Introduction
Epithelial ovarian carcinoma (EOC) is the most
aggressive tumor among gynecologic malignancies; it can be treated
effectively with surgery and chemotherapy during the early stage
(1,2). However, the difficulty of early
diagnosis and the rapid metastasis represent outstanding clinical
challenges associated with EOC, which lead to the frequent failure
of current treatment strategies (3). To date, the mechanism of cancer
metastasis has yet to be clarified. To improve the 5-year survival
rate of EOC patients, further investigations into EOC pathogenesis
and the development of novel treatment agents are required.
The mucin-type O-glycosylation is a common
post-translational modification of proteins, which is initiated by
the family members of polypeptide
N-acetylgalactosaminyltransferases (GALNTs) that transfer
UDP-N-acetylgalactosamine (UDP-GalNAc) to the hydroxyl group of
serine (S) or threonine (T) residues on the target proteins forming
Tn antigen (GalNAca-S/T) (4,5). The
GALNTs family consists of at least 20 members in humans, i.e.
GALNT1 to 14 and GALNTL1 to L6 (6).
Aberrant glycosylation is a characteristic of most types of human
cancer and effects several cellular properties, such as cell
proliferation, apoptosis, differentiation, migration, invasion,
transformation and immune responses (7). Studies have shown that O-glycans and
GALNT genes play important roles in various tumors. For example,
GALNT2 mediates the malignant character of hepatocellular carcinoma
by modifying epidermal growth factor receptor (EGFR) (8). Upregulated GALNT3 promotes pancreatic
cancer cell growth (9). The
overexpression of GALNT6 in breast cancer disrupts mammary acinar
morphogenesis through O-glycosylation of fibronectin (10). GALNT14 expression is a potential
biomarker for breast cancer, and may be involved in modulating the
apoptotic activity of insulin-like growth factor binding protein-3
(IGFBP-3) (11,12). Death-receptor O-glycosylation by
GALNT14 mediates tumor-cell sensitivity to tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL) (13).
Mucin 13 (MUC13), a membrane-bound mucin, is
normally expressed in the large intestine, kidney, trachea, small
intestine and gastric epithelium (14). It has a large 151-amino acid tandem
repeat domain, which is rich in serine and threonine residues that
act as glycosylation sites, a sea urchin sperm protein enterokinase
arginine domain, and three epidermal growth factor-like domains in
the extracellular component, followed by a short transmembrane
domain and a 69-amino acid cytoplasmic domain (15–17).
In recent studies, aberrant expression of MUC13 has been shown in
ovarian, gastric, pancreatic and colorectal cancer, and has been
involved in carcinogenesis and tumor progression (17–20).
However, the precise molecular mechanisms by which MUC13 regulates
EOC properties remain largely unknown.
MUC13 is one of the substrates of GALNT14 (21). In EOC cells, the expression pattern
and function of GALNT14 have not been reported, although
O-glycosylation can mediate multiple cellular properties. In the
present study, we reported that GALNT14 is frequently upregulated
in ovarian cancer cells. Moreover, GALNT14 modifies MUC13
O-glycosylation, and plays critical roles in migration and
cytoskeletal regulation of ovarian cancer cells.
Materials and methods
Cell lines and materials
Human ovarian carcinoma cell lines (SKOV-3, OVCAR-3
and HO8910PM) were kindly donated by the Institute of Ultrasound of
the Chongqing Medical University. HO8910 cells were a gift from the
Institute of the Pathology of Chongqing Medical University.
RPMI-1640 medium was supplied by Gibco (Grand Island, NY, USA).
Recombinant human IL-8 was purchased from Noroprotein (Shanghai,
China), Takara Taq™ and PrimeScript® RT Reagent kit were
supplied by Takara Biotechnology (Dalian, China).
3-(4,5-dimethylthiazol-2-thiazyl)-2,5-diphenyltetrazolium bromide
(MTT), dimethylsulfoxide (DMSO), ERK1/2 inhibitor (PD98059), p38
MAPK inhibitor (SB203580) and PI3K inhibitor (LY294002) were
obtained from Sigma (St. Louis, MO, USA). Rabbit anti-β-actin and
RIPA buffer were purchased from Cell Signaling Technology (Danvers,
MA, USA). Mouse anti-MUC13 was from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). Rabbit anti-GALNT14 was purchased from Sigma.
Biotinylated Vicia villosa agglutinin (VVA) was supplied by
Vector Laboratories, Inc. (Burlingame, CA, USA).
Peroxidase-conjugated AffiniPure goat anti-rabbit IgG (H+L), goat
anti-mouse IgG (H+L), horseradish peroxidase streptavidin were from
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing,
China). Effectene Transfection Reagent was purchased from Qiagen
(Germantown, MD, USA).
Cell culture and siRNA transfection
All cell lines were maintained in RPMI-1640 medium
containing 10% FBS, 50 IU/ml penicillin, 50 μg/ml streptomycin in a
cell culture incubator at 37°C, 5% CO2. Cells were
tested to have no mycoplasma contamination prior to the
experiments. Cell transfections were performed with Effectene
Transfection Reagent according to the manufacturer’s instructions
using 25 nM siRNA targeting GALNT14 in a 6-well plate or 50-ml
flask. The three GALNT14 target sequences used were: siRNA1, 5′-GAU
CCG GGA AAU CAU AUU ATT-3′ (sense) and 5′-UAA UAU GAU UUC CCG GAU
CTT-3′ (antisense); siRNA2, 5′-GCC AAC ACG UAU AUA AAG ATT-3′
(sense) and 5′-UCU UUA UAU ACG UGU UGG CTT-3′ (antisense); siRNA3,
5′-CCA UCC AGA AGG GCA AUA UTT-3′ (sense) and 5′-AUA UUG CCC UUC
UGG AUG GTT-3′ (antisense). Negative control (NC) siRNA and β-actin
siRNA were included. Cells were incubated for 48 h after
transfection and used for further experiments.
Cell viability assay
Cell viability was evaluated by the MTT reduction
assay. SKOV-3 cells were seeded in 96-well plates with
7.5×103 cells/well. After growing to confluence, the
cells were treated with or without IL-8 (25, 50, 100 and 500 μg/l).
Following incubation for 24 h, the medium was discarded, and cells
were incubated with MTT (5 mg/ml) in culture medium at 37°C for 4
h. Then, culture medium was removed, and 100 μl of DMSO per well
was added for formazan dissolution. The absorbance was measured at
a wavelength of 490 nm by a Sunrise Remote Microplate Reader
(Grodig, Austria) and then normalized the value to the control
group.
Wound healing assay
HO8910PM cells were counted and seeded at a density
of 1×105 cells/well in 24-well plates to reach 80–90%
confluence. A sterile 10-μl plastic pipette tip was used to create
three artificial wounds across the cell monolayer per well, and the
debris was removed by washing the cells three times with PBS. Cells
that migrated into the wounded area were visualized and
photographed randomly in each well at 0 and 24 h with a Nikon TEU
2000 inverted microscope. The cell migration ability was estimated
by the relative distance of wound closure.
In vitro migration assays
A total of 5×104 cells were suspended in
100 μl RPMI-1640 medium without serum or growth factors, and plated
in the upper chamber with the non-Matrigel-coated polycarbonate
membrane (24-well insert; 8-μm pore size, Corning Life Sciences).
RPMI-1640 medium (600 μl) containing 10% FBS was added to the lower
chamber as a chemoattractant. Following incubation for 24 h at 37°C
under 5% CO2, cells that had not migrated through the
pores were removed with a cotton swab, whereas cells on the lower
surface of the membrane were fixed in 4% paraformaldehyde and
stained with hematoxylin. The number of stained cells was counted
by an inverted microscope (Nikon TEU 2000).
RNA extraction and reverse
transcription-PCR (RT-PCR) analysis
Total RNA was isolated from the ovarian cancer cell
lines using TRIzol (Takara, Dalian, China) according to the
manufacturer’s specifications. The quantity of RNA samples was
measured by UV absorbance at 260–280 nm using a DNA/RNA GeneQuant
Calculator (Amersham Biosciences, Piscataway, NJ, USA). Total cDNA
was synthesized from 2 μg total RNA in 20 μl of reaction mixture
containing 50 pmol of oligo-dT, 5.0 units of AMV reverse
transcriptase, 40 units of RNase inhibitor, 40 nmol of dNTP, 4 μl
of 5X RT buffer (Bioer, Hangzhou, China). One microliter of the
resulting cDNA samples was taken for the amplification of the
different transcripts by the different primers. The amplification
conditions were: 94°C for 4 min, followed by 30 cycles of 94°C for
30 sec, 54°C for 45 sec and 72°C for 30 sec, and finally 5 min at
72°C. The PCR reactions were performed in 20 μl volumes in the
presence of 10 μM of each of the sense and the antisense primers
using 2 units of BioReady rTaq Polymerase (Bioer). The following
primers for RT-PCR were used: GALNT14 (474 bp): sense 5′-ACC TGG
ACA CCT TCA CCT ACA T-3′; antisense 5′-CCA ATC TGC TCT CAA CAT
TCC-3′. GAPDH (230 bp): sense 5′-CTC TCT GCT CCT CCT GTT CGA
CAG-3′; antisense 5′-GTG GAA TCA TAT TGG AAC ATG T-3′. The PCR
products were resolved by electrophoresis on 1.5% agarose gel
containing 1% Goldview™.
Western blot, Vicia villosa agglutinin
(VVA) lectin blot and immunoprecipitation
For extraction of total protein, ovarian cancer cell
lines were harvested and lysed in 1X RIPA (CST) lysis buffer
supplemented with 5 mM Na fluoride, 1 mM PMSF and 1 mM Na
orthovanadate protease inhibitors. Extracted protein samples were
measured by BCA protein assay (Pierce Chemical Co., Rockford, IL,
USA). Total cell lysates containing 1 mg of protein were
immunoprecipitated with primary antibodies and agarose beads at
4°C. Then, the agarose beads were washed. Prior to western
blotting, cell lysates or immunoprecipitated protein mixed with 5X
SDS sample buffer were boiled for 10 min, electrophoresed on a 10%
polyacrylamide minigel with equal amount (20 μg) and then
transferred onto PVDF membranes. After blocking with 5% non-fat
milk or 3% BSA in TBS for 1 h at room temperature, membranes
blotted with proteins were incubated with the biotinylated VVA
lectin or primary antibodies overnight at 4°C. After washing three
times (for 10 min) in TBST, the membranes were incubated with
HRP-conjugated anti-mouse or anti-rabbit secondary antibodies
(dilution 1:4,000) or HRP-conjugated streptavidin (dilution 1:500)
for 1 h at room temperature. After washing three times (for 10 min)
in TBST, protein bands were visualized using ECL reagents and
quantitated with a Fluor-S (Bio-Rad) instrument.
Statistical analysis
Statistical analysis was performed using SPSS
version 10.0 package (SPSS Inc., Chicago, IL, USA). Student’s
t-test and one-way analysis of variance (ANOVA) were used for
comparisons between groups. Data are presented as the means ± SD of
3 independent experiments. P<0.05 was considered to indicate a
statistically significant difference.
Results
GALNT14 and Tn antigen expression in
ovarian cancer cell lines
The mRNA expression profile of GALNT14 has been
examined in primary normal and malignant tissue samples from ovary,
skin, lung, pancreas, breast, endometrium, bladder and lymphoid
cancer. Up to 30% of tissue samples from various types of human
cancer including ovarian carcinomas showed GALNT14 mRNA
overexpression (13). However, the
GALNT14 functions in ovarian cancer remain largely unknown. Under
these circumstances, we first investigated the GALNT14 expression
in a panel of four EOC cell lines at the mRNA and protein levels by
RT-PCR and western blot analysis to select suitable cell lines for
the subsequent functional studies. Of these cell lines, SKOV-3 and
OVCAR-3 cells showed a faint-GALNT14 expression, whereas HO8910 and
HO8910PM showed a relatively high expression (Fig. 1A and B). Therefore, SKOV-3 and
HO8910 were selected as representative of high- and low-GALNT14
expressing cell lines, respectively. HO8910 and HO8910PM were used
to knock down the endogenous expression of GALNT14. Vicia
villosa lectin (VVA) blot analysis, which preferentially
recognizes terminal N-acetylgalactosamine residue linked to serine
or threonine in a polypeptide (Tn antigen), was also performed to
detect the activity of GALNTs (8,22). The
expression profile of Tn antigen was simultaneously determined in
these cell lines (Fig. 1B). We
found that Tn antigen expression levels in each cell line were
clearly different, which suggested the activities of GALNTs in
different ovarian cancer cells were discrepant.
Knockdown of endogenous expression of
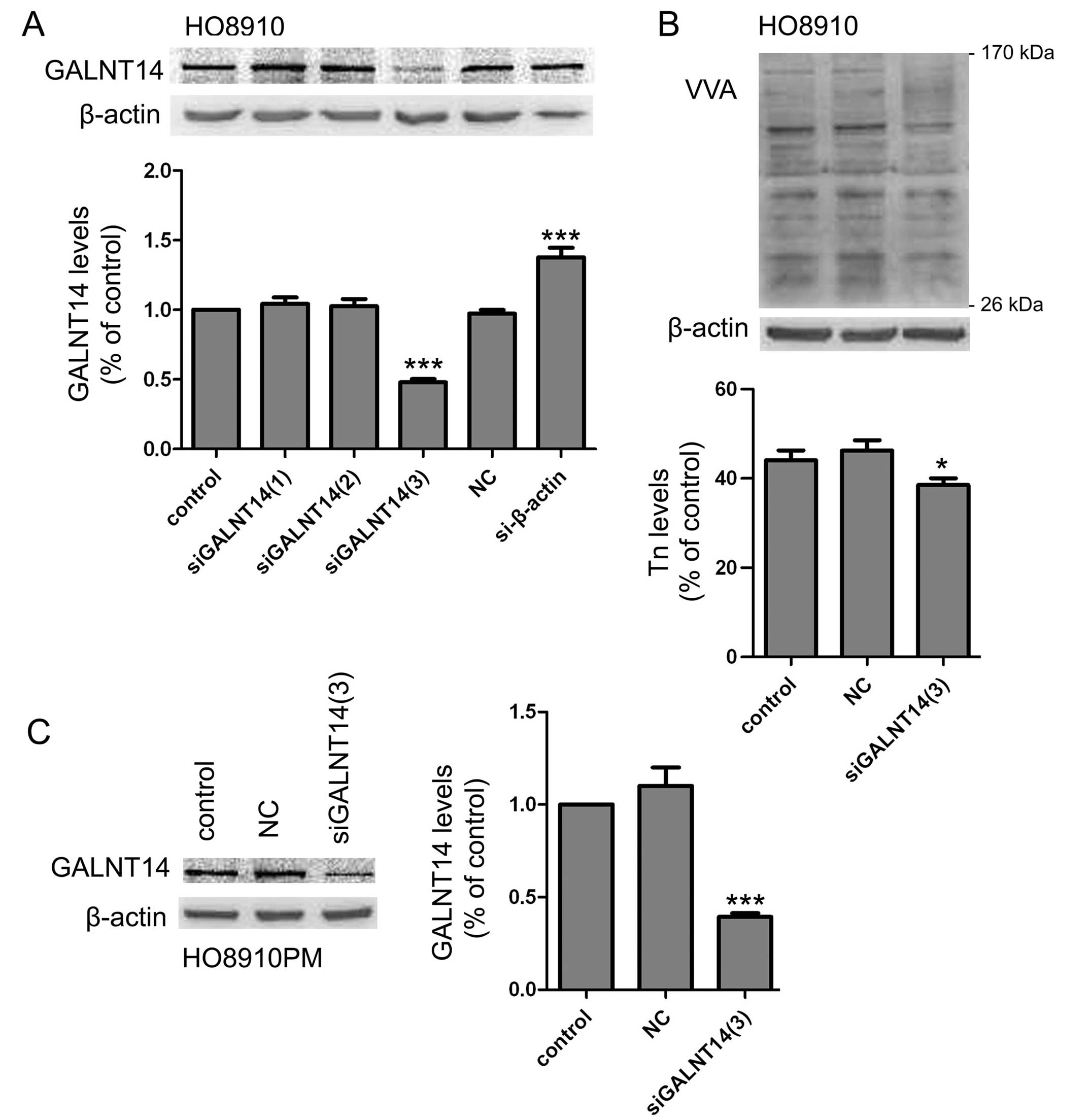
GALNT14 reduces O-glycosylation protein expression
To assay the biological significance of GALNT14 in
ovarian cancer cells, we adopted GALNT14 siRNA to transfect into
HO8910 cells and to make cell mode which was knockdown endogenous
expression of GALNT14. Results showed that the introduction of
siGALNT14(3) resulted in a significant reduction of GALNT14
expression (Fig. 2A). The decreased
glycoproteins were also observed by VVA lectin blot in cell lysates
(Fig. 2B). To further confirm the
efficiency of siGALNT14(3) interference, we measured another
GALNT14-positive ovarian cancer cell line, HO8910PM, by western
blot analysis (Fig. 2C). Therefore,
siGALNT14(3) was selected for subsequent experiments.
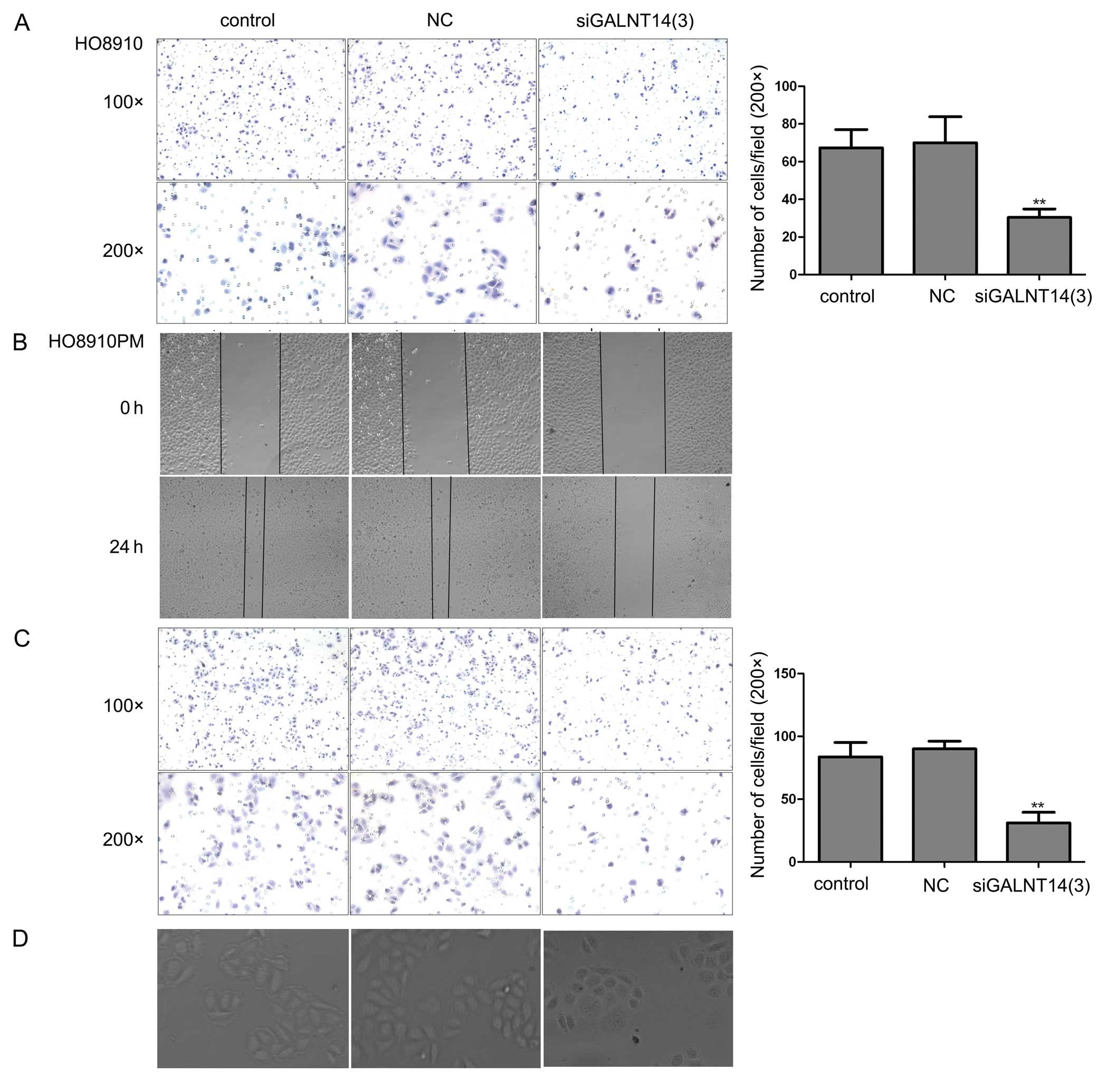
GALNT14 knockdown reduces cellular
migration and alters cellular morphologic characteristics
Increased cellular migration capability is required
for a cancer cell to be competent for metastasis. Thus, we
evaluated the effect of GALNT14 on the migration of HO8910 and
HO8910PM cells by applying Transwell cell migration assay in
vitro. As shown in Fig. 3A and
C, knockdown of GALNT14 significantly suppressed cellular
migration ability in HO8910 and HO8910PM cells (P<0.05). To
further verify the role of GALNT14 in cellular migration, we
performed wound healing assay in 24-well plates. Consistent with
the above results, the number of cells migrating toward the center
of the wound area was markedly depleted in the GALNT14 siRNA group
compared with the control and NC siRNA groups (Fig. 3B). Notably, 2 days after
transfection of siRNA, the clear, bipolar, elongated cell shape was
converted to a relatively round morphology (Fig. 3D). These results suggest that
GALNT14 is closely related to ovarian cancer metastasis.
MUC13 is a substrate of GALNT14 in
ovarian cancer cell lines
MUC13, a transmembrane mucin, is highly expressed in
ovarian cancer tissues compared with the normal/benign ovary
samples. Moreover, the marked changes in cell-cell adhesion, cell
motility, proliferation, and tumorigenesis are observed upon
exogenous MUC13 expression (20),
which are partly similar to the function of GALNT14. In addition, a
panel of mucin-derived peptide substrates such as MUC13, MUC2,
MUC5AC and MUC7 could be glycosylated by glycosyltransferase
GALNT14, which transfers GalNAc to the Ser/Thr residues on the
target substrates (21). Moreover,
according to previous research on the critical roles of MUC1
glycosylation by polypeptide N-acetylgalactosaminyltransferase 6 in
mammary carcinogenesis (23), we
proposed that MUC13 increased the migration of ovarian cancer cell
lines by the glycosylating function of GALNT14. To verify this
hypothesis, we first measured the expression levels of these two
molecules in ovarian cancer cell lines by western blot analysis,
and found that GALNT14 and MUC13 proteins were co-expressed in
ovarian cancer cells (Fig. 4A).
This finding indicated that GALNT14 might contribute to ovarian
carcinogenesis through stabilization of the MUC13 protein. To
investigate the interaction of GALNT14 and MUC13 in detail, we
knocked down GALNT14 expression by siRNA and examined its effect on
the MUC13 protein in HO8910PM cells. The results revealed that the
knockdown of GALNT14 did not affect the expression of MUC13 at the
protein level (Fig. 4B). However,
compared to the control and NC groups, significantly lower levels
of terminal N-acetylgalactosamine residue modified MUC13 were
immunoprecipitated from HO8910PM cell lysates in the GALNT14 siRNA
group, without any changes in the total expression of MUC13 protein
(Fig. 4C). Collectively, these
results suggested that GALNT14 may influence the post-translational
modification and stabilization of MUC13 protein in ovarian cancer
cells.
GALNT14 regulation of the migration
ability of tumor cells is not associated with the IL-8 pathway
It has been demonstrated that CXC-chemokine
interleukin-8 (IL-8) mediates the metastasis of various tumor cells
(24–26). Groux-Degroote et al(27) reported that IL-6 and IL-8 contribute
to the increased expression of some glycosyltransferases and
sulfotransferases participating in the biosynthesis of
sialyl-Lewisx and 6-sulfo-sialyl-Lewisx
epitopes in the human bronchial mucosa. However, the correlation
between IL-8 and GALNT14 has not been reported. Thus, we analyzed
the expression of GALNT14 in ovarian cancer cell lines which were
stimulated by recombinant human IL-8. First, to select the suitable
concentration of IL-8, its inhibitory efficiency on the
proliferation of SKOV-3 cells was examined using MTT assay.
Treatment with IL-8 did not suppress cell proliferation at a low
dose (25–100 μg/l) for 24 h, but it induced marked cytotoxicity at
a dose up to 500 μg/l compared with the control group (Fig. 5A). Furthermore, IL-8 (50–100 μg/l,
24 h) successfully increased the migration of SKOV-3 and HO8910
cell lines (Fig. 5B). However, the
incubation of IL-8 for 24 h in two cell lines did not alter the
expression of GALNT14 protein and Tn antigen (Fig. 5C). To further confirm this result,
we extended the stimulation time of IL-8 to 72 h, but the
expression levels of GALNT14 were not markedly different (Fig. 5D). These data suggest the GALNTs
family including GALNT14 may not be involved in the role of IL-8 in
cellular migration.
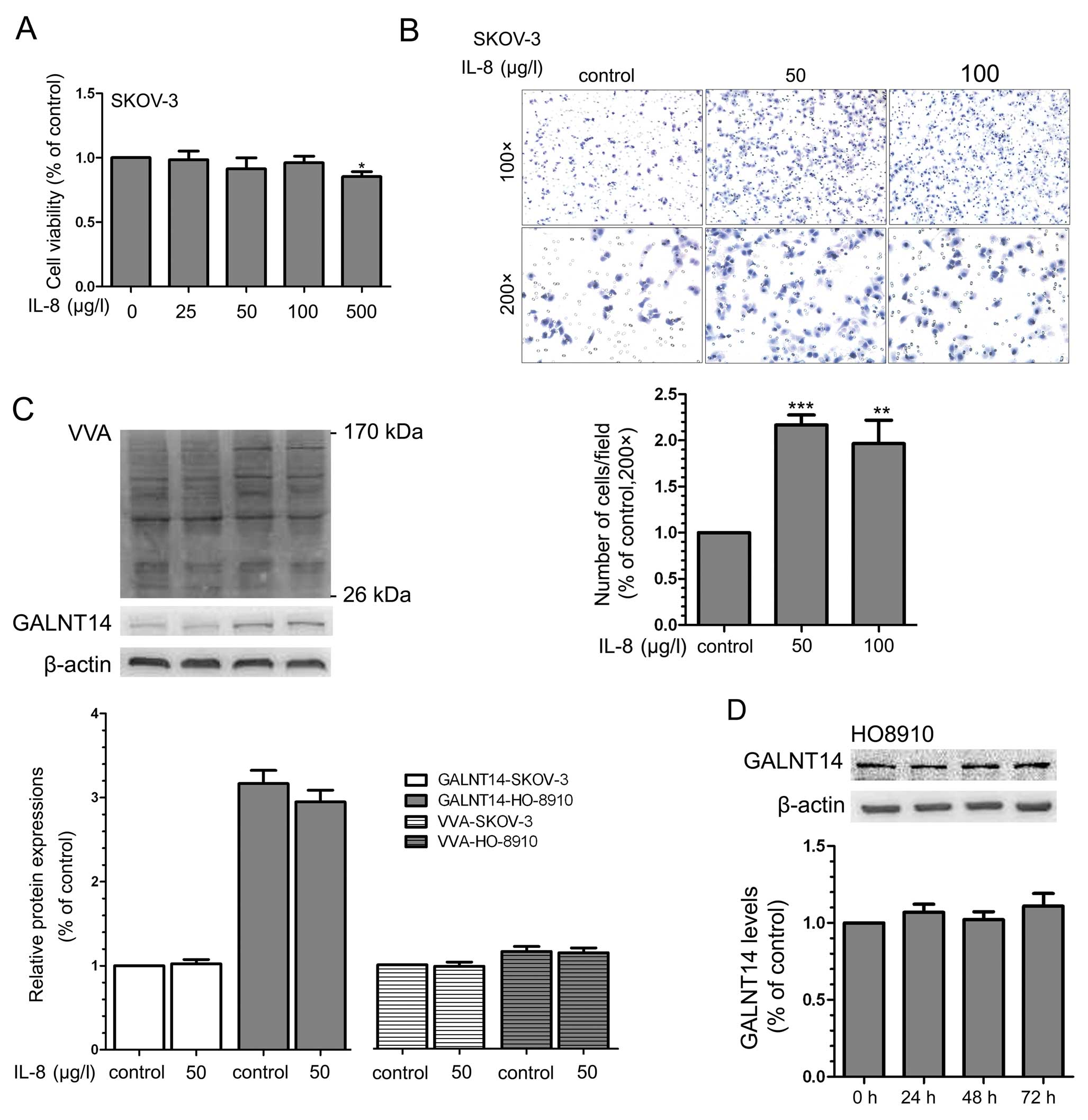 | Figure 5Effects of IL-8 on cell migration
ability and the expression of GALNT14 and Tn antigen. (A) SKOV-3
cells were treated with various concentrations of IL-8 (0, 25, 50,
100 and 500 μg/l) for 24 h, and cell viability was analyzed by MTT
analysis (n=5) as described in Materials and methods.
*P<0.05. (B) Effects of various concentrations of
IL-8 (0, 50 and 100 μg/l) on cell migration by Transwell migration
assays. SKOV-3 and HO8910 (data not shown) cells were treated with
various concentrations of IL-8 for 24 h, and cells migrating
through the pores were counted in five random images and compared.
Results are presented as means ± SD from 3 independent experiments.
(Original magnification, ×100 and ×200). **P<0.01;
***P<0.001. (C) Effects of IL-8 (0 and 50 μg/l; 24 h)
on the expression of GALNT14 and Tn antigen in SKOV-3 and HO8910
cell lines by western blotting. Representative results are shown.
The protein bands were quantified from 3 separate experiments.
Error bars, means ± SD. (D) Effects of IL-8 (50 μg/l; 0, 24, 48 and
72 h) on the expression of GALNT14 in HO8910 cell lines by western
blot analysis. The relative level of GALNT14 was normalized to
β-actin and obtained from 3 separate experiments. Error bars, means
± SD. |
ERK1/2 regulates the expression of
GALNT14 and Tn
Aberrant glycosylation modification affects the
function of specific substrates targeted by these enzymes and the
signaling pathways mediated by these substrates (8,23,28).
However, the regulation of Golgi glycosyltransferases by signaling
pathway mechanisms has not been studied extensively. Seales et
al(29) recently found that the
protein kinase C/Ras/ERK signaling pathway activates myeloid
fibronectin receptors by altering β1 integrin sialylation. Thus, to
find the relevant signaling pathways involved in regulating GALNT14
expression, we analyzed potential pathways using inhibitors of the
p38 MAPK (SB203580), the ERK1/2 (PD98059), and the PI3K (LY294002)
pathway in HO8910 cell lines. Among them, the ERK1/2 (PD98059) was
the only compound to effectively attenuate the expression of
GALNT14 at the mRNA and protein levels (Fig. 6A-D). Furthermore, the expression of
Tn antigen, which existed in O-glycosylated substrates, was
accordingly decreased (Fig. 6C). To
further demonstrate these findings, we repeated the same
experiments in another EOC cell line, HO8910PM (Fig. 6E). The result was consistent with
the above findings.
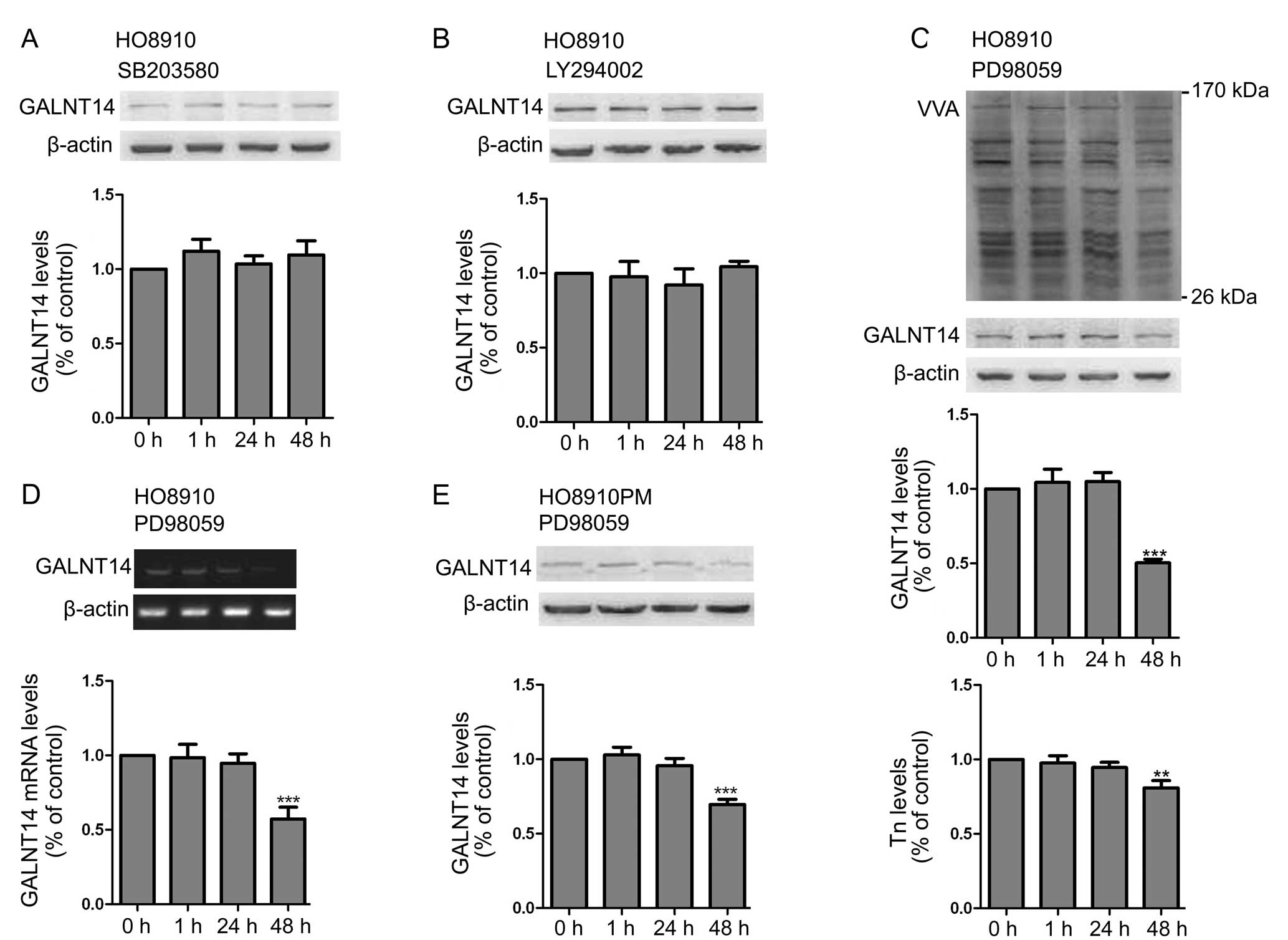 | Figure 6The ERK1/2 inhibitor PD98059
suppresses the expression of GALNT14 and Tn antigen. (A) HO8910
cells were treated with the p38 MAPK inhibitor SB203580 (10 μmol/l)
for 0, 1, 24 and 48 h, and the expression profile of GALNT14 was
detected by western blot analysis. (B) Effect of the PI3K inhibitor
LY294002 (10 μmol/l; 0, 1, 24 and 48 h) on the expression of
GALNT14 in HO8910 cell lines. (C) Effect of the ERK1/2 inhibitor
PD98059 (10 μmol/l; 0, 1, 24 and 48 h) on the expression of GALNT14
protein and Tn antigen in HO8910 cell lines. The relative level of
GALNT14 protein and Tn antigen were normalized to β-actin. n=3;
error bars, means ± SD. **P<0.01;
***P<0.001. (D) Effect of the ERK1/2 inhibitor
PD98059 (10 μmol/l; 0, 1, 24 and 48 h) on the expression of GALNT14
mRNA in HO8910 cell lines. The relative level of GALNT14 mRNA was
normalized to GAPDH. n=3; error bars, means ± SD. (E) Another type
of EOC cell line, HO8910PM, was treated with the ERK1/2 inhibitor
PD98059 (10 μmol/l) for 0, 1, 24 and 48 h, and the expression of
GALNT14 protein was detected by western blot analysis. Data are
expressed as means ± SD. ***P<0.001; n=3. |
Discussion
Mucin type O-glycosylation is initiated by members
belonging to the GALNT family present in the Golgi apparatus, and
is one of the common modifications that have various functions in
the folding, stability and targeting of multiple glycoproteins
(30). Accumulating evidence
demonstrates that the GALNT family members are related to several
cellular functions by catalyzing their specific substrates. For
example, GALNT2 regulates the concentrations of plasma lipids by
O-glycosylating angiopoietin-like protein 3 (31). The O-glycosyltransferase
pgant3 promotes cell adhesion during eukaryotic development
by affecting the secretion and localization of the extracellular
matrix integrin ligand, Tiggrin (32). Glycosyltransferase GALNT2 regulates
the malignant character of hepatocellular carcinoma by modifying
the epidermal growth factor receptor (8). Polypeptide
N-acetylgalactosaminyltransferase 6 modulates mammary
carcinogenesis through glycosylating MUC1 (23). It has been reported that the
expression of GALNT14 mRNA is markedly higher in tumor tissues of
the ovary, lung, breast, endometrium and bladder compared to these
normal tissues (13). Accordingly,
we investigated the GALNT14 expression in a panel of EOC cell lines
by RT-PCR and western blot analysis. As expected, varying degrees
of the expression of GALNT14 were detected in the four types of
ovarian cancer cells. Subsequent functional analyses of GALNT14
revealed that GALNT14 could regulate cellular migration and
cellular morphologic characteristics in ovarian cancer cells. In
the present study, we showed for the first time that GALNT14 can
mediate the malignant behavior of ovarian cancer cells.
Altered O-linked oligosaccharides expressed by
cancer cells have several important functions in malignant
transformation and tumor progression, including cell adhesion,
invasion and metastasis (33).
Short O-glycan Tn antigen is generally shielded by covalently bound
terminal carbohydrate moieties in healthy and benign-diseased
tissues, but it is unmasked in approximately 90% of human
carcinomas, including ovarian cancer, due to defective
O-glycosylation (34–36). Similarly, we showed different
degrees of the expression of tumor-associated carbohydrate epitope,
Tn antigen, in the four types of ovarian cancer cells. Tn antigen
is rarely expressed in normal tissues, but it is widely expressed
in human carcinomas (37). Thus, Tn
antigen has attracted significant interest as a molecule target for
tumor diagnosis and immunotherapy. A large number of anti-Tn IgG
and IgM antibodies have been produced and analyzed for their
potential feasibilities and antitumor activities (38–40).
Although anti-Tn antibodies have been generated, some issues such
as reduced effectiveness in vivo, immunogenicity and
cross-reactivity against type-A blood antigen have to be resolved
before applying to clinical therapy. Therefore, production of
available anti-Tn antibodies requires further research and
considerable clinical significance. It is also the focus and the
follow-up research points of our laboratory.
Mucin glycosylating enzyme GALNT6 contributes to
mammary carcinogenesis through abnormal glycosylation and
stabilization of specific substrate MUC1 (23). GALNT2, sharing a high amino acid
sequence homology with GALNT14, regulates the malignant character
of hepatocellular carcinoma by modulating the structure of short
O-glycan on substrate EGFR and the phosphorylation levels of EGFR
and its downstream signaling molecules (8,21).
Similar to other N-acetylgalactosaminyltransferases, GALNT14
has specific substrates including MUC13 (21,41).
MUC13, a transmembrane mucin, is overexpressed in ovarian cancer
tissues vs. the normal/benign ovary samples, and modulates
cell-cell adhesion, cell motility, proliferation and tumorigenesis
(20). We found that GALNT14 and
MUC13 proteins were almost co-expressed in ovarian cancer cells.
Accordingly, we hypothesized that GALNT14 contributes to ovarian
carcinogenesis through aberrant glycosylation of MUC13. Consistent
with our speculation, knockdown of GALNT14 had no marked influence
on the expression profile of MUC13 protein, but the structure of
short O-glycan on MUC13 was clearly attenuated. Moreover, the
knockdown of GALNT14 was followed by the decrease of Tn antigen
with different ranges of molecular weights. These suggest that
GALNT14 may have additional functions through glycosylation of its
other unidentified substrates in addition to MUC13 in EOC cells.
Therefore, an in depth investigation of novel substrates of GALNT14
is warranted to elucidate unveiled pathophysiologic roles of
GALNT14 in ovarian cancer.
It has been reported that CXC-chemokine
interleukin-8 (IL-8), a pro-inflammatory cytokine initially
described as a monocyte and neutrophil chemoattractant, mediates
the metastasis of various tumor cells (24–26).
Moreover, IL-6 and IL-8 promote the expression of
glycosyltransferases and sulfotransferases involved in the
biosynthesis of sialyl-Lewisx and
6-sulfo-sialyl-Lewisx epitopes in the human bronchial
mucosa (27). Thus, to elucidate
the relationship between IL-8 stimulation and the expression of
N-acetylgalactosaminyltransferase GALNT14 and tumor-associated
carbohydrate epitope Tn in ovarian cancer cells, we made the
corresponding detections. Our findings indicated that, although
IL-8 could regulate the migration ability of EOC cells, it had no
marked effect on the expression of GALNT14 and Tn antigen. Thus,
IL-8 modulates the migration ability of EOC cells but not by the
GALNTs pathway. Considering the common mediating function of IL-8
and GALNTs in cellular biological behavior, we inferred that IL-8
may be the downstream signaling molecule of GALNTs pathway, or that
IL-8 pathway and GALNTs pathway may be completely independent of
one another. Therefore, further analysis is required.
To investigate the signaling pathways involved in
GALNT14-induced alterations in cellular biological behavior, we
determined enzyme activity of GALNT14 after using several common
signaling pathway inhibitors. Seales et al(29) found that the protein kinase
C/Ras/ERK signaling pathway activates myeloid fibronectin receptors
by altering β1 integrin sialylation. Similarly, we found that
ERK1/2 inhibitor modulated the expression level of GALNT14, which
suggested that GALNT14 was the downstream signaling molecule of
ERK1/2 in the regulation of cellular biological behavior. Based on
the above results, we inferred that ERK1/2 inhibitor could also
mediate the glycosylation of MUC13 and the cellular biological
behavior of EOC cells. Additional studies are required to further
verify our findings and our inferences. To our knowledge, the
present study is the first to show that GALNT14 is modulated by the
ERK pathway, which may provide novel insights into the
pathophysiologic roles of GALNT14 in ovarian cancer
progression.
In conclusion, the results of the present study
suggest that GALNT14 could modulate MUC13 O-glycosylation and
stabilization, and thereby mediate the malignant behavior of
ovarian cancer cells. This study not only verifies a
pathophysiologic role of GALNT14 in EOC cells but also provides
insight into the significance of the regulation of the ERK pathway
on GALNT14 expression in EOC tumor progression, although further
verification tests are required to elucidate the exact mechanism of
the ERK-GALNT14-MUC13 pathway in ovarian cancer cells.
Understanding the correlative function and further mechanisms of
O-glycosylation on the specific substrates by GALNT family genes
may offer novel insights into the development of EOC anticancer
drugs. These contain anti-microRNAs, carbohydrate mimetics, siRNAs,
or small molecule compounds that can regulate GALNT gene expression
or enzyme activity.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81070222) and the Natural Science
Foundation of Chongqing (no. CSTC, 2009BA5083).
Abbreviations:
|
GALNT14
|
polypeptide
N-acetylgalactosaminyltransferase 14
|
|
MUC13
|
mucin 13
|
|
IL-8
|
interleukin-8
|
|
ERK1/2
|
extracellular signal-regulated kinase
1/2
|
|
EOC
|
epithelial ovarian cancer
|
|
EGFR
|
epidermal growth factor receptor
|
|
IGFBP-3
|
insulin-like growth factor binding
protein-3
|
|
TRAIL
|
tumor necrosis factor-related
apoptosis-inducing ligand
|
References
|
1
|
Auersperg N, Edelson MI, Mok SC, Johnson
SW and Hamilton TC: The biology of ovarian cancer. Semin Oncol.
25:281–304. 1998.
|
|
2
|
Auersperg N, Wong AS, Choi KC, Kang SK and
Leung PC: Ovarian surface epithelium: biology, endocrinology, and
pathology. Endocr Rev. 22:255–288. 2001.PubMed/NCBI
|
|
3
|
Lengyel E: Ovarian cancer development and
metastasis. Am J Pathol. 177:1053–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tian E and Ten Hagen KG: Recent insights
into the biological roles of mucin-type O-glycosylation. Glycoconj
J. 26:325–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ten Hagen KG, Fritz TA and Tabak LA: All
in the family: the UDP-GalNAc:polypeptide
N-acetylgalactosaminyltransferases. Glycobiology. 13:1–16.
2003.PubMed/NCBI
|
|
6
|
Tarp MA and Clausen H: Mucin-type
O-glycosylation and its potential use in drug and vaccine
development. Biochim Biophys Acta. 1780:546–563. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hakomori S: Glycosylation defining cancer
malignancy: new wine in an old bottle. Proc Natl Acad Sci USA.
99:10231–10233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu YM, Liu CH, Hu RH, Huang MJ, Lee JJ,
Chen CH, Huang J, Lai HS, Lee PH, Hsu WM, Huang HC and Huang MC:
Mucin glycosylating enzyme GALNT2 regulates the malignant character
of hepatocellular carcinoma by modifying the EGF receptor. Cancer
Res. 71:7270–7279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Taniuchi K, Cerny RL, Tanouchi A, Kohno K,
Kotani N, Honke K, Saibara T and Hollingsworth MA: Overexpression
of GalNAc-transferase GalNAc-T3 promotes pancreatic cancer cell
growth. Oncogene. 30:4843–4854. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park JH, Katagiri T, Chung S, Kijima K and
Nakamura Y: Polypeptide N-acetylgalactosaminyltransferase 6
disrupts mammary acinar morphogenesis through O-glycosylation of
fibronectin. Neoplasia. 13:320–326. 2011.PubMed/NCBI
|
|
11
|
Wu C, Shan Y, Liu X, Song W, Wang J, Zou
M, Wang M and Xu D: GalNAc-T14 may be involved in regulating the
apoptotic action of IGFBP-3. J Biosci. 34:389–395. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu C, Guo X, Wang W, Wang Y, Shan Y, Zhang
B, Song W, Ma S, Ge J, Deng H and Zhu M:
N-Acetylgalactosaminyltransferase-14 as a potential biomarker for
breast cancer by immunohistochemistry. BMC Cancer. 10:123–130.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wagner KW, Punnoose EA, Januario T,
Lawrence DA, Pitti RM, Lancaster K, Lee D, von Goetz M, Yee SF,
Totpal K, Huw L, Katta V, Cavet G, Hymowitz SG, Amler L and
Ashkenazi A: Death-receptor O-glycosylation controls tumor-cell
sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med.
13:1070–1077. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Packer LM, Williams SJ, Callaghan S,
Gotley DC and McGuckin MA: Expression of the cell surface mucin
gene family in adenocarcinomas. Int J Oncol. 25:1119–1126.
2004.PubMed/NCBI
|
|
15
|
Maher DM, Gupta BK, Nagata S, Jaggi M and
Chauhan SC: Mucin 13: structure, function, and potential roles in
cancer pathogenesis. Mol Cancer Res. 9:531–537. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Williams SJ, Wreschner DH, Tran M, Eyre
HJ, Sutherland GR and McGuckin MA: Muc13, a novel human cell
surface mucin expressed by epithelial and hemopoietic cells. J Biol
Chem. 276:18327–18336. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shimamura T, Ito H, Shibahara J, Watanabe
A, Hippo Y, Taniguchi H, Chen Y, Kashima T, Ohtomo T, Tanioka F,
Iwanari H, Kodama T, Kazui T, Sugimura H, Fukayama M and Aburatani
H: Overexpression of MUC13 is associated with intestinal-type
gastric cancer. Cancer Sci. 96:265–273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Walsh MD, Young JP, Leggett BA, Williams
SH, Jass JR and McGuckin MA: The MUC13 cell surface mucin is highly
expressed by human colorectal carcinomas. Hum Pathol. 38:883–892.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chauhan SC, Ebeling MC, Maher DM, Koch MD,
Watanabe A, Aburatani H, Lio Y and Jaggi M: MUC13 mucin augments
pancreatic tumorigenesis. Mol Cancer Ther. 11:24–33. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chauhan SC, Vannatta K, Ebeling MC,
Vinayek N, Watanabe A, Pandey KK, Bell MC, Koch MD, Aburatani H,
Lio Y and Jaggi M: Expression and functions of transmembrane mucin
MUC13 in ovarian cancer. Cancer Res. 69:765–774. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang H, Tachibana K, Zhang Y, Iwasaki H,
Kameyama A, Cheng L, Guo J, Hiruma T, Togayachi A, Kudo T, Kikuchi
N and Narimatsu H: Cloning and characterization of a novel
UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase,
pp-GalNAc-T14. Biochem Biophys Res Commun. 300:738–744. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagao M, Nakamura M, Oka N, Akiguchi I and
Kimura J: Abnormal glycosylation of motor neurons with
N-acetyl-D-galactosamine in a case of subacute motor neuronopathy
associated with lymphoma. J Neurol. 241:372–375. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park JH, Nishidate T, Kijima K, Ohashi T,
Takegawa K, Fujikane T, Hirata K, Nakamura Y and Katagiri T:
Critical roles of mucin 1 glycosylation by transactivated
polypeptide N-acetylgalactosaminyltransferase 6 in mammary
carcinogenesis. Cancer Res. 70:2759–2769. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singh RK, Gutman M, Radinsky R, Bucana CD
and Fidler IJ: Expression of interleukin 8 correlates with the
metastatic potential of human melanoma cells in nude mice. Cancer
Res. 54:3242–3247. 1994.PubMed/NCBI
|
|
25
|
Luca M, Huang S, Gershenwald JE, Singh RK,
Reich R and Bar-Eli M: Expression of interleukin-8 by human
melanoma cells up-regulates MMP-2 activity and increases tumor
growth and metastasis. Am J Pathol. 151:1105–1113. 1997.PubMed/NCBI
|
|
26
|
De Larco JE, Wuertz BR, Rosner KA,
Erickson SA, Gamache DE, Manivel JC and Furcht LT: A potential role
for interleukin-8 in the metastatic phenotype of breast carcinoma
cells. Am J Pathol. 158:639–646. 2001.PubMed/NCBI
|
|
27
|
Groux-Degroote S, Krzewinski-Recchi MA,
Cazet A, Vincent A, Lehoux S, Lafitte JJ, Van Seuningen I and
Delannoy P: IL-6 and IL-8 increase the expression of
glycosyltransferases and sulfotransferases involved in the
biosynthesis of sialylated and/or sulfated Lewisx
epitopes in the human bronchial mucosa. Biochem J. 410:213–223.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Freire-de-Lima L, Gelfenbeyn K, Ding Y,
Mandel U, Clausen H, Handa K and Hakomori SI: Involvement of
O-glycosylation defining oncofetal fibronectin in
epithelial-mesenchymal transition process. Proc Natl Acad Sci USA.
108:17690–17695. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Seales EC, Shaikh FM, Woodard-Grice AV,
Aggarwal P, McBrayer AC, Hennessy KM and Bellis SL: A protein
kinase C/Ras/ERK signaling pathway activates myeloid fibronectin
receptors by altering beta1 integrin sialylation. J Biol Chem.
280:37610–37615. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carraway KL III, Funes M, Workman HC and
Sweeney C: Contribution of membrane mucins to tumor progression
through modulation of cellular growth signaling pathways. Curr Top
Dev Biol. 78:1–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schjoldager KT, Vester-Christensen MB,
Bennett EP, Levery SB, Schwientek T, Yin W, Blixt O and Clausen H:
O-glycosylation modulates proprotein convertase activation of
angiopoietin-like protein 3: possible role of polypeptide
GalNAc-transferase-2 in regulation of concentrations of plasma
lipids. J Biol Chem. 285:36293–36303. 2010. View Article : Google Scholar
|
|
32
|
Zhang L, Tran DT and Ten Hagen KG: An
O-glycosyltransferase promotes cell adhesion during development by
influencing secretion of an extracellular matrix integrin ligand. J
Biol Chem. 285:19491–19501. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baldus SE, Engelmann K and Hanisch FG:
MUC1 and the MUCs: a family of human mucins with impact in cancer
biology. Crit Rev Clin Lab Sci. 41:189–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Springer GF, Desai PR, Ghazizadeh M and
Tegtmeyer H: T/Tn pancarcinoma autoantigens: fundamental,
diagnostic, and prognostic aspects. Cancer Detect Prev. 19:173–182.
1995.PubMed/NCBI
|
|
35
|
Springer GF: Immunoreactive T and Tn
epitopes in cancer diagnosis, prognosis, and immunotherapy. J Mol
Med. 75:594–602. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Q, Anver MR, Butcher DO and
Gildersleeve JC: Resolving conflicting data on expression of the Tn
antigen and implications for clinical trials with cancer vaccines.
Mol Cancer Ther. 8:971–979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Babino A, Oppezzo P, Bianco S, Barrios E,
Berois N, Navarrete H and Osinaga E: Tn antigen is a pre-cancerous
biomarker in breast tissue and serum in n-nitrosomethylurea-induced
rat mammary carcinogenesis. Int J Cancer. 86:753–759. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ando H, Matsushita T, Wakitani M, Sato T,
Kodama-Nishida S, Shibata K, Shitara K and Ohta S: Mouse-human
chimeric anti-Tn IgG1 induced anti-tumor activity against Jurkat
cells in vitro and in vivo. Biol Pharm Bull. 31:1739–1744. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takahashi HK, Metoki R and Hakomori S:
Immunoglobulin G3 monoclonal antibody directed to Tn antigen
(tumor-associated alpha-N-acetylgalactosaminyl epitope) that does
not cross-react with blood group A antigen. Cancer Res.
48:4361–4367. 1988.
|
|
40
|
Zhang M, Yao Z, Saga T, Sakahara H,
Nakamoto Y, Sato N, Nakada H, Yamashina I and Konishi J: Improved
intratumoral penetration of radiolabeled streptavidin in
intraperitoneal tumors pretargeted with biotinylated antibody. J
Nucl Med. 39:30–33. 1998.
|
|
41
|
Elhammer AP, Poorman RA, Brown E, Maggiora
LL, Hoogerheide JG and Kézdy FJ: The specificity of UDP-GalNAc:
polypeptide N-acetylgalactosaminyltransferase as inferred from a
database of in vivo substrates and from the in vitro glycosylation
of proteins and peptides. J Biol Chem. 268:10029–10038. 1993.
|