results in the closure group as well as the difference
between conventional clips and OTSC clips using group. The closure
group had a longer operation time; however, this difference was not
significantly different among the groups. We used conventional
clips in 18 cases, and OTSC system in 9 cases for closing a large
mucosal defect after colorectal ESD. The mean closure procedure
time is significantly shorter in the conventional clips using group
compared to OTSC using group. The resected tumor diameter tends to
be larger in the OTSC using group than in the conventional clip
using group with no significant differences. Complications related
to prophylactic closure did not occur in either group.
 | Table IIClosure technique results. |
Table II
Closure technique results.
| Closure technique
results |
|---|
| No. of lesions | 27 |
| Technique,
conventional clip:OTSC | 18:9 |
| Closure completion
rate, n (%) | 26/27 (96) |
| Closure procedure
time, median (range), min | 16.6
(8.3–37.7) |
| Total procedure
time, median (range), min | 124.8 (60–250) |
| Closure related to
complication |
|
| Perforation, n
(%) | 0/27 (0) |
| Bleeding, n
(%) | 0/27 (0) |
| Stenosis, n
(%) | 0/27 (0) |
 | Table IIIConventional clips and OTSC technique
results. |
Table III
Conventional clips and OTSC technique
results.
| Conventional clips
(n=18) | OTSCs (n=9) | P-value |
|---|
| Using OTSC and clip
no., median (range) | 8 (4–12) | 1 (1–3) | <0.0001 |
| Closure procedure
time, median (range) | 11.1 (8.3–30) | 25.3
(8.5–37.7) | 0.0013 |
| Resected specimen
diameter, median (range) | 28 (16–45) | 32 (20–55) | 0.0641 |
The outcome data are summarized in Table IV. Abdominal pain was present in 1
patient (3.7%) in the closure group, whereas, abdominal pain was
present in 12 patients (29.3%) in the non-closure group. The
closure group had a significantly lower ratio of abdominal pain
after colorectal ESD compared to non-closure group. However, the
closure group had a lower WBC count (post operative day 1) and
C-reaction protein levels (post operative day 4) with significant
difference between the two groups.
 | Table IVPatient responses and
measurements. |
Table IV
Patient responses and
measurements.
| Findings | Closure group | Non-closure
group | P-value |
|---|
| No. of lesions | 27 | 41 | |
| Abdominal pain, n
(%) | 1/27 (3.7) | 12/41 (24.4) | 0.0046 |
| White blood cell
count (POD1), median (range) | 6170
(1780–11860) | 7320
(3960–14190) | 0.0421 |
| White blood cell
count (POD4), median (range) | 4750
(2210–7870) | 5335
(2950–14670) | 0.1258 |
| C-reactive protein
(POD1), median (range) | 0.45
(0.03–3.28) | 0.43
(0.02–5.35) | 0.5175 |
| C-reactive protein
(POD4), median (range) | 0.17
(0.02–2.07) | 1.01
(0.01–21.86) | 0.0456 |
| Maximum body
temperature, median (range) | 36.9
(36.4–38.5) | 37.1
(36.5–38.9) | 0.0341 |
| Postoperative
bleeding, n (%) | 0/27 (0) | 2/41 (4.9) | 0.1725 |
| Perforation, n
(%) | 0/27 (0) | 1/41 (2.4) | 0.4136 |
| Hospitalization,
mean (range) | 8 (7–11) | 8 (5–19) | 0.6483 |
| Total procedure
time, median (range), min | 128 (60–250) | 105 (45–240) | 0.3330 |
| en bloc R0
resection, n (%) | 26/27 (96) | 41/41 (100) | 0.7551 |
| en bloc
curative resection, n (%) | 23/27 (85) | 36/41 (88) | 0.7551 |
The median hospitalization period was similar among
the groups. In the non-closure group, perforation occurred in one
case and postoperative bleeding occurred in two cases, of which one
bleeding case underwent an emergency endoscopy. Immediately after
colorectal ESD with complete removal one perforation was
recognized, and needed emergency surgery because the endoscopic
treatment was ineffective. The lesion was LST-NG, and located in
the cecum with severe fibrosis. The en bloc R0 resection
rate and en bloc curative resection rate were similar
between the groups.
Discussion
The major complications of colorectal ESD are
postoperative perforation and hemorrhage. To standardize the
colorectal ESD procedure, decreasing the rate of perforation and
other complications are urgently needed. In the present study, in
fact, we experienced the development of abdominal pain, fever,
leukocytosis and peritoneal inflammation in the absence of a frank
perforation. These common clinical complications are also reported
by previous studies after colorectal ESD (8,25).
These symptoms are similar to postpolypectomy electrocoagulation
syndrome.
The majority of patients after colorectal ESD
complain about abdominal pain and tenderness in the region of the
ESD site. Some patients have localized abdominal tenderness,
rigidity, fever, leukocytosis and tachycardia (20), a symptom complex that closely
resembles colonic perforation. Usually patients complained such
symptoms within 12 h after ESD; however, symptoms may appear up to
five days after the procedure (21).
Postpolypectomy syndrome and transmural burn
syndrome are characterized by a local peritoneal inflammation in
the absence of frank perforation, which occurs after colorectal
ESD, and they are related to excessive coagulation in the
muscularis propria (2) and a large
mucosal defect after colorectal ESD. According to previous reports
such events occur in 0.5–1.2% patients undergoing polypectomy
(22,23), however, we experienced these events
more often after colorectal ESD than after polypectomy and EMR.
In the present study, we had 6 patients (14%) with
moderate and severe peritoneal inflammation, and they experienced a
prolonged fasting period, duration of antibiotics administration
and hospitalization. In a previous report, the mean amount of
C-reactive protein 2 days after the ESD was 5.82±12.10 mg/l in
cases with perforation and 1.27±2.00 mg/l in cases without
perforation (8). These results
suggesting that the endoscopic closure was more effective in
preventing inflammatory reactions.
Although various closure devices and methods have
been reported for the closure of artificial wound after ESD
(25,26), it still has greater technical
difficulty. In addition, we could not close large mucosal defects
(tumor size >35 mm) using only conventional clips. Because of
the limitations to commercially available clips, is a low closure
force that is suboptimal for compressing scarred and hardened
tissues (27). The over-the-scope
clip system offers the advantage of capturing nitinol clip loaded
at the tip of the endoscope which can capture leaks and fistula
orifices and compress them constantly until healed (28).
An over-the-scope clip (OTSC) (Ovesco Endoscop) has
been developed for the closure of small mural defects and bleeding
ulcers (29). The OTSC produces
more durable closure than standard endoclips (30) because of its ability to grasp more
tissue, include the entire thickness of the visceral wall and apply
a greater compressive force. We used the over-the-scope clip system
(OTSC, Ovesco Endoscopy) for: i) a large mucosal defect (tumor size
>30 mm); ii) flexure of the colon; iii) excessive coagulation in
the muscularis propria; and iv) an inability to close with
conventional clips. The closure group with OTSC system required
more time for the closure compared with the conventional group. The
reason is that in 5 cases the tumor was located at a sharp bend in
the sigmoid colon, difficult to close a large mucosal defect using
a Twin Grasper (Ovesco Endoscopy). Complications related to the use
of OTSC are perforation (31),
mucosal laceration (32),
postprocedural pain (32), which
did not occur in closure groups of the present study.
The present study shows that endoscopic closure
using conventional clips and the OTSC system was effective in
preventing local peritoneal inflammation and in relieving the
patient’s abdominal symptoms after colorectal ESD. However, the
present study did not demonstrate that endoscopic closure decreased
the occurrence of perforation and postoperative bleeding.
The present study has some limitations. First, the
small patient sample size was a limiting factor. Therefore, we need
more prospective studies involving larger numbers of patients to
establish the effectiveness of the endoscopic closure. Second, this
study was not a prospective randomized study that directly compared
the closure group with the non-closure group. A prospective
randomized study is needed to confirm the efficacy and safety of
this method in the near future. The third limitation of the present
study was the non-standardization of polyps removed by three
experts.
In conclusion, the prophylactic closure efficiently
reduced the inflammatory reaction and abdominal symptoms of
colorectal ESD in patients with large superficial colorectal
neoplasms without increasing adverse effects.
Abbreviations:
|
ESD
|
endoscopic submucosal dissection
|
|
EMR
|
endoscopic mucosal resection
|
|
LST
|
lateral spreading tumor
|
|
CRP
|
C-reactive protein
|
|
WBC
|
white blood cell
|
|
OTSC
|
over-the-scope clip system
|
References
|
1
|
Hurlstone DP, Sanders DS, Cross SS, et al:
Colonoscopic resection of lateral spreading tumours: a prospective
analysis of endoscopic mucosal resection. Gut. 53:1334–1339. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoshida N, Yagi N, Naito Y, et al: Safe
procedure in endoscopic submucosal dissection for colorectal tumors
focused on preventing complications. World J Gastroenterol.
16:1688–1695. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raju GS, Saito Y, Matsuda T, et al:
Endoscopic management of colonoscopic perforations. Gastrointest
Endosc. 74:1380–1388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujishiro M, Yahagi N, Kakushima N, et al:
Outcomes of endoscopic submucosal dissection for colorectal
epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol
Hepatol. 5:678–683. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Isomoto H, Nishiyama H, Yamaguchi N, et
al: Clinicopathological factors associated with clinical outcomes
of endoscopic submucosal dissection for colorectal epithelial
neoplasms. Endoscopy. 41:679–683. 2009. View Article : Google Scholar
|
|
6
|
Anderson ML, Pasha TM and Leighton JA:
Endoscopic perforation of the colon: lessons from a 10-year study.
Am J Gastroenterol. 95:3418–3422. 2000.PubMed/NCBI
|
|
7
|
Onogi F, Araki H, Ibuka T, et al:
‘Transmural air leak’: a computed tomographic finding following
endoscopic submucosal dissection of gastric tumors. Endoscopy.
42:441–447. 2010.
|
|
8
|
Yoshida N, Kanemasa K, Sakai K, et al:
Experience of endoscopic submucosal dissection (ESD) to colorectal
tumor-especially about clinical course of cases with perforation
(Japanese literature with English abstract). Gastroenterol Endosc.
50:1472–1483. 2008.
|
|
9
|
Kashida H and Kudo SE: Early colorectal
cancer: concept, diagnosis, and management. Int J Clin Oncol.
11:1–8. 2006. View Article : Google Scholar
|
|
10
|
Saito Y, Fujii T, Kondo H, et al:
Endoscopic treatment for laterally spreading tumors in the colon.
Endoscopy. 33:682–686. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanaka S, Haruma K, Oka S, et al:
Clinicopathologic features and endoscopic treatment of
superficially spreading colorectal neoplasms larger than 20 mm.
Gastrointest Endosc. 54:62–66. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Uraoka T, Saito Y, Matsuda T, et al:
Endoscopic indications for endoscopic mucosal resection of
laterally spreading tumors in the colorectum. Gut. 55:1592–1597.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saito Y, Uraoka T, Matsuda T, et al:
Endoscopic treatment of large superficial colorectal tumors: a case
series of 200 endoscopic submucosal dissections (with video).
Gastrointest Endosc. 66:966–973. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishiguro A, Uno Y, Ishiguro Y, et al:
Correlation of lifting versus non-lifting and microscopic depth of
invasion in early colorectal cancer. Gastrointest Endosc.
50:329–333. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kobayashi N, Saito Y, Sano Y, et al:
Determining the treatment strategy for colorectal neoplastic
lesions: endoscopic assessment or the non-lifting sign for
diagnosing invasion depth? Endoscopy. 39:701–705. 2007. View Article : Google Scholar
|
|
16
|
Participants in the Paris workshop. The
Paris endoscopic classification of superficial neoplastic lesions:
esophagus, stomach, and colon: November 30 to December 1, 2002.
Gastrointest Endosc. 58:S3–S43. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kudo S, Lambert R, Allen IJ, et al:
Nonpolypoid neoplastic lesions of the colorectal mucosa.
Gastrointest Endosc. 68:S3–S47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schlemper RJ, Riddell RH, Kato Y, et al:
The Vienna classification of gastrointestinal epithelial neoplasia.
Gut. 47:251–255. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dixon MF: Gastrointestinal epithelial
neoplasia: Vienna revisited. Gut. 51:130–131. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Waye JD: The postpolypectomy coagulation
syndrome. Gastro-intest Endosc. 27:1841981. View Article : Google Scholar
|
|
21
|
Waye JD, Lewis BS and Yessayan S:
Colonoscopy: a prospective report of complications. J Clin
Gastroenterol. 15:347–351. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waye JD, Kahn O and Auerbach ME:
Complications of colonoscopy and flexible sigmoidoscopy.
Gastrointest Endosc Clin N Am. 6:343–377. 1996.PubMed/NCBI
|
|
23
|
Nelson DB, McQuaid KR, Bond JH, et al:
Procedural success and complications of large-scale screening
colonoscopy. Gastrointest Endosc. 55:307–314. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Toyonaga T, Mani M, Fujita T, et al:
Retrospective study of technical aspects and complications of
endoscopic submucosal dissection for laterally spreading tumors of
the colorectum. Endoscopy. 42:714–722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sakamoto N, Beppu K, Matsumoto K, et al:
‘Loop Clip’, a new closure device for large mucosal defects after
EMR and ESD. Endoscopy. 40:E97–E98. 2008.
|
|
26
|
Yosuke O, Yutaka S, Taku S, et al: New
closure technique for large mucosal defects after endoscopic
submucosal dissection of colorectal tumors (with video).
Gastrointest Endosc. 75:663–667. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Voermans RP, Vergouwe F, Breedveld P, et
al: Comparison of endoscopic closure modalities for standardized
colonic perforations in a porcine colon model. Endoscopy.
43:217–222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schurr MO, Hartmann C, Ho CN, et al: An
over-the-scope clip (OTSC) system for closure of iatrogenic colon
perforations: results of an experimental survival study in pigs.
Endoscopy. 40:584–588. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kirschniak A, Kratt T, Stuker D, et al: A
new endoscopic over-the-scope clip system for treatment of lesions
and bleeding in the GI tract: first clinical experiences.
Gastrointest Endosc. 66:162–167. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
von Renteln D, Vassiliou MC and Rothstein
RI: Randomized controlled trial comparing endoscopic clips and
over-the-scope clips for closure of natural orifice transluminal
endoscopic surgery gastrotomies. Endoscopy. 41:1056–1061. 2009.
|
|
31
|
Albert JG, Friedrich-Rust M, Woeste G, et
al: Benefit of a clipping device in use in intestinal bleeding and
intestinal leakage. Gastrointest Endosc. 74:389–397. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seebach L, Bauerfeind P and Gubler C:
‘Sparing the surgeon’: clinical experience with over-the-scope
clips for gastrointestinal perforation. Endoscopy. 42:1108–1111.
2010.
|