Introduction
Angiogenesis is important in sustaining tumor
growth. As a tumor grows, the upregulation of angiogenic factors
results in the sprouting of new blood vessels from pre-existing
vessels to supply the tumor, but these new vessels fail to mature
into a normally functioning vasculature (1). As a consequence, neoangiogenic vessels
are fragile, leaky and dysfunctional, and they are targets for
various therapeutic modalities (2).
Ultrasound has been used for clinical imaging, as well as for its
therapeutic actions in physical therapy. It was previously reported
that vascular endothelium was destroyed after treatment with
ultrasound combined with microbubble contrast agent (UCA) (3). Others found microvessel rupture in
vivo in response to acoustic treatment of tissues containing
UCAs (4). Continuous 1-MHz
low-intensity ultrasound was able to affect the fragile and leaky
angiogenic blood vessels in a tumor (5)., and 3-MHz ultrasound was more
efficacious than 1 MHz for antivascular cancer therapy, in which
direct heating of the tumor due to ultrasound absorption is the
main mechanism (6). However, in
low-frequency ultrasound (7), it is
ultrasound-induced cavitation that may play an important role in
the antitumor treatment (8).
Collapse cavitation causes capillary destruction and, thus, may be
therapeutically beneficial for tumor (9). Cavitation, in a broad sense, refers to
ultrasonically induced activity occurring in a liquid or
liquid-like material that contains bubbles or pockets of gas or
vapor. Soft tissue is viscoelastic material, not a pure liquid, and
the cavitation thresholds in soft tissue are higher than those in
liquid, as endogenous cavitation nuclei are rare in most soft
tissues (10). Microbubbles in
ultrasound contrast agent (UCA) provide preexisting nuclei and,
thus, cavitation threshold of the soft tissue is decreased and
microbubbles increase the possibility for ultrasound-induced
cavitation and the potential for bioeffects. In vitro
studies have been carried out with regard to the effects of
low-frequency ultrasound combined with contrast agent (11). However, few researchers have studied
these effects in vivo. The objective of the present study
was to explore the vascular bioeffects in the tumor tissue of nude
mice treated with low-frequency ultrasound combined with a UCA.
Materials and methods
Animal protocol
The study included three parts, each with 25–35 male
nude mice aged 4 weeks and weighing 15–20 g purchased from the
Animal Center of the Shanghai Institute of Chinese Academy of
Science. All mice were treated and housed according to the approved
guidelines (Guidelines for the Care and Use of Laboratory Animals).
Following anesthesia by intraperitoneal injection of 0.004 g
ketamine, the mice were secured to a superclean bench according to
the principles of aseptic operation. Each mouse was then
subcutaneously inoculated with 2×106 cells from the
DU145 cell line into the flank after local sterilization. The mice
continued to be raised at specified pathogen free (SPF)
qualification after operation, and were observed at 2-day
intervals. Two weeks later, experiments were initiated when the
tumors had reached a size of 5–8 mm. Finally, each study included
20 tumor-bearing mice. In the first study, all subcutaneous tumors
were examined by contrast-enhanced ultrasonography (CEUS) at the
initiation (0 week) and completion (2 weeks) of the experiments. In
the second study, following completion of the experiment, the
tumors were excised and examined by immunohistochemistry and
confocal laser microscopy to assess the response to low frequency
US. In the third study, cell apoptosis of tumor of nude mice was
examined by terminal deoxynucleotidyl transferase-mediated dUTP
nick end labeling (TUNEL) and tumor cells and micro-vessel were
explored by transmission electron microscopy (TEM).
Experimental groupings for tumor therapy
and experimental protocol
In each study, 20 tumor-bearing nude mice were
randomly divided into four groups, with five mice in each group.
The groups were: the A group, negative control (sham treatment);
the B group, UCA only; the C group, low-frequency ultrasound (US);
and the D group, US+UCA. A microbubble UCA (SonoVue, Bracco SpA,
Milan, Italy) was used. The mice were anesthetized by
intraperitoneal injection using 0.3 ml 1% pentobarbital sodium.
After successful anesthesia, the tumor xenografts were subsequently
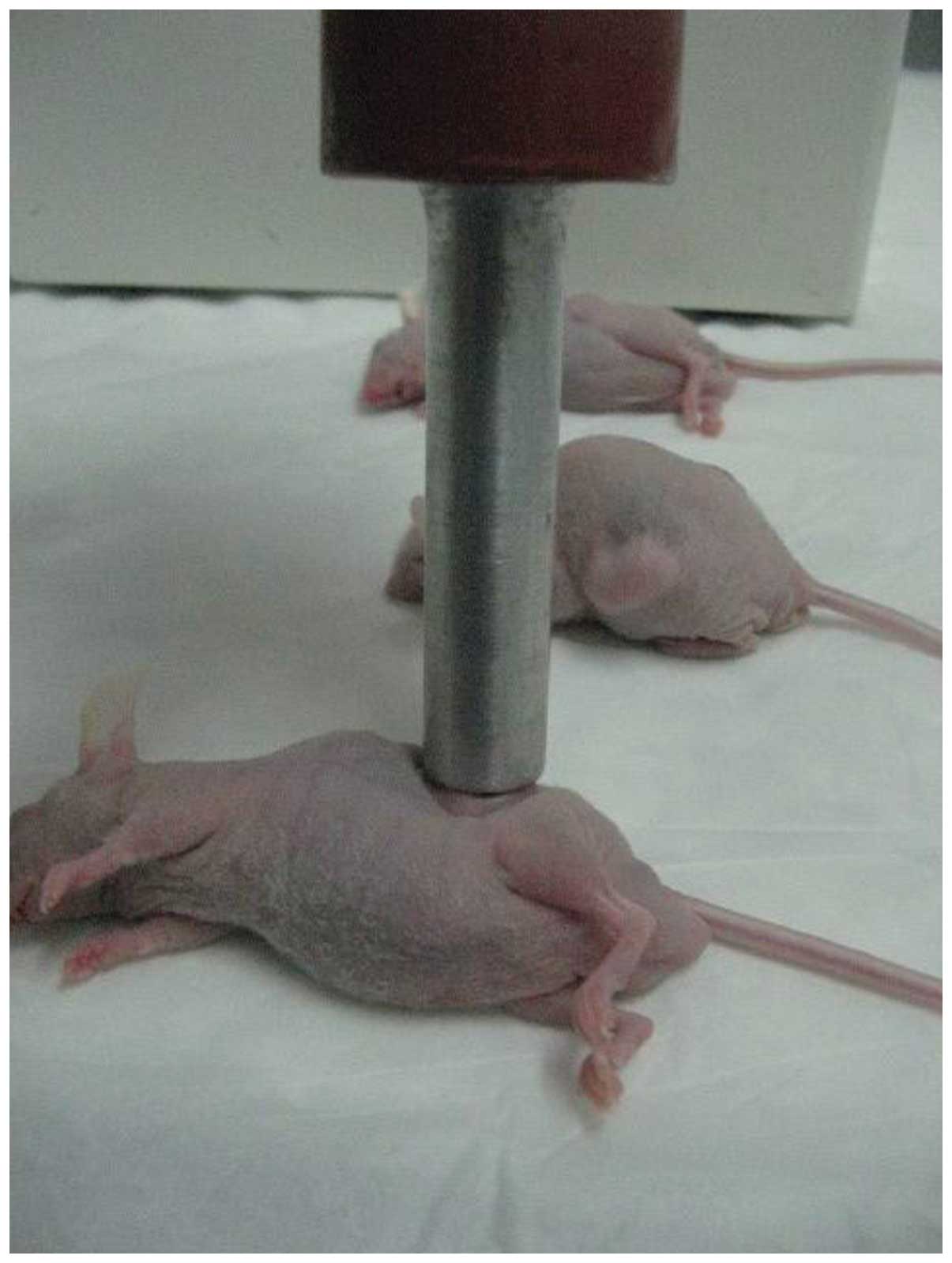
sonicated using a transducer (Fig.
1) manufactured in the Shanghai Institute of Ultrasound in
Medicine at Shanghai Jiaotong University placed on the skin with
contact gel (Aquasonic 100; Parker Laboratories Inc., Fairfield,
NJ, USA). The diameter of the therapeutic ultrasound transducer was
~13 mm, which could cover the entire tumor (Fig. 2). Low-frequency ultrasound
parameters were set at 21 kHz, 26 mW/cm2, duty cycle 40%
(on 2 sec, off 3 sec) and duration 3 min once every other day for
two weeks. The intrinsic frequency of the probe is 21 kHz. In the
pilot study, with the power adjustment to 26 mW/cm2, no
significant heat effect was found on the skin of nude mice, so the
cavitation effect on the tumor by low-frequency ultrasound was
explored. As in vivo transit time of contrast agents in
subcutaneous tumor in CEUS is ~3 min, we set the treatment
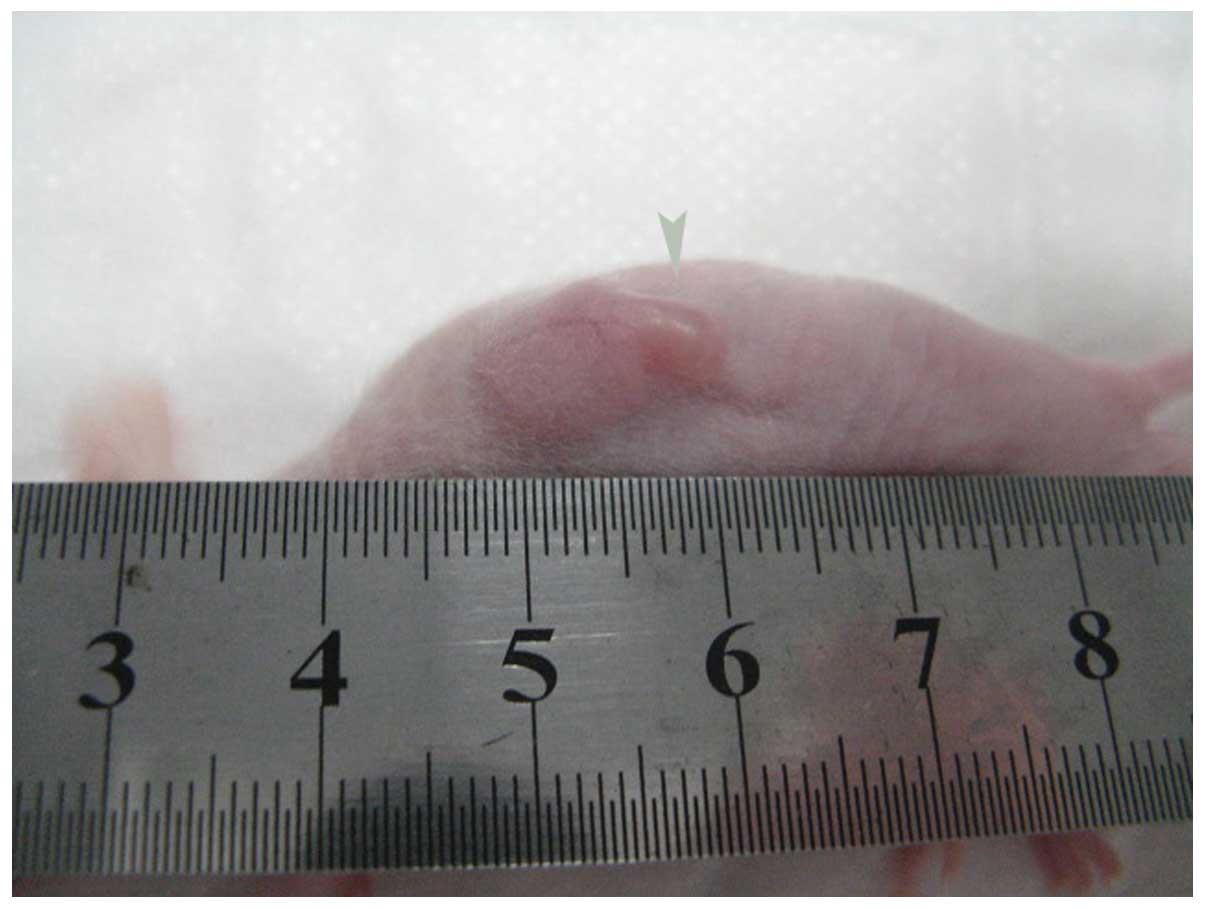
procedure for 3 min. On the sixteenth day in the US and the US+UCA
group, necrosis appeared at the edge of the tumor (arrow) in the
control group (Fig. 3), thus, we
set the experiment duration to 2 weeks (14 days), to ensure the
same experimental conditions with maximum exclusion from other
interference factors. The UCA was administered via the tail veins
of nude mice by bolus injection. It was composed of a phospholipid
shell containing sulfur hexafluoride microbubbles. The dose of
contrast agent administered was 0.2 ml per mouse for each
treatment. The concentration of contrast agent is
1.8×109 microbubbles/ml.
Contrast-enhanced ultrasonography
CEUS is a useful tool for assessing tumor
neovascularity and also for monitoring anti-angiogenic therapies
(12). In the first study, at the
initiation (0 week) and completion (2 weeks) of the experiment, the
subcutaneous prostate cancer of nude mice was examined by CEUS.
CEUS images of the tumors were obtained using Mylab90 instrument
(Baisden Medical Co., Italy) by an experienced examiner. The
frequency of the probe used was 15 MHz. The UCA used was SonoVue, a
sulfur hexafluoride UCA (Bracco). The agent (25 mg) was shaken for
a~1 min with 5 ml of 0.9% saline solution, and 0.2 ml of this
suspension was injected as a bolus manually through a 1-ml syringe
placed in the tail vein. Following the bolus injection, the
real-time enhancement pattern of contrast agent inside the tumor
was observed for 3–5 min and the imaging video was recorded. Images
were recorded digitally on an optical disc and analyzed offline.
The video was replayed and the area of the whole tumors was chosen
as the manually outlined region of interest (ROI) (13). The time-intensity curve (TIC) was
drawn automatically with quantitative imaging analysis software
(Qontraxt) to obtain the following parameters under intralesion
contrast perfusion: time to peak intensity (TTP), peak intensity
(PI) and the area under the curve (AUC). Data were processed in the
same conditions with the same ultrasound system. The enhancement
patterns and TIC results were analyzed by one physician. Qontraxt
is an easy-to-use software that reads a time sequence of perfusion
images stored in digital format, and allows the objective
evaluation of quantitative perfusion parameters. It performs a full
map parametric analysis of any portion of an organ during a
selected set of frames. For this, the software makes a combination
of all the selected frames into a unique set of parametric images.
The loop of images is automatically processed after the tissue
region and the perfusion period are defined. The resulting
parametric maps allow a visual assessment of perfusion properties
over the entire selected ROI at once.
Immunohistochemistry
In the second study, the samples of tumors were
fixed with formaldehyde, dehydrated with a graded alcohol series
and embedded in paraffin. The sections were incubated with primary
antibodies against cyclooxygenase-2 (COX-2) and vascular
endothelial growth factor (VEGF; Santa Cruz Biotechnology Inc.,
Santa Cruz, CA, USA) at a 1:100 dilution, and subsequently
incubated with appropriate biotinylated secondary antibody as
previously detailed (14).
Colorimetric detection was performed using a DAB detection kit
(Wuhan Boster Biological Technology Co., Ltd., Wuhan, China).
Images were acquired using an Olympus BX51 microscope. The
percentage of cells expressing the marker was classified
qualitatively, based on the intensity of the immunohistochemical
staining and the percent of cells that were stained as follows
(15): score 0, low intensity
staining in 0–24% of cells; score 1, low to moderate intensity
staining in 25–49% of cells; score 2, moderate to strong staining
in 50–74% of cells; and score 3, strong intensity staining in
75–100% of cells.
Confocal laser microscopy
Microassessment of vascular endothelium can be
achieved in selected reasonable sized areas from tissue sections
using laser confocal microscopy. Fluorescence expression and
distribution pattern were observed using an Olympus FluoView FV500
confocal laser microscope. The digital image subtraction method was
devised to eliminate auto fluorescence. Slices were coded so that
analyses could be performed without knowing which treatment each
individual animal had received. For each sample, RFP expression and
therapy efficiency were evaluated in six randomly chosen fields per
section. After thorough washing in normal Tyrode’s solution,
samples were directly embedded in optimal cutting temperature
compound and quickly frozen in liquid nitrogen for VEGF and COX-2
determination using confocal microscopy. Frozen 4 μm-thick sections
were spread over glass slides, allowed to dry for 1 h and stored
desiccated at −80°C until use. Tumor preparations were loaded with
either 12.5 μM fluo-3 acid or 10 μM sodium green acid for 15 min at
room temperature. After several washes in Tyrode’s buffer, sections
were mounted with a coverslip in 50% glycerol in phosphate-buffered
saline (PBS). Excitation and emission wavelengths were 488 nm (10%)
and >530 nm, respectively.
TUNEL staining assay
Apoptosis is an organized process of cell death,
which occurs naturally. The induction of apoptosis is of interest
in research, as it can be used to evaluate the efficiency of tumor
treatment (16). Ultrasound
exposure can result in cellular and tissue damage and cell
apoptosis (17,18). The third study was performed to
examine the potential apoptosis of sonication to tumor-bearing nude
mice. Apoptotic cells were detected in deparaffinized tissue
sections using a Fluorescein-based In Situ Cell Death Detection Kit
(Roche Applied Science, Indianapolis, IN, USA) according to the
manufacturer’s protocols. Sections were imaged by confocal
microscopy for the presence of fluorescein-positive nuclei. The
populations were quantified as a percentage of the total cells
present in the samples.
Transmission electron microscopy
Also in the third study, each tumor sample ~1
mm3 for transmission electron microscopy (TEM) was fixed
in 2% glutaraldehyde and PBS for 2 h at 4°C followed by PBS buffer
and washed twice for 10 min. After treatment with 1% osmium
tetroxide in PBS specimens were fixed in 4°C for 2 h and dehydrated
with 30%, followed by 50%, followed by 70% ethanol three times each
for 10 min. The samples were then embedded in propylene oxide for 2
h and stained with lead citrate E. Finally, after sectioning,
specimens were examined using TEM (Philips CM-120; Philips,
Eindhoven, The Netherlands).
Statistical analysis
Statistical analysis was performed using SPSS
version 11.0 (SPSS Inc., Chicago, IL, USA). The Student’s t-test
was used to make a statistical comparison between groups. All
testing was carried out using Prism 3.0 (GraphPad, San Diego, CA,
USA). Error bars were displayed as standard error above the mean.
Statistical significance was determined using P=0.05.
Results
Contrast-enhanced ultrasonography
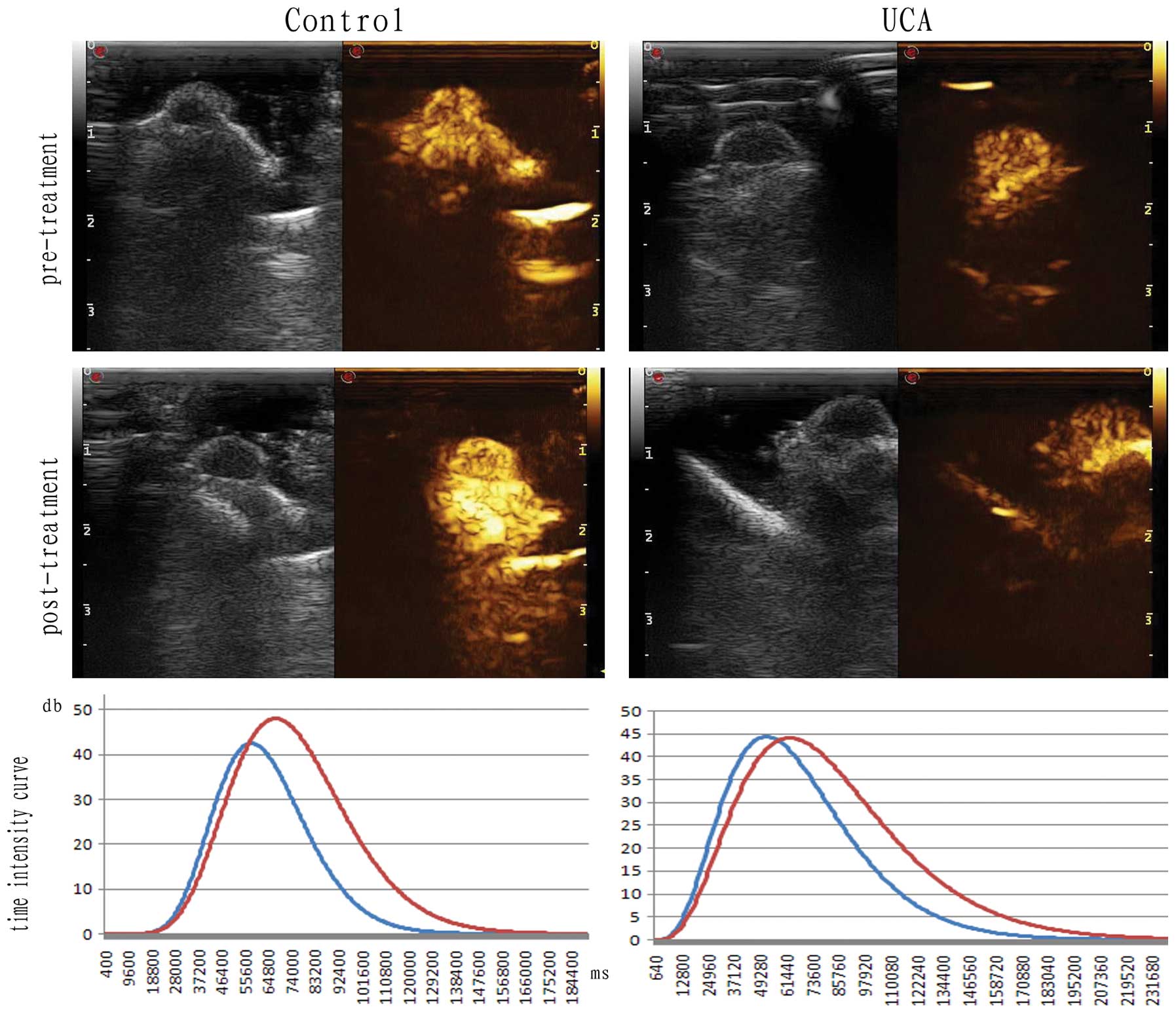
In the first study, only in the US+UCA group, PI and
AUC decreased. The PI of the US+UCA group at the initiation and
completion of the experiments was 40.2±2.311 dB and 22.8±3.527 dB
with t=4.127, P=0.0033. The AUC of the US+UCA group at the
initiation and completion of the experiments was 5.78±0.4831 1/sec
and 3.38±0.3262 1/sec with t=4.117, P=0.0034. There were no
significant changes of PI and AUC in the control, UCA and US
groups. There were no significant changes of TTP in the 4 groups
(Figs. 4–8; Tables
I–III). Insonation of the
tumors by US+UCA may be effective in reducing the blood supply of
tumors.
 | Table IPeak intensity on CEUS prior to and
following treatment in each group (dB). |
Table I
Peak intensity on CEUS prior to and
following treatment in each group (dB).
| Group | 0 weeks | 2 weeks | t | P-value |
|---|
| Control | 39.4±1.435 | 43.6±2.993 | 1.265 | 0.2414 |
| UCA | 45.4±1.288 | 42.1±2.408 | 1.245 | 0.2484 |
| US | 40.4±1.288 | 38.2±2.478 | 0.788 | 0.4536 |
| US+UCA | 40.2±2.311 | 22.8±3.527 | 4.127 | 0.0033 |
 | Table IIIArea under curve on CEUS prior to and
following treatment in each group (1/sec). |
Table III
Area under curve on CEUS prior to and
following treatment in each group (1/sec).
| Group | 0 weeks | 2 weeks | t | P-value |
|---|
| Control | 4.54±0.3614 | 5.12±0.3992 | 1.077 | 0.3129 |
| UCA | 4.52±0.4375 | 5.2±0.4087 | 1.136 | 0.2889 |
| US | 4.58±0.4532 | 4.32±0.3693 | 0.4447 | 0.6683 |
| US+UCA | 5.78±0.4831 | 3.38±0.3262 | 4.117 | 0.0034 |
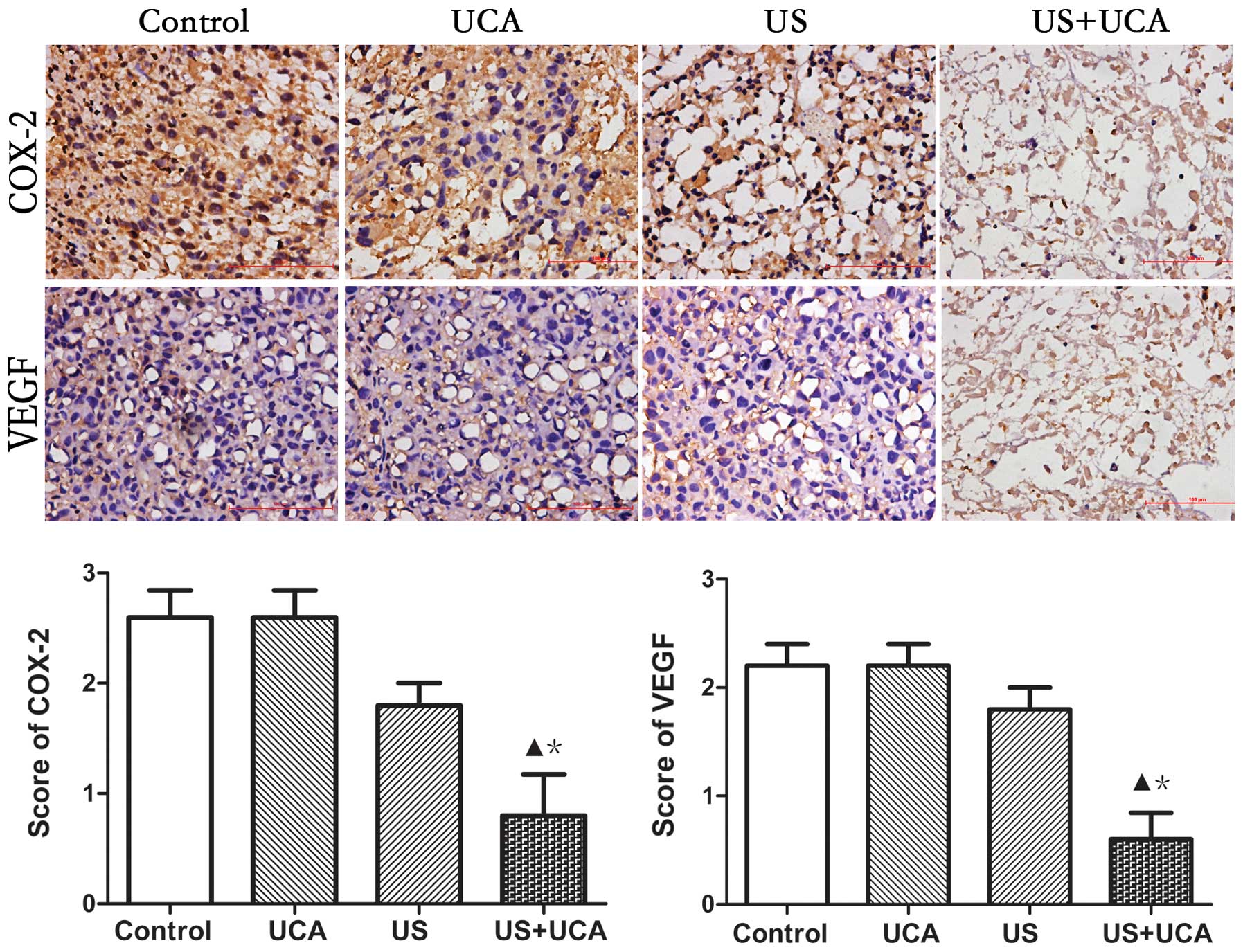
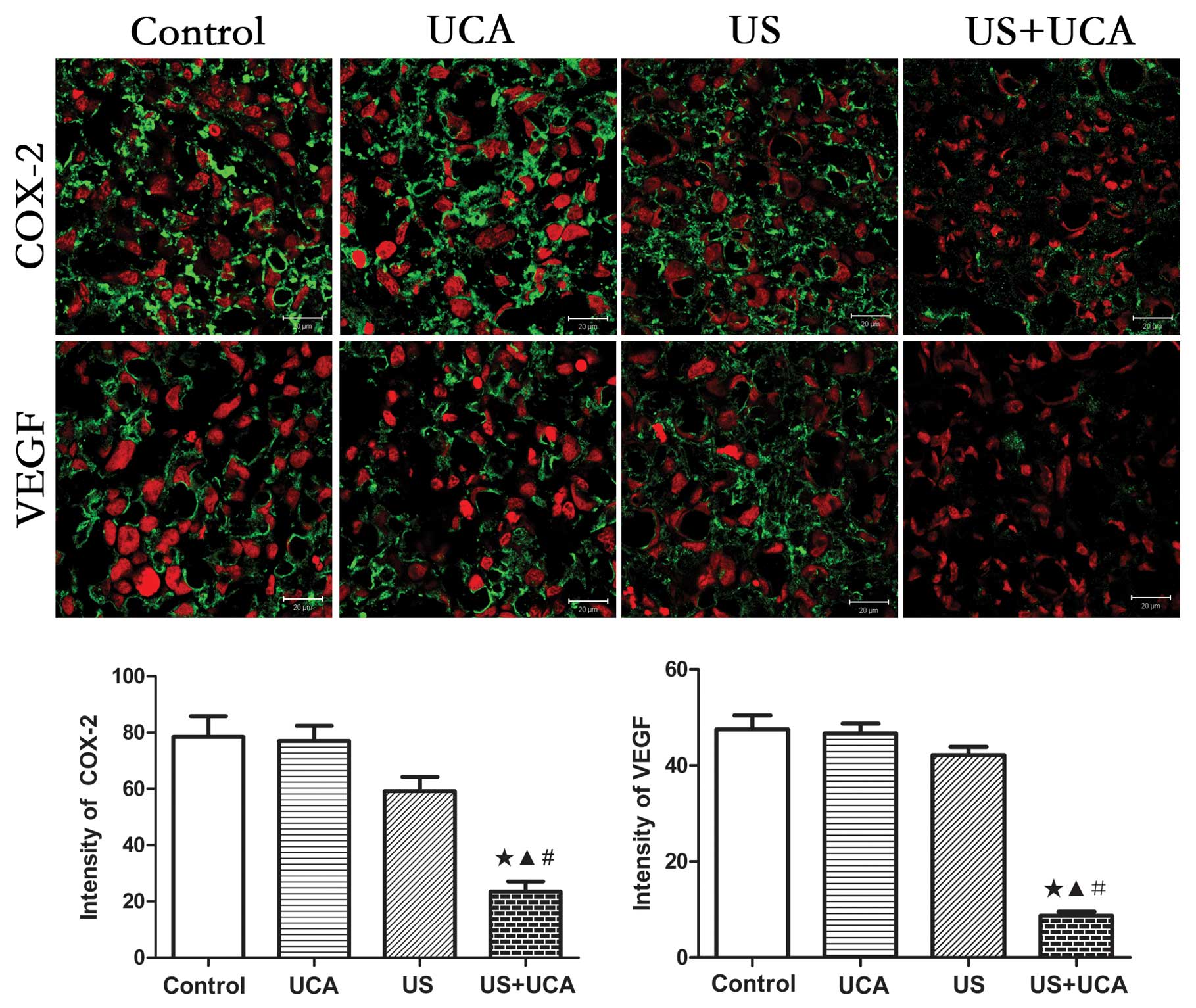
Immunohistochemistry
Scores were classified as 0 to 3, based on the
intensity of staining and the percentage of positive cells. The
results indicated that the intensity score for the UCA group was
the same as that for the control group. This indicated that the UCA
alone had no effect on protein expression in the tumor vascular
endothelium. In the US group the staining intensity decreased, but
there was no significant difference as compared with the control
and UCA groups (P>0.05). However, in the US+UCA group, the
staining intensity of COX-2 and VEGF was lower than in the other 3
groups (P<0.05) (Fig. 9).
Therefore, the combination of US and UCA could lead to significant
downregulation of the intensity of staining of COX-2 and VEGF
protein.
Confocal laser microscopy
In the control group, COX-2 and VEGF protein
expression was stronger than in the other 3 groups. The mean
intensity values for COX-2 in vascular endothelial cells and
cytoplasm in the control, UCA, US and US+UCA groups were
78.44±7.367, 76.95±5.518, 59.21±5.113 and 23.43±3.608,
respectively. There were significant differences in protein
expression among the 4 groups as determined using the ANOVA test,
with F=21.44 and P<0.0001. Using the Newman-Keuls multiple
comparison test, a significant difference was found between the
US+UCA group and other 3 groups (p<0.001), but there was no
significant difference between the control, UCA and US groups
(P>0.05) (Fig. 10). The mean
intensity values of VEGF in the vascular endothelial cells and
cytoplasm for the control, UCA, US and US+UCA groups were
47.51±2.905, 46.66±2.046, 42.17±1.733 and 8.71±0.8691,
respectively. There were significant differences in protein
expression among the four groups as determined using the ANOVA
test, with F=83.67 and P<0.0001. Using the Newman-Keuls multiple
comparison test, a significant difference was found between the
US+UCA group and the other 3 groups (P<0.001), but there was no
significant difference between the control, UCA and US groups
(P>0.05) (Fig. 10).
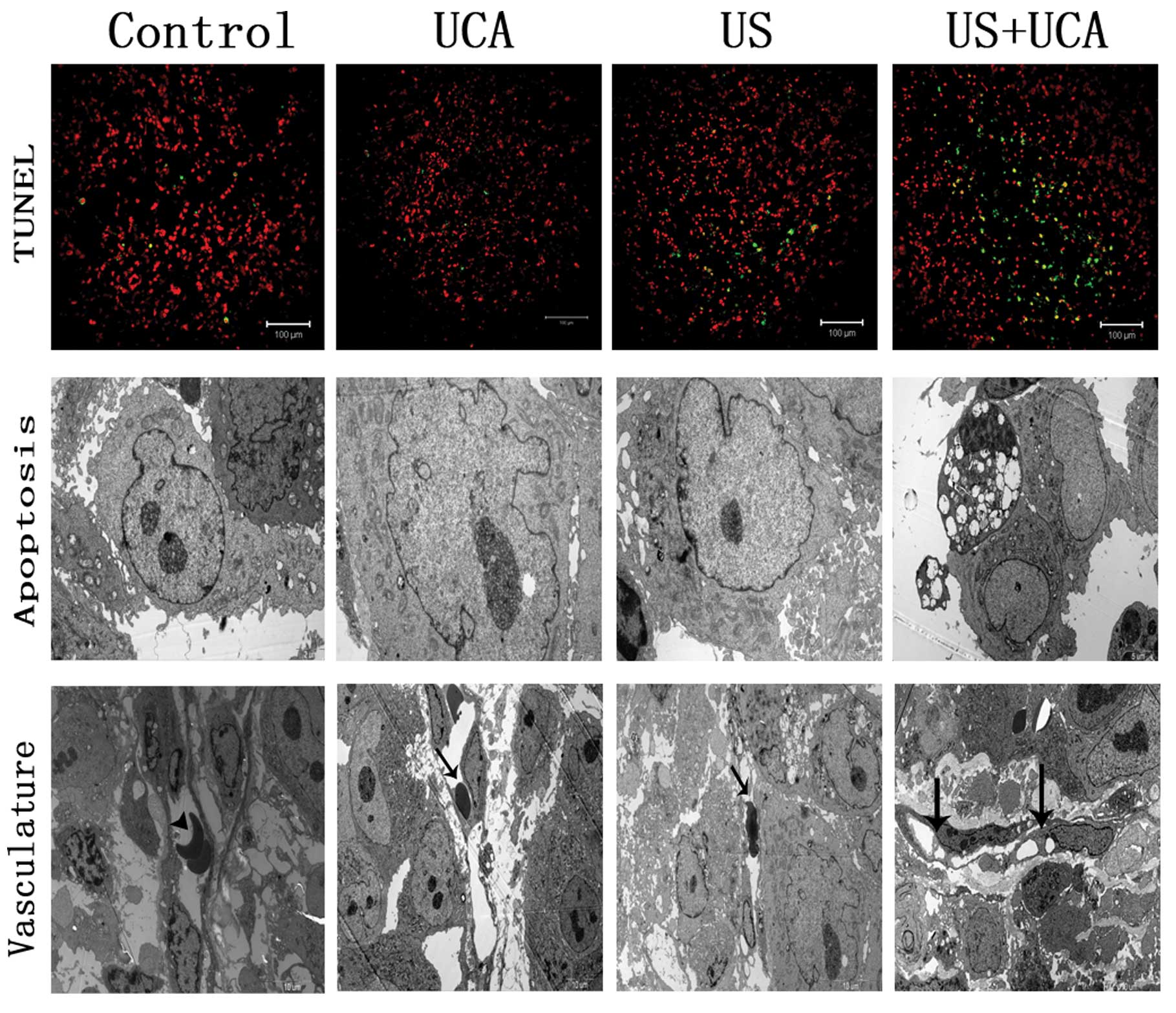
TUNEL staining
In the third study, the average apoptotic rates of
tumor cells in the control, UCA, US and US+UCA groups were: 4.6±1,
6.2±1.72, 11±1.9 and 47.4±8.3%, respectively. There was a
significant difference between the US+UCA and the control, UCA, US
groups with t=5.108, P=0.0009; t=4.849, P=0.0013 and t=4.267,
P=0.0027, respectively (Fig.
11).
Transmission electron microscopy
TEM revealed apparent apoptotic bodies and vascular
lumen occlusion in the US+UCA group. Most tumor cells were found
normal in the other 3 groups. Intact vascular lumen and normal
erythrocytes in the tumor vessels were also found in the control,
UCA and US groups (Fig. 12).
Discussion
A critical event in tumor growth and progression is
the upregulation of angiogenesis. Thus, targeting angiogenesis has
become an attractive treatment modality in cancer medicine. In the
present study, we carried out a novel antitumor study in which
subcutaneous tumors implanted in nude mice were exposed to 21 kHz
ultrasound, with a pressure amplitude of 26 mW/cm2, for
3 min in the presence of the microbubble contrast agent SonoVue.
After 2 weeks of treatment, we used CEUS, immunohistochemistry,
confocal laser microscopy, TUNEL and TEM, respectively to assess
the outcome of the treatment.
Clinical trials have shown that CEUS can be used to
assess the anticancer efficacy of antiangiogenic treatments
(12). The PI calculated from the
TIC is considered to represent flux per unit volume of a scanning
lesion. Thus, analyzing the PI using CEUS in tumor parenchyma may
aid in the evaluation of the efficacy of anti-angiogenesis
treatment of tumors (19). TIC on
the CEUS can be used to quantitatively assess tumor
microcirculation and reduce the subjectivity of ultrasonography
examinations. PI and AUC on the TIC on CEUS can provide
non-invasive parameters for evaluation of tumor vascularity as PI
and AUC were positively correlated with microvessel density (MVD)
(20). In the present study, a
decreased PI and AUC in the contrast curve were observed following
treatment with low-frequency ultrasound combined with contrast
agent. According to a study by Wilhelm et al(21), the anti-angiogenic agent can inhibit
angiogenesis and reduce MVD. Lavisse et al(22) also reported that the PI was
decreased using an anti-angiogenesis treatment. Tumors can induce
the growth of new blood vessels to obtain oxygen and nutrition for
their growth, and as a consequence the total blood flow in the
tumor increases, which is known as angiogenesis. Following
treatment, if the tumor has a poor parenchymal vascular network due
to decreased angiogenic activity, atrophy of arterioles in the
parenchyma can occur, and decreased blood perfusion per unit volume
may decrease the onset of tumor enhancement. This may be the reason
why PI and AUC were reduced on CEUS after US+UCA treatment of
subcutaneous tumors of nude mice.
In the second study, in order to evaluate the
results of the treatment, we used immunohistochemistry and confocal
laser microscopy to detect angiogenesis-associated gene proteins,
such as VEGF and COX-2 of tumor tissue. VEGF is a primary stimulant
for tumor angiogenesis, making it a critical target for cancer
therapy. COX-2 is associated with carcinogenesis due to stimulation
of cell proliferation, inhibition of apoptosis and enhancement of
angiogenesis (23). Inhibition of
VEGF and COX-2 can be seen as an attractive therapeutic target in
the treatment of cancer. Confocal microscopy constitutes a powerful
state-of-the-art technique in the investigation of vessel structure
and function in normal and pathological conditions, in relation to
tumor treatment. In contrast to conventional wide-field fluorescent
and light microscopy, images captured using confocal laser scanning
microscopes showed much improved clarity with successful
elimination of out-of-focus background noise via the pinhole
(24). Confocal fluorescent
microscopic studies of tumor tissue have been made possible by the
intrinsic green autofluorescent properties of tissue. The results
of the present study showed that, following intravenous injection
of ultrasound contrast agent, there was a clearly decreased VEGF
and COX-2 gene expression in the irradiated tumors of nude
mice.
In the third study, tumor cells in the US+UCA group
had substantially higher apoptosis, compared with the control, UCA
and US groups. Low-frequency ultrasound exposure combined with
contrast agent induced substantial apoptosis for tumor cells in the
tumor-bearing mice. The use of UCA in addition to low-frequency
ultrasound is of significance in antitumor therapeutic
applications. In previous research, exposure of cells to ultrasonic
cavitation was shown to induce apoptosis in addition to the
conventionally reported instantaneous cell lysis and necrotic
disintegration (25). Other studies
(26,27) also reported that the induction of
apoptosis by ultrasound exposure has been directly linked to
inertial cavitation by its dependence on the presence of a UCA,
which provides cavitation nuclei, and by the influence of different
dissolved gases. Using TEM in the present study, lumen occlusion
were observed in irradiated vessels in the US+UCA group. However,
there were no obvious vascular changes in control, UCA and US
groups. UCAs, which are artificially augmented population
cavitation nuclei, play an important role in the treatment of
murine tumors during anti-vasculature therapy. Insonified by
low-frequency ultrasound pressure, bubbles become unstable,
collapse and fragment in tissue, and this phenomenon is referred to
as the bioeffects of acoustic cavitation. Acoustic cavitation
involves the concentration of acoustical energy and its conversion
into local mechanical perturbation, which can also damage nearby
biological cells and structures such as vascular endothelium and
vessel lumens. In our study, observed lumen occlusion of vessel
leading to the decreased blood supply may be the major reason why
PI and AUC were decreased on CEUS and COX-2 and VEGF expression
declined in immunohistochemistry and confocal laser microscopy.
Sonicated by ultrasound, bubble expansion
significantly distended the vessel to ~2.7 times its original
diameter (28). The bubble then
collapsed at 1.05 μs leading to almost axially symmetric vessel
invagination. The diameter of the vessel at maximum invagination is
~0.4 times its original diameter. Invagination which generates
higher strains on the vessel wall than distention, was commonly
observed when bubbles collapsed near the vessel wall, which pulled
the vessel inward toward the lumen (28). Sonicated by low frequency
ultrasound, the non-linear effect of microbubble cavitation is
rather strong, and even low intensity US could cause strong
biological effects to cells (29).
We hypothesize that cumulative effects of vessel invagination
produced by substantial microbubble fragmentation irradiated by 21
kHz US, had a tendency to vascular stenosis, and long-term effects
will eventually lead to vascular occlusion. In the future,
high-speed photomicrography system may be used to study the
relationship between the microbubble sonicated by 21kHz ultrasound
and the blood vessel in vivo.
In the UCA alone group in our study, COX-2 and VEGF
expression was the same as that in the control group when assayed
using immunohistochemistry and laser confocal microscopy. The
reason for this may be that after the UCA microbubbles were
injected through the tail vein, they went through the whole body
and were excreted through the respiratory tract. The diameter of
bubbles was about 2.5 μm, which was smaller than the red blood
cells, but larger than the vascular endothelial gap. Thus, the
bubbles seldom penetrated into the tissue spaces. Therefore, in the
UCA alone group these bubbles had little effect on vascular
endothelium and tumor tissue. The protein expression detected by
immunohistochemistry and laser confocal microscopy was similar to
the control group.
There were some limitations to our study. First,
although US in combination with UCA had an effect on the vessel
that was related to protein expression in the tumor tissue, US
alone had a few effects on the tumor tissue. Thus, the exact
mechanism of vascular damage has not been fully elucidated in our
study and requires further research. Second, potential adverse
effects on blood vessels in normal tissues were not investigated in
the US+UCA group and thus require further exploration in the
future.
In general, low-frequency ultrasound in combination
with contrast agent was found to be effective in decreasing the PI
and AUC of contrast ultrasound imaging and in reducing the
expression of VEGF or COX-2 in the vascular endothelium and
cytoplasm. More apoptosis was also found in the US+UCA group.
Changes to blood vessels may be induced by insonation of 21 kHz
ultrasound when they contain exogenous intraluminal UCA. However,
the exact mechanisms of low-frequency ultrasound and contrast agent
on tumor angiogenesis remain to be identified. The interaction of
microbubbles with tissue remains a subject of extensive theoretical
and experimental study, and is particularly geared towards the
optimization of local tumor therapy.
Acknowledgements
This study was supported in part by the National
Natural Science Foundation of China (81271597) and the Key Basic
Research Project of Shanghai Science and Technology Commission
(10JC1412600). The authors thank Mao Xin and Xie Guo Ming for
helping with the tumor cell culture and tumor inoculation. We also
thank Zhen Ming Xia and Luan Yan Yan for their help with the
contrast-enhanced ultrasonography.
References
|
1
|
Samant RS and Shevde LA: Recent advances
in anti-angiogenic therapy of cancer. Oncotarget. 2:122–134.
2011.PubMed/NCBI
|
|
2
|
Sherwood LM, Parris EE and Folkman J:
Tumor angiogenesis: therapeutic implications. N Engl J Med.
285:1182–1186. 1971. View Article : Google Scholar
|
|
3
|
Hwang JH, Brayman AA, Reidy MA, Matula TJ,
Kimmey MB and Crum LA: Vascular effects induced by combined 1-MHz
ultrasound and microbubble contrast agent treatments in vivo.
Ultrasound Med Biol. 31:553–564. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Skyba DM, Price RJ, Linka AZ, Skalak TC
and Kaul S: Direct in vivo visualization of intravascular
destruction of microbubbles by ultrasound and its local effects on
tissue. Circulation. 98:290–293. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wood AKW, Ansaloni S, Ziemer LS, Lee WMF,
Feldman MD and Sehgal CM: The antivascular action of physiotherapy
ultrasound on murine tumors. Ultrasound Med Biol. 31:1403–1410.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wood AKW, Bunte RM, Price HE, et al: The
disruption of murine tumor neovasculature by low-intensity
ultrasound-comparison between 1- and 3-MHz sonication frequencies.
Acad Radiol. 15:1133–1141. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wollina U, Heinig B, Naumann G, Scheibe A,
Schmidt WD and Neugebauer R: Effects of low-frequency ultrasound on
microcirculation in venous leg ulcers. Indian J Dermatol.
56:174–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barnett SB, Rott HD, ter Haar GR, Ziskin
MC and Maeda K: The sensitivity of biological tissue to ultrasound.
Ultrasound Med Biol. 23:805–812. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johnson CA, Sarwate S, Miller RJ and
O’Brien WD Jr: A temporal study of ultrasound contrast
agent-induced changes in capillary density. J Ultrasound Med.
29:1267–1275. 2010.PubMed/NCBI
|
|
10
|
Yang X and Church CC: A model for the
dynamics of gas bubbles in soft tissue. J Acoust Soc Am.
118:3595–3606. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hutcheson J, Schlicher R, Hicks H and
Prausnitz M: Saving cells from ultrasound-induced apoptosis:
quantification of cell death and uptake following sonication and
effects of targeted calcium chelation. Ultrasound Med Biol.
36:1008–1021. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lassau N, Chami L, Benatsou B, Peronneau P
and Roche A: Dynamic contrast-enhanced ultrasonography (DCE-US)
with quantification of tumor perfusion: a new diagnostic tool to
evaluate the early effects of antiangiogenic treatment. Eur Radiol.
17:F89–F98. 2007. View Article : Google Scholar
|
|
13
|
Paprottka P, Cyran C, Zengel P, et al:
Non-invasive contrast enhanced ultrasound for quantitative
assessment of tumor microcirculation. Contrast mixed mode
examination vs only contrast enhanced ultrasound examination. Clin
Hemorheol Microcirc. 46:149–158. 2010.
|
|
14
|
Yu SM and Kim SJ: Endoplasmic reticulum
stress (ER-stress) by 2-deoxy-D-glucose (2DG) reduces
cyclooxygenase-2 (COX-2) expression and N-glycosylation and induces
a loss of COX-2 activity via a Src kinase-dependent pathway in
rabbit articular chondrocytes. Exp Mol Med. 42:777–786. 2010.
View Article : Google Scholar
|
|
15
|
Forsberg F, Dicker AP, Thakur ML, et al:
Comparing contrast-enhanced ultrasound to immunohistochemical
markers of angiogenesis in a human melanoma xenograft model:
preliminary results. Ultrasound Med Biol. 28:445–451. 2002.
View Article : Google Scholar
|
|
16
|
Kotturi H, Li J, Branham-O’Connor M, et
al: Tumor cells expressing a fusion protein of MULT1 and Fas are
rejected in vivo by apoptosis and NK cell activation. Gene Ther.
15:1302–1310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feril LB Jr and Kondo T: Biological
effects of low intensity ultrasound: the mechanism involved, and
its implications on therapy and on biosafety of ultrasound. J
Radiat Res. 45:479–489. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miller DL and Dou C: Induction of
apoptosis in sonoporation and ultrasonic gene transfer. Ultrasound
Med Biol. 35:144–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Forsberg F, Ro RJ, Fox TB, et al: Contrast
enhanced maximum intensity projection ultrasound imaging for
assessing angiogenesis in murine glioma and breast tumor models: a
comparative study. Ultrasonics. 51:382–389. 2011. View Article : Google Scholar
|
|
20
|
Wang J, Lv F, Fei X, et al: Study on the
characteristics of contrast-enhanced ultrasound and its utility in
assessing the microvessel density in ovarian tumors or tumor-like
lesions. Int J Biol Sci. 7:600–606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wilhelm SM, Carter C, Tang LY, et al: BAY
43-9006 exhibits broad spectrum oral antitumor activity and targets
the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in
tumor progression and angiogenesis. Cancer Res. 64:7099–7109. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lavisse S, Lejeune P, Rouffiac V, et al:
Early quantitative evaluation of a tumor vasculature disruptive
agent AVE8062 using dynamic contrast-enhanced ultrasonography.
Invest Radiol. 43:100–111. 2008. View Article : Google Scholar
|
|
23
|
Chan TA: Nonsteroidal anti-inflammatory
drugs, apoptosis, and colon-cancer chemoprevention. Lancet Oncol.
3:166–174. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu J, Min W, Conchello JA, Xie XS and
Lichtman JW: Super-resolution laser scanning microscopy through
spatiotemporal modulation. Nano Lett. 9:3883–3889. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ashush H, Rozenszajn LA, Blass M, et al:
Apoptosis induction of human myeloid leukemic cells by ultrasound
exposure. Cancer Res. 60:1014–1020. 2000.
|
|
26
|
Honda H, Zhao QL and Kondo T: Effects of
dissolved gases and an echo contrast agent on apoptosis induced by
ultrasound and its mechanism via the mitochondria-caspase pathway.
Ultrasound Med Biol. 28:673–682. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feril LB, Kondo T, Zhao QL, et al:
Enhancement of ultrasound-induced apoptosis and cell lysis by
echo-contrast agents. Ultrasound Med Biol. 29:331–337. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen H, Brayman AA, Bailey MR and Matula
TJ: Blood vessel rupture by cavitation. Urol Res. 38:321–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tian ZM, Wan MX, Lu MZ, Wang XD and Wang
L: The alteration of protein profile of Walker 256 carinosarcoma
cells during the apoptotic process induced by ultrasound.
Ultrasound Med Biol. 31:121–128. 2005. View Article : Google Scholar : PubMed/NCBI
|