Introduction
OK-432 is a penicillin-killed and lyophilized
preparation of a low-virulence strain (Su) of Streptococcus
pyogenes (group A) that was developed by Okamoto et
al(1). OK-432 is a major cancer
immunotherapy agent and has been shown to be effective against
several types of cancer (2–5). We have also reported that OK-432 shows
a strong antitumor effect against oral cancer in combination with
radiotherapy and UFT, an oral fluoropyrimidine formulation
combining tegafur and uracil in a 1:4 ratio (6,7).
The main action mechanism of OK-432 is the induction
and activation of cytotoxic macrophages, cytotoxic T lymphocytes,
and antitumor effector cells such as natural killer (NK) cells and
lymphokine-activated killer (LAK) cells. OK-432 also reportedly
increases antitumor effects by increasing production of antitumor
cytokines such as interferon (IFN)-γ, interleukin (IL)-2, IL-12,
and tumor necrosis factor (TNF)-α by Th1 cells, NK cells, and
monocytes/macrophages (8–12), and displays antitumor effects by
inducing cancer antigen-specific cytotoxic T lymphocytes via
maturation of antigen-presenting dendritic cells (13).
We have successfully isolated a lipoteichoic
acid-related molecule, OK-PSA, which is an active component of
OK-432 (14,15). We have also demonstrated that OK-PSA
exhibits antitumor effects by the induction of antitumor
cytokine-producing Th1 cells (16–20).
Moreover, we have clarified that the receptors against OK-PSA are
Toll-like receptor (TLR) 4/MD-2 complexes that are expressed on the
surface of immunocompetent cells (21–24).
In addition, when the expression of either TLR4 or MD-2 is lost in
peripheral blood mononuclear cells (PBMCs) in patients with oral
cancer, the sensitivity to OK-432 is decreased (25). However, after administration of
OK-432 in patients with oral cancer expressing both TLR4 and MD-2,
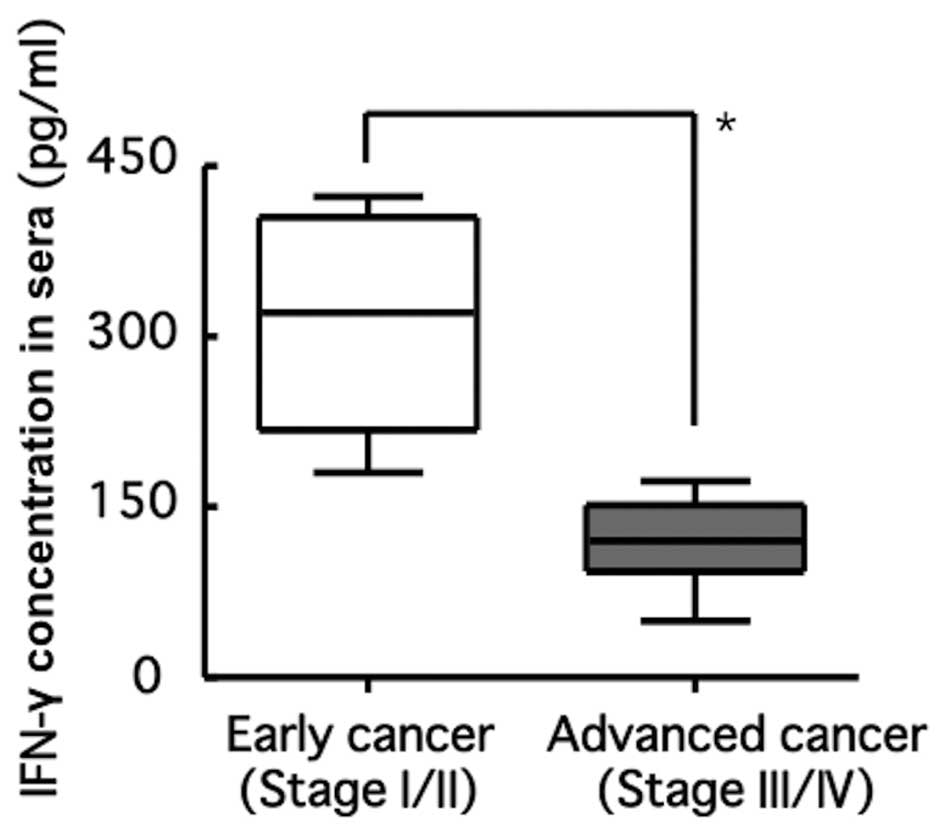
diverse serum concentrations of IFN-γ were detected, at ranges of
50–433 pg/ml (Fig. 1). Moreover,
serum concentrations of IFN-γ in patients with advanced cancer were
lower than those in patients with early cancer. These results
suggest that the responsiveness of OK-432 against patients with
oral cancer is dependent on cancer progression and growth. Thus, we
hypothesized that some types of soluble factors secreted by cancer
cells may decrease the production of IFN-γ with the administration
of OK-432.
In the present study, we established an in
vitro simulation model for immune status in cancer-burden
patients by the addition of conditioned medium (CM) derived from
oral cancer cells to PBMCs derived from healthy volunteers. Whether
or not soluble factors derived from oral cancer cells affected
IFN-γ production of PBMCs following stimulation with OK-432 was
then investigated.
Materials and methods
Investigation of the association between
stage of oral cancer and serum concentration following OK-432
administration
We re-analyzed the clinical data described by
Okamoto et al(25) on the
association between serum levels of IFN-γ and expression of TLR4
and MD-2 following administration of OK-432 in patients with oral
cancer. According to the International Union against Cancer (UICC)
criteria (26) for
tumor-node-metastasis (TNM) classification and cancer stage, 4
patients were stage II, 7 were stage III, and 2 were stage IV.
Patients with oral cancer expressing both TLR4 and MD-2 were
divided into early cancer (stages I and II) and advanced cancer
(stages III and IV), and serum levels of IFN-γ 24 h after
administration of OK-432 were compared.
Cells and cell culture
B88 (27) and HNt
cells (28), established in our
laboratory, were derived from patients with oral squamous cell
carcinoma (SCC), and TYS cells (29) were a human oral adenoid squamous
carcinoma cell line derived from a minor salivary gland in the oral
mucosa. All cells were maintained in DMEM (Sigma-Aldrich, St.
Louis, MO, USA) supplemented with 10% (V/V) FBS (Bio-Whittaker,
Walkersville, MD, USA), 100 μg/ml streptomycin, and 100 U/ml
penicillin (Invitrogen, Carlsbad, CA, USA) in a humidified
atmosphere of 95% air and 5% CO2 at 37°C. K-562 cells
(30), a human erythroblastic
leukemic cell line, Daudi cells (31), a human Burkitt’s lymphoma cell line,
and PBMCs derived from a healthy volunteer were maintained in
RPMI-1640 (Sigma-Aldrich) supplemented with 10% FBS.
Preparation of PBMCs
PBMCs were isolated from heparinized venous blood
derived from a healthy volunteer by Ficoll-Hypaque gradient density
centrifugation according to Boyüm’s standard procedures (32).
Preparation of CM
Oral cancer cells (2.5×106 cells) were
grown in 100-mm dishes (Falcon; Becton-Dickinson Labware, Lincoln
Park, NJ, USA) in complete culture medium for 48 h up to attaching
the dishes. Then, cells were washed 3 times using
phosphate-buffered saline (−) and were cultured for an additional
72 h in 10 ml of serum-free media. Media were collected and
purified by passing through 0.2 μm polyethersulfone membranes
(Corning Costar, Rochester, NY, USA), followed by subjection to
experiments as CM.
In vitro simulation model of patients
with oral cancer
PBMCs (1×106 cells/ml) were seeded in
RPMI-1640 containing 10% FBS. Media were replaced with
CM-containing media at a CM volume of 1/8, 1/4 or 1/2. FBS and
OK-432 (Chugai Pharmaceutical, Tokyo, Japan) were added to a final
concentration of 10% and 1 μg/ml, respectively.
Enzyme-linked immunosorbent assay
(ELISA)
Immunosuppressive cytokines [IFN-γ, IL-12, IL-4,
IL-6, IL-10, transforming growth factor-β (TGF-β) and vascular
endothelial growth factor (VEGF)] in cell culture supernatant and
CM were measured by an ELISA kit according to the manufacturer’s
instructions (BioSource International, Inc., Camarillo, CA,
USA).
Measurement of cytotoxic activities
The cytotoxic activity of PBMCs was measured with a
lactate dehydrogenase (LDH) release assay (33–35)
using K-562 cells that exhibited sensitivity to human NK and Daudi
cells without sensitivity to NK cells as target cells. Target cells
were suspended in RPMI-1640 medium containing 1% FBS. After seeding
in a proportion of 5×103 cells/50 μl per well in 96-well
round-bottomed microplates (Corning Costar), 1×105
cells/μl of PBMCs were added as effector cells. After 4 h of
co-culture, the enzymatic activity of LDH released from the
breakdown of target cells as a result of cytotoxic activity was
measured using a Cytotoxicity Detection Kitplus (Roche
Diagnostics GmbH, Mannheim, Germany). Cytotoxic activity was
calculated using the following formula: Cytotoxicity (%) = (A − low
control)/(high control − low control) × 100; A = (effector-target
cell mix − effector cell control).
High control indicates that cells showed absorbance
in supernatant when Triton X-100 (Sigma-Aldrich) was dissolved in
target cells. Low control cells showed absorbance in supernatant
when only target cells were cultured. The effector-target cell mix
shows absorbance in supernatant from a mixed culture of effector
cells and target cells, and effector cell control shows absorbance
in supernatant when only effector cells were cultured.
Treatment of PBMCs with neutralizing
antibodies (Abs)
CM was treated for 2 h at 37°C with the following
neutralizing Abs; anti-IL-4 Ab (clone 3007; 750 ng/ml), anti-IL-6
Ab (clone 6708; 75 ng/ml), anti-IL-10 Ab (clone 25209; 250 ng/ml),
anti-TGF-β Ab (clone 27235; 500 ng/ml), or anti-VEGF Ab (clone
26503; 15 ng/ml) to neutralize each cytokine. After neutralized-CM
was added to PBMCs, cells were stimulated with OK-432 for 24 h and
then the amount of IFN-γ and IL-12 in culture supernatant was
measured with an ELISA kit (BioSource International, Inc.). All
neutralizing antibodies used were purchased from R&D Systems
(Minneapolis, MN, USA).
Statistical analysis
The data obtained are expressed as the means ±
standard deviation, using analysis of variance (ANOVA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Association between cancer stage and
serum concentration of IFN-γ in patients with oral cancer following
administration of OK-432
Twenty-four hours after the administration of
OK-432, serum concentrations of IFN-γ in patients with advanced
cancer were significantly lower than those in patients with early
cancer (Fig. 1).
Effect of CM on the expression of IFN-γ
mRNA in PBMCs following treatment with OK-432
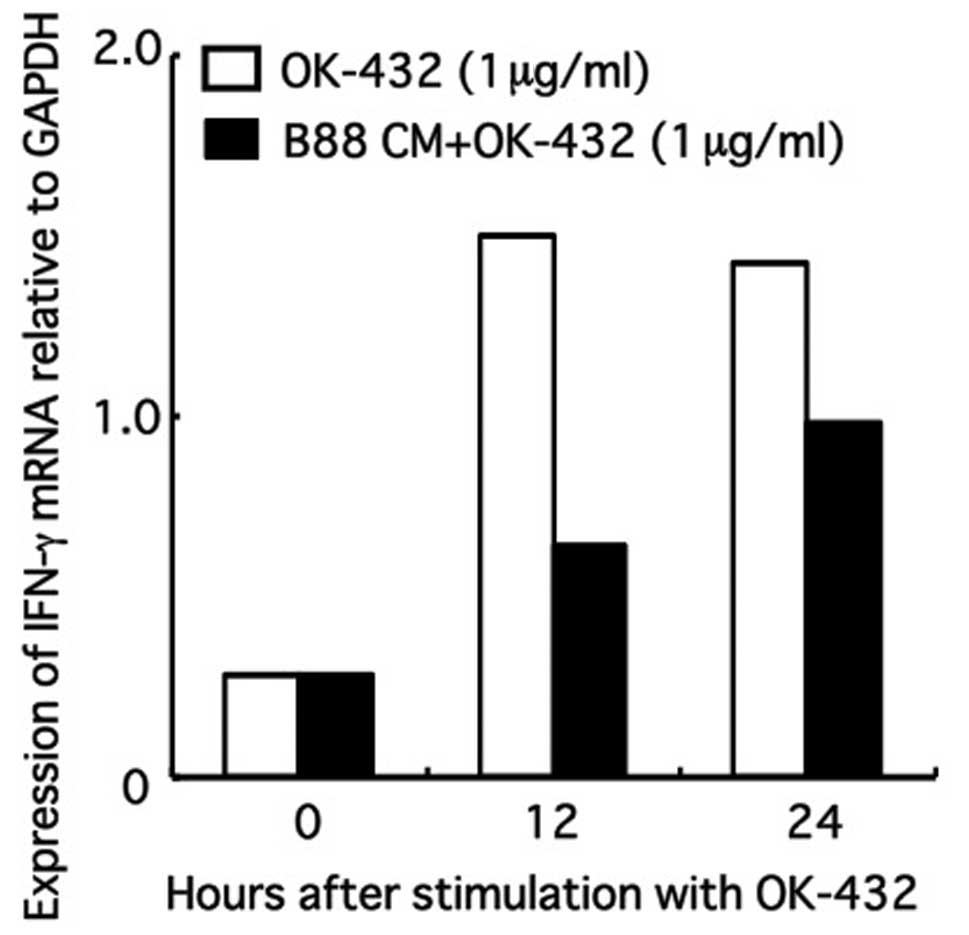
IFN-γ mRNA expression of PBMCs increased following
stimulation with OK-432, but was significantly inhibited after
replacement of CM-containing media at a CM (derived from B88 cells)
volume of 1/2 (Fig. 2).
Effect of CM on IFN-γ production from
PBMCs following treatment with OK-432
IFN-γ produced by PBMCs increased after stimulation
with OK-432, but decreased in the presence of CM in a CM
concentration-dependent manner. Inhibition of IFN-γ production from
PBMCs in the presence of CM was detected in all oral cancer cells
used (Fig. 3).
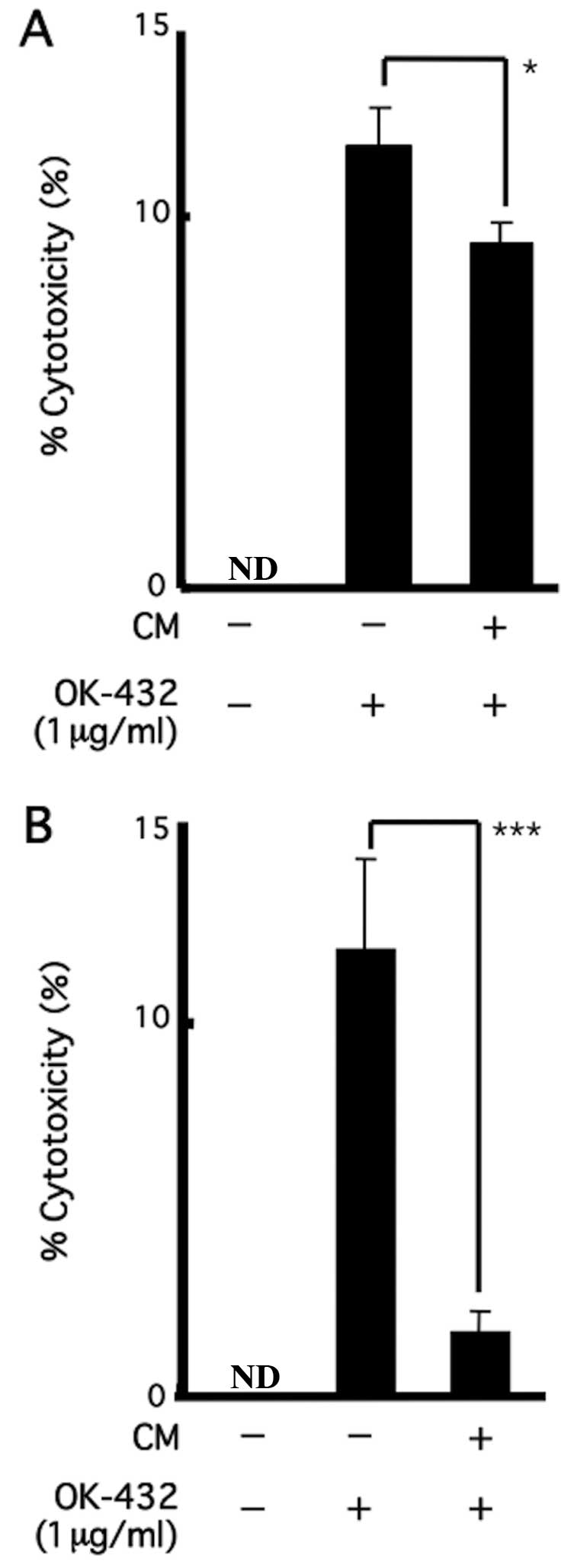
Effect of CM on the cytotoxic activity of
PBMCs following treatment with OK-432
Cytotoxic activities of PBMCs against K-562 cells
(Fig. 4A) and Daudi cells (Fig. 4B) were enhanced in both cells
following treatment with OK-432; however, activities were
significantly inhibited after replacement of CM-containing media at
a CM (derived from B88 cells) volume of 1/2 (Fig. 4).
Effect of immunosuppressive cytokines on
IFN-γ production from PBMCs following treatment with OK-432
Table I shows the
concentration of each cytokine in CM derived from B88, TYS, and HNt
cells. Of these cytokines, neutralization tests against
representative immunosuppressive cytokines, IL-4, IL-6, IL-10,
TGF-β and VEGF, were conducted to elucidate the inhibitory
mechanism of IFN-γ production from PBMCs in the presence of CM.
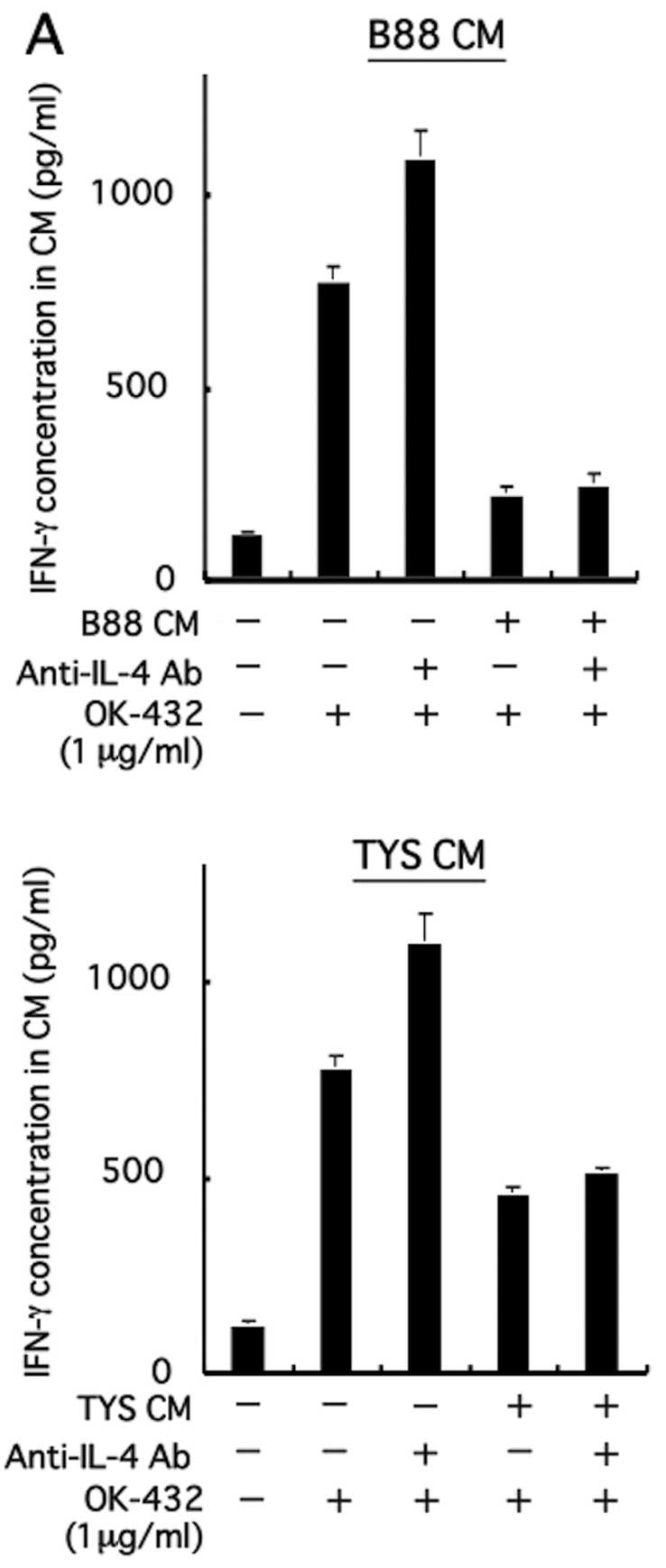
Despite the addition of CM derived from B88 cells and TYS cells
pretreated with neutralizing Abs against these immunosuppressive
cytokines, inhibition of IFN-γ production from PBMCs was not
restored (Fig. 5). By the addition
of neutralizing Abs against IL-6 and VEGF to CM derived from TYS
cells, inhibition of IFN-γ production from PBMCs appears to have
been restored (Fig. 5B and E).
However, induction of IFN-γ production from PBMCs was also observed
only with the addition of neutralizing antibody in the absence of
CM. These results indicate that IL-6 and VEGF are likely not the
main cytokines affecting the inhibition of IFN-γ production from
PBMCs with CM.
 | Table IConcentration of cytokines in CM from
oral cancer cell lines (pg/ml). |
Table I
Concentration of cytokines in CM from
oral cancer cell lines (pg/ml).
| Cells | IFN-γ | IL-12 | IL-4 | IL-6 | IL-10 | TGF-β | VEGF |
|---|
| B88 | ND | 28.4 | 34.5 | 910 | ND | 107 | 4662 |
| TYS | ND | 23.8 | 23.4 | 1437 | ND | 113 | 5019 |
| HNt | ND | 24.4 | - | 1385 | ND | 70 | 3891 |
Discussion
In the present study, we established an in
vitro simulation model for immune status in cancer-burden
patients by the addition of CM derived from oral cancer cells to
PBMCs isolated from a healthy volunteer. Whether or not soluble
factors derived from oral cancer cells affected IFN-γ production
from PBMCs following stimulation with OK-432 was then investigated.
We found that, in the presence of CM, enhanced production of IFN-γ
and cytotoxic activities of PBMCs following stimulation with OK-432
decreased in a CM concentration-dependent manner. Although IL-4,
IL-6, IL-10, TGF-β and VEGF were representative immunosuppressive
cytokines that play critical roles in the inhibition of IFN-γ
production, they did not contribute to the inhibition of IFN-γ
production by CM.
In general, diverse immunosuppressive cytokines
produced by cancer cells are known to contribute to the immune
suppression of patients with cancer. For example, IL-6 is generally
released by macrophages, but is also produced by cancer cells,
which inhibits production of IFN-γ from PBMCs (36). In the present study, high levels of
IL-6 production were detected in all oral cancer cells used. It was
also reported that the immunosuppressive status of patients with
cancer caused by cachexia is induced by inhibition of IFN-γ and
acceleration of IL-6 production. TGF-β is also known as an
immunosuppressive cytokine produced by several types of cancer
cells. It was demonstrated that by the neutralization of TGF-β in
CM and the addition of recombinant TGF-β, the immunosuppressive
effect of TGF-β was based on the inhibition of CD4+
T-cell function (37). It was also
clarified that TGF-β in CM suppressed IFN-γ production from PBMCs
using pancreatic and liver cancer cells (38,39).
It is well known that VEGF is also secreted from cancer cells and
stimulates growth and metastasis of cancer via angiogenesis. The
immunosuppressive effects of VEGF are considered to occur by
blocking the differentiation of bone marrow stem cells into
dendritic cells and the maturation of dendritic cells and by
inhibiting the antigen-presenting ability and cytokine production
of dendritic cells (40,41). The oral cancer cells used in the
present study also secreted these representative immunosuppressive
cytokines; however, these cytokines were not involved in the
inhibition of IFN-γ production from PBMCs by CM.
Immunosuppressive cytokines produced by PBMCs
stimulated by cancer cell-derived soluble factors are also known to
induce immunosuppression of patients with cancer. It has been
reported that CM from kidney cancer cells or malignant melanoma
cells enhanced the production of immunosuppressive cytokines such
as IL-4, IL-6 and IL-10 by PBMCs (42,43).
IL-10 is also known to be produced by PBMCs that are stimulated by
OK-432 (44), followed by the
inhibition of IFN-γ production (45). In the present study, IL-10 levels
were not detectable in CM derived from oral cancer cells, but were
markedly induced by stimulation of OK-432, and were further induced
with the addition of CM (data not shown). However, even if IL-10 is
blocked by the neutralizing Ab, inhibition of IFN-γ production from
PBMCs by the addition of CM could not recover. Among the other
immunosuppressive cytokines investigated in the present study,
production of IL-4, TGF-β, or VEGF was not detected in PBMCs even
after stimulation with OK-432 (data not shown). While production of
IL-6 was induced in PBMCs following stimulation with OK-432 (data
not shown), IFN-γ production from PBMCs inhibited by the addition
of CM following stimulation with OK-432 did not recover even by the
neutralization of IL-6. We thus concluded that representative
immunosuppressive cytokines produced by cancer cells or PBMCs are
not involved in the suppression of IFN-γ production from PBMCs by
the addition of CM. These results suggest that unknown molecules,
excluding these representative immunosuppressive cytokines, may be
involved in the inhibition of IFN-γ production. Clarifying these
unknown molecules may be a way to develop effective immunotherapy
against oral cancer.
References
|
1
|
Okamoto H, Shoin S, Koshimura S and
Shimizu R: Studies on the anticancer and streptolysin S-forming
abilities of hemolytic streptococci. Jpn J Microbiol. 11:323–326.
1967. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Watanabe Y and Iwa T: Clinical value of
immunotherapy with the streptococcal preparation OK-432 in
non-small cell lung cancer. J Biol Response Mod. 6:169–180.
1987.PubMed/NCBI
|
|
3
|
Katano M and Torisu M: New approach to
management of malignant ascites with a streptococcal preparation,
OK-432. II Intraperitoneal inflammatory cell-mediated tumor cell
destruction. Surgery. 93:365–373. 1983.PubMed/NCBI
|
|
4
|
Uchida A and Micksche M: Intrapleural
administration of OK-432 in cancer patients: activation of NK cells
and reduction of suppressor cells. Int J Cancer. 31:1–5. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uchida A, Micksche M and Hoshino T:
Intrapleural administration of OK-432 in cancer patients:
augmentation of autologous tumor killing activity of
tumor-associated large granular lymphocytes. Cancer Immunol
Immunother. 18:5–12. 1984. View Article : Google Scholar
|
|
6
|
Sato M, Yoshida H, Yanagawa T, Yura Y,
Urata M, Atsumi M, Hayashi Y and Takegawa Y: Effects of intradermal
administration of streptococcal preparation OK-432 on interferon
and natural killer cell activities in patients with oral cancer.
Int J Oral Surg. 13:7–15. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sato M, Harada K, Yoshida H, Yura Y, Azuma
M, Iga H, Bando T, Kawamata H and Takegawa Y: Therapy for oral
squamous cell carcinoma by tegafur and streptococcal agent OK-432
in combination with radiotherapy: association of the therapeutic
effect with differentiation and apoptosis in the cancer cells.
Apoptosis. 2:227–238. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oshimi K, Kano S, Takaku F and Okumura K:
Augmentation of mouse natural killer cell activity by a
streptococcal preparation, OK-432. J Natl Cancer Inst.
65:1265–1269. 1980.PubMed/NCBI
|
|
9
|
Oshimi K, Wakasugi H, Seki H and Kano S:
Streptococcal preparation OK-432 augments cytotoxic activity
against an erythroleukemic cell line in humans. Cancer Immunol
Immunother. 9:187–192. 1980. View Article : Google Scholar
|
|
10
|
Matsubara S, Suzuki F and Ishida N:
Induction of immune interferon in mice treated with a bacterial
immunopotentiator, OK-432. Cancer Immunol Immunother. 6:41–45.
1979. View Article : Google Scholar
|
|
11
|
Sato M, Hayashi Y, Yashida H, Yanagawa T,
Yura Y, Urata M and Furumoto N: Effect of immunotherapy with a
streptococcal preparation, OK-432, on the peripheral killer
lymphocyte population in patients with head and neck cancer.
Immunopharmacological Aspects of OK-432 in Humans. 1st edition.
Excerpta Medica; Tokyo: pp. 78–88. 1986
|
|
12
|
Kaji R, Yoshida H, Yanagawa T and Sato M:
Monoclonal antibody to a human salivary adenocarcinoma cell line:
augmentation of antibody-dependent cell-mediated cytotoxity
activity by streptococcal preparation OK-432 in human salivary
adenocarcinoma-bearing nude mice given the antibody. J Biol
Response Mod. 8:488–500. 1989.
|
|
13
|
Nakahara S, Tsunoda T, Baba T, Asabe S and
Tahara H: Dendritic cells stimulated with a bacterial products,
OK-432, efficiently induce cytotoxic T lymphocytes specific to
tumor rejection peptide. Cancer Res. 63:4112–4118. 2003.PubMed/NCBI
|
|
14
|
Okamoto M, Kaji R, Kasetani H, Yoshida H,
Moriya Y, Saito M and Sato M: Purification and characterization of
interferon-γ-inducing molecule of OK-432, a penicillin-killed
streptococcal preparation, by monoclonal antibody neutralizing
interferon-γ-inducing activity of OK-432. J Immunother Emphasis
Tumor Immunol. 13:232–242. 1993.
|
|
15
|
Okamoto M, Ohe G, Oshikawa T, Furuichi S,
Nishikawa N, Tano T, Ahmed SU, Yoshida H, Moriya Y, Saito S and
Sato M: Induction of Th1-type cytokines by lipoteichoic
acid-related preparation isolated from OK-432, a penicillin-killed
streptococcal agent. Immunopharmacology. 49:363–376. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okamoto M and Sato M: Toll-like receptor
signaling in anti-cancer immunity. J Med Invest. 50:9–24. 2003.
|
|
17
|
Okamoto M and Sato M: Effective molecule
of a streptococcal preparation OK-432 and its molecular targets:
significance for cancer immunotherapy. Rec Res Dev Infect Immun.
1:29–44. 2003.
|
|
18
|
Takada H, Kawabata Y, Arakaki R, Kusumoto
S, Fukase K, Suda Y, Yoshimura T, Kokeguchi S, Kato K and Komuro T:
Molecular and structural requirements of a lipoteichoic acid from
Enterococcus hirae ATCC 9790 for cytokine-inducing,
antitumor, and antigenic activities. Infect Immun. 63:57–65.
1995.PubMed/NCBI
|
|
19
|
Okamoto M, Oshikawa T, Furuichi S,
Nishikawa N, Tano T, Ahmed SU, Yoshida H, Matsubara S, Matsuno T
and Sato M: Comparison of cytokine-inducing activity in a
lipoteichoic acid-related molecule isolated from a
penicillin-killed group A Streptococcus and from untreated
bacteria. Int Immunopharmacol. 1:1957–1968. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oshikawa T, Okamoto M, Ohe G, Furuichi S,
Nishikawa H, Ahmed SU, Yoshida H, Moriya Y, Matsubara S, Ryoma Y,
Saito M and Sato M: Isolation of a Th1-inducing molecule from
OK-432, a streptococcal preparation, by a monoclonal antibody TS-2
that neutralizes the interferon-γ-inducing activity of OK-432:
comparison of the enhancement of anti-tumor immunity between the
TS-2-binding and TS-2-unbinding fraction. Int Immunopharmacol.
3:643–655. 2003.PubMed/NCBI
|
|
21
|
Okamoto M, Ohe G, Furuichi S, Nishikawa N,
Tano T, Ahmed SU, Yoshida H and Sato M: Enhancement of anti-cancer
immunity by a lipoteichoic-acid-related molecule isolated from a
penicillin-killed group A Streptococcus. Cancer Immunol
Immunother. 50:408–416. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okamoto M, Ohe G, Furuichi S, Nishikawa N,
Tano T, Ahmed SU, Yoshida H and Sato M: Enhancement of anti-tumor
immunity by lipoteichoic acid-related molecule isolated from
OK-432, a streptococcal agent, in athymic nude mice bearing human
salivary adenocarcinoma: Role of natural killer cells. Anticancer
Res. 226:3229–3240. 2002.
|
|
23
|
Shimazu R, Akashi S, Ogata H, Nagai Y,
Fukudome K, Miyake K and Kimoto M: MD-2, a molecule that confers
lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp
Med. 189:1777–1782. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okamoto M, Oshikawa T, Ohe G, Furuichi S,
Nishikawa N, Tano T, Ahmed SU, Yoshida H and Sato M: Severe
impairment of anti-cancer effect of lipoteichoic acid-related
molecule isolated from a penicillin-killed Streptococcus
pyogenes in toll-like receptor 4-deficient mice. Int
Immunopharmacol. 1:1789–1795. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Okamoto M, Oshikawa T, Tano T, Ohe G,
Furuichi S, Nishikawa H, Ahmed SU, Akashi S, Miyake K, Takeuchi O,
Akira S, Moriya Y, Matsubara S, Ryoma Y, Saito M and Sato M:
Involvement of Toll-like receptor 4 signaling in interferon-γ
production and anti-tumor effect by a streptococcal agent OK-432. J
Natl Cancer Inst. 95:316–326. 2003.
|
|
26
|
UICC International Union Against Cancer.
TNM Classification of Malignant Tumours. Sobin LH and Wittekind C:
5th edition. Wiley-Liss; New York, NY: pp. 17–50. 1997
|
|
27
|
Uchida D, Begum NM, Almofti A, Nakashiro
K, Kawamata H, Tateishi Y, Hamakawa H, Yoshida H and Sato M:
Possible role of stromal cell-derived factor-1/CXCR4 signaling on
lymph-node metastasis of oral squamous cell carcinoma. Exp Cell
Res. 290:289–302. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kawamata H, Nakashiro K, Uchida D, Harada
K, Yoshida H and Sato M: Possible contribution of active MMP2 to
lymph-node metastasis and secreted cathepsin L to bone invasion of
newly established human oral-squamous-cancer cell lines. Int J
Cancer. 70:120–127. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yanagawa T, Hayashi Y, Yoshida H, Yura Y,
Nagamine S, Bando T and Sato M: An adenoid squamous
carcinoma-forming cell line established from an oral keratinizing
squamous cell carcinoma expressing carcinoembryonic antigen. Am J
Pathol. 124:496–509. 1986.
|
|
30
|
Anderson LC, Nilsson K and Gahmberg CG:
K-562, a human erythroleukemic cell line. Int J Cancer. 23:143–147.
1979. View Article : Google Scholar
|
|
31
|
Klein E, Klein G, Nadkarmi JS, Nadkarmi
JJ, Wigzell H and Clifford P: Surface IgM-kappa specificity on a
Burkitt lymphoma cell in vivo and in derived culture lines. Cancer
Res. 28:1300–1310. 1968.PubMed/NCBI
|
|
32
|
Boyüm A: Isolation of mononuclear cells
and granulocytes from human blood. Isolation of mononuclear cells
by one centrifugation, and of granulocytes by combining
centrifugation and sedimentation at 1g. Scand J Clin Lab Invest
Suppl. 97:77–89. 1968.PubMed/NCBI
|
|
33
|
Nachlas MM, Margulies SI, Goldberg JD and
Seligman AM: The determination of lactic dehydrogenase with a
tetrazolium salt. Anal Biochem. 1:317–326. 1960. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Korzeniewski C and Callewaert DM: An
enzyme-release assay for natural cytotoxicity. J Immunol Methods.
64:313–320. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Decker T and Lohmann-Matthes ML: A quick
and simple method for the quantitation of lactate dehydrogenase
release in measurements of cellular cytotoxicity and tumor necrosis
factor (TNF) activity. J Immunol Methods. 115:61–69. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pasare C and Medzhitov R: Toll
pathway-dependent blockade of CD4+CD25+ T
cell-mediated suppression by dendritic cells. Science.
299:1033–1036. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tada T, Ohzeki S, Utsumi K, Takiuchi H,
Muramatsu M, Li XF, Shimizu J, Fujiwara H and Hamaoka T:
Transforming growth factor-β-induced inhibition of T cell functions
and its relevance to immunosuppression in the tumor-bearing state.
J Immunol. 146:1077–1082. 1991.
|
|
38
|
Bellone G, Turletti A, Artusio E, Mareschi
K, Carbone A, Tibaudi D, Robecchi A, Emanuelli G and Rodeck U:
Tumor-associated transforming growth factor-β and interleukin-10
contribute to a systemic Th2 immune phenotype in pancreatic
carcinoma patients. Am J Pathol. 155:537–547. 1999.
|
|
39
|
Mouri H, Sakaguchi K, Sawayama T, Senoh T,
Ohta T, Nishimura M, Fujiwara A, Terao M, Shiratori Y and Tsuji T:
Suppressive effects of transforming growth factor-β1 produced by
hepatocellular carcinoma cell lines on interferon-γ production by
peripheral blood mononuclear cells. Acta Med Okayama. 56:309–315.
2002.
|
|
40
|
Wang T, Niu G, Kortylewski M, Burdelya L,
Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola
D, Dalton W, Jove R, Pardoll D and Yu H: Regulation of the innate
and adaptive immune responses by Stat-3 signaling in tumor cells.
Nat Med. 10:48–54. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gabrilovich D, Ishida T, Oyama T, Ran S,
Kravtsov V, Nadaf S and Carbone DP: Vascular endothelial growth
factor inhibits the development of dendritic cells and dramatically
affects the differentiation of multiple hematopoietic lineages in
vivo. Blood. 92:4150–4166. 1998.
|
|
42
|
Smyth GP, Stapleton PP, Barden CB, Mestre
JR, Freeman TA, Duff MD, Maddali S, Yan Z and Daly JM: Renal cell
carcinoma induces prostaglandin E2 and T-helper type 2 cytokine
production in peripheral blood mononuclear cells. Ann Surg Oncol.
10:455–462. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
McCarter M, Clarke J, Richter D and Wilson
C: Melanoma skews dendritic cells to facilitate a T helper 2
profile. Surgery. 138:321–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fujimoto T, Duda RB, Szilvasi A, Chen X,
Mai M and O’Donnell MA: Streptococcal preparation OK-432 is a
potent inducer of IL-12 and a T helper cell 1 dominant state. J
Immunol. 158:5619–5626. 1997.PubMed/NCBI
|
|
45
|
Fiorentino DF, Zlotnic A, Vieira P,
Mosmann TR, Howard M, Moore KW and Ogarra A: IL-10 acts on the
antigen-presenting cell to inhibit cytokine production by Th1
cells. J Immunol. 146:3444–3451. 1991.PubMed/NCBI
|