Introduction
Gastrectomy is a risk factor for the long-term
development of gastric stump carcinoma (GSC) (1). The formation of a gastric stump after
surgery is considered a precancerous condition (2). Many factors appear to be involved in
the etiopathogenesis of GSC, including achlorhydria,
hypergastrinemia and biliary reflux, Epstein-Barr virus and
Helicobacter pylori (H. pylori) infection, atrophic
gastritis, and polymorphisms in the genes encoding interleukin-1β
and cyclooxygenase-2 (3–5).
Patients may develop GSC following distal
gastrectomy for benign disease. For example, a large
population-based study showed that patients who underwent gastric
resection for benign disease had an increased risk of cancer in the
gastric remnant ≥30 years later (6). In contrast, a small case series showed
that patients may develop GCS following distal gastrectomy for
cancer (7,8). Early detection of GSC is important,
and strict surveillance for a minimum of 10 years has been
recommended after initial gastrectomy for gastric cancer (9,10). The
incidence of GSC may be higher after the Billroth II procedure than
after the Billroth I procedure (7,11),
because higher amounts of duodenal contents containing bile persist
in the gastric stump after undergoing Billroth II gastrectomy than
after Billroth I gastrectomy (12).
These findings therefore suggest that duodenogastric reflux (DGR)
may be associated with the development of GSC.
H. pylori infection is thought to be a
significant risk factor for gastric cancer (13,14),
with recent epidemiologic evidence suggesting the involvement of
H. pylori in the carcinogenic process (15). In addition, H. pylori
eradication has been associated with a reduced likelihood of
metachronous cancer development and with tumor growth inhibition,
since eradication is associated with the healing of background
gastric mucosa (16).
GSC may also be related to H. pylori
infection. Patients who undergo distal gastric resection have an
increased risk of developing GSC, primarily because of DGR. DGR
correlates with spontaneous eradication of H. pylori
infection (17,18), and facilitates the survival of H.
pylori in the gastric stump, after distal gastrectomy (19).
Proton pump inhibitor (PPI)-based standard therapy
is just as effective for eradicating H. pylori from the
remnant stomach as from the non-surgically treated stomach
(20). Eradication therapy results
in significant improvements in inflammation and atrophy of the
mucosal layer in the remnant stomach after early gastric cancer
surgery (21). These findings
support the role of H. pylori in gastric carcinogenesis and
suggest that H. pylori eradication therapy may prevent the
development of metachronous gastric cancer after gastric resection
(22).
Pylorus-preserving gastrectomy (PPG), including
transectional resection (TR) and local resection (LR), in patients
with early gastric cancer along with lymphatic basin dissection,
has been found to modulate gastric emptying and prevent DGR
(23,24). Moreover, sleeve gastrectomy has been
reported to lead to H. pylori eradication (25). We investigated the prevalence of
H. pylori in the residual stomach after PPG for gastric
cancer, as well as the correlations between H. pylori
positivity and the clinical characteristics and severity of
gastritis in the residual stomach.
Materials and methods
Patients
Patients who underwent PPG, including transectional
resection (TR) and local resection (LR), at least 1 year prior to
this study and were followed up as outpatients in our department
were selected for this study. All patients were positive for H.
pylori infection on endoscopic biopsy before surgery. Subjects
taking H2 receptor antagonists, proton pump inhibitors (PPIs),
non-steroidal anti-inflammatory drugs, antibiotics, or bismuth
salts, and those who had undergone H. pylori-eradication
therapy were excluded. Seventy-two patients agreed to participate
in the trial, with all providing prior, written, informed consent.
The median patient age was 62 years (range 31–85 years).
Forty-eight subjects were male and 24 were female. All patients had
undergone PPG for early gastric cancer. The median time from
surgery was 5 years (range 1–10 years). None of these patients had
experienced local recurrence or metastasis of their original tumor.
Two patients developed GSC 9 years after PPG. Of the 72 patients,
46 had undergone TR (23) and 26
had undergone LR; these 2 groups did not differ in regards to
patient characteristics, including mean age, gender, distribution,
or mean time following surgery.
DGR
The degree of DGR was assessed by using
hepatobiliary scintigraphy, by monitoring pH, and by endoscopic
examination.
Hepatobiliary scintigraphy
Eighteen patients were selected. After an overnight
fast, each patient received an intravenous injection of 37 MBq of
99mTc N-pyridoxyl-5-methyltryptophan
(99mTc-PMT; Japan Medi-Physics, Japan). Patients then
underwent serial hepatobiliary scanning in the sitting position
using a gamma camera, with images taken at 0, 10, 20, 30, 45, 60,
90 and 120 min. A region of interest (ROI) corresponding to the
remnant stomach was outlined in anterior views, and the
radioactivity in each ROI was measured and expressed as a
percentage of the radioactivity at time 0 (i.e., reflux
amount).
Measurement of pH in the remnant
stomach
Before assessment, the pH monitor was calibrated,
i.e., the tip of the probe was introduced into a liquid and
stabilized. The calibrated probe was placed transnasally in the
remnant stomach and taped in place, with measurements started after
assuring that the equipment was correctly placed. The pH was
measured for 24 h, following which, all data from the device were
transferred to a personal computer and analyzed.
Endoscopic examination
All patients underwent endoscopic examination every
6 months following surgery. During upper gastrointestinal
endoscopy, performed after an overnight fast, tissue specimens were
sampled from the greater curvature of the antrum and the
fornix.
Detection of H. pylori
Stool samples of all patients were collected and
analyzed for H. pylori antigen using enzyme immunoassays
(HpSA, Premier Platinum HpSA; Meridian Diagnostics Inc.,
Cincinnati, OH, USA) in accordance with the manufacturer's
instructions. Briefly, diluted fecal samples and a
peroxidase-conjugated polyclonal antibody were added to the
microwells containing polyclonal antibodies to H. pylori and
incubated for 1 h at room temperature. The wells were washed to
remove unbound materials. Substrate was added and the wells were
incubated for 10 min at room temperature. The presence of bound
H. pylori antigens was demonstrated by a change in color
from blue to yellow. A stop solution was added, and
spectrophotometric analysis was performed at 450 nm. Absorbances
<0.140 were considered negative; those from 0.140–0.159 were
considered equivocal; and those >0.160 were considered positive.
In these assays, buffer mixed with inactivated H. pylori
antigen was used as a positive control, and buffer mixed with
preservative was used as a negative control.
Histological assessment of biopsy
specimens
Biopsy specimens from each site of the stomach were
oriented on filter paper and immediately fixed in 10% buffered
formalin. Paraffin-processed sections were cut at 3 levels and
stained with hematoxylin and eosin (H&E). The sections were
examined in a blinded manner by a single pathologist and
specifically assessed for severity of gastritis using the updated
Sydney system. The degree of inflammation, activity, atrophy and
intestinal metaplasia at each site was graded as normal, mild,
moderate or severe.
Statistical analysis
Differences between groups were analyzed using the
Student's t-test, the Mann-Whitney rank sum test, Fisher's exact
test, or the log-rank test, as appropriate. All statistical
analyses were performed using StatView software (SAS Co., Berkeley,
CA, USA), with p-value <0.05 considered to indicate a
statistically significant result.
Ethics
The study was performed according to the Declaration
of Helsinki and approved by the Regional Ethics Committee of
Kanazawa University.
Results
Environment of the remnant stomach after
PPG
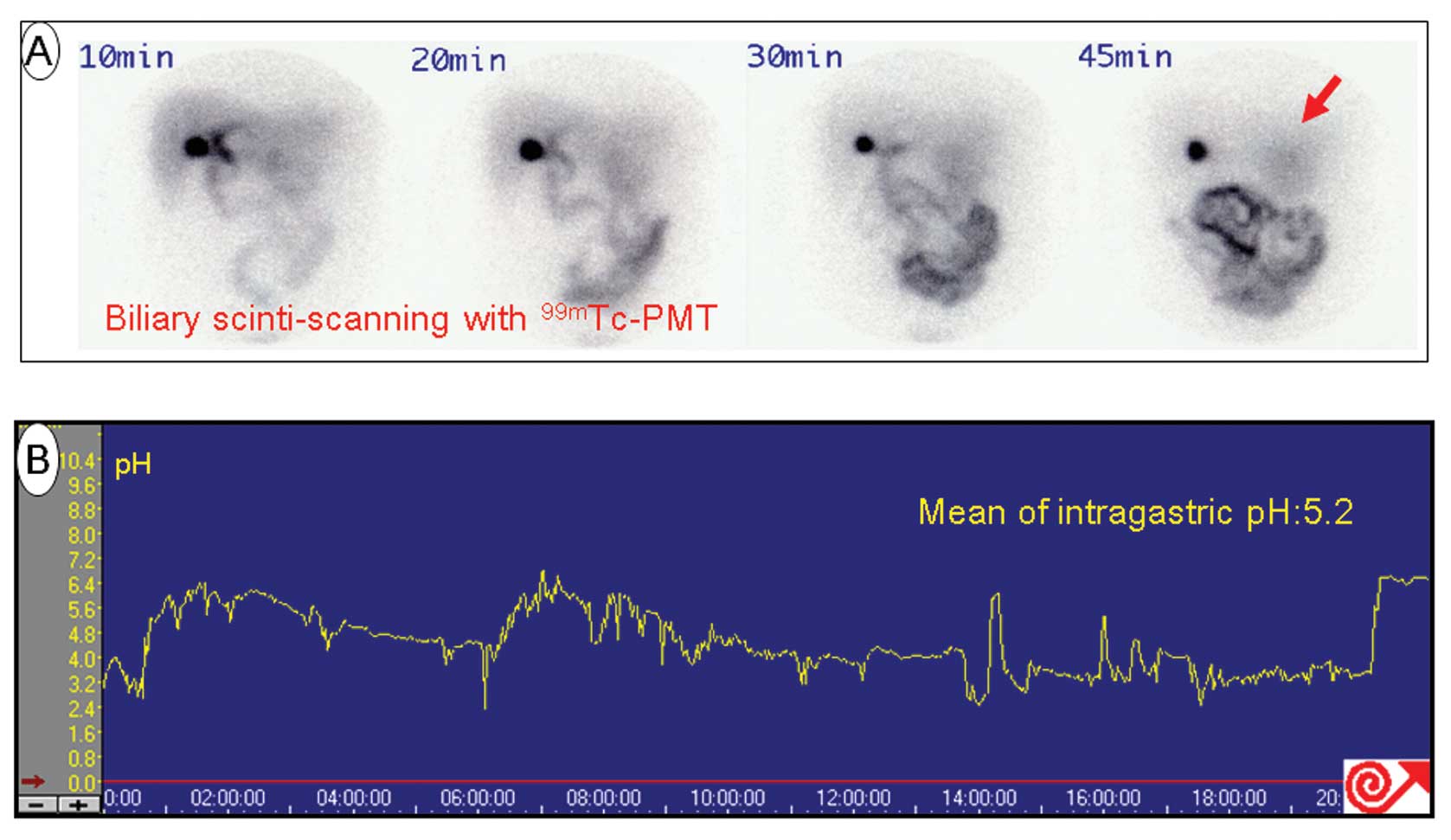
Of the 18 patients tested, none showed detectable
99mTc-PMT in the remnant stomach, indicating an absence
of bile reflux in patients who had undergone PPG at least 1 year
earlier. Representative findings of 99mTc-PMT and pH
monitoring in a post-LR patient showed no DGR and a mean
intragastric pH of 5.2 over 24 h (Fig.
1).
Upper gastrointestinal endoscopy showed that 2 of
the 72 (3%) patients had mild DGR.
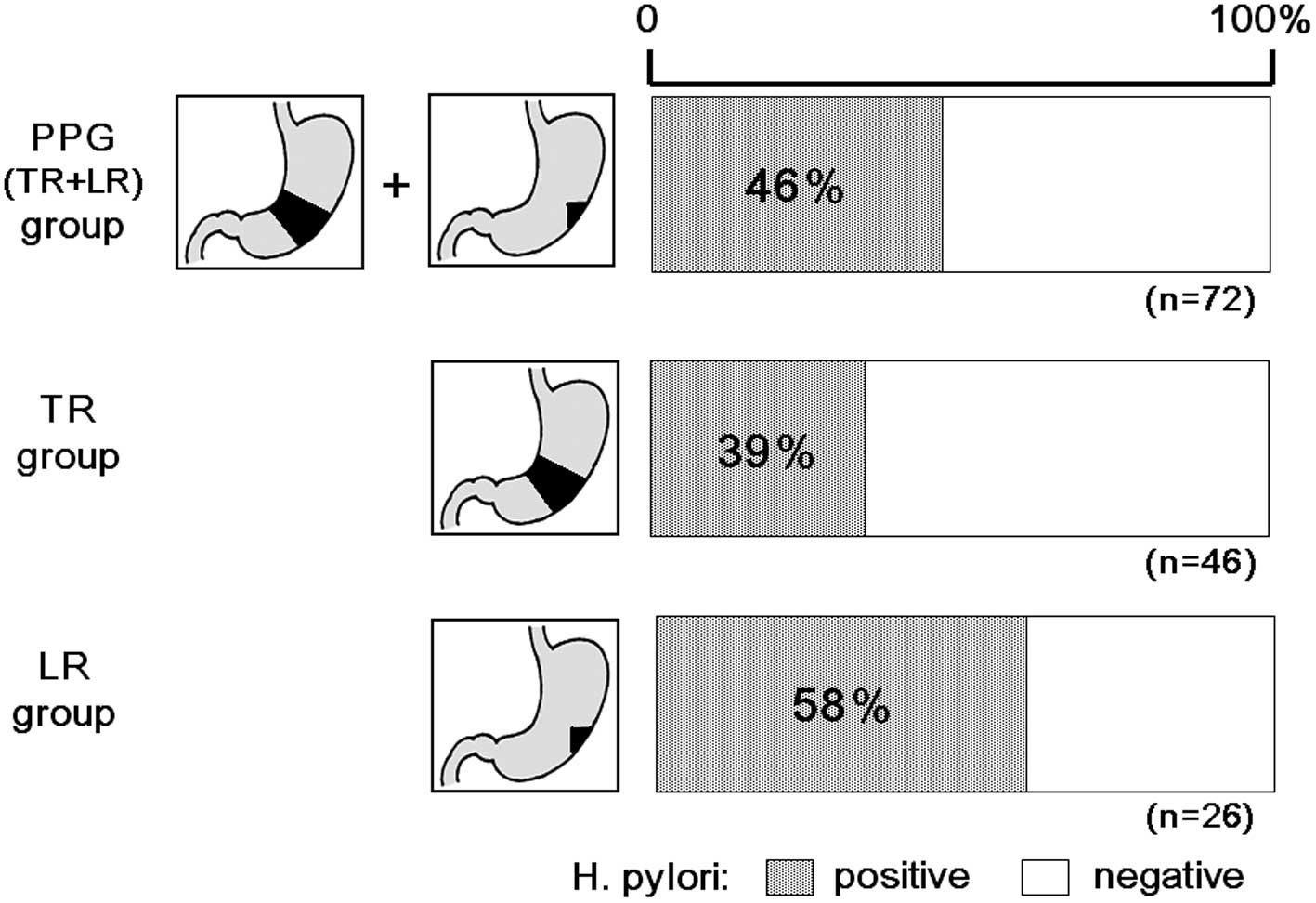
Presence of H. pylori
Of the 72 patients, 33 (46%) were positive for HpSA,
including 18 of the 46 (39%) patients who had undergone TR and 16
of the 26 (58%) patients who had undergone LR. The overall
prevalence of H. pylori infection was significantly lower
after PPG than before PPG (Fig.
2).
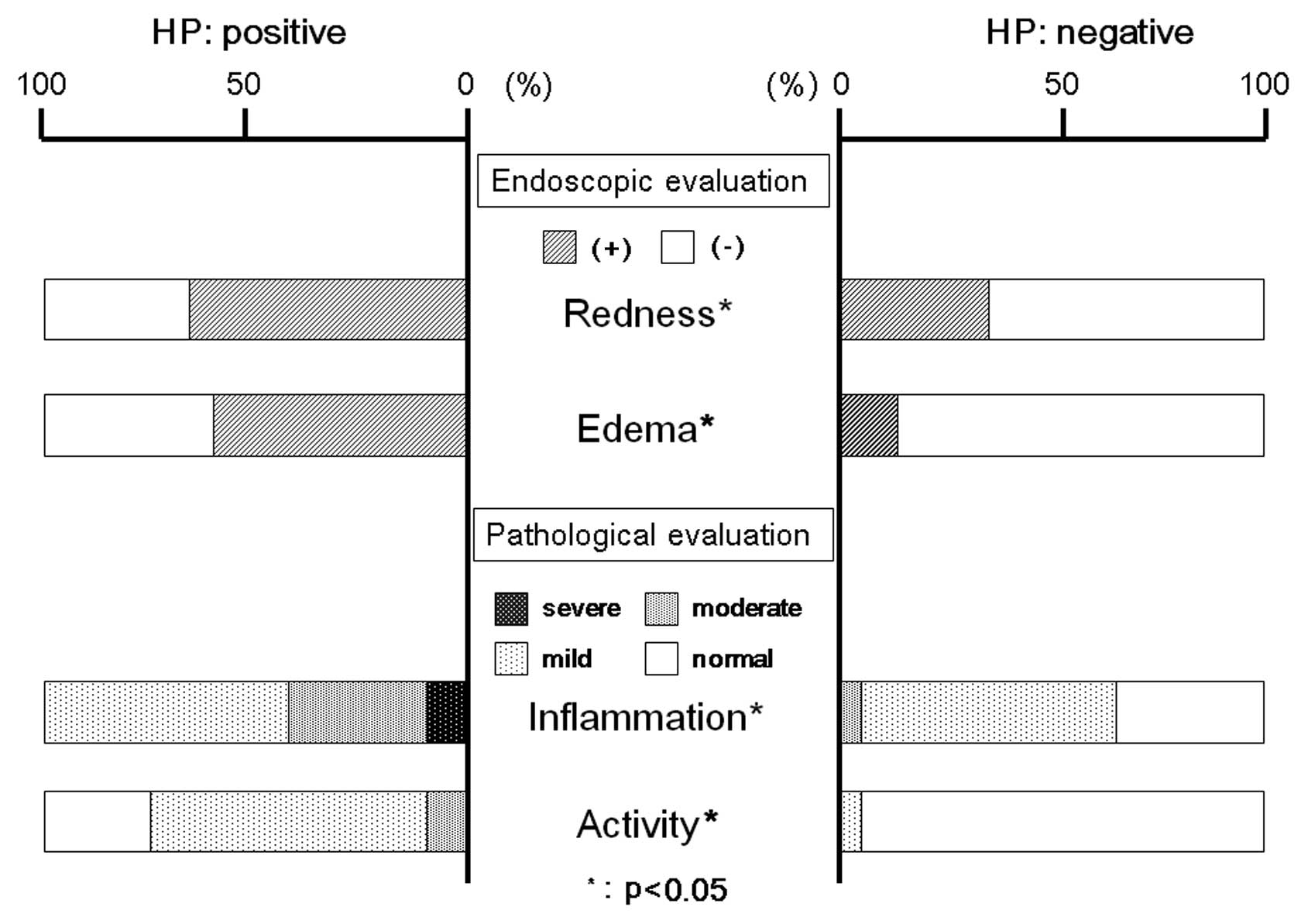
Degree of gastritis
We assessed the relationship between H.
pylori infection and the endoscopic severity of remnant
gastritis. Endoscopically, we observed redness and edema throughout
the remnant gastric mucosa, with the incidence of both being higher
in H. pylori-positive than in H. pylori-negative
patients. Moreover, histological evaluation of the biopsy specimens
taken from the greater curvature of the antrum and the fornix
showed that both inflammation and activity were higher in H.
pylori-positive than in the H. pylori-negative patients
(Fig. 3).
Outcomes in post-PPG patients with and
without H. pylori infection
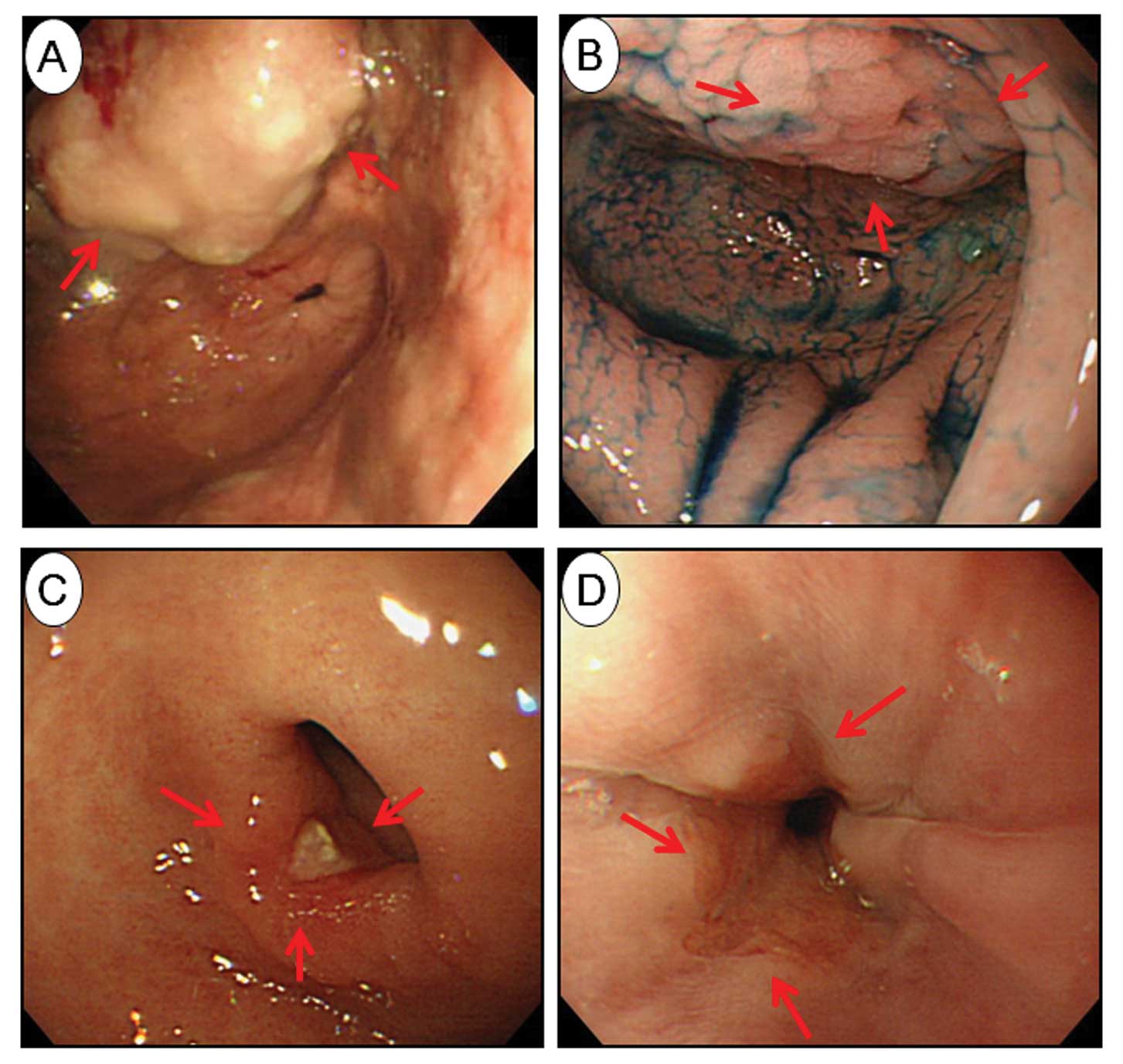
We found that 2 patients had GSC with persistent
H. pylori infection 9 years after PPG (Fig. 4A and B), but both were negative for
bile reflux using 99mTc-PMT.
Of the 33 patients positive for H. pylori
after PPG, 6 underwent H. pylori eradication using
standardized methods, with eradication in all 6 being successful.
One of these 6 patients had an ulcer, and another had reflux
esophagitis after eradication (Fig. 4C
and D), but both were successfully treated with PPIs.
Discussion
To the best of our knowledge, this is the first
study to compare the spontaneous reduction in H. pylori
infection and the presence of mucosal lesions in the remnant
stomach after PPG for gastric cancer.
The presence of residual mucosa in the gastric stump
is considered a risk factor for GSC, as well as a precancerous
condition, independent of indications for surgery (22). Although there are more sensitive
methods for detecting H. pylori, we assessed H.
pylori infection status using stool HpSA tests. The use of
stool tests may explain the lower prevalence of H. pylori
infection among our patients than in a previous study (26). However, bacterial culture and
histopathological examinations are direct but more invasive
methods, whereas the urea breath and stool HpSA tests are both
indirect and non-invasive methods of detecting H.
pylori(27). Comparisons of
urease and stool HpSA tests with histopathology showed that the
sensitivity and specificity of the urease tests were 62.2 and 100%
respectively, whereas the sensitivity and specificity of the stool
tests were 68.9 and 100% respectively, indicating that the results
obtained with biopsy urease and HpSA tests were generally similar
(28), and that stool HpSA tests
are generally useful for the diagnosis of H. pylori
infection.
Because it constitutes a precancerous condition, the
resected stomach offers an opportunity to assess the factors
involved in gastric carcinogenesis. Bile reflux interferes with the
prevalence of H. pylori infection (17,18).
Roux-en-Y reconstruction following distal gastrectomy has been
shown to be superior to Billroth I and II reconstruction in
preventing remnant gastritis, as the former reduces DGR (29). Therefore, Roux-en-Y reconstruction
may reduce H. pylori infection by preventing bile reflux and
gastritis (30). The prevalence of
H. pylori infection was found to be low following jejunal
pouch interposition and Roux-en-Y reconstruction, which prevent
bile reflux, but high in patients who underwent Billroth I and II
reconstruction. DGR was rare in patients who underwent jejunal
pouch interposition and Roux-en-Y reconstruction, but was often
observed in patients who underwent Billroth I and II
reconstruction, suggesting a positive relationship between DGR and
H. pylori infection (19).
We found that the PPG procedure prevented bile reflux, as shown by
99mTc-PMT, and reduced the prevalence of H.
pylori significantly when compared with its prevalence prior to
PPG, providing further evidence of the positive relationship
between DGR and H. pylori infection.
Routine post-surgical treatment with the antibiotic
cephalosporin may affect H. pylori infection. However, an
evaluation of 8 H. pylori-positive colorectal cancer
patients showed that none became H. pylori-negative after
surgery and antibiotic treatment. These findings suggest that
post-operative antibiotic treatment is not associated with
eradication of H. pylori infection after surgery.
We found that 2 patients had residual gastric cancer
with persistent H. pylori infection 9 years after PPG. Of
the 33 H. pylori-positive patients after PPG, 6 underwent
H. pylori eradication using standardized methods, with
eradication in all 6 being successful. One of these patients had
GERD, and another had an ulcer after eradication, but both were
successfully treated with PPIs, suggesting that eradication therapy
may prevent GCS. PPI-based therapy was as effective in eradication
H. pylori in remnant stomachs as in unoperated stomachs,
with eradication therapy significantly decreasing inflammatory cell
infiltration of the mucosal layer (20,31).
In addition, eradication therapy decreased the Ki-67 labeling index
and almost normalized tissue IL-8 levels, suggesting that H.
pylori eradication may reduce the risk of H.
pylori-associated carcinogenesis in patients who have undergone
gastrectomy for early gastric cancer (32).
Our findings suggest that PPG itself may lead to
H. pylori eradication. Further clinical studies on larger
populations are needed to address this issue and to formulate
appropriate guidelines for this relatively new procedure.
Abbreviations:
|
H. pylori
|
Helicobacter pylori
|
|
GSC
|
gastric stump carcinoma
|
|
PPG
|
pylorus-preserving gastrectomy
|
|
PPI
|
Proton pump inhibitor
|
|
DGR
|
duodenogastric reflux
|
References
|
1
|
Sinning C, Schaefer N, Standop J, Hirner A
and Wolff M: Gastric stump carcinoma - epidemiology and current
concepts in pathogenesis and treatment. Eur J Surg Oncol.
33:133–139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Rees BP, Saukkonen K, Ristimäki A, et
al: Cyclooxygenase-2 expression during carcinogenesis in the human
stomach. J Pathol. 196:171–179. 2002.PubMed/NCBI
|
|
3
|
Sitarz R, Maciejewski R, Polkowski WP and
Offerhaus GJ: Gastroenterostoma after Billroth antrectomy as a
premalignant condition. World J Gastroenterol. 18:3201–3206.
2012.PubMed/NCBI
|
|
4
|
Zur Hausen A, van Rees BP, van Beek J, et
al: Epstein-Barr virus in gastric carcinomas and gastric stump
carcinomas: a late event in gastric carcinogenesis. J Clin Pathol.
57:487–491. 2004.PubMed/NCBI
|
|
5
|
Johannesson KA, Hammar E and Staël von
Holstein C: Mucosal changes in the gastric remnant: long-term
effects of bile reflux diversion and Helicobacter pylori
infection. Eur J Gastroenterol Hepatol. 15:35–40. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lagergren J, Lindam A and Mason RM:
Gastric stump cancer after distal gastrectomy for benign gastric
ulcer in a population-based study. Int J Cancer. 131:E1048–E1052.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sowa M, Onoda N, Nakanishi I, et al: Early
stage carcinoma of the gastric remnant in Japan. Anticancer Res.
13:1835–1838. 1993.PubMed/NCBI
|
|
8
|
Kaminishi M, Shimizu N, Yamaguchi H,
Hashimoto M, Sakai S and Oohara T: Different carcinogenesis in the
gastric remnant after gastrectomy for gastric cancer. Cancer.
77:1646–1653. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohashi M, Katai H, Fukagawa T, Gotoda T,
Sano T and Sasako M: Cancer of the gastric stump following distal
gastrectomy for cancer. Br J Surg. 94:92–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaneko K, Kondo H, Saito D, et al: Early
gastric stump cancer following distal gastrectomy. Gut. 43:342–344.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thorban S, Böttcher K, Etter M, Roder JD,
Busch R and Siewert JR: Prognostic factors in gastric stump
carcinoma. Ann Surg. 231:188–194. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lorusso D, Linsalata M, Pezzolla F, et al:
Duodenogastric reflux and gastric mucosal polyamines in the
non-operated stomach and in the gastric remnant after Billroth II
gastric resection. A role in gastric carcinogenesis? Anticancer
Res. 20:2197–2201. 2000.
|
|
13
|
Goodwin CS, Armstrong JA and Marshall BJ:
Campylobacter pyloridis, gastritis, and peptic ulceration. J
Clin Pathol. 39:353–365. 1986. View Article : Google Scholar
|
|
14
|
Matsumoto Y, Marusawa H, Kinoshita K, et
al: Helicobacter pylori infection triggers aberrant
expression of activation-induced cytidine deaminase in gastric
epithelium. Nat Med. 13:470–476. 2007. View
Article : Google Scholar
|
|
15
|
Takahashi S: Long-term Helicobacter
pylori infection and the development of atrophic gastritis and
gastric cancer in Japan. J Gastroenterol. 37(Suppl 13): 24–27.
2002.
|
|
16
|
Uemura N and Okamoto S: Effect of
Helicobacter pylori eradication on subsequent development of
cancer after endoscopic resection of early gastric cancer in Japan.
Gastroenterol Clin North Am. 29:819–827. 2000.
|
|
17
|
Fukuhara K, Osugi H, Takada N, et al:
Duodenogastric reflux eradicates Helicobacter pylori after
distal gastrectomy. Hepatogastroenterology. 51:1548–1550.
2004.PubMed/NCBI
|
|
18
|
Abe H, Murakami K, Satoh S, et al:
Influence of bile reflux and Helicobacter pylori infection
on gastritis in the remnant gastric mucosa after distal
gastrectomy. J Gastroenterol. 40:563–569. 2005.PubMed/NCBI
|
|
19
|
Nakagawara H, Miwa K, Nakamura S and
Hattori T: Duodenogastric reflux sustains Helicobacter
pylori infection in the gastric stump. Scand J Gastroenterol.
38:931–937. 2003. View Article : Google Scholar
|
|
20
|
Matsukura N, Tajiri T, Kato S, et al:
Helicobacter pylori eradication therapy for the remnant
stomach after gastrectomy. Gastric Cancer. 6:100–107. 2003.
|
|
21
|
Kato S, Matsukura N, Matsuda N, Tsuchiya
S, Naito Z and Tajiri T: Normalization of pH level and gastric
mucosa after eradication of H. pylori in the remnant
stomach. J Gastroenterol Hepatol. 23(Suppl 2): S258–S261. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giuliani A, Galati G, Demoro M, Scimò M,
Pecorella I and Basso L: Screening of Helicobacter pylori
infection after gastrectomy for cancer or peptic ulcer: results of
a cohort study. Arch Surg. 145:962–967. 2010.
|
|
23
|
Fujimura T, Fushida S, Kayahara M, Ohta T,
Kinami S and Miwa K: Transectional gastrectomy: an old but renewed
concept for early gastric cancer. Surg Today. 40:398–403. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kitagawa Y and Kitajima M: Diagnostic
validity of radio-guided sentinel node mapping for gastric cancer:
a review of current status and future direction. Surg Technol Int.
15:32–36. 2006.PubMed/NCBI
|
|
25
|
Keren D, Matter I, Rainis T, Goldstein O,
Stermer E and Lavy A: Sleeve gastrectomy leads to Helicobacter
pylori eradication. Obes Surg. 19:751–756. 2009. View Article : Google Scholar
|
|
26
|
Deguchi R, Matsushima M, Suzuki T, et al:
Comparison of a monoclonal with a polyclonal antibody-based enzyme
immunoassay stool test in diagnosing Helicobacter pylori
infection after eradication therapy. J Gastroenterol. 44:713–716.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hooton C, Keohane J, Clair J, et al:
Comparison of three stool antigen assays with the 13C-urea breath
test for the primary diagnosis of Helicobacter pylori
infection and monitoring treatment outcome. Eur J Gastroenterol
Hepatol. 18:595–599. 2006.PubMed/NCBI
|
|
28
|
Ceken N, Yurtsever SG, Baran N, Alper E,
Buyrac Z and Unsal B: Comparison of Helicobacter pylori
antibody detection in stool with other diagnostic tests for
infection. Asian Pac J Cancer Prev. 12:1077–1081. 2011.
|
|
29
|
Osugi H, Fukuhara K, Takada N, Takemura M
and Kinoshita H: Reconstructive procedure after distal gastrectomy
to prevent remnant gastritis. Hepatogastroenterology. 51:1215–1218.
2004.PubMed/NCBI
|
|
30
|
Chan DC, Fan YM, Lin CK, Chen CJ, Chen CY
and Chao YC: Roux-en-Y reconstruction after distal gastrectomy to
reduce enterogastric reflux and Helicobacter pylori
infection. J Gastrointest Surg. 11:1732–1740. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Onoda N, Katsuragi K, Sawada T, et al:
Efficacy of Helicobacter pylori eradication on the chronic
mucosal inflammation of the remnant stomach after distal
gastrectomy for early gastric cancer. J Exp Clin Cancer Res.
24:515–521. 2005.
|
|
32
|
Hamaguchi K, Ogawa K, Katsube T, Konno S
and Aiba M: Does eradication of Helicobacter pylori reduce
the risk of carcinogenesis in the residual stomach after
gastrectomy for early gastric cancer? Comparison of mucosal lesions
in the residual stomach before and after Helicobacter pylori
eradication. Langenbecks Arch Surg. 389:83–91. 2004.
|