Introduction
Gastric cancer (GC) is one of the most common causes
of cancer-related death. Every year, 1 million new cases of GC are
diagnosed and 700,000 die of this disease worldwide (1,2). Most
patients with GC are diagnosed with advanced GC and overall
survival remains poor. GC is generally considered to be an
age-related disease and although more than half of GC patients are
≥70 years of age, some studies have revealed that 2.0–8.0% of
patients with GC are ≤40 years of age (3–5).
Although the incidence of advanced GC has steadily decreased
because of recent developments in medical screening, GC in young
people remains a serious problem as routine screening in Japan does
not include people <40 years of age. GC is difficult to diagnose
in young people and is asymptomatic even in the advanced stages of
the disease.
The prognosis for young patients remains
controversial. Clinicopathological features of GC are reported to
differ between younger and older patients and it is thought that
the prognosis of the disease is worse for younger patients due to
delayed diagnosis and more aggressive tumor behavior (6–9).
However, other reports state that tumor staging and prognosis for
younger patients is similar to older patients and is dependent upon
whether or not the patient undergoes a curative resection (5,10,11).
In order to clarify the prognosis of younger patients with GC, we
analyzed the differences in demographic and clinicopathological
characteristics between younger (≤40 years of age) and older
(>40 years of age) GC patients.
Materials and methods
From 1977 to 2006, a total of 3,818 patients with
pathologically confirmed primary gastric adenocarcinoma were
consulted and 3,563 underwent gastric resection at the Department
of Surgery at Kurume University School of Medicine. Patients were
monitored for at least 5 years after surgery every 1–3 months and
examined by computed tomography (CT) scan, ultrasound and upper
endoscopy at least once a year. Patients with diagnoses of squamous
cell carcinoma, adenosquamous cell carcinoma, small cell carcinoma,
carcinoid tumor, lymphoma or gastrointestinal stromal tumors were
excluded.
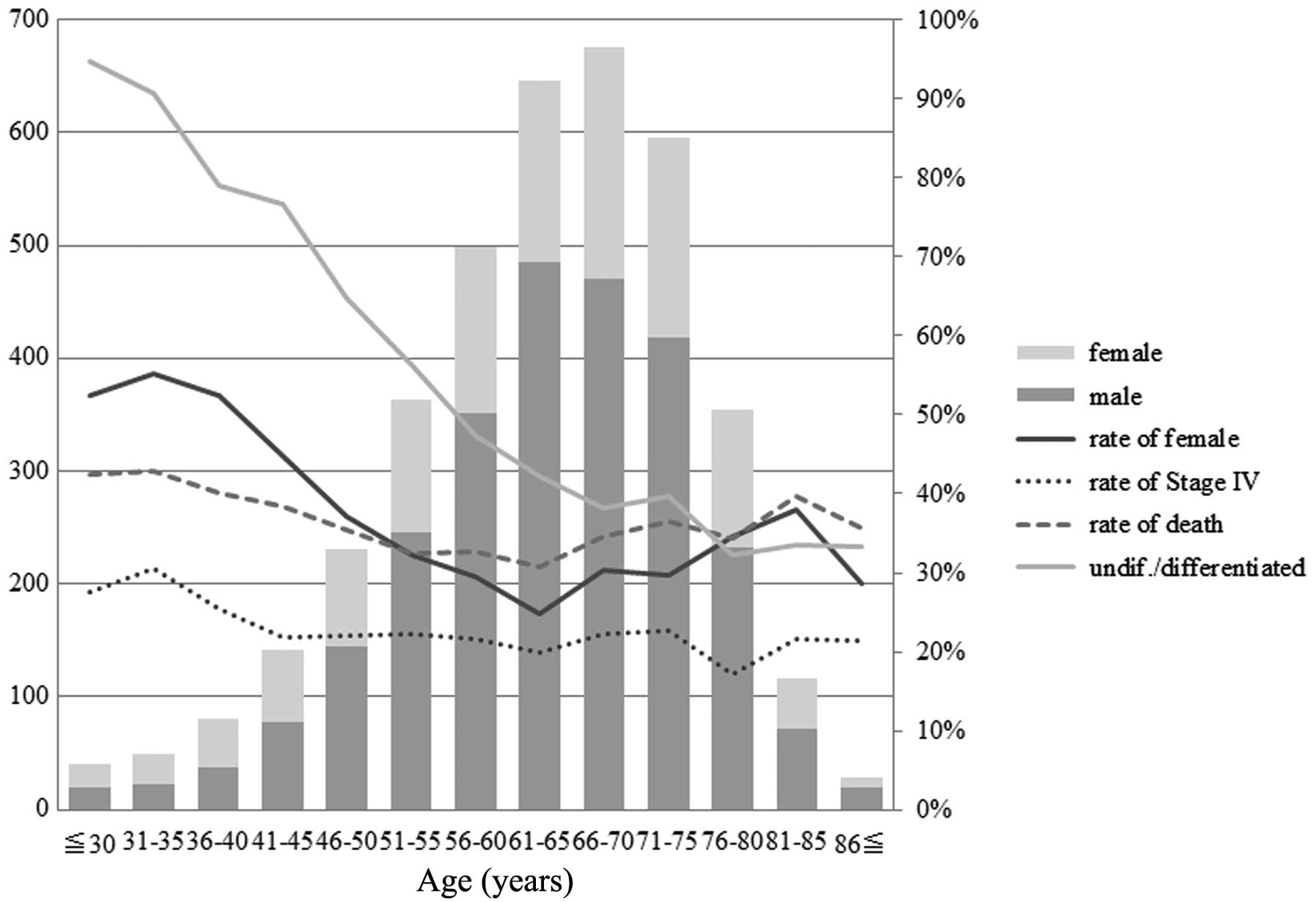
The distribution of gender frequency,
undifferentiated cancer type, stage IV disease and survival at 5
years was examined to define appropriate age groups for comparison
(Fig. 1). These demographic and
clinicopathological features tended to be different between the
patients aged 40 years or less and those aged over 40 years. Thus,
we divided our population into 2 groups according to age with a
cut-off of 40 years. The younger group (YG) was comprised of 169
patients (4.43%) ≤40 years of age while the older group (OG) was
comprised of the remaining 3,649 patients >40 years of age. In
the YG, 79 were male (2.07%) and 90 were female (2.36%), while in
the OG 2,518 were male (66.0%) and 1,131 were female (29.6%). The
median ages were 34.5±4.8 years (range, 20–40 years) for the YG and
64.5±10.0 years (range, 41–92) for the OG. The male-to-female
ratios in the YG and OG were 1:1.14 and 1:0.45, respectively, with
a significantly higher proportion of females in the YG compared
with the OG (P<0.0001). Patient records retrospectively examined
for gender, family history of GC, clinicopathological factors,
surgical procedures and survival. The tumors were staged according
to the guidelines of the Japanese Classification of Gastric
Carcinoma (Japanese Gastric Cancer Association) (12).
Total or distal (subtotal) gastrectomy was performed
according to the tumor size and location, status of resection
margins and lymph node involvement. The standard procedure was a
spleen- and pancreas-preserving D2 or D3 lymph node dissection.
Surgery was considered curative when all resection margins were
clear, there was no or minimal serosal invasion, nodal involvement
was N2 or less, there was an absence of tumor invasion in the last
lymph node resected barrier, and there was no evidence of spread to
the liver, peritoneum, or ovaries at laparotomy. Sixty-nine (40.8%)
of the YG patients and 1,180 (32.3%) of the OG patients were
randomly assigned to various regimens of chemotherapy (P=0.021),
including 5-fluorouracil, cisplatin, mitomycin C, and paclitaxel,
among others. Nine (0.2%) patients in the OG and no patients in the
YG received neoadjuvant chemotherapy (5-fluorouracil/cisplatin
combination).
Clinical records were compared by either Fisher’s
exact test or Pearson’s χ2 test, as appropriate.
Survival rate was calculated by the Kaplan-Meier method, and
univariate analyses used the log-rank test. Factors that were
deemed of potential importance to the univariate analysis were
included in the multivariate analysis using the Cox proportional
hazard model. A P<0.05 was considered a statistically
significant result. Data analysis was performed using the
statistical program JMP® 8 (SAS Institute, Cary, NC,
USA).
Results
The clinicopathological characteristics of the GC
patients are compared in Table I.
There was no statistically significant difference between the YG
and OG regarding family history of GC (5.9 vs. 6.3%, P=0.851). The
proportion of tumor lesions located in the middle third or
involving the whole stomach was significantly higher in the YG than
in the OG (41.4 vs. 28.7%, P=0.0004; 13.6 vs. 9.0%, P=0.044,
respectively), while the occurrence of tumor lesions in the lower
third of the stomach was significantly higher in the OG than that
in the YG (23.7 vs. 36.8%, P=0.0005). There was no statistically
significant difference among the proportion of esophageal or
duodenal invasion or of the occurrence of gastric stump (P=0.556,
P=0.312 and P=0.071, respectively). Regarding macroscopic lesion
types, Borrmann type 4 (diffuse infiltrative) lesions were more
common in the YG compared with the OG (23.7 vs. 9.5%, P<0.0001),
while Borrmann type 0 (superficial), type 1 (mass), type 2
(ulcerative) lesions were more common in the OG compared with the
YG (36.7 vs. 46.2%, P=0.016; 0 vs. 2.4%, P=0.042; 6.5 vs. 11.8%,
P=0.035, respectively). With regard to histological type,
significantly more patients in the YG had poorly differentiated
adenocarcinoma and signet ring cell carcinoma (39.1 vs. 25.4%,
P=0.0002; 44.4 vs. 16.4%, P<0.0001, respectively), while more
patients in the OG had papillary adenocarcinoma and tubular
adenocarcinoma (0 vs. 4.5%, P=0.008; 14.2 vs. 51.1%, P<0.0001,
respectively). Depth of invasion, peritoneal metastasis and stage
of disease status had a significantly greater incidence in the YG
than in the OG (P=0.010, P=0.0014 and P=0.019, respectively). Both
groups had similar distributions with respect to lymph node
metastasis, the mean number of metastatic lymph nodes, hepatic
metastasis and distant metastasis.
 | Table IClinicopathological features of the
gastric cancer patients. |
Table I
Clinicopathological features of the
gastric cancer patients.
| Groups | |
|---|
|
| |
|---|
| Factors | ≤40 year (n=169) n
(%) | >40 year (n=3,649)
n (%) | P-valuea |
|---|
| Gender |
| Male | 79 (46.8) | 2,518 (69.0) | <0.0001a |
| Female | 90 (53.3) | 1,131 (31.0) | |
| Family history of
GC | 10 (5.9) | 229 (6.28) | 0.851 |
| Tumor location |
| Upper third | 34 (20.1) | 790 (21.7) | 0.636 |
| Middle third | 70 (41.4) | 1,047 (28.7) | 0.0004a |
| Lower third | 40 (23.7) | 1,341 (36.8) | 0.0005a |
| Whole stomach | 23 (13.6) | 329 (9.0) | 0.044a |
| Gastric stump | 2 (1.2) | 142 (3.9) | 0.071 |
| Esophageal
invasion | 16 (9.5) | 299 (8.2) | 0.556 |
| Duodenal
invasion | 4 (2.4) | 141 (3.9) | 0.312 |
| Macroscopic type |
| Type 0 | 62 (36.7) | 1,685 (46.2) | 0.016a |
| Type 1 | 0 (0) | 87 (2.4) | 0.042a |
| Type 2 | 11 (6.5) | 431 (11.8) | 0.035a |
| Type 3 | 41 (24.3) | 864 (23.7) | 0.862 |
| Type 4 | 40 (23.7) | 347 (9.5) | <0.0001a |
| Type 5 | 15 (8.9) | 235 (6.4) | 0.211 |
| Histological
type |
| pap | 0 (0) | 163 (4.5) | 0.008a |
| tub | 24 (14.2) | 1,861 (51.0) | <0.0001a |
| por | 66 (39.1) | 943 (25.4) | 0.0002a |
| sig | 75 (44.4) | 600 (16.4) | <0.0001a |
| muc | 4 (2.4) | 82 (2.2) | 0.836 |
| Depth of invasion
(T) |
| 1a | 42 (24.9) | 910 (24.9) | 0.010a |
| 1b | 18 (10.7) | 757 (20.7) | |
| 2 | 10 (5.9) | 242 (6.6) | |
| 3 | 8 (4.7) | 252 (6.9) | |
| 4a | 57 (33.7) | 1,019 (27.9) | |
| 4b | 17 (10.1) | 231 (6.3) | |
| x | 17 (10.1) | 238 (6.5) | |
| LN metastasis
(N) |
| 0 | 96 (56.8) | 2,033 (55.7) | 0.292 |
| 1 | 11 (6.5) | 365 (10.0) | |
| 2 | 10 (5.9) | 337 (9.2) | |
| 3a | 20 (11.8) | 346 (9.5) | |
| 3b | 13 (7.7) | 311 (8.5) | |
| x | 19 (11.2) | 257 (7.0) | |
| No. of metastatic
LNs | 4.0±8.0 | 4.1±9.0 | 0.939 |
| Hepatic metastasis
(H) | 4 (2.4) | 203 (5.6) | 0.072 |
| Peritoneal
metastasis (P) | 33 (19.6) | 414 (11.5) | 0.0014a |
| Distant metastasis
(M) | 20 (11.8) | 341 (9.4) | 0.280 |
| Stage |
| I | 68 (40.2) | 1,765 (48.4) | 0.019a |
| II | 30 (17.6) | 471 (12.9) | |
| III | 23 (13.6) | 628 (17.2) | |
| IV | 48 (28.4) | 782 (21.5) | |
Surgical characteristics are summarized in Table II. In the YG, 152 patients (89.9%)
had surgical resection while 3,411 (93.5%) of the OG patients had
surgical resection (P=0.072); 112 (73.7%) YG patients and 2,728
(80.0%) OG patients had curative resection. The curative resection
rate in the YG tended to be lower than that in the OG (P=0.059).
The proportion of ‘open and closure’ in the YG was higher than that
in the OG, due to the unresectable situation (P=0.037). The
incidence of total gastrectomy and distal gastrectomy were similar
in the YG and OG (P=0.138 and P=0.879, respectively). Proximal
gastrectomy or partial resection (segmental or wedge gastrectomy),
so-called reduction surgery, was frequently performed in the OG due
to the comorbidities or general conditions present in this group
(P=0.041 and P=0.005, respectively). There was a higher proportion
of D2 and D3 lymphadenectomy in the YG compared with the OG
(P=0.0015). Regarding the combined resection, pancreas tail,
spleen, transverse colon and ovary were highly resected in the YG
compared with the OG (P=0.0001, P=0.010, P<0.0001 and
P<0.0001, respectively).
 | Table IISurgical characteristics of the
gastric cancer patients. |
Table II
Surgical characteristics of the
gastric cancer patients.
| Groups | |
|---|
|
| |
|---|
| Factors | ≤40 year (n=169) n
(%) | >40 year
(n=3,649) n (%) | P-valuea |
|---|
| Operation
procedure |
| Total | 52 (30.8) | 936 (25.7) | 0.138 |
| Proximal | 3 (1.9) | 195 (5.3) | 0.041a |
| Distal | 97 (57.4) | 2,116 (58.0) | 0.879 |
| Partial
resection | 0 (0) | 164 (4.5) | 0.005a |
| Bypass | 0 (0) | 49 (1.3) | 0.130 |
| Open and
closure | 8 (4.7) | 82 (2.3) | 0.037a |
| Non-surgery | 9 (5.3) | 107 (2.9) | 0.076 |
| Combined
resection | 45 (26.6) | 789 (21.6) | 0.124 |
| Pancreas | 21 (12.4) | 197 (5.4) | 0.0001a |
| Spleen | 40 (23.7) | 588 (16.1) | 0.010a |
| Liver | 1 (0.6) | 16 (0.4) | 0.770 |
| Gallbladder | 5 (3.0) | 199 (5.5) | 0.159 |
| Large
intestine | 8 (4.7) | 37 (1.0) | <0.0001a |
| Ovary | 3 (1.8) | 4 (0.1) | <0.0001a |
| Diaphragm | 2 (1.2) | 22 (0.6) | 0.351 |
| Others | 1 (0.6) | 10 (0.3) | 0.451 |
|
Lymphadenectomy |
| D0 | 3 (2.0) | 217 (6.4) | 0.0015a |
| D1 | 30 (19.7) | 988 (29.0) | |
| ≥D2 | 119 (78.3) | 2,205 (64.7) | |
| Gastric
resection | 152 (89.9) | 3,411 (93.5) | 0.072 |
| Non-gastric
resection | 17 (10.1) | 238 (6.5) | |
| Curative
resection | 112 (73.7) | 2,728 (80.0) | 0.059 |
| Non-curative
resection | 40 (26.3) | 683 (20.0) | |
| Chemotherapy | 69 (40.8) | 1,180 (32.3) | 0.021a |
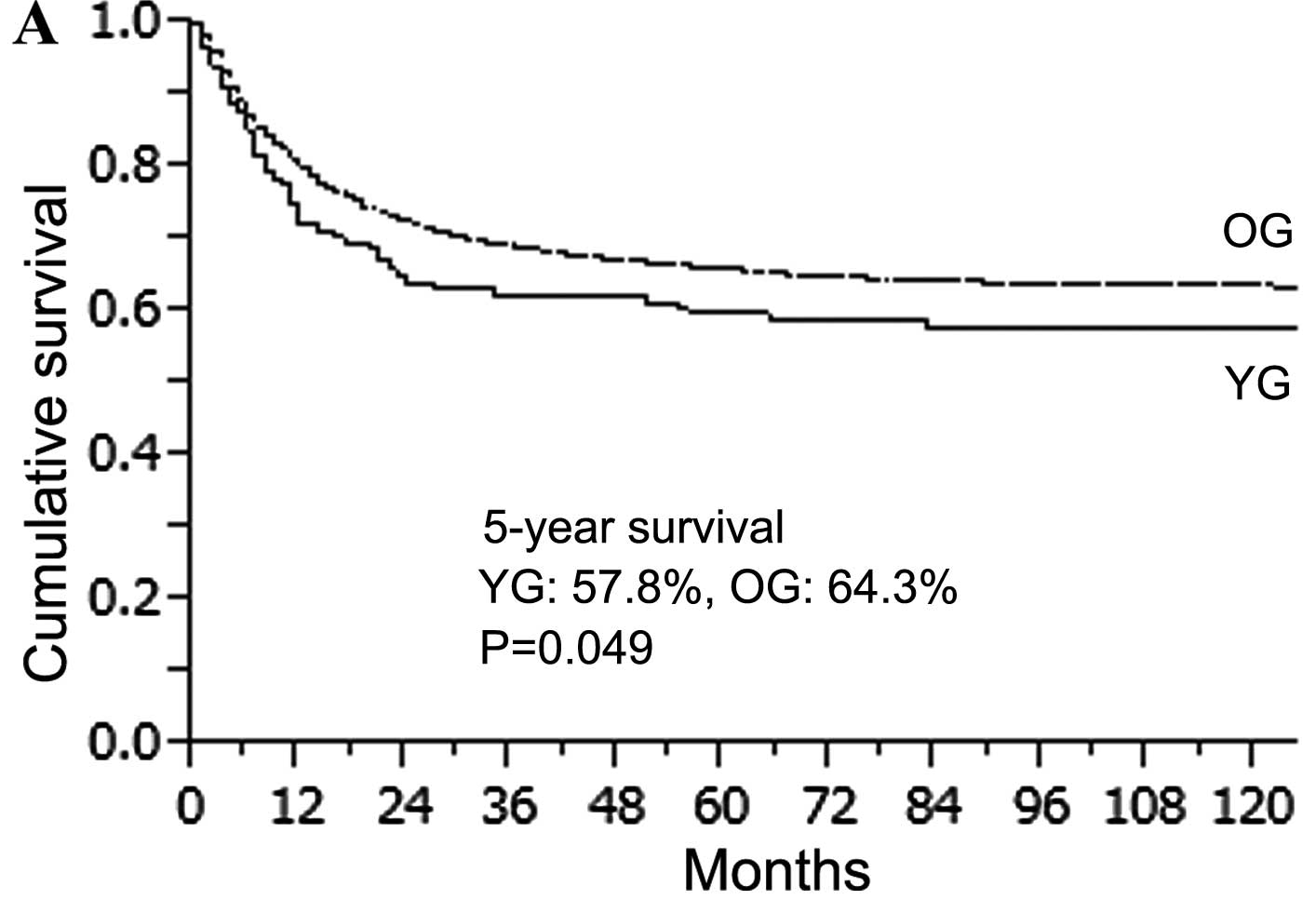
The overall median follow-up was 65.1 months (range
0–256 months). The 5-year overall survival rate in the YG and OG
was 57.8 and 64.3%, respectively (Fig.
2A). The OG survival rate was significantly higher than that of
the YG (P=0.049). However, patients in the YG with curative
resection had a similar 5-year survival rate to those in the OG
with curative resection (88.0 vs. 85.8%, P=0.547) (Fig. 2B). When the 5-year survival rate was
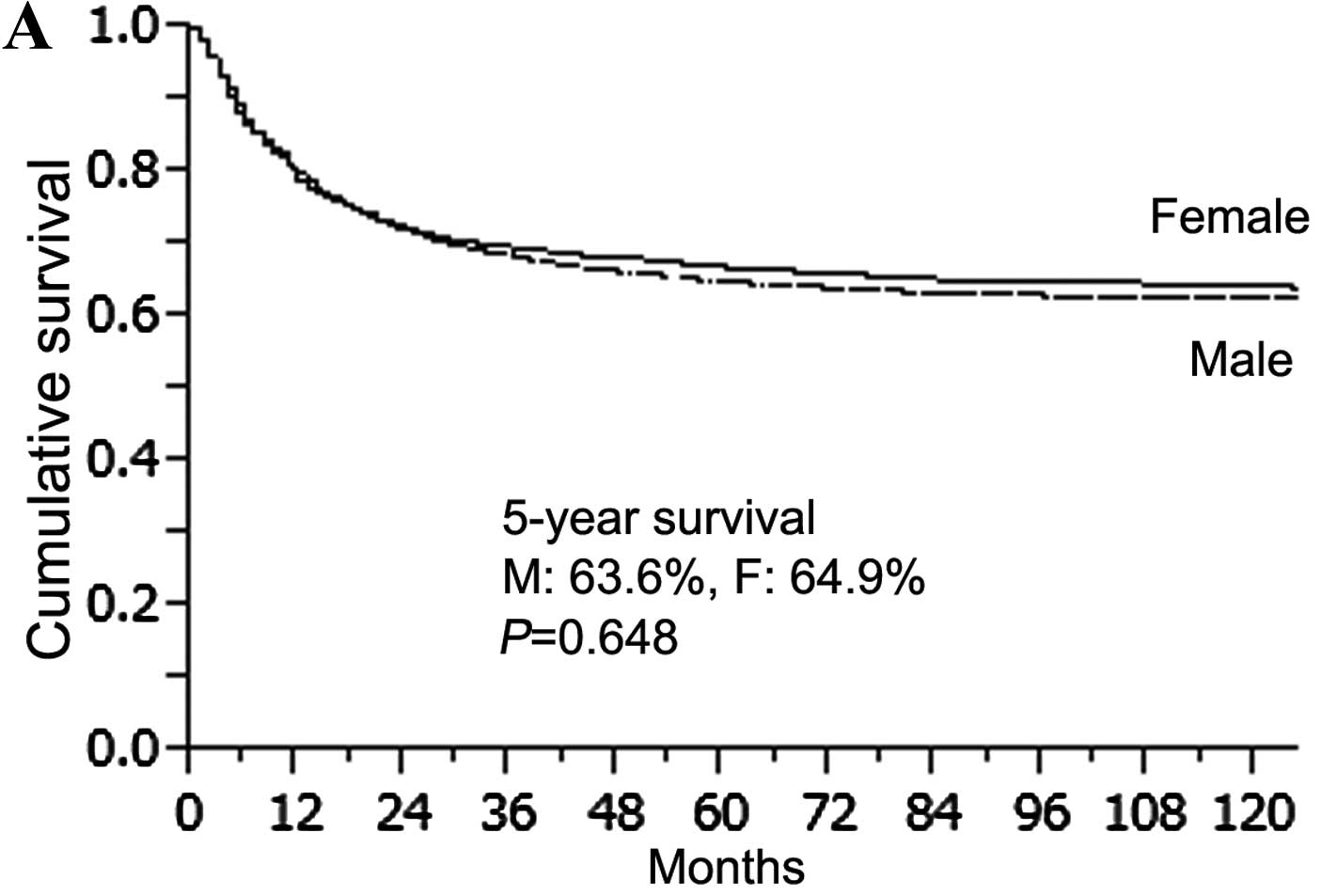
compared with gender, there was no significant difference in
survival for all patients or those in the OG (male 63.6% vs. female
64.9%, P=0.648; male 63.2% vs. female 66.6%, P=0.141) (Fig. 3A and B). However, female patients in
the YG showed a significantly lower survival rate than males in the
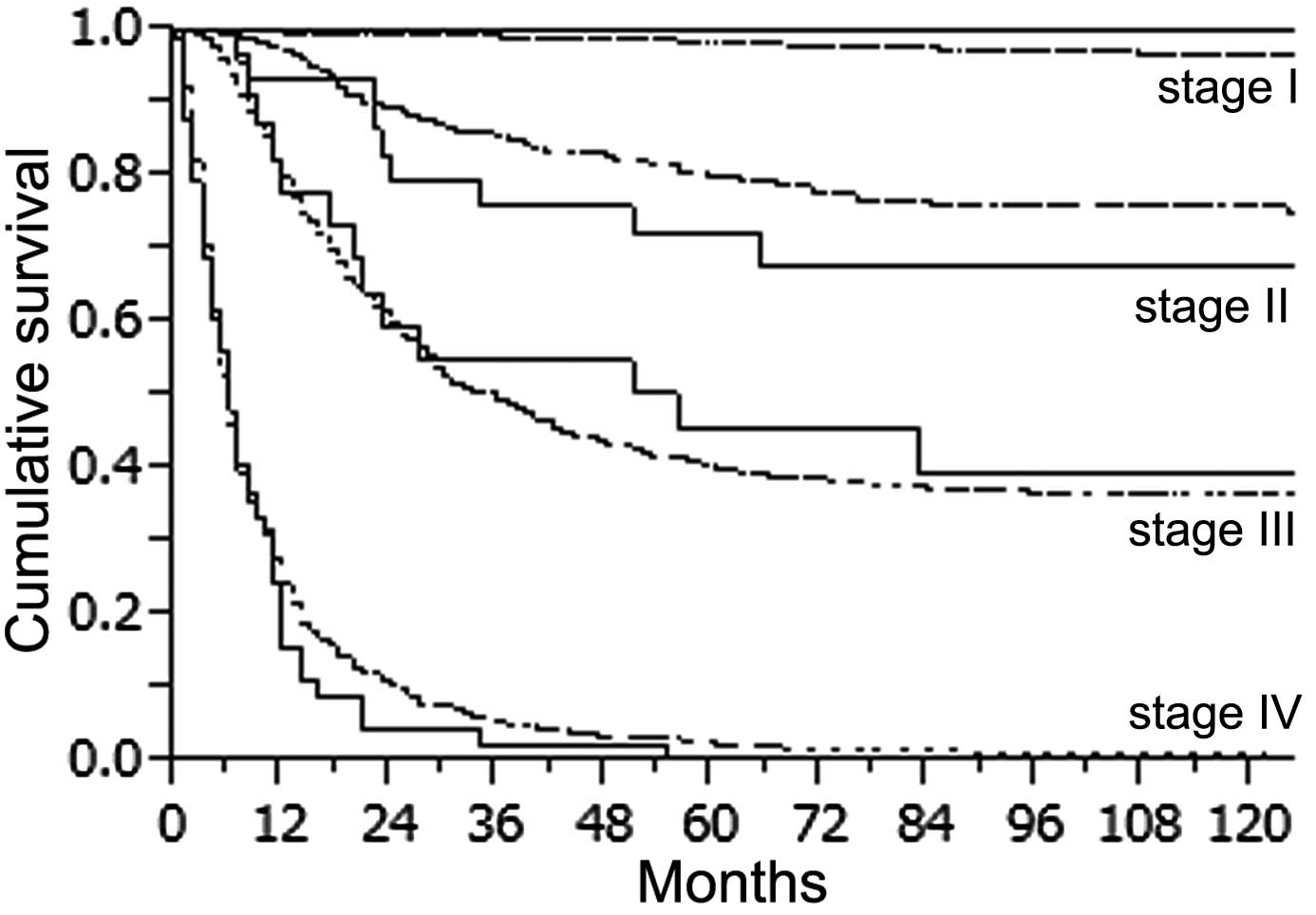
YG (female 44.3% vs. male 73.1%, P=0.0002) (Fig. 3C). When survival was determined
according to the stage of the disease, there was no statistically
significant difference in survival rate for all stages between the
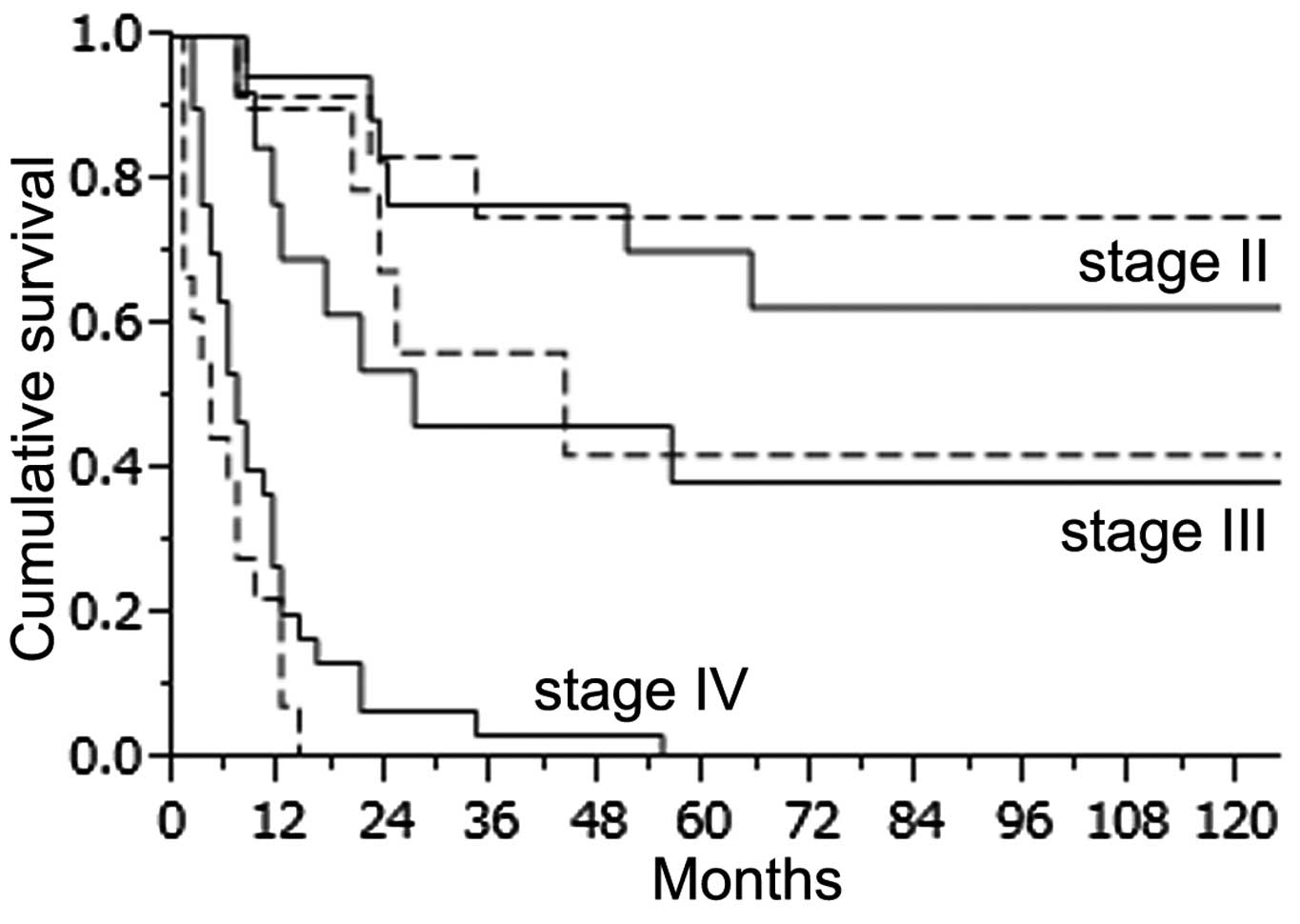
2 groups (Fig. 4). However, stage
IV patients in the YG had a slightly worse outcome than the
pacients in the OG. The 1-year survival rate in the YG and OG was
15.6 and 24.2%, respectively and the 2-year survival rate was 4.4
and 10.4%, respectively. We also compared survival in the YG
determined according to the stage (II–IV) between patients treated
with chemotherapy (CG) and those not treated with chemotherapy
(NCG). The 5-year survival rate of CG and NCG patients were as
follows: stage II (70.3 vs. 75.0%, P=0.646); stage III (38.5 vs.
42.2%, P=0.568). The 2-year survival rate of CG and NCG patients at
stage IV was 6.7 vs. 0% (P=0.612). There was no significant
difference in survival rate for all stages between the 2 groups
(Fig. 5).
Analyses of the prognostic factors for the YG in GC
are presented in Table III.
Macroscopic type, depth of invasion, peritoneal metastasis, distant
metastasis and curative resection emerged as independent prognostic
factors (P=0.014, P=0.041, P=0.001, P=0.018 and P=0.021,
respectively).
 | Table IIIAnalyses for prognostic factors for
gastric cancer; in young patients. |
Table III
Analyses for prognostic factors for
gastric cancer; in young patients.
| Multivariate
analysis |
|---|
|
|
|---|
| Factors | Hazard ratio | 95% CI | P-valuea |
|---|
| Gender |
| Male vs.
female | | | 0.971 |
| Tumor location |
| L, M vs. U | | | 0.917 |
| Macroscopic
type |
| Type 1–2 vs. type
3–4 | 8.684 | 1.451–169.115 | 0.014a |
| Histological
type |
| Differ. vs.
undiffer | | | 0.814 |
| Depth of
invasion |
| T1–2 vs. T3–4 | 3.346 | 1.054–10.838 | 0.041a |
| LN metastasis |
| N0–1 vs. N2–3 | | | 0.175 |
| Hepatic
metastasis |
| H(−) vs. H(+) | | | 0.083 |
| Peritoneal
metastasis |
| P(−) vs. P(+) | 7.229 | 2.241–24.323 | 0.001a |
| Distant
metastasis |
| M(−) vs. M(+) | 4.271 | 1.284–15.842 | 0.018a |
|
Lymphadenectomy |
| D0–1 vs. D2–3 | | | 0.736 |
| Curative
resection |
| No vs. Yes | 6.322 | 2.423–21.255 | 0.021a |
Discussion
GC is usually a disease of the aged, with the mean
patient age ranging between 50 and 70 years. It is thought that GC
results from a combination of environmental factors and an
accumulation of generalized and specific genetic alterations,
consequently affecting primarily older patients after a long period
of atrophic gastritis (13).
Intestinal-type cancers develop as a result of chronic atrophic
gastritis and subsequent intestinal metaplasia is primarily
associated with chronic Helicobacter pylori infection
(13,14). Younger patients have fewer years to
develop intestinal metaplasia, which may partially explain why they
disproportionately present with a higher proportion of diffuse
cancers. The diffuse type of GC is common in young patients with
genetic predisposition (presence of CDH1), one of the major
factors involved in the development of GC (2,15,16).
Although the underlying genetic events are not always known, they
can involve CDH1 germline mutations, which encode an
aberrant form of E-cadherin, resulting in hereditary diffuse GC
(17–19). In this study, 5.9% of the YG
patients with GC had a positive family history of GC and there was
no statistically significant difference compared with the OG
(P=0.851). The proportion of tumor lesions located in the middle
third and involving the whole stomach was significantly higher in
the YG than in the OG, although the presence of tumor lesions in
the lower third of the stomach was significantly higher in the OG
than in the YG. Regarding macroscopic types, Borrmann type 4
lesions were more common in the YG, while Borrmann type 0–2 lesions
were more common in the OG. With regard to histological type, we
found that poorly differentiated adenocarcinoma and signet ring
cell carcinoma were more common in the YG, while more patients in
the OG had evidence of papillary adenocarcinoma and tubular
adenocarcinoma. The macroscopic and histological results presented
here were comparable to other reports (5,20,21).
In the present study, there was a higher proportion
of D2 and D3 lymphadenectomy in the YG compared with the OG. Both
groups had similar distributions with respect to lymph node
metastasis and the mean number of metastatic lymph nodes.
Furthermore, reduction surgeries were frequently performed in the
OG. These results may be due to the comorbidities or general
conditions in the OG. There were 186 patients ≥80 years of age in
this study (D0, 34; D1, 110; D2, 42). In Japan, radical gastrectomy
with extended lymphadenectomy (D2) is commonly employed for GC, as
this procedure results in higher stage-stratified survival compared
to the Western method (22).
However, in elderly patients, limited operations are usually
employed to reduce surgical stress (23). However, other studies found that
postoperative survival was not significantly different following D1
or D2 gastrectomy in GC patients over 80 years of age (24,25).
Some studies report female predominance among young
patients with GC (26–28) and indeed, we found a female-to-male
ratio of 1.14:1 in young patients (0.45:1 in older patients). In
this study, there was no significant difference in the 5-year
overall survival regarding gender in all patients (male 63.6% vs.
female 64.9%, P=0.648). However, females had a lower survival rate
than males in the YG (female 44.3% vs. male 73.1%, P=0.0002). This
predominance of females is considered by some to be due to hormonal
factors, such as the harmful role of estrogens, as well as higher
percentages of estrogen receptor-positive cells in young females
and in patients with poorly differentiated GC (29–31).
The relationship between gender hormones and the prognosis of GC
remains controversial. Further studies are needed to determine
whether gender affects prognosis in younger patients.
The 5-year overall survival rate for the YG and OG
was 59.7 and 65.9%, respectively. Survival in the OG was
significantly higher than that in the YG (P=0.049). In previous
reports, the prognosis of younger patients was poor and the
survival rate was low, particularly in patients with advanced GC
(32–34). Delay in diagnosis and the more
aggressive biological behavior of GC in younger patients have been
suggested as possible causes of poor prognosis (3–5). In
our study, we found higher proportions of T4 invasion, peritoneal
metastasis, distant metastasis and stage IV in young patients.
However, we also found that younger patients have similar outcomes
to older patients when matched for tumor stage. Whether the
prognosis of GC patients undergoing resection is influenced by age
remains unclear, but curative resection is the only chance for
long-term survival for GC patients. Some analysis has indicated
that younger patients undergoing curative resection have a better
prognosis than those who do not undergo the procedure (6–9). Our
study also found that patients in the YG who underwent curative
resection had a similar 5-year survival rate to patients in the OG
who underwent curative resection.
We also compared survival in the YG according to the
stage (II–IV) of CG or NCG patients. In our study, there was no
benefit regarding survival for all stages between the 2 groups.
However, because numerous drugs and regimens have been used in our
institution over a period of 30 years, accurate evaluation was
difficult. The role of chemotherapy in prolonging life, either with
adjuvant or palliative intent, is controversial. There were many
prospective randomized trials for adjuvant chemotherapy after
curative resection and for unresectable cases. Recently, the
large-scale Japanese phase III trial by the Adjuvant Chemotherapy
Trial of S-1 for Gastric Cancer (ACTS-GC) group reported the
superiority of S-1 as an adjuvant chemotherapy over surgery alone
after D2 lymph node dissection (35). Its applicability outside of East
Asia is uncertain and the First-Line Advanced Gastric Cancer Study
(FLAGS) in advanced disease that compared cisplatin and S-1 vs.
cisplatin and fluoropyridines in non-Asian countries was negative
(36). Median survival has
gradually improved, but is still <1 year and standard treatment
remains a matter of debate.
Other studies have suggested various
clinicopathological factors that contribute to poorer survival
outcomes (2,4,5,7,10,29).
In this study, macroscopic type, depth of invasion, peritoneal
metastasis, distant metastasis and curative resection were
independent factors in younger patients for reduced survival by
multivariate analysis. These results suggest that a more aggressive
surgical attitude and early diagnosis should be carried out in
younger patients with GC to achieve curative resection, which may
improve patient outcomes.
In conclusion, this study demonstrated that young
patients with GC who undergo curative resection do not have a worse
prognosis than older patients. Early diagnosis, particularly in
young females, is vital for a successful curative resection and a
better prognosis.
References
|
1
|
Parkin DM, Bray FI and Devesa SS: Cancer
burden in the year 2000. The global picture. Eur J Cancer. 37(Suppl
8): S4–S66. 2001.PubMed/NCBI
|
|
2
|
Santoro R, Carboni F, Lepiane P, Ettorre
GM and Santoro E: Clinicopathological features and prognosis of
gastric cancer in young European adults. Br J Surg. 94:737–742.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Theuer CP, Kurosaki T, Taylor TH and
Anton-Culver H: Unique features of gastric carcinoma in the young:
a population-based analysis. Cancer. 83:25–33. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kulig J, Popiela T, Kolodziejczyk P,
Sierzega M, Jedrys J and Szczepanik AM: Clinicopathological profile
and long-term outcome in young adults with gastric cancer:
multicenter evaluation of 214 patients. Langenbecks Arch Surg.
393:37–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim DY, Ryu SY, Kim YJ and Kim SK:
Clinicopathological characteristics of gastric carcinoma in young
patients. Langenbecks Arch Surg. 388:245–249. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JH, Boo YJ, Park JM, et al: Incidence
and long-term outcome of young patients with gastric carcinoma
according to sex: does hormonal status affect prognosis? Arch Surg.
143:1062–1067. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Llanos O, Butte JM, Crovari F, Duarte I
and Guzmán S: Survival of young patients after gastrectomy for
gastric cancer. World J Surg. 30:17–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maconi G, Kurihara H, Panizzo V, et al:
Gastric cancer in young patients with no alarm symptoms: focus on
delay in diagnosis, stage of neoplasm and survival. Scand J
Gastroenterol. 38:1249–1255. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simsa J, Leffler J, Hoch J, Linke Z and
Pádr R: Gastric cancer in young patients - is there any hope for
them? Acta Chir Belg. 104:673–676. 2004.PubMed/NCBI
|
|
10
|
Park JC, Lee YC, Kim JH, et al:
Clinicopathological aspects and prognostic value with respect to
age: an analysis of 3,362 consecutive gastric cancer patients. J
Surg Oncol. 99:395–401. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramos-De la Medina A, Salgado-Nesme N,
Torres-Villalobos G and Medina-Franco H: Clinicopathologic
characteristics of gastric cancer in a young patient population. J
Gastrointest Surg. 8:240–244. 2004.PubMed/NCBI
|
|
12
|
Japanese Gastric Cancer Association.
Japanese classification of gastric carcinoma. 3rd English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tavares A, Gandra A, Viveiros F, Cidade C
and Maciel J: Analysis of clinicopathologic characteristics and
prognosis of gastric cancer in young and older patients. Pathol
Oncol Res. 19:111–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bani-Hani KE: Clinicopathological
comparison between young and old age patients with gastric
adenocarcinoma. Int J Gastrointest Cancer. 35:43–52. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Correa P: The biological model of gastric
carcinogenesis. IARC Sci Publ. 157:301–310. 2004.PubMed/NCBI
|
|
16
|
Lynch HT, Grady W, Suriano G and Huntsman
D: Gastric cancer: new genetic developments. J Surg Oncol.
90:114–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huntsman DG, Carneiro F, Lewis FR, et al:
Early gastric cancer in young, asymptomatic carriers of germ-line
E-cadherin mutations. N Engl J Med. 344:1904–1909. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suriano G, Oliveira C, Ferreira P, et al:
Identification of CDH1 germline missense mutations
associated with functional inactivation of the E-cadherin protein
in young gastric cancer probands. Hum Mol Genet. 12:575–582.
2003.
|
|
19
|
Suriano G, Yew S, Ferreira P, et al:
Characterization of a recurrent germ line mutation of the
E-cadherin gene: implications for genetic testing and clinical
management. Clin Cancer Res. 11:5401–5409. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Katai H, Sasako M, Sano T and Maruyama K:
Gastric carcinoma in young adults. Jpn J Clin Oncol. 26:139–143.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakamura T, Yao T, Niho Y and Tsuneyoshi
M: A clinicopathological study in young patients with gastric
carcinoma. J Surg Oncol. 71:214–219. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Murayama K, Sasako M, Kinoshita T, Sano T
and Katai H: Optimum resection with lymph node dissection for
gastric cancer. Surgery for Gastrointestinal Cancer. Wanebo HJ:
Lippincott-Raven; Philadelphia: pp. 319–325. 1997
|
|
23
|
Tsujitani S, Katano K, Oka A, Ikeguchi M,
Maeta M and Kaibara N: Limited operation for gastric cancer in the
elderly. Br J Surg. 83:836–839. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Korenaga D, Baba H, Kakeji Y, et al:
Comparison of R1 and R2 gastrectomy for gastric cancer in patients
over 80 years of age. J Surg Oncol. 48:136–141. 1991.PubMed/NCBI
|
|
25
|
Haga Y, Yagi Y and Ogawa M: Less-invasive
surgery for gastric cancer prolongs survival in patients over 80
years of age. Surg Today. 29:842–848. 1999.PubMed/NCBI
|
|
26
|
Grabiec J and Owen DA: Carcinoma of the
stomach in young persons. Cancer. 56:388–396. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Holburt E and Freedman SI: Gastric
carcinoma in patients younger than age 36 years. Cancer.
60:1395–1399. 1987.PubMed/NCBI
|
|
28
|
Mori M, Sugimachi K, Ohiwa T, Okamura T,
Tamura S and Inokuchi K: Early gastric carcinoma in Japanese
patients under 30 years of age. Br J Surg. 72:289–291.
1985.PubMed/NCBI
|
|
29
|
Derakhshan MH, Liptrot S, Paul J, Brown
IL, Morrison D and McColl KE: Oesophageal and gastric
intestinal-type adenocarcinomas show the same male predominance due
to a 17 year delayed development in females. Gut. 58:16–23.
2009.PubMed/NCBI
|
|
30
|
Ebert MP and Malfertheiner P: Review
article: Pathogenesis of sporadic and familial gastric cancer -
implications for clinical management and cancer prevention. Aliment
Pharmacol Ther. 16:1059–1066. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lindblad M, Ye W, Rubio C and Lagergren J:
Estrogen and risk of gastric cancer: a protective effect in a
nationwide cohort study of patients with prostate cancer in Sweden.
Cancer Epidemiol Biomarkers Prev. 13:2203–2207. 2004.PubMed/NCBI
|
|
32
|
Chung HW, Noh SH and Lim JB: Analysis of
demographic characteristics in 3242 young age gastric cancer
patients in Korea. World J Gastroenterol. 16:256–263. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lai JF, Kim S, Li C, et al:
Clinicopathological characteristics and prognosis for young gastric
adenocarcinoma patients after curative resection. Ann Surg Oncol.
15:1464–1469. 2008. View Article : Google Scholar
|
|
34
|
Theuer CP, de Virgilio C, Keese G, et al:
Gastric adenocarcinoma in patients 40 years of age or younger. Am J
Surg. 172:473–476. 1996.PubMed/NCBI
|
|
35
|
Sakuramoto S, Sasako M, Yamaguchi T, et
al: Adjuvant chemotherapy for gastric cancer with S-1, an oral
fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ajani JA, Rodriguez W, Bodoky G, et al:
Multicenter phase III comparison of cisplatin/S-1 with
cisplatin/infusional fluorouracil in advanced gastric or
gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin
Oncol. 28:1547–1553. 2010. View Article : Google Scholar
|