Introduction
Lung cancer remains the leading cause of
cancer-related mortality in several countries, including China
(1,2). Although significant improvements have
been made in the diagnosis and treatment of lung cancer, the
prognosis for lung cancer is poor, with an overall five-year
survival rate of approximately 16% (3). Lung adenocarcinoma (AdC) is a common
histological type of lung cancer. In recent years, the frequency of
lung AdC has increased and its prognosis remains poor (4,5). This
high mortality rate is often attributed to the presence of
advanced-stage metastasis, with more than two thirds of patients
showing lymph node involvement and metastasis at the initial
diagnosis. Therefore, to develop effective new strategies for the
prediction, diagnosis and treatment of lung cancer metastasis,
molecular mechanisms controlling metastasis must be identified.
Several groups have successfully used gene
expression profiling techniques and model systems with different
invasive or metastatic ability to identify genes that correlate
with invasiveness or metastatic potential (6–8).
Proteomics, particularly quantitative proteomics, has introduced a
new approach to cancer research which aims at identifying
differential expression proteins associated with carcinogenesis,
providing new opportunities to reveal the molecular mechanism
underlying this disease. Identification of differentially expressed
proteins in lung AdC using proteomics revealed that expression
levels of proteins may have some predictive power for metastasis
and prognosis (9–12). In our lab, comparative proteomic
studies of primary lung AdC with and without lymph node metastasis
suggest that Annexin A3 and Annexin A1 are potential biomarkers for
lymph node metastasis and prognosis in lung AdC (13,14).
However, in the past years, lung AdC proteomic
studies have focused on whole cellular proteomic analysis.
Subcellular proteomic analysis has advantages over whole cellular
proteomic analysis, including its ability to identify low-abundance
proteins that may play a crucial role in tumors and provides a
deeper insight into cellular events as protein abundances can be
revealed on the level of different subcellular compartments and
also protein translocations between different cell parts can be
detected (15,16). The cell membrane possesses a number
of important biological functions, such as signaling transduction
into and out of the cells, ion transport and cell-cell and
cell-matrix interactions and communications (17,18).
Plasma membrane (PM) proteins are known to have implications in
cell proliferation, cell adhesion, cell motility and tumor cell
invasion (19–21) and account for more than two thirds
of currently known drug targets (22,23).
Therefore, the cell membrane is of substantial interest with regard
to various aspects of tumor, from carcinogenic and metastatic
mechanisms to molecular diagnosis and therapeutics. A membrane
proteomic analysis offers unprecedented possibilities for
identification of tumor biomarkers and therapeutic targets and for
understanding carcinogenic mechanisms.
In the present study, isobaric tags for relative and
absolute quantitation (iTRAQ) labeling followed by 2D-LC-MS/MS was
performed to identify differential PM proteins in AdC tissues and
paired normal lung tissues adjacent to tumors. Two different
proteins, caveolin-1 and integrin β1, identified by the
quantitative proteomics, were selected for validation by western
blotting. Furthermore, the clinicopathological significance of
integrin β1 was further evaluated using immunohistochemistry of
paraffin-embedded archival tissue specimens and statistical
analyses. Our data facilitate an understanding of AdC
carcinogenesis and mining biomarkers for the diagnosis and
treatment of this disease.
Materials and methods
Tissue specimens
Twenty cases of fresh primary lung AdCs and paired
paraneoplastic normal lung tissues (PNLTs) adjacent to tumors from
the lung AdC patients undergoing curative surgery were obtained
from the Department of Cardiothoracic Surgery, Xiangya Hospital of
Central South University, China, and stored at −80°C until use. The
patients signed an informed consent form for the study which was
approved by the local Ethics Committee. Two pairs of matched tumor
and normal tissues were used for iTRAQ labeling and eighteen pairs
of matched tumor and normal tissues were used for western blotting.
An independent set of formalin-fixed and paraffin embedded archival
tissue specimens used for immunohistochemistry were obtained from
the Department of Pathology, Xiangya Hospital of Central South
University and included 42 cases of PNLT, 46 cases of without lymph
node metastasis primary AdC (non-LNM AdC) and 62 cases of with
lymph node metastatic (LNM) AdC between January 2004 and May 2006
from the AdC patients undergoing curative surgery. The patients
recruited in this study had not received chemotherapy, radiotherapy
prior to the surgery. The clinicopathological characteristics of
the patients used in the present study are noted in Table I.
 | Table IRelationship between integrin β1
expression and clinicopathological factors in lung
adenocarcinoma. |
Table I
Relationship between integrin β1
expression and clinicopathological factors in lung
adenocarcinoma.
| | Integrin β1 |
|---|
| |
|
|---|
| N | Low (0–2) | Moderate (3–4) | High (5–6) | P-value |
|---|
| Age | | | | | 0.87 |
| <50 | 20 | 7 | 6 | 7 | |
| ≥50 | 88 | 31 | 29 | 28 | |
| Gender | | | | | 0.908 |
| Male | 60 | 25 | 20 | 15 | |
| Female | 48 | 13 | 15 | 20 | |
|
Differentiation | | | | | 0.893 |
| Well | 22 | 8 | 7 | 7 | |
| Moderate | 23 | 8 | 8 | 7 | |
| Poor | 63 | 22 | 20 | 21 | |
| pT stage | | | | | 0.072 |
| T1 | 22 | 11 | 10 | 1 | |
| T2 | 63 | 29 | 33 | 1 | |
| T3–4 | 23 | 4 | 6 | 13 | |
| pN stage | | | | | 0.024a |
| pN0 | 34 | 27 | 6 | 1 | |
| pN1 | 32 | 9 | 21 | 2 | |
| pN2 | 42 | 1 | 8 | 33 | |
| Clinical stage | | | | | 0.016a |
| I | 42 | 30 | 11 | 1 | |
| II | 24 | 6 | 18 | 0 | |
| III–IV | 42 | 2 | 6 | 34 | |
| Recurrence | | | | | 0.000a |
| Negative | 26 | 16 | 7 | 3 | |
| Positive | 82 | 12 | 28 | 32 | |
Purification of PM
PM was purified using sucrose density centrifugation
in combination with aqueous two-phase partition as described by Cao
et al(24). Ten samples were
pooled to purified PM for each AdC and PNLT. The purified PM
fractions were pelleted by centrifugation and frozen at −80°C until
used for protein extraction.
Protein extraction, digestion and
labeling with iTRAQ reagents
The PMs were dissolved in lysis buffer (7 M urea, 2
M thiourea, 65 mM DTT, 0.1 mM PMSF) at 4°C for 1 h and then
centrifuged at 12,000 rpm for 30 min at 4°C. The supernatant was
collected and desalted using 2D Cleanup Kit (Amersham Biosciences).
The protein concentration was determined by 2D Quantification Kit
(Amersham Biosciences). Trypsin digestion and iTRAQ labeling were
performed according to the manufacturer’s protocol (Applied
Biosystems). Briefly, 100 μg protein sample was reduced and
alkylated and then digested overnight at 37°C with 1 mg/ml trypsin
solution and labeled with iTRAQ™ Reagents (Applied Biosystems). The
iTRAQ labeling was labeled with 114 and 116 iTRAQ tags for lung AdC
samples and 115 and 117 iTRAQs for normal lung tissue samples. Four
labeled digests were then mixed and dried using a rotary vacuum
concentrator.
LC-MS/MS
The mixed peptides were fractionated by strong
cation exchange (SCX) chromatography on an LC-20AD HPLC system
(Shimadzu) using a polySulfoethyl column (2.1×100 mm, 5 μm, 300 Å;
The Nest Group, Inc.) as previously described by us (25). Briefly, the mixed peptides were
desalted with Sep-Pak Cartridge (Waters), diluted with the loading
buffer [10 mM KH2PO4 in 25% acetonitrile
(ACN), pH 2.8] and loaded onto the column. Buffer A was identical
in composition to the loading buffer and buffer B was the same as
buffer A except that it contained 350 mM KCl. Separation was
performed using a linear binary gradient of 0–80% buffer B in
buffer A at a flow rate of 200 μl/min for 60 min. The absorbance at
214 and 280 nm was monitored and a total of 30 SCX fractions were
collected along the gradient.
Each SCX fraction was dried down by the rotary
vacuum concentrator, dissolved in buffer C (5% ACN, 0.1% FA) and
analyzed on Qstar XL (Applied Biosystems) as previously described
by us (25). Briefly, peptides were
separated on a reverse-phase (RB) column (ZORBAX 300SB-C18 column,
5 μm, 300 Å, 0.1×15 mm; Micromass) using an LC-20AD HPLC system.
The HPLC gradient was 5–35% buffer D (95% ACN, 0.1% FA) in buffer C
at a flow rate of 0.2 μl/min for 65 min. Survey scans were acquired
from 400–1,800 with up to 4 precursors selected for MS/MS from m/z
100–2,000 using a dynamic exclusion of 30 S. The iTRAQ labeled
peptides fragmented under CID conditions to give reporter ions at
114.1, 115.1, 116.1 and 117.1 Th. The ratios of peak areas of the
iTRAQ reporter ions reflect the relative abundances of the peptides
and, consequently, the proteins in the samples. Larger
sequence-information-rich fragment ions were also produced under
these MS/MS conditions and gave the identity of the protein from
which the peptide originated.
Data processing
The software used for data acquisition was Analyst
QS 1.1 (Applied Biosystems). The software used for protein
identification and quantitation was ProteinPilot™ 3.0 software
(Applied Biosystems). The software compares relative intensity of
proteins present in samples based on the intensity of reporter ions
released from each labeled peptide and automatically calculates
protein ratios and P-values for each protein. The data from
LC-MS/MS analyses were merged and searched against combined human
Swiss-Prot protein sequence database. The following search
parameters were used: iTRAQ 4-plex as the sample type, digestion
with trypsin and cystein alkylation with methyl methane
thiosulfate. The precursor tolerance was set to 150 ppm and the
iTRAQ fragment tolerance was set to 0.2 Da, one of missed cleavages
permitted, fixed and variable modifications as well as the peak
list generating parameters are built-in functions of ProteinPilot.
Identified proteins were grouped by the software to minimize
redundancy. All peptides used for the calculation of protein ratios
were unique to the given protein or proteins within the group and
peptides that were common to other isoforms or proteins of the same
family were ignored. The protein confidence threshold cutoff was
set to 1.3 (unused) with at least one peptide above the 95%
confidence level. The average iTRAQ ratios from the two experiments
were calculated for each protein. In addition, false discovery rate
for the protein identification was calculated by searching against
a reversed database.
Bioinformatics analysis
Predictions for putative transmembrane domains
(TMDs) in all identified proteins were carried out using the
transmembrane hidden Markov model (TMHMM) algorithm available at
http://www.cbs.dtu.dk/services/TMHMM
(26). The average hydropathy for
identified proteins and peptides was calculated using the ProtParam
software available at http://www.expasy.org (27). Proteins with positive grand average
of hydropathicity (GRAVY) values were considered to be hydrophobic
and those with negative values, hydrophilic.
Differential protein validation
Eighteen pairs of matched lung AdC and normal lung
tissues were used for western blotting. Briefly, 50 μg of lysates
were separated by 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to a PVDF membrane.
Blots were blocked with 5% nonfat dry milk for 2 h at room
temperature and then incubated with primary anti-caveolin-1, or
anti-integrin β1 antibody overnight at 4°C, followed by incubation
with a horseradish peroxidase-conjugated secondary antibody
(1:3,000; Amersham Biosciences) for 1 h at room temperature. The
signal was visualized with an ECL detection reagent and quantities
by densitometry using Image J software (http://rsb.info.nih.gov/ij). β-actin was detected
simultaneously as a loading control.
Immunohistochemical analysis
Immunohistochemistry was performed on formalin-fixed
and paraffin-embedded tissue sections using a standard
immunohistochemical technique. Briefly, 4 μm of tissue sections
were deparaffinized, rehydrated and treated with an antigen
retrieval solution (10 mmol/l sodium citrate buffer, pH 6.0). The
sections were incubated with anti-integrin β1 antibody (1:40)
overnight at 4°C and were then incubated with 1:1,000 dilution of
biotinylated secondary antibody followed by avidin-biotin
peroxidase complex (DAKO) according to the manufacturer’s
instructions. Finally, tissue sections were incubated with
3′,3′-diaminobenzidine (Sigma-Aldrich) until a brown color
developed and counterstained with Harris modified hematoxylin. In
negative controls, primary antibodies were omitted.
Immunostaining was blindly evaluated by two
investigators in an effort to provide a consensus on staining
patterns by light microscopy. A quantitative score was performed by
adding the score of staining area and the score of staining
intensity for each case to assess the expression levels of the
proteins as previously described by us (28). A combined staining score of ≤2 was
considered to be low staining (no expression), a score between 3
and 4 was considered to be moderate staining (expression), and a
score between 5 and 6 was considered to be strong staining (high
expression).
Statistical analysis
All statistical analyses were performed using SPSS
13.0 Software. The significant difference integrin β1 expression
between the tumor and normal tissues and primary and metastatic
tumors was determined by using the Mann-Whitney U test. Significant
differences between the expression of those two factors and
clinical variables, including age, gender, histologic type/grade,
primary tumor (T) stage, regional lymph node (N) metastasis,
clinical stage and recurrence, were compared by the Mann-Whitney U
test or ANOVA test. All patients underwent postoperative
chemotherapy and were followed-up by telephone to obtain the
information of patient outcome. The follow-up period lasted up to
60 months. Relapse-free duration was calculated from the time of
surgery to the time of first recurrence after surgery. Overall
survival was calculated from the time of surgery to the time of
death. Mortality due to lung AdC was considered as outcome;
mortality due to other causes was censored and the missing values
were replaced by the series mean method. Relapse-free probability
curves and overall survival curves were obtained by the
Kaplan-Meier method and log-rank testing was used to evaluate the
statistical significance of differences. Cox regression analysis
was used to evaluate the prognostic significance of
clinicopathological factors. A difference of P<0.05 was
considered statistically significant.
Results
Identification of differentially
expressed proteins in lung AdC and normal lung tissue using
iTRAQ-2D-LC-MS/MS
The 2D-LC-MS/MS analysis resulted in the
identification of 353 proteins (data not shown) in iTRAQ labeling
experiment, with ≥95% confidence, using one or more peptides. We
also analyzed identified proteins on the basis of subcellular
location and predicted TMDs. Among the proteins with their location
annotated, proteins located on PM are known as PM proteins. Of the
353 identified proteins by MS/MS analysis, 224 (63.5%) proteins are
PM or PM-related proteins. Of these, 91 (25.8%) are predicted to
have one or more TMDs. The GRAVY values of identified PM proteins
range from −1.165 to 1.027.
In the present study, the proteins that met the
following criteria were considered as differential proteins between
the two types of tissues: i) proteins were identified based on ≥2
peptides in the iTRAQ labeling experiments; ii) proteins were
quantified with at least two peptides; iii) P-value of identified
proteins <0.05; and iv) proteins showed an averaged
ratio-fold-change ≥1.5 or ≤0.66. According to these criteria, a
total of 45 differentially expressed proteins were found in the two
types of tissues, 21 proteins upregulated and 24 downregulated
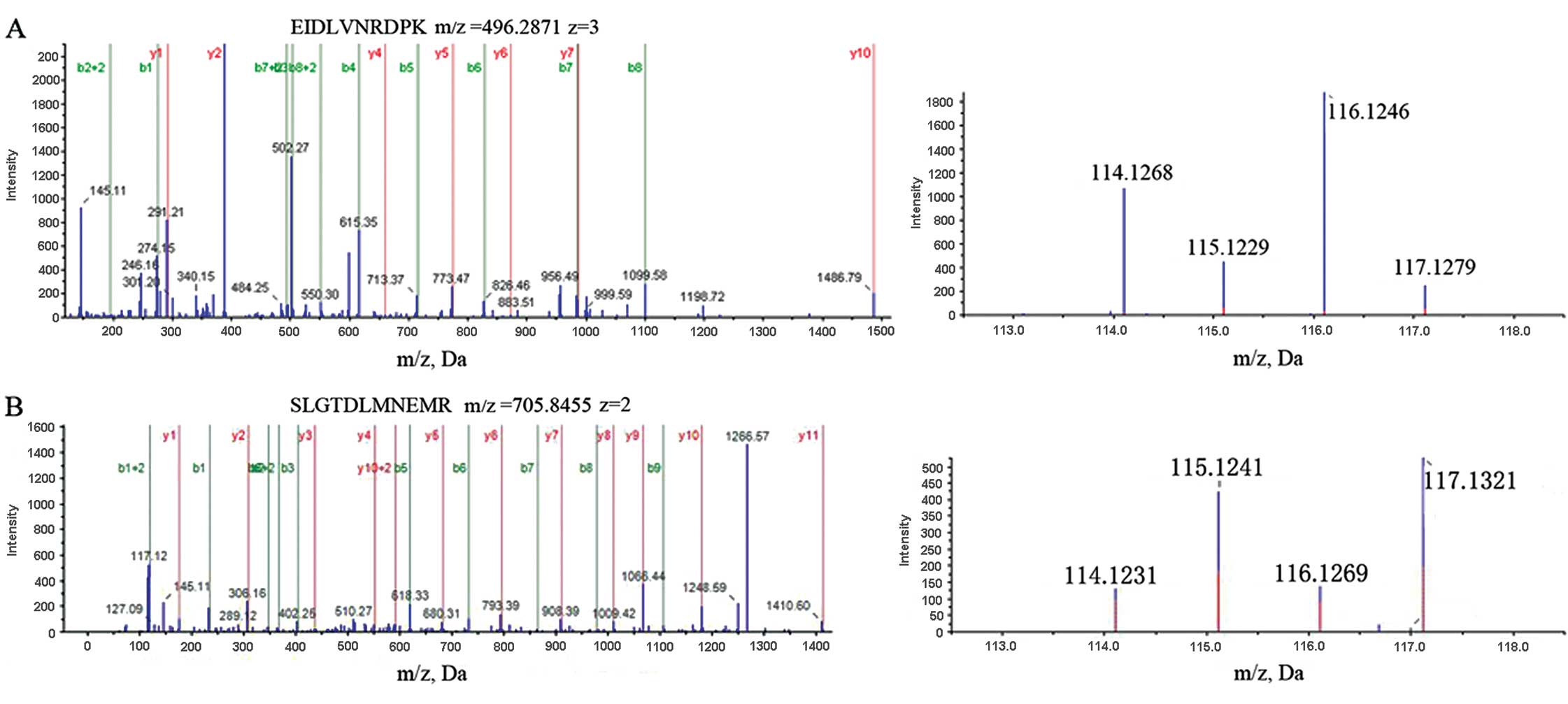
(Table II). MS/MS spectra used for
the identification and quantitation of caveolin-1 and integrin β1
are shown in Fig. 1.
 | Table IIForty-five differentially expressed
proteins in AdC vs. matched PNLT identified by iTRAQ labeling
combined with 2D-LC-MS/MS. |
Table II
Forty-five differentially expressed
proteins in AdC vs. matched PNLT identified by iTRAQ labeling
combined with 2D-LC-MS/MS.
| Accession no. | Protein name | AdC vs. PNLT | Located | TMDs | GRAVY |
|---|
| Q9UGM3 | Deleted in
malignant brain tumor 1 protein | 40.005 | Secreted | 0 | −0.346 |
| P06731 | Carcinoembryonic
antigen-related cell adhesion molecule 5 | 13.094 | PM | 0 | −0.411 |
| P15941 | Mucin-1 | 9.713 | PM | 1 | −0.501 |
| P05362 | Intercellular
adhesion molecule 1 | 9.034 | PM | 1 | −0.326 |
| P01833 | Polymeric
immunoglobulin receptor | 6.916 | PM | 1 | −0.405 |
| Q99828 | Calcium and
integrin-binding protein 1 | 5.191 | PM | 0 | −0.329 |
| Q14764 | Major vault
protein | 3.965 | Cytoplasm | 0 | −0.361 |
| P04406 |
Glyceraldehyde-3-phosphate
dehydrogenase | 3.837 | Cytoplasm | 1 | −0.114 |
| O96009 | Napsin-A | 3.768 | Secreted | 0 | 0.193 |
| P08575 | Leukocyte common
antigen | 3.375 | PM | 2 | −0.628 |
| A7Y9J9 | Mucin-5AC | 3.150 | Secreted | 0 | −0.379 |
| P98088 | Mucin-5AC
(fragments) | 2.959 | Secreted | 0 | −0.379 |
| O00299 | Chloride
intracellular channel protein 1 | 2.619 | PM | 0 | −0.293 |
| Q00610 | Clathrin heavy
chain 1 | 2.606 | M | 0 | −0.244 |
| P08311 | Cathepsin G | 2.503 | M | 0 | −0.562 |
| P05023 |
Sodium/potassium-transporting ATPase
subunit α-1 | 2.456 | PM | 10 | 0.010 |
| P04083 | Annexin A1 | 2.453 | M Related | 0 | −0.426 |
| O75955 | Flotillin-1 | 2.281 | PM | 0 | −0.338 |
| P01871 | Ig mu chain C
region | 2.240 | PM | 0 | −0.326 |
| P06733 | α-enolase | 1.720 | M | 0 | −0.226 |
| P05556 | Integrin β1 | 1.583 | PM | 1 | −0.455 |
| P27105 | Erythrocyte band 7
integral membrane protein | 0.496 | PM | 1 | 0.037 |
| Q99536 | Synaptic vesicle
membrane protein VAT-1 homolog | 0.490 | Cytoplasm | 0 | −0.043 |
| Q9BVC6 | Transmembrane
protein 109 | 0.442 | PM | 5 | 0.411 |
| P11233 | Ras-related protein
Rap-1A | 0.436 | PM | 0 | −0.683 |
| Q969L2 | Protein MAL2 | 0.436 | PM | 4 | 0.728 |
| Q99758 | ATP-binding
cassette sub-family A member 3 | 0.393 | PM | 11 | 0.089 |
| P07099 | Epoxide hydrolase
1 | 0.387 | PM | 0 | −0.261 |
| P21333 | Filamin-A | 0.341 | PM | 0 | −0.318 |
| P62158 | Calmodulin | 0.285 | PM | 0 | −0.671 |
| O60437 | Periplakin | 0.284 | M | 0 | −0.982 |
| P21964 | Catechol
O-methyl-transferase | 0.236 | PM | 1 | 0.16 |
| Q9UGT4 | Sushi
domain-containing protein 2 | 0.229 | PM | 1 | −0.275 |
| P00387 | NADH-cytochrome b5
reductase 3 | 0.223 | PM | 0 | −0.187 |
| P08758 | Annexin A5 | 0.223 | M related | 0 | −0.337 |
| P02730 | Band 3 anion
transport protein | 0.160 | PM | 11 | 0.213 |
| O00159 | Myosin-Ic | 0.148 | PM | 0 | −0.387 |
| Q16853 | Membrane primary
amine oxidase | 0.147 | PM | 1 | −0.135 |
| P50148 | Guanine
nucleotide-binding protein G(q) subunit α | 0.123 | PM | 0 | −0.440 |
| Q03135 | Caveolin-1 | 0.115 | PM | 1 | 0.054 |
| P10301 | Ras-related protein
R-Ras | 0.086 | PM | 0 | −0.408 |
| Q9NZN4 | EH
domain-containing protein 2 | 0.057 | PM | 0 | −0.309 |
| Q09666 | Neuroblast
differentiation-associated protein AHNAK | 0.057 | Nucleus | 0 | −0.499 |
| Q6NZI2 | Polymerase I and
transcript release factor | 0.053 | M | 0 | −0.272 |
| P22748 | Carbonic anhydrase
4 | 0.024 | PM | 1 | −0.576 |
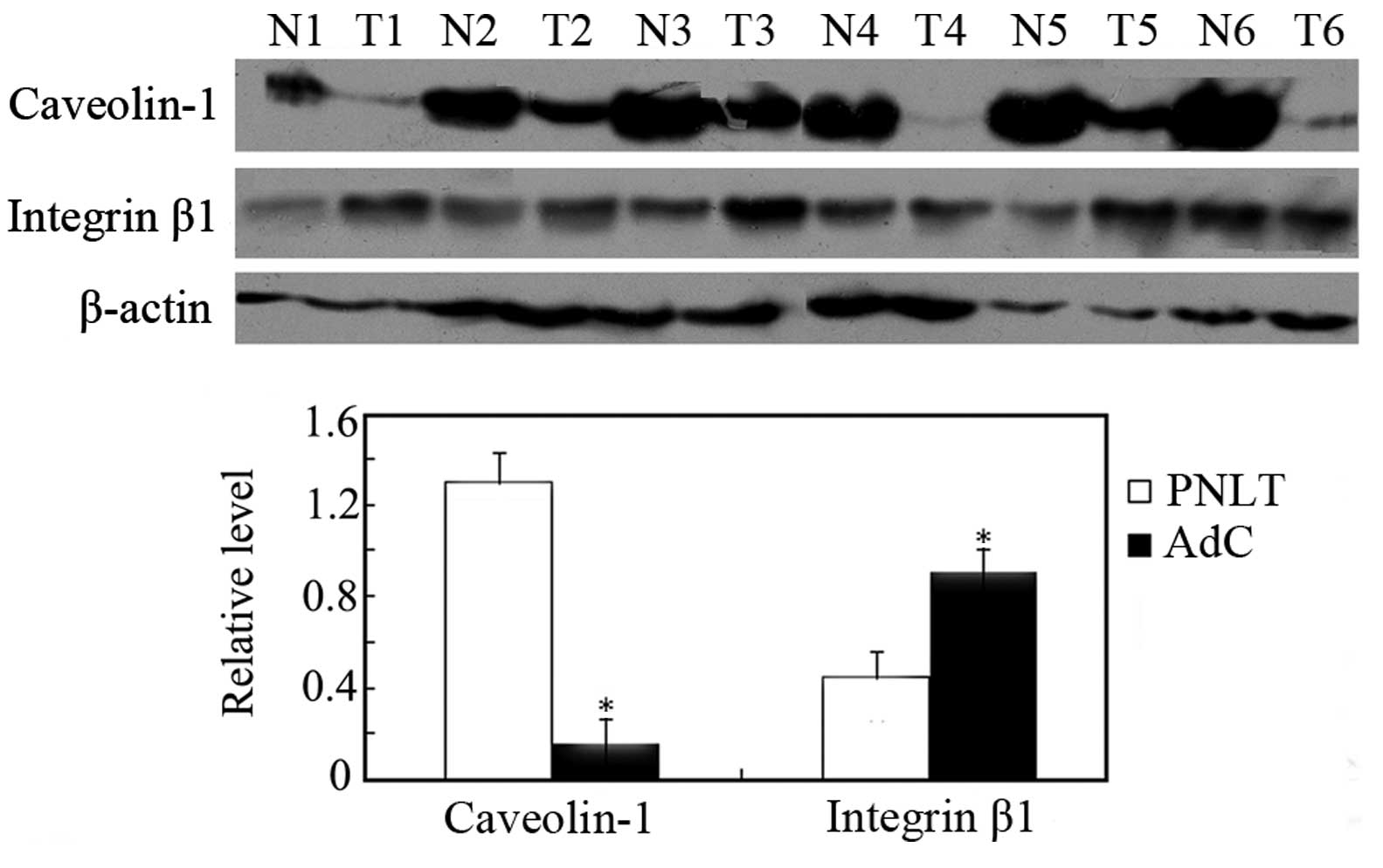
Validation of differentially expressed
proteins identified by quantitative proteomics
To confirm the expression levels of the differential
proteins identified by a proteomics approach, expressions of
caveolin-1 and integrin β1 in 18 pairs of AdC and matched PNLT
adjacent to tumors were detected by western blotting. As shown in
Fig. 2, caveolin-1 was
downregulated, whereas integrin β1 was upregulated in AdC compared
with PNLT, which is consistent with the findings in MS/MS
analysis.
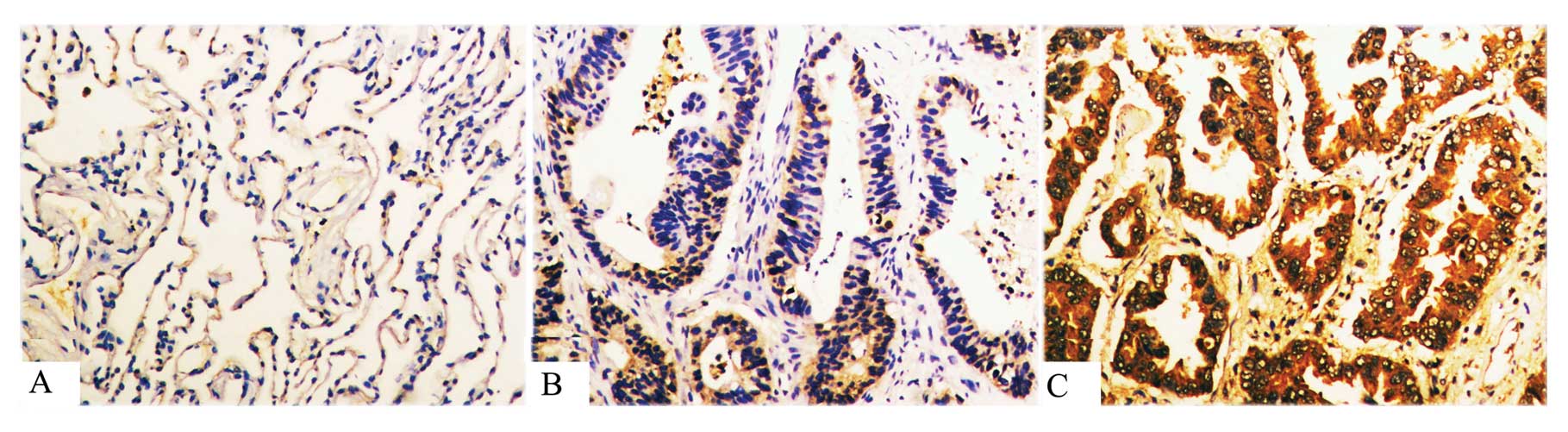
Expression of integrin β1 in PNLT,
primary lung AdC and lymph node metastases
We detected the expression of integrin β1 using
immunohistochemical staining in 46 cases of non-LNM AdC, 62 cases
of LNM AdC and 42 cases of PNLT. As shown in Fig. 3 and Table III, integrin β1 was significantly
upregulated in non-LNM AdC vs. PNLT and in LNM AdC vs. non-LNM
AdC.
 | Table IIIDifference of integrin β1 expression
in PNLT, non-LNM AdC and LNM AdC. |
Table III
Difference of integrin β1 expression
in PNLT, non-LNM AdC and LNM AdC.
| | Score | |
|---|
| |
| |
|---|
| n | Low (0–2) | Moderate (3–4) | High (5–6) | P-value |
|---|
| Integrin β1 |
| PNLT | 42 | 34 | 6 | 2 | 0.001a,b |
| Non-LNM AdC | 46 | 21 | 20 | 5 | |
| LNM AdC | 62 | 17 | 15 | 30 | 0.001a,c |
Correlation of integrin β1 expression in
primary AdC with clinicopathological factors
Table I shows the
correlation of several clinical pathological factors with integrin
β1 expression status in 108 cases of primary AdC (including 46
cases of non-LNM AdC and 62 cases of LNM AdC). Integrin β1
expression levels were significantly correlated with clinical
stage, recurrence and lymph node metastasis. Tumors with the
upregulation of integrin β1 tended to have a more advanced clinical
stage and more frequent recurrence and lymph node metastasis. No
significant correlations were found between the expression of
integrin β1 and other characteristics, including gender, age, tumor
size and tumor differentiation.
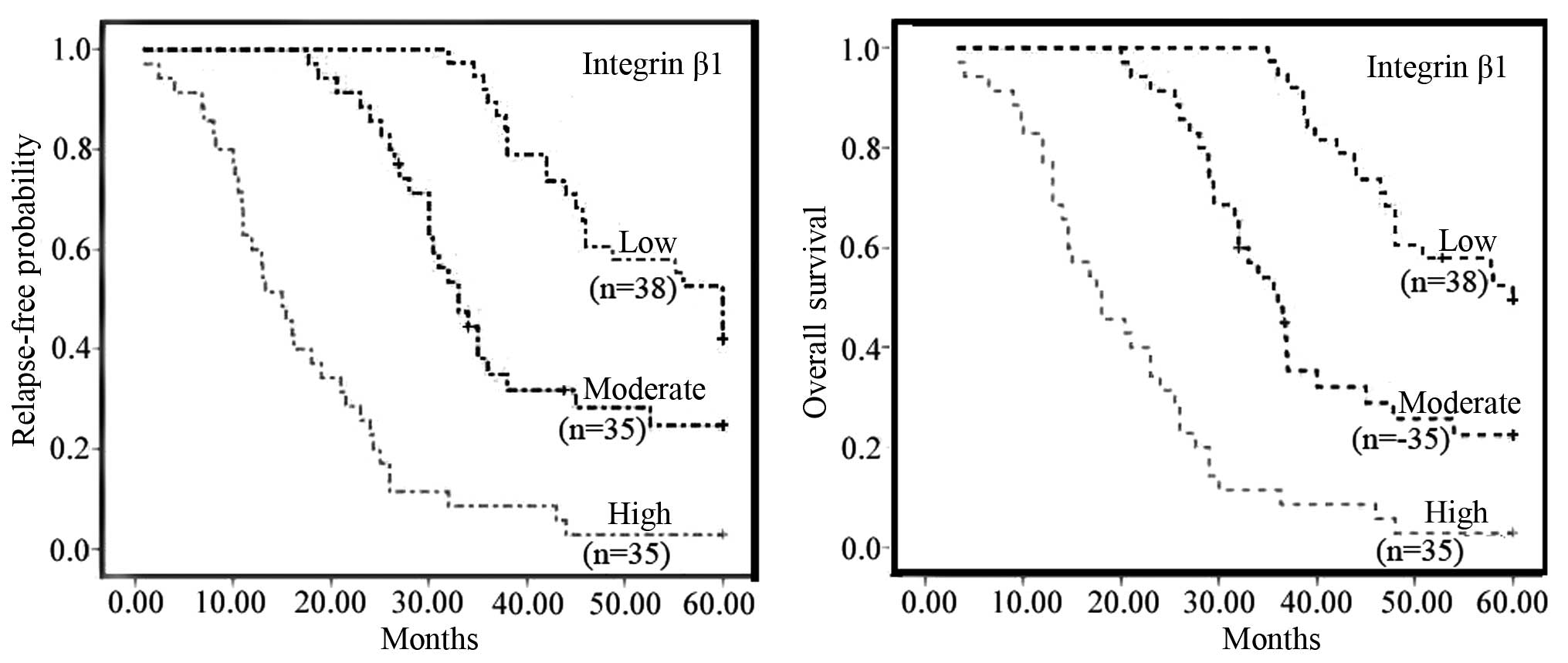
Correlation of postoperative relapse and
survival with integrin β1 expression
By the end of the study, 82 of the 108 patients had
died, 24 patients were still alive and 2 patients had been lost to
follow-up. The relapse-free times of the patients with low,
moderate and high expression of integrin β1 were 51.6±10.1,
47.1±13.3 and 16.5±10 months, respectively. The relapse-free
probability curve showed that the relapse rate was significantly
increased along with increasing integrin β1 expression (Fig. 4, left). The mean survival rates of
the patients with low, moderate and high expression of integrin β1
were 53±8.7, 38.4±12.9 and 17.8±9.5 months, respectively. The
survival curves showed that the overall survival rate was
significantly decreased along with increasing expressions of
integrin β1 (Fig. 4, right). In
univariate analysis (Table IV),
increased postoperative relapse and decreased survival were
correlated with advanced clinical stage, lymph node metastasis,
increasing expressions of integrin β1. In multivariate analysis
(Table V), advanced clinical stage,
lymph node metastasis and increasing expressions of integrin β1
remained the significant independent prognostic factors of
increased relapse rate and decreased overall survival rate.
 | Table IVUnivariate Cox regression analysis of
relapse-free and overall survival for integrin β1 expression. |
Table IV
Univariate Cox regression analysis of
relapse-free and overall survival for integrin β1 expression.
| Relapse-free
probability | Overall
survival |
|---|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age |
0.501(0.308–0.813) | 0.094 | 0.465
(0.287–0.753) | 0.120 |
| Gender |
0.926(0.596–1.438) | 0.731 | 0.929
(0.596–1.452 | 0.747 |
| Male
(reference) | 1.000 | | 1.000 | |
| Female |
0.926(0.596–1.438) | 0.731 | 0.929
(0.595–1.452) | 0.747 |
| Tumor
differentiation | | 0.700 | | 0.502 |
| Well
(reference) | 1.000 | | 1.000 | |
| Moderate | 0.871
(0.486–1.56) | 0.426 | 1.384
(0.681–2.811) | 0.369 |
| Poor | 1.504
(0.618–1.796) | 0.750 | 1.210
(0.769–1.904) | 0.410 |
| pT stage | | 0.513 | | 0.779 |
| T1
(reference) | 1.000 | | 1.000 | |
| T2 | 0.675
(0.341–1.336) | 0.611 | 1.009
(0.352–2.895) | 0.861 |
| T3–4 | 0.781
(0.449–1.360) | 0.257 | 1.288
(0.560–2.962) | 0.551 |
| pN stage | | 0.000a | | 0.000a |
| N0
(reference) | 1.000 | | 1.000 | |
| N1 | 14.812
(6.024–36.421) | 0.000 | 16.639
(6.513–42.507) | 0.000 |
| N2–3 | 11.357
(6.244–20.660) | 0.000 | 11.988
(6.518–22.045) | 0.006 |
| Clinical stage | | 0.000a | | 0.000a |
| I (reference) | 1.000 | | 1.000 | |
| II | 19.744
(7.291–53.464) | 0.000 | 18.973
(7.073–50.899) | 0.000 |
| III–IV | 21.445
(10.799–42.585) | 0.000 | 20.501
(13.340–44648) | 0.000 |
| Integrin β1
expression | | 0.000a | | 0.000a |
| Low | 1.000 | | 1.000 | |
| Moderate | 2.202
(1.231–3.935) | 0.008 | 2.626
(1.442–4.780) | 0.002 |
| High | 6.592
(4.106–10.584) | 0.000 | 6.183
(3.851–9.929) | 0.000 |
 | Table VMultivariate Cox regression analysis
of relapse-free and overall survival for integrin β1
expression. |
Table V
Multivariate Cox regression analysis
of relapse-free and overall survival for integrin β1
expression.
| Relapse-free
probability | Overall
survival |
|---|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| pN stage | | 0.011a | | 0.006a |
| N0
(reference) | 1.000 | | 1.000 | |
| N1 |
5.354(1.537–18.644) | 0.008 |
7.484(2.040–27.463) | 0.002 |
| N2–3 |
3.508(1.493–8.240) | 0.004 |
3.551(1.477–8.537) | 0.005 |
| Clinical stage | | 0.003a | | 0.005a |
| I (reference) | 1.000 | | 1.000 | |
| II |
4.440(1.186–16.619) | 0.027 |
4.165(1.130–15.348) | 0.032 |
| III–IV |
5.167(2.028–13.163) | 0.001 |
4.745(1.862–12.094) | 0.001 |
| Integrin β1
expression | | 0.000a | | 0.000a |
| Low | 1.000 | | 1.000 | |
| Moderate |
2.086(1.010–4.310) | 0.047 |
2.866(1.311–6.265) | 0.008 |
| High |
4.045(2.169–7.543) | 0.000 |
3.557(1.879–6.734) | 0.000 |
Discussion
In the present study, iTRAQ labeling combined with
2D-LC-MS/MS was performed to identify differential PM proteins in
AdC and PNLT. As a result, 45 differentially expressed proteins
were identified and differential PM proteins, caveolin-1 and
integrin β1, were selectively validated. Next, the
clinicopathological significance of integrin β1 was further
evaluated using immunohistochemistry of paraffin-embedded archival
tissue specimens and statistical analysis. Results show that
integrin β1 is a potential biomarker for LNM and prognosis of
AdC.
Integrins, a large family of membrane receptors, are
α/β-heterodimeric transmembrane adhesion molecules which bind to
specific ECM ligands (29,30). Integrin β1 is an important subunit
of integrins and recognizes the sequence R-G-D in a wide array of
ligands. Integrin β1 comprises at least eight isoform members and
plays a role in cell signaling and thereby defines cellular shape,
mobility and regulates the cell cycle (31). Integrin β1 has been shown to
regulate signaling through transmembrane growth factor receptors
such as epidermal growth factor receptor and transforming growth
factor-β receptor (32,33). Integrin β1 has been reported as
critical for TGF-β1-mediated transcription and epithelial cell
plasticity in vitro(34).
Integrin β1 was involved in the development and progression of
carcinogenesis in several types of cancer, including kidney cancer,
breast cancer and fibrosarcoma, bladder and colon carcinoma
(34,35). Bredin et al(36) found that integrin β1 was involved in
lung cancer cell migration in vitro towards fibronectin,
laminin and type IV collagen. Upregulation of integrin β1 is an
important factor for gefitinib resistance in the NSCLC cell line
(37) and overexpression of
integrin β1 is correlated with the invasion and metastasis events
of HCC in patients (38). Integrin
β1 is correlated with highly invasive and metastatic behavior and
is a poor prognostic factor in patients with SCLC (39,40).
Herein, an increase in integrin β1 expression level was associated
with advanced clinical stage and lymph node metastases, suggesting
that integrin β1 is associated with the progression and LNM of lung
AdC. In addition, a univariate analysis indicated that high
integrin β1 expression is strongly associated with increased tumor
relapse and a multivariate analysis further indicated that high
integrin β1 expression is an independent relapse factor for
increased tumor relapse in lung AdC.
In conclusion, the present study not only confirmed
expression of caveolin-1 and integrin β1 by proteomic approaches in
primary AdC and paired normal lung tissues adjacent to tumors, but
also showed that primary AdC with higher integrin β1 expression
tended to have later clinical stage, more frequent recurrence and
LNM. Furthermore, survival curves showed that the AdC patients with
integrin β1 upregulation had a poor prognosis. Multivariate
analysis confirmed that integrin β1 expression was an independent
prognostic indicator. Findings of the present study may have
clinical value in predicting the prognosis of AdC and identifying
AdC patients that are at high risk of metastasis and
recurrence.
Acknowledgements
The authors acknowledge the grants from the Major
New Drug Discovery Science and Technology of China
(2012ZX09303013-006) and the National Natural Science Foundation of
China (81272609, 21105129, 81102046). We also acknowledge the
Institutes of Biomedical Sciences of Fudan University Xiao-Hui Liu
for her assistance with iTRAQ analysis.
Abbreviations:
|
AdC
|
lung adenocarcinoma
|
|
PM
|
plasma membrane
|
|
PNLT
|
paraneoplastic normal lung tissue
|
|
non-LNM AdC
|
without lymph node metastasis primary
AdC
|
|
LNM AdC
|
with lymph node metastatic primary
AdC
|
|
SDS-PAGE
|
sodium dodecyl sulfate-polyacrylamide
gel electrophoresis
|
|
SCX
|
strong cation exchange
|
|
ACN
|
acetonitrile
|
|
GRAVY
|
grand average of hydropathicity
|
|
iTRAQ
|
isobaric tags for relative and
absolute quantitation
|
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Yang L, Parkin DM, Li LD, Chen YD and Bray
F: Estimation and projection of the national profile of cancer
mortality in China: 1991–2005. Br J Cancer. 90:2157–2166.
2004.PubMed/NCBI
|
|
3
|
Howe HL, Wingo PA, Thun MJ, Ries LA,
Rosenberg HM, Feigal EG and Edwards BK: Annual report to the nation
on the status of cancer (1973 through 1998), featuring cancers with
recent increasing trends. J Natl Cancer Inst. 93:824–842. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Little AG, Gay EG, Gaspar LE and Stewart
AK: National survey of non-small cell lung cancer in the United
States: epidemiology, pathology and patterns of care. Lung Cancer.
57:253–260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jemal A, Siegel R, Ward E, Murray T, Xu J,
Smigal C and Thun MJ: Cancer statistics, 2006. CA Cancer J Clin.
56:106–130. 2006. View Article : Google Scholar
|
|
6
|
Steeg PS, Bevilacqua G, Kopper L,
Thorgeirsson UP, Talmadge JE, Liotta LA and Sobel ME: Evidence for
a novel gene associated with low tumor metastatic potential. J Natl
Cancer Inst. 80:200–204. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen JJ, Peck K, Hong TM, Yang SC, Sher
YP, Shih JY, Wu R, et al: Global analysis of gene expression in
invasion by a lung cancer model. Cancer Res. 61:5223–5230.
2001.PubMed/NCBI
|
|
8
|
Nakamura N, Kobayashi K, Nakamoto M, Kohno
T, Sasaki H, Matsuno Y and Yokota J: Identification of tumor
markers and differentiation markers for molecular diagnosis of lung
adenocarcinoma. Oncogene. 25:4245–4255. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tian T, Hao J, Xu A, Hao J, Luo C, Liu C,
Huang L, Xiao X and He D: Determination of metastasis-associated
proteins in non-small cell lung cancer by comparative proteomic
analysis. Cancer Sci. 98:1265–1274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen G, Gharib TG, Huang CC, Thomas DG,
Shedden KA, Taylor JM, Kardia SL, et al: Proteomic analysis of lung
adenocarcinoma: identification of a highly expressed set of
proteins in tumors. Clin Cancer Res. 8:2298–2305. 2002.PubMed/NCBI
|
|
11
|
Chen G, Gharib TG, Thomas DG, Huang CC,
Misek DE, Kuick RD, Giordano TJ, et al: Proteomic analysis of
eIF-5A in lung adenocarcinomas. Proteomics. 3:496–504. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rho JH, Roehrl MH and Wang JY: Tissue
proteomics reveals differential and compartment-specific expression
of the homologs transgelin and transgelin-2 in lung adenocarcinoma
and its stroma. J Proteome Res. 8:5610–5618. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu YF, Xiao ZQ, Li MX, Li MY, Zhang PF,
Li C, Li F, et al: Quantitative proteome analysis reveals annexin
A3 as a novel biomarker in lung adenocarcinoma. J Pathol.
217:54–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu YF, Zhang PF, Li MY, Li QQ and Chen
ZC: Identification of annexin A1 as a proinvasive and prognostic
factor for lung adenocarcinoma. Clin Exp Metastasis. 28:413–425.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Emmott E, Wise H, Loucaides EM, Matthews
DA, Digard P and Hiscox JA: Quantitative proteomics using SILAC
coupled to LC-MS/MS reveals changes in the nucleolar proteome in
influenza A virus-infected cells. J Proteome Res. 9:5335–5345.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qattan AT, Mulvey C, Crawford M, Natale DA
and Godovac-Zimmermann J: Quantitative organelle proteomics of
MCF-7 breast cancer cells reveals multiple subcellular locations
for proteins in cellular functional processes. J Proteome Res.
9:495–508. 2010. View Article : Google Scholar
|
|
17
|
Wu CC, MacCoss MJ, Howell KE and Yates JR
III: A method for the comprehensive proteomic analysis of membrane
proteins. Nat Biotechnol. 21:532–538. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu CC and Yates JR III: The application of
mass spectrometry to membrane proteomics. Nat Biotechnol.
21:262–267. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dowling P, Meleady P, Dowd A, Henry M,
Glynn S and Clynes M: Proteomic analysis of isolated membrane
fractions from superinvasive cancer cells. Biochim Biophys Acta.
1774:93–101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang X, Zhao J, Hajivandi M, Wu R, Tao J,
Amshey JW and Pope RM: Quantification of membrane and
membrane-bound proteins in normal and malignant breast cancer cells
isolated from the same patient with primary breast carcinoma. J
Proteome Res. 5:2632–2641. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stockwin LH, Blonder J, Bumke MA, Lucas
DA, Chan KC, Conrads TP, Issaq HJ, et al: Proteomic analysis of
plasma membrane from hypoxia-adapted malignant melanoma. J Proteome
Res. 5:2996–3007. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, et al: Use of chemotherapy plus
a monoclonal antibody against HER2 for metastatic breast cancer
that overexpresses HER2. N Engl J Med. 344:783–792. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oh P, Li Y, Yu J, Durr E, Krasinska KM,
Carver LA, Testa JE and Schnitzer JE: Subtractive proteomic mapping
of the endothelial surface in lung and solid tumors for
tissue-specific therapy. Nature. 429:629–635. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cao R, Li X, Liu Z, Peng X, Hu W, Wang X,
Chen P, et al: Integration of a two-phase partition method into
proteomics research on rat liver plasma membrane proteins. J
Proteome Res. 5:634–642. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiao Z, Li G, Chen Y, Li M, Peng F, Li C,
Li F, et al: Quantitative proteomic analysis of formalin-fixed and
paraffin-embedded nasopharyngeal carcinoma using iTRAQ labeling,
two-dimensional liquid chromatography, and tandem mass
spectrometry. J Histochem Cytochem. 58:517–527. 2010. View Article : Google Scholar
|
|
26
|
Krogh A, Larsson B, von Heijne G and
Sonnhammer EL: Predicting transmembrane protein topology with a
hidden Markov model: application to complete genomes. J Mol Biol.
305:567–580. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kyte J and Doolittle RF: A simple method
for displaying the hydropathic character of a protein. J Mol Biol.
157:105–132. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng AL, Huang WG, Chen ZC, Peng F, Zhang
PF, Li MY, Li F, et al: Identification of novel nasopharyngeal
carcinoma biomarkers by laser capture microdissection and proteomic
analysis. Clin Cancer Res. 14:435–445. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tarone G, Hirsch E, Brancaccio M, De
Acetis M, Barberis L, Balzac F, Retta SF, et al: Integrin function
and regulation in development. Int J Dev Biol. 44:725–731.
2000.PubMed/NCBI
|
|
30
|
Hollenbeck ST, Itoh H, Louie O, Faries PL,
Liu B and Kent KC: Type I collagen synergistically enhances
PDGF-induced smooth muscle cell proliferation through
pp60src-dependent crosstalk between the α2β1 integrin
and PDGFβ receptor. Biochem Biophys Res Commun. 325:328–337. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moro L, Venturino M, Bozzo C, Silengo L,
Altruda F, Beguinot L, Tarone G and Defilippi P: Integrins induce
activation of EGF receptor: role in MAP kinase induction and
adhesion-dependent cell survival. EMBO J. 17:6622–6632. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhowmick NA, Zent R, Ghiassi M, McDonnell
M and Moses HL: Integrin β1 signaling is necessary for transforming
growth factor-β activation of p38MAPK and epithelial plasticity. J
Biol Chem. 276:46707–46713. 2001.
|
|
33
|
Frish SM and Francis H: Disruption of
epithelial cell-matrix interaction induces apoptosis. J Cell Biol.
124:619–626. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aoudjit F and Vuori K: Integrin signaling
inhibits paclitaxel-induced apoptosis in breast cancer cells.
Oncogene. 20:4995–5004. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamada KM, Kennedy DW, Yamada SS, Gralnick
H, Chen WT and Akiyama SK: Monoclonal antibody and synthetic
peptide inhibitors of human tumor cell migration. Cancer Res.
50:4485–4496. 1990.PubMed/NCBI
|
|
36
|
Bredin CG, Sundqvist KG, Hauzenberger D
and Klominek J: Integrin dependent migration of lung cancer cells
to extracellular matrix components. Eur Respir J. 11:400–407. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ju LX, Zhou CC, Li W and Yan LH: Integrin
beta1 over-expression associates with resistance to tyrosine kinase
inhibitor gefitinib in non-small cell lungcancer. J Cell Biochem.
111:1565–1574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao G, Cui J, Qin Q, Zhang J, Liu L, Deng
S, Wu C, et al: Mechanical stiffness of liver tissues in relation
to integrin β1 expression may influence the development of hepatic
cirrhosis and hepatocellular carcinoma. J Surg Oncol. 102:482–489.
2010.PubMed/NCBI
|
|
39
|
Oshita F, Kameda Y, Ikehara M, Tanaka G,
Yamada K, et al: Increased expression of integrin beta1 is a poor
prognostic factor in small-cell lung cancer. Anticancer Res.
22:1065–1070. 2002.PubMed/NCBI
|
|
40
|
Chang MH, Lee K, Lee KY, Kim YS, Kim YK
and Kang JH: Prognostic role of integrin β1, E-cadherin, and rac1
expression in small cell lung cancer. APMIS. 120:28–38. 2012.
|