Introduction
Endometrial cancer (EC) is the most common
gynecological malignancy affecting women in the Western world. It
was predicted that 47,130 new cases would be diagnosed in the USA
in 2012 resulting in 8,010 disease-related deaths (1). Localized disease is preferentially
treated surgically by hysterectomy with or without adjuvant
treatment. This results in 5-year survival rates of approximately
96%. However, 30% of all EC cases remain undiagnosed until regional
or distant metastasis are present, resulting in much poorer
prognosis and survival (2). Thus,
there is a need to improve our understanding of the molecular and
cellular mechanisms responsible for the development of EC, and to
develop novel therapeutic strategies to prevent EC progression.
The tumor microenvironment plays an important role
in the development and progression of EC. The extracellular matrix
(ECM) is extensively remodeled or digested by matrix
metalloproteinases and other proteases produced by cancer and
stromal cells. The process is mediated by a number of different
proteases that catalyze the degradation of the basement membrane
that otherwise would confine the spread of the solid primary tumor.
These enzymes include cysteine, serine and aspartic proteases
(3), matrix metalloproteinases
(4), vascular endothelial growth
factors (5) and kallikreins
(6).
Cathepsins are believed to play a housekeeping role
in terminal protein degradation within the lysosome. Previous
evidence indicates that extracellular cathepsins play a central
role in cancer metastasis by altering ECM remodeling and
facilitating invasion (3,7,8).
Cathepsin B is a lysosomal cysteine protease which
belongs to a family of 11 cysteine proteases (Cathepsins B, C, H,
F, K, L, O, S, V, W and X/Z) (9).
In benign cells, it is synthesized as a pre-pro-enzyme that is
mainly stored in the lysosome (10). In malignant cells, Cathepsin B is
secreted from the lysosomes and contributes to degradation of the
basement membrane of the cell, thereby facilitating invasion
(11). Accumulating evidence has
implicated Cathepsin B in human breast (12), lung (13), colorectal (14) and ovarian (15) cancer.
It has recently been reported that increased
Cathepsin B acts as an unfavorable and independent tumor marker for
EC (16). However, little
information is available about the precise function of Cathepsin B
in EC carcinogenesis. In the present study, we investigated the
association between Cathepsin B expression and clinical outcome in
patients with EC. We also investigated the role of Cathepsin B in
promoting EC carcinogenesis in vitro and in vivo.
Materials and methods
Patients and samples
Samples were collected from 76 patients diagnosed
with uterine EC at the International Peace Maternity and Child
Health Hospital Affiliated to Shanghai Jiao Tong University School
of Medicine, between August 2009 and April 2011. None of the
patients had undergone hormone therapy, radiotherapy, or
chemotherapy prior to surgery. All cases were classified and graded
according to the criteria of the International Federation of
Obstetrics and Gynecology (FIGO 2009) (17). The characteristics of all EC tissue
samples are provided in Table
I.
 | Table IRelationship between Cathepsin B
expression and clinicopathological factors in endometrial
cancer. |
Table I
Relationship between Cathepsin B
expression and clinicopathological factors in endometrial
cancer.
| | Cathepsin B
expression | |
|---|
| |
| |
|---|
| Variable | No. of patients
(%) | Negative | Positive | P-value |
|---|
| Total | 76 (100) | 11 | 65 | |
| Age (years) |
| ≤50 | 15 (19.7) | 3 | 12 | 0.446 |
| >50 | 61 (80.3) | 8 | 53 | |
| FIGO stage |
| I | 60 (78.9) | 6 | 54 | 0.091 |
| II | 6 (7.8) | 2 | 4 | |
| III | 8 (10.5) | 3 | 5 | |
| IV | 2 (2.8) | 0 | 2 | |
| Grade
(endometrioid, n=64) |
| G1 | 30 (46.8) | 5 | 25 | 0.867 |
| G2 | 24 (37.5) | 4 | 20 | |
| G3 | 10 (15.7) | 1 | 9 | |
| Histological
type |
| Endometrioid | 64 (84.2) | 10 | 54 | 1.000 |
|
Non-endometrioid | 12 (15.8) | 1 | 11 | |
| Myometrial
invasion |
| <1/2 | 61 (80.2) | 7 | 54 | 0.212 |
| ≥1/2 | 15 (19.8) | 4 | 11 | |
| Lymph node
metastasis |
| No | 67 (89.1) | 11 | 56 | 0.342 |
| Yes | 9 (10.9) | 0 | 9 | |
| Lymphovascular
space involvement |
| No | 58 (76.3) | 10 | 48 | 0.442 |
| Yes | 18 (23.7) | 1 | 17 | |
| ER expression |
| Negative | 17 (22.4) | 3 | 14 | 0.702 |
| Positive | 59 (77.6) | 8 | 51 | |
| PR expression |
| Negative | 13 (17.1) | 3 | 10 | 0.388 |
| Positive | 63 (82.9) | 8 | 55 | |
Twenty normal endometrial samples were obtained from
patients undergoing hysterectomy for other conditions such as myoma
or adenomyosis. Fifteen endometrial atypical hyperplasia (EAH)
tissues were collected from patients undergoing hysteroscopic
examination for irregular bleeding.
The study was approved by the Ethics Committee of
the Medical Faculty of Shanghai Jiao Tong University. Informed
consent was obtained from all patients.
Immunohistochemistry
Tissue sections (4 μm) were processed for
hematoxylin and eosin (H&E) staining or immunohistochemistry
(IHC) as previously described (18). Rabbit monoclonal antibodies
Cathepsin B (3547-1) were purchased from Epitomics (Burlingame, CA,
USA).
Cathepsin B expression was evaluated in terms of
staining intensity, scored as 0 (negative), 1 (weak), 2 (medium),
or 3 (strong). The extent of staining was scored as 0 (0%), 1
(1–25%), 2 (26–50%), 3 (51–75%), or 4 (76–100%), according to the
percentage of the positively stained areas in relation to the whole
tumor area. The sum of the intensity score and extent scores was
used as the final staining score (0–7) (19).
The results were assessed by two pathologists who
were blinded to details regarding patient background.
The formalin-fixed, paraffin-embedded sections of
xenografted tumors from nude mice were analyzed using standard
avidin-biotin immunohistochemical techniques following exposure to
anti-Ki-67 antibody and anti-PCNA antibody (1:100; Wuhan Boster
Bio-Engineering Co., Wuhan, China) according to the manufacturer’s
instructions. Labeled cell nuclei in tumor sections were regarded
as positive.
Vector construction
Four short hairpin RNA (shRNA) oligonucleotides
targeting Cathepsin B and one non-target oligonucleotide were
designed and inserted into lentiviral vector pMAGic 4.0 at the
sites of AgeI (R0552S; New England Biolabs UK Ltd., UK) and
EcoRI (R0101S; New England Biolabs UK). The sequences used
are shown in Table II.
 | Table IIshRNA oligo sequences. |
Table II
shRNA oligo sequences.
|
Oligonucleotide | Sequences |
|---|
| shRNA-CTSB#1 | F:
CCGGGGATCACTGTGGAATCGAATTCAAGAGATTCGATTCCACAGTGATCCTTTTTTG |
| shRNA-CTSB#1 | R:
AATTCAAAAAAGGATCACTGTGGAATCGAATCTCTTGAATTCGATTCCACAGTGATCC |
| shRNA-CTSB#2 | F:
CCGGCCACATTTGTCACAGAAATTTCAAGAGAATTTCTGTGACAAATGTGGTTTTTTG |
| shRNA-CTSB#2 | R:
AATTCAAAAAACCACATTTGTCACAGAAATTCTCTTGAAATTTCTGTGACAAATGTGG |
| shRNA-CTSB#3 | F:
CCGGCCAACACGTCACCGGAGAGATCTCGAGATCTCTCCGGTGACGTGTTGGTTTTTTG |
| shRNA-CTSB#3 | R:
AATTCAAAAAACCAACACGTCACCGGAGAGATCTCGAGATCTCTCCGGTGACGTGTTGG |
| shRNA-CTSB#4 | F:
CCGGGCTGGTCAACTATGTCAACAACTCGAGTTGTTGACATAGTTGACCAGCTTTTTTG |
| shRNA-CTSB#4 | R:
AATTCAAAAAAGCTGGTCAACTATGTCAACAACTCGAGTTGTTGACATAGTTGACCAGC |
| shRNA-NT | F:
CCGGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTG |
| shRNA-NT | R:
AATTCAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAA |
Cell culture and lentiviral
infection
The human EC cell line HEC-1A was obtained from the
Shanghai Cell Bank of the Chinese Academy of Sciences and cultured
with DMEM/F12 supplemented with 10% FBS. To generate EC cell lines
expressing shRNAs, HEC-1A cells were infected with viral
supernatant containing non-target (NT) or CTSB-specific shRNA
lentiviral particles, in the presence of polybrene (6 μg/ml). Cells
were treated with puromycin (2 μg/ml) to generate stable Cathepsin
B knockdown clones.
Real-time PCR analysis
Total RNAs were extracted using TRIzol reagent
(Invitrogen, Life Technologies, Shanghai, China). For Cathepsin B
mRNA detection, RNAs were reverse transcribed according to the
manufacturer’s protocol (Takara, Dalian, China). Real-time PCR
analysis was performed using SYBR-Green (Takara) on an ABI Prism
700 thermal cycler (Applied Biosystems, Foster City, CA, USA).
Gene expression was calculated using the
2−ΔΔCt formula. The following primers were used:
Cathepsin B, 5′-CTG TCG GAT GAG CTG GTC AAC-3′ (sense) and 5′-TCG
GTA AAC ATA ACT CTC TGG GG-3′ (antisense); GAPDH, 5′-CCA CCC ATG
GCA AAT TCC ATG GCA-3′ (sense) and 5′-TCT AGA CGG CAG GTC AGG TCC
ACC-3′ (antisense).
Western blot analysis
Cells were washed with PBS once and harvested in 10%
SDS. The extracted proteins were separated by 12%
SDS-polyacrylamide gel electrophoresis and transferred to PVDF
membranes. The membranes were first blocked with 5% BSA in TBST and
then probed with the indicated primary antibodies at room
temperature for 1.5 h. After washing three times, the membranes
were incubated with the appropriate peroxidase-conjugated secondary
antibodies for 1 h. The signals were detected using an enhanced
chemiluminescence kit (GE Healthcare). The antibodies used were
Cathepsin B (1:1,000; Epitomics), GAPDH (1:1,000; Epitomics) and
peroxidase-conjugated anti-rabbit IgG secondary antibodies
(1:5,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Cell proliferation assay
Cells were plated into 96-well plates including
three control wells with medium alone that provided blanks for
absorbance readings. After 48 or 72 h,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) reagent
was added to each well (including the control wells) according to
the manufacturer’s protocol (Sigma, St. Louis, MO, USA). After
exposure for 24, 48, 72, 96 and 120 h the cells were incubated for
4 h after which the medium was discarded. DMSO (150 μl) was added
to all wells and absorbance was measured in 490 nm (Bio-Rad
Laboratories).
Migration and invasion assays
Cell migration and invasive ability was examined
using 24-well Transwell plates with 8.0 μm pore, polycarbonate
membrane inserts. The experiments were performed according to the
manufacturer’s protocol (Corning).
For the invasion assay, the upper side of the
membranes was coated with 100 μg Matrigel (BD), while the migration
assay was not. Then, 1×105 cells per well (200
μl/chamber) were seeded into the top chamber in serum-free media;
600 μl of complete medium was added to the lower chamber. Cells
that invaded through the surface of the membrane were fixed with
methanol and stained with crystal violet after 48 or 72 h.
Non-invasive cells were scraped from the top of the
Transwell plate with a cotton swab. Cells from five random
microscope field per filter were selected for counting.
Xenograft tumor formation assays
Eighteen female BALB/c nude mice (5 weeks of age)
were obtained from the Chinese Academy of Sciences, Shanghai,
China. The mice were housed under a laminar flow hood in an
isolated room using protocols approved by the Animal Care and Use
Committee of Shanghai Jiao Tong University School of Medicine.
HEC-1A and two other stable HEC-1A derived cell-lines (HEC-1A NT
and HEC-1A sh-CTSB) were harvested and resuspended at a density of
5×106 cells/200 μl of sterile saline.
Six mice per group were subcutaneously injected with
different EC cell lines in the subdermal space on the medial side
of the neck. Tumor volume was measured weekly for 4 weeks, until
the end of the experiment. Tumor volume was calculated using the
formula: largest diameter × smallest diameter2 × 0.5.
Tumor weight was determined after the animals were sacrificed at
the end of the xenograft experiments.
Statistical analysis
Statistical analyses were performed using SPSS
version 17.0 software (SPSS Inc., Chicago, IL, USA). Data are
represented as means and standard deviations (±SD). Numerical data
were analyzed using unpaired Student’s t-tests. One-way analysis of
variance (ANOVA) was used for multiple comparisons. Chi-square
tests were used to compare the categorical data. Values of
P<0.05 were considered to indicate statistically significant
differences.
Results
Cathepsin B is highly expressed in EC
tissues
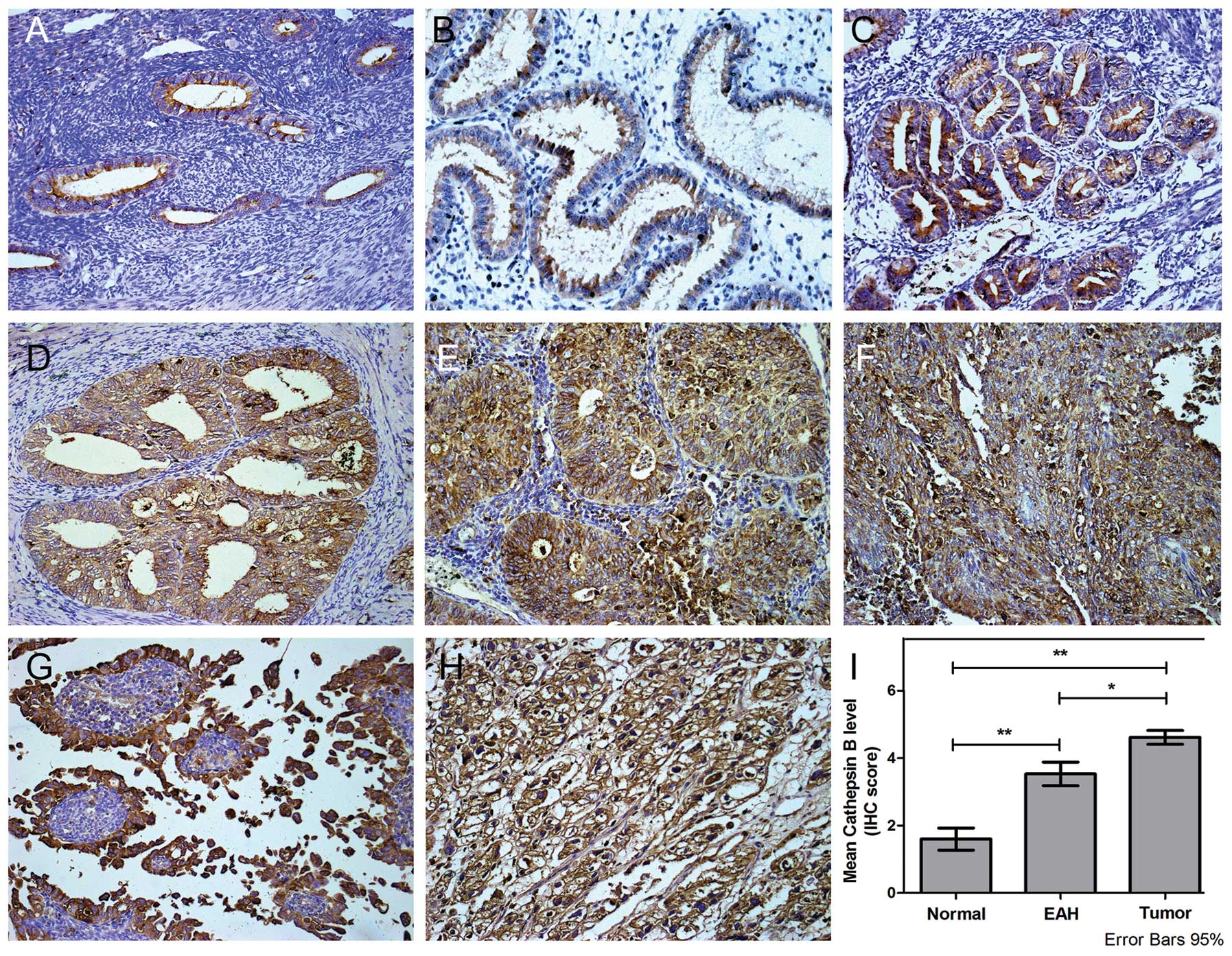
The expression of Cathepsin B protein in EC cells
was analyzed by IHC. Diffuse positive Cathepsin B immunostaining
was observed throughout the cytoplasm and occasionally on the cell
membrane. Extracellular localization was also noted. Moderate and
weak Cathepsin B immunoreactivity was seen in EAH and normal
endometrial tissues (Fig. 1A-C),
while strong immunoreactivity was observed in type I (Fig. 1D-F) and type II EC (Fig. 1G-H) cancer.
Of the 76 tumor samples analyzed, 64 tumors showed
cytoplasmic and cell membrane expression of Cathepsin B (IHC score
≥4). In all cases this was significantly higher than that in normal
endometrium or EAH tissue (Fig.
1I). No significant association was found between patient age,
FIGO staging, pathological grade, histological type, myometrial
invasion, lymph node metastasis, lymphovascular space involvement
or expression of estrogen (ER) or progesterone receptor (PR)
(P>0.05; Table I).
Cathepsin B expression is suppressed by
RNA interference
RNA interference oligonucleotides (shRNA-CTSB#1,
shRNA-CTSB#2, shRNA-CTSB#3 and shRNA-CTSB#4) which targeted
Cathepsin B and non-target shRNA-NT were synthesized and built into
a lentiviral vector. HEC-1A cells were infected by viral
supernatant, enabling stable cell lines to be established.
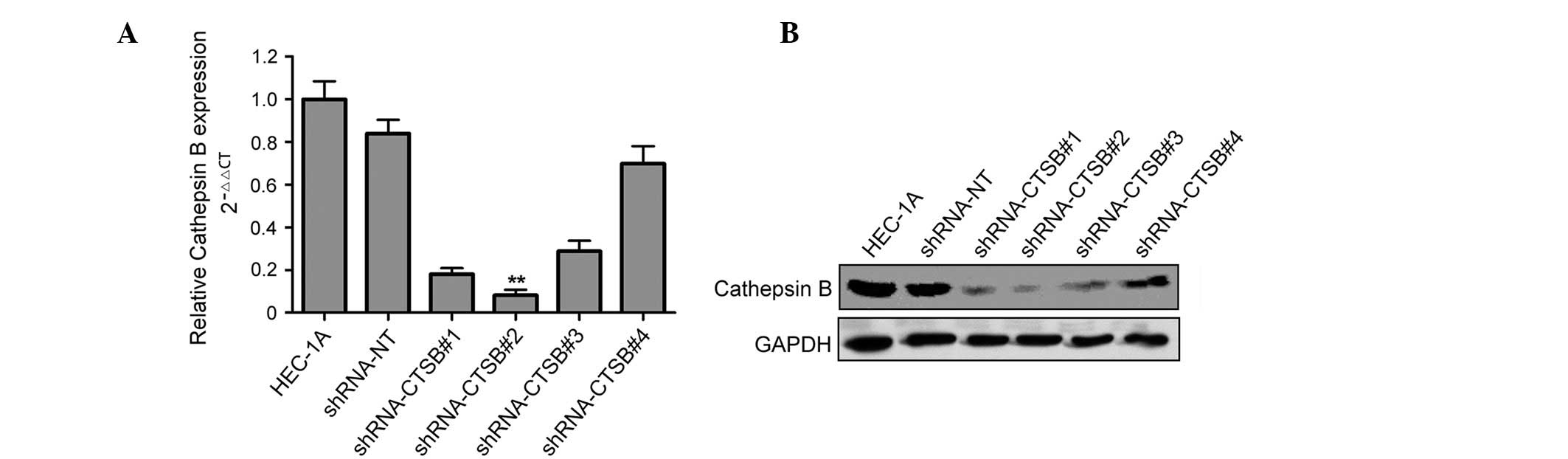
Western blot analysis and qRT-PCR indicated that
both mRNA and protein levels of Cathepsin B were suppressed by
Cathepsin B shRNA (Fig. 2A and B).
Among four shRNA oligonucleotides which were designed to target
Cathepsin B, shRNA-CTSB#2 showed maximum inhibition efficiency at
both mRNA and protein levels and was chosen for subsequent
studies.
Suppression of Cathepsin B inhibits the
proliferation, migration and invasion of EC cell lines
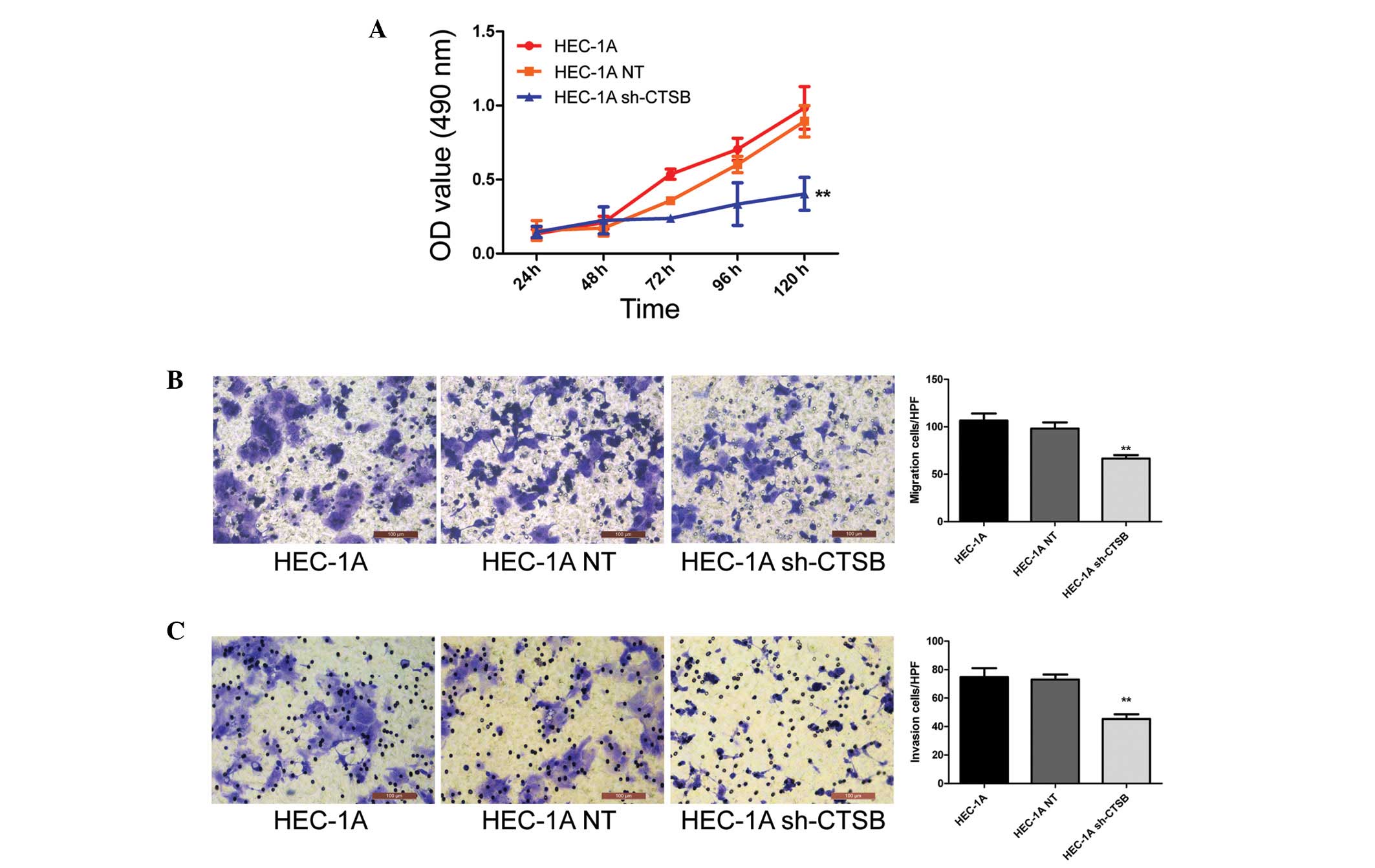
To explore whether the suppression of Cathepsin B
inhibits the growth of EC cell lines, HEC-1A, HEC-1A NT and HEC-1A
sh-CTSB cells were seeded into 96-well plates. MTT assays performed
every 24 h demonstrated that HEC-1A sh-CTSB inhibited cell
proliferation compared with both HEC-1A and HEC-1A NT cells
(P<0.01) (Fig. 3A). We also
performed Transwell migration and invasion assays to investigate
the effects of Cathepsin B on the migratory and invasive behaviors
of EC cells in vitro. The results showed that cells in the
HEC-1A sh-CTSB group had a much lower penetration rate than cells
in the HEC-1A and HEC-1A NT groups (P<0.01) (Fig. 3B and C). No significant differences
were seen between the HEC-1A and HEC-1A NT groups.
Suppression of Cathepsin B inhibits tumor
growth in vivo
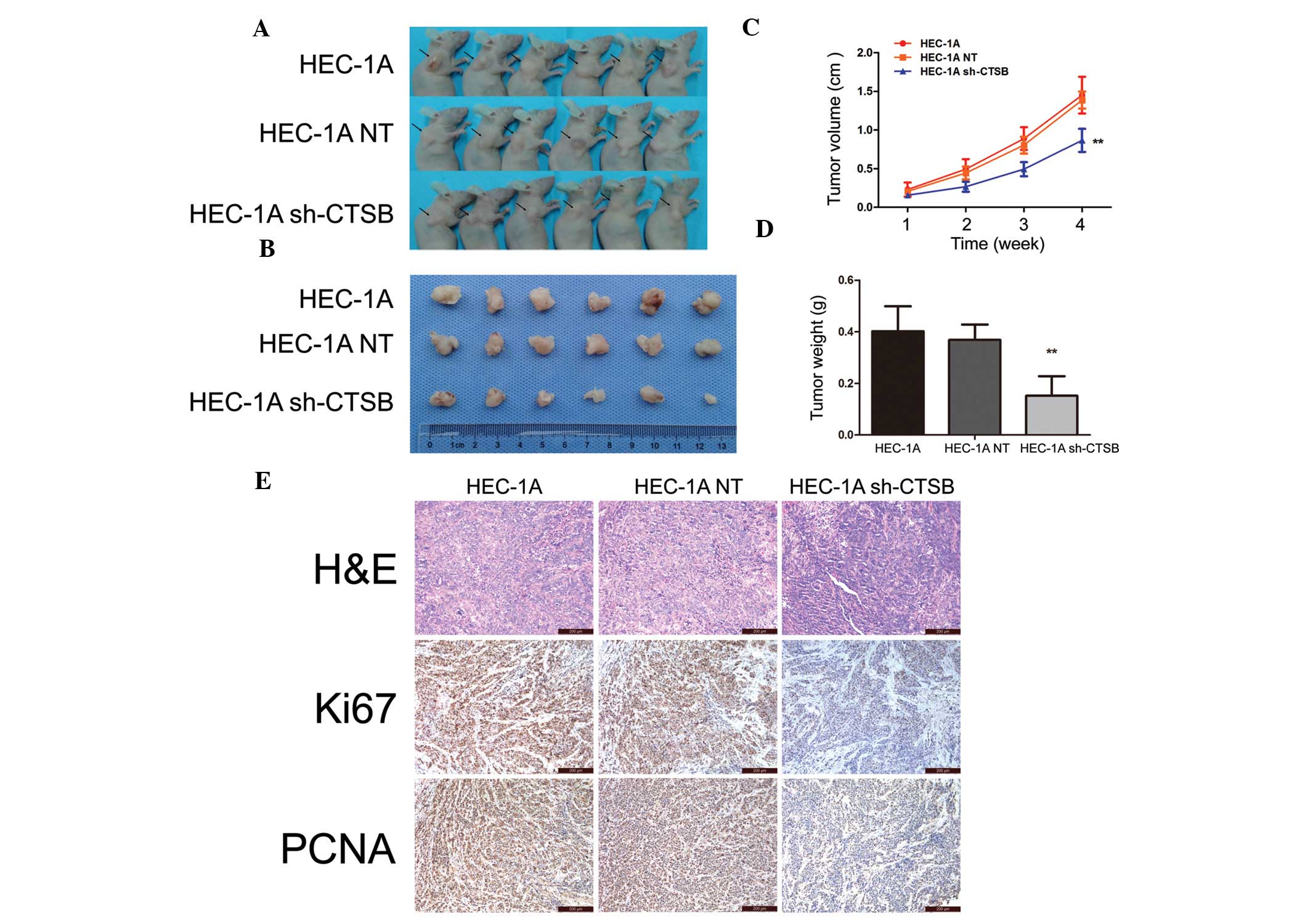
Animal studies were conducted to evaluate the effect
of Cathepsin B on tumor growth in nude mice. HEC-1A, HEC-1A NT and
HEC-1A sh-CTSB cells (5×106 cells/200 μl of sterile
saline) were subcutaneously injected in the subdermal space on the
medial side of the neck. After 4 weeks, tumor volume was smaller in
the HEC-1A sh-CTSB group than in the HEC-1A and HEC-1A NT groups
(P<0.01) (Fig. 4A-C). Tumor
weight determined after sacrificing the animals at 4 weeks showed
the same results as tumor volume (P<0.01) (Fig. 4D). In addition, nuclear expression
of Ki-67 and PCNA was lower in the HEC-1A sh-CTSB group than in the
HEC-1A and HEC-1A NT groups (Fig.
4E).
Discussion
Endometrial cancer (EC) spreads by direct extension
through the myometrium, by exfoliation of cells that are shed
through the fallopian tubes or by lymphatic and/or hematogenous
dissemination (20). The most
common route of EC spread is the direct extension of the tumor to
the myometrium (21). Localized
invasion and metastasis of EC results from several interdependent
processes involving proteolytic enzymes (22). Among several cancer invasion-related
characteristics, release of tumor-derived proteases is thought to
break the basement membrane and extracellular matrix, thereby
promoting cancer cell invasion into surrounding normal tissues and
facilitating distant metastasis through lymphovascular channels
(23).
In the present study, we showed that Cathepsin B is
highly expressed in human EC. Using preclinical models, we
demonstrated specific knockdown of Cathepsin B in the EC cell line
HEC-1A led to inhibition of tumor growth and invasion in
vitro and in vivo. These results suggest that Cathepsin
B may be a useful diagnostic biomarker of metastatic potential in
EC.
Previous studies have demonstrated that Cathepsin B
immunostaining in EC is associated with cancer progression.
Immunohistochemical studies (24)
reported that the malignant endometrium displays higher Cathepsin B
activity than benign tissue samples. A recent study (16) examined Cathepsin B protein levels in
64 paraffin-embedded endometrial tumor tissues, and found 27 of the
46 tumors (42.2%) were Cathepsin B positive.
In the present study, we found that the normal
endometrium produces weak levels of Cathepsin B (mean IHC score
<2), whereas extensive expression was detected in 76 cases of EC
(mean IHC score >4). Previous studies have reported that
Cathepsin B positivity is significantly associated with the FIGO
stage of the disease as well as cervical and stromal invasion
(16). However, our results showed
no evidence of a relationship between high Cathepsin B levels and
clinicopathological factors in EC. Differences in racial
background, and sample sizes between the two studies may explain
these apparently discrepant findings. Thus, further investigation
is required to determine whether Cathepsin B is an independent
prognostic factor for EC.
Cathepsin B has been associated with enhanced
malignant potential (such as proliferation, migration and invasion)
in several different types of cancer (12,18,25).
However, its precise role in the carcinogenesis of EC remains
uncertain. In the present study, we investigated the malignant
characteristics of Cathepsin B in the EC cell line HEC-1A using
shRNA transfection. We showed that inhibition of Cathepsin B
significantly decreased the proliferation of cancer cells, and that
suppression of Cathepsin B significantly attenuated migration and
invasive activity of HEC-1A cells. These findings are in agreement
with those reported for other types of tumors (26,27).
Taken together, these results suggest Cathepsin B has metastatic
potential in EC cells.
The tumor microenvironment is known to modulate the
expression of cathepsin B in tumor cells and in other cell types
(such as stromal fibroblasts) associated with tumors (28). Our in vivo experiments
indicated that tumor volume and weight were significantly reduced
by suppression of Cathepsin B. This finding is consistent with a
recent study of Cathepsin B in breast cancer (29). We also demonstrated lower
proliferation indexes (Ki-67 and PCNA) in xenograft tumor tissues
from the Cathepsin B knockdown group. This finding is consistent
with our results in vitro, and suggests that Cathepsin B
overexpression may facilitate tumor growth, while reduced
expression may suppress EC growth and development.
It has previously been shown that cathepsin D
converts pro-cathepsin B into active cathepsin B (30). Overexpression of cathepsin D has
been reported in adenomatous hyperplasia, but not in endometrial
adenocarcinomas, and thus it has been regarded as a possible index
for malignant transformation (3).
Additional evidence suggests that Cathepsin B can be activated by
other proteases, including cathepsin G, urokinase-type plasminogen
activator (uPA), tissue-type plasminogen activator (tPA) and
elastase, all of which interact with Cathepsin B in the role of
modulating tumor invasion (31,32).
Other investigators have shown that Cathepsin B undergoes
auto-activation under certain conditions, adding to the possible
mechanisms that might regulate cathepsin B (33). Thus, further studies are required to
delineate the regulation of Cathepsin B functions and to elucidate
the mechanisms that underlie its oncogene activities in EC.
In summary, our results show that Cathepsin B
expression is higher in EC than in normal endometrium and that
knockdown of Cathepsin B inhibits the proliferation, migration and
invasion of EC cell lines both in vitro and in vivo.
High expression of Cathepsin B, therefore, appears to play an
important role in tumorigenesis and progression of EC. These
findings indicate that Cathepsin B has potential as a new molecular
target for EC therapy.
Acknowledgements
The study was supported by the Science and
Technology Commission of Shanghai Municipality (no. 12ZR1451400),
the Young Scientific Research Project of Shanghai Municipal Health
Bureau (no. 20124Y045) and the Project by Shanghai Jiao Tong
University School of Medicine (no. 31010421). We express our thanks
to Qin Huang (The Centre of Research Laboratory, International
Peace Maternity and Child Health Hospital Affiliated to Shanghai
Jiao Tong University School of Medicine, Shanghai, China) for the
technical assistance.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Abal M, Llaurado M, Doll A, et al:
Molecular determinants of invasion in endometrial cancer. Clin
Transl Oncol. 9:272–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mylonas I, Makovitzky J, Richter DU,
Jeschke U, Briese V and Friese K: Cathepsin D expression in normal,
hyperplastic and malignant endometrial tissue: an
immunohistochemical analysis. Acta Histochem. 105:245–252. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aglund K, Rauvala M, Puistola U, et al:
Gelatinases A and B (MMP-2 and MMP-9) in endometrial cancer-MMP-9
correlates to the grade and the stage. Gynecol Oncol. 94:699–704.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sanseverino F, Santopietro R, Torricelli
M, et al: pRb2/p130 and VEGF expression in endometrial carcinoma in
relation to angiogenesis and histopathologic tumor grade. Cancer
Biol Ther. 5:84–88. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Santin AD, Diamandis EP, Bellone S, et al:
Human kallikrein 6: a new potential serum biomarker for uterine
serous papillary cancer. Clin Cancer Res. 11:3320–3325. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roshy S, Sloane BF and Moin K:
Pericellular cathepsin B and malignant progression. Cancer
Metastasis Rev. 22:271–286. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nasu K, Kai K, Fujisawa K, Takai N,
Nishida Y and Miyakawa I: Expression of cathepsin L in normal
endometrium and endometrial cancer. Eur J Obstet Gynecol Reprod
Biol. 99:102–105. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vasiljeva O and Turk B: Dual contrasting
roles of cysteine cathepsins in cancer progression: apoptosis
versus tumour invasion. Biochimie. 90:380–386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sinha AA, Jamuar MP, Wilson MJ, Rozhin J
and Sloane BF: Plasma membrane association of cathepsin B in human
prostate cancer: biochemical and immunogold electron microscopic
analysis. Prostate. 49:172–184. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jedeszko C and Sloane BF: Cysteine
cathepsins in human cancer. Biol Chem. 385:1017–1027. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Victor BC, Anbalagan A, Mohamed MM, Sloane
BF and Cavallo-Medved D: Inhibition of cathepsin B activity
attenuates extracellular matrix degradation and inflammatory breast
cancer invasion. Breast Cancer Res. 13:R1152011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Q, Fei J, Wu L, et al: Detection of
cathepsin B, cathepsin L, cystatin C, urokinase plasminogen
activator and urokinase plasminogen activator receptor in the sera
of lung cancer patients. Oncol Lett. 2:693–699. 2011.PubMed/NCBI
|
|
14
|
Talieri M, Papadopoulou S, Scorilas A, et
al: Cathepsin B and cathepsin D expression in the progression of
colorectal adenoma to carcinoma. Cancer Lett. 205:97–106. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scorilas A, Fotiou S, Tsiambas E, et al:
Determination of cathepsin B expression may offer additional
prognostic information for ovarian cancer patients. Biol Chem.
383:1297–1303. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Devetzi M, Scorilas A, Tsiambas E, et al:
Cathepsin B protein levels in endometrial cancer: potential value
as a tumour biomarker. Gynecol Oncol. 112:531–536. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Creasman W: Revised FIGO staging for
carcinoma of the endometrium. Int J Gynaecol Obstet. 105:1092009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu D, Wang H, Li Z, et al: Cathepsin B may
be a potential biomarker in cervical cancer. Histol Histopathol.
27:79–87. 2012.PubMed/NCBI
|
|
19
|
Kyo S, Sakaguchi J, Ohno S, et al: High
Twist expression is involved in infiltrative endometrial cancer and
affects patient survival. Hum Pathol. 37:431–438. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sato R, Jobo T and Kuramoto H: Parametrial
spread is a prognostic factor in endometrial carcinoma. Eur J
Gynaecol Oncol. 24:241–245. 2003.PubMed/NCBI
|
|
22
|
Jedinak A and Maliar T: Inhibitors of
proteases as anticancer drugs. Neoplasma. 52:185–192.
2005.PubMed/NCBI
|
|
23
|
Hornebeck W, Emonard H, Monboisse JC and
Bellon G: Matrix-directed regulation of pericellular proteolysis
and tumor progression. Semin Cancer Biol. 12:231–241. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bradley WH, Lima PH, Rodgers L, Blomquist
CH and Downs LS: Endometrial carcinoma expresses an increased
cathepsin B/D ratio. Gynecol Oncol. 108:84–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gopinathan A, Denicola GM, Frese KK, et
al: Cathepsin B promotes the progression of pancreatic ductal
adenocarcinoma in mice. Gut. 61:877–884. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nomura T and Katunuma N: Involvement of
cathepsins in the invasion, metastasis and proliferation of cancer
cells. J Med Invest. 52:1–9. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shiose Y, Ochi Y, Kuga H, Yamashita F and
Hashida M: Relationship between drug release of DE-310,
macromolecular prodrug of DX-8951f, and cathepsins activity in
several tumors. Biol Pharm Bull. 30:2365–2370. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sloane BF, Yan S, Podgorski I, et al:
Cathepsin B and tumor proteolysis: contribution of the tumor
microenvironment. Semin Cancer Biol. 15:149–157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vasiljeva O, Korovin M, Gajda M, et al:
Reduced tumour cell proliferation and delayed development of
high-grade mammary carcinomas in cathepsin B-deficient mice.
Oncogene. 27:4191–4199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van der Stappen JW, Williams AC, Maciewicz
RA and Paraskeva C: Activation of cathepsin B, secreted by a
colorectal cancer cell line requires low pH and is mediated by
cathepsin D. Int J Cancer. 67:547–554. 1996.PubMed/NCBI
|
|
31
|
Skrzydlewska E, Sulkowska M, Koda M and
Sulkowski S: Proteolytic-antiproteolytic balance and its regulation
in carcinogenesis. World J Gastroenterol. 11:1251–1266. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mason SD and Joyce JA: Proteolytic
networks in cancer. Trends Cell Biol. 21:228–237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Caglic D, Pungercar JR, Pejler G, Turk V
and Turk B: Glycosaminoglycans facilitate procathepsin B activation
through disruption of propeptide-mature enzyme interactions. J Biol
Chem. 282:33076–33085. 2007. View Article : Google Scholar : PubMed/NCBI
|