Introduction
Breast cancer is the most frequently diagnosed
cancer and the leading cause of cancer-related death among females
worldwide, accounting for 23% of the total new cancer cases and 14%
of the total cancer deaths (1). The
main causes of death of patients with breast cancer are due to the
excessive proliferation and metastasis of cancer cells (2,3). To
date, various anticancer agents which are natural products derived
from plants, have been used clinically as first-line option drugs,
such as paclitaxel, camptothecins and vinorelbine (4–6).
However, there is still the need for more effective drugs with low
toxic effects for breast cancer therapy. Hence, it is a challenge
to discover an agent that can efficiently suppress the abnormal
growth and metastatic progression of breast cancer cells.
Evodiamine, a quinolone alkaloid, is a bioactive
component isolated from the fruit of Evodia rutaecarpa,
which is extracted from medicinal plants such as Evodia
rutaecarpa (Juss.) Benth, Evodia rutaecarpa (Juss.)
Benth. var. officinalis (Dode) Huang and Evodia
rutaecarpa (Juss.) Benth. var. bodinieri (Dode) Huang.
Evodiamine has been shown to exhibit comprehensive pharmacological
activities including antiobesity, anti-IAV, protection against
myocardial ischemia-reperfusion injury and regulation of
testosterone secretion (7–10); and particularly, its anticancer
bioactivity is indispensable.
Evodiamine arrests cancer cell cycle distributions
and induces cell apoptosis to play a role in antitumor growth.
Previous studies have indicated that evodiamine-induced S phase
arrest in LoVo cells is associated with a marked decrease in the
protein expression of cyclin A, cyclin A-dependent kinase 2 and
cdc25c (11). Evodiamine caused
blockage of cells in the G2/M phase, accompanied by an increase in
the protein expression of cyclin B1 and the phosphorylated form of
p34(cdc2) (Thr 161) in prostate cancer LNCaP cells (12). Evodiamine inhibited the growth of
thyroid cancer ARO cells, arrested the cells at the M phase, and
induced apoptosis through inducing the activation of caspase-3, -8
and -9, and the cleavage of polyADP-ribose polymerase (13). Moreover, evodiamine was found to
increase the expression of Bax and p53, decreased the expression of
Bcl-2, lowered the mitochondrial transmembrane potential and
induced the activation of caspase-3 in colorectal carcinoma
COLO-205 cells (14).
Previous studies have revealed that evodiamine and
paclitaxel may be regarded as leading compounds for use as
antimetastatic agents acting through the inhibition of colon cancer
cell migration without cytotoxicity (15). Evodiamine concentration-dependently
inhibited the invasion of Lewis lung carcinoma (LLC) and B16–F10
melanoma in addition to colon 26-L5 carcinoma (16). Pretreatment of tumor cells with
evodiamine before inoculation into mice as well as administration
into mice after tumor inoculation both caused reduction in lung
metastasis formation (17).
Evodiamine was found to be a highly potent inhibitor of NF-κB
activation, and it abrogated both inducible and constitutive NF-κB
activation. NF-κB-regulated gene products such as c-Myc, COX-2,
MMP-9, ICAM-1, MDR1, survivin and Bfl-1/A1 were all downregulated
by evodiamine (18).
Research has revealed that evodiamine has potent
anticancer effects on various types of cancer cells, yet the
targets and underlying mechanisms of evodiamine-induced cytotoxic
and anti-metastatic actions in highly metastatic human breast
cancer MDA-MB-231 cells remain unclear. Therefore, in this study,
we aimed to ascertain whether evodiamine induces apoptosis and
inhibits migration and invasion in MDA-MB-231 cells in vitro
and in vivo.
Materials and methods
Drugs and reagents
The compound evodiamine was purchased from Shanghai
Research and Development Centre for Standardization of Traditional
Chinese Medicine (Shanghai, China), and dissolved in dimethyl
sulfoxide (DMSO). Dulbecco’s modified Eagle’s medium (DMEM),
trypsin, penicillin and streptomycin were purchased from Gibco-BRL
(Grand Island, NY, USA). 3-(4, 5-Dimethylthiazol-2yl)-2,
5-diphenyltetrazolium bromide (MTT), DMSO, propidium iodide (PI),
acridine orange (AO) and Matrigel were purchased from Sigma-Aldrich
(St. Louis, MO, USA). Antibodies against Bcl-2, cyclin D1,
p27Kip1, CDK6, p38 MAPK, p-p38 MAPK, p-ERK, p-SAPK/JNK,
SAPK/JNK and GAPDH were purchased from Cell Signaling Technology
Inc. (Danvers, MA, USA). Bax and ERK were purchased from Abgent
(Flanders Ct, San Diego, CA, USA). Urokinase-type plasminogen
activator (uPA) and uPAR were purchased from Santa Cruz
Biotechnology Inc. (Santa Cruz, CA, USA). MMP-2 and -9 were
purchased from Abcam (Cambridge, UK). IRDye™fluorescence antibodies
were obtained from LI-COR Biosciences (Lincoln, NE, USA). SB203580
and PD98059 were obtained from Biomol (Philadelphia, PA, USA).
Cell lines and culture conditions
The human breast cancer cell line MDA-MB-231 was
purchased from the Cell Bank of the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China) and cultured in DMEM
supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin
and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 5%
CO2.
MTT assay
Cell viability was measured by the MTT assay.
MDA-MB-231 cells (5×104/ml) were plated in 96-well
plates overnight, and then treated with various concentrations of
evodiamine. After 24, 48, 72 h of drug exposure, 20 μl of MTT (5
mg/ml) was added to incubate for 4 h. Then 150 μl DMSO was added to
dissolve the formazan crystals. The optical density (OD) was
detected at 490 nm by an ELISA plate reader (BioTek Instruments,
Inc., Winooski, VT, USA). The percentage of cell survival was
calculated as follows: Cell survival rate (%) = (OD sample/OD
control) × 100%.
Wound healing assay
MDA-MB-231 cells (2×105/ml) were seeded
in a 6-well culture plate to form a confluent monolayer. The
monolayer of cells was then scrape-wounded with a sterile
micropipette tip to create a wound of constant (~1 mm) width. After
removal of the cellular debris with phosphate-buffered saline (PBS)
twice, cells were treated with medium containing the indicated
concentration of evodiamine at 37°C. After incubation for 24 h,
MDA-MB-231 cells that had migrated to the wounded region were
observed (x100 magnification).
Migration and invasion assays
Migration and invasion assays were performed in
8-μm-diameter pore size Transwell chambers in 24-well plates
(Corning Incorporated, Corning, NY, USA). For the cell invasion
assay, the internal surface of each polycarbonate membrane was
coated with Matrigel (30 μg) for 30 min at 37°C for gel formation
and then blocked with serum-free DMEM containing 0.2% bovine serum
albumin (BSA). In brief, MDA-MB-231 cells (5×105/ml)
were seeded onto the upper chamber in 200 μl of serum-free medium
containing evodiamine at concentrations of 0, 15, 30, 60 μM,
respectively; the lower compartment of the chamber was filled with
600 μl DMEM supplemented with 0.2% BSA and 10% FBS. After
incubation for 24 h at 37°C, the cells in the upper surface of the
membrane were carefully removed with a cotton swab, and cells that
had migrated or invaded to the lower surface of the membrane were
fixed with methanol and stained with 0.1% crystal violet for 30
min. The migrated or invaded cells were then visualized and counted
from 6 randomly selected fields (x200 magnification).
Cell cycle analysis
Cell cycle phase analysis was performed by PI
staining. MDA-MB-231 cells were treated with different
concentrations of evodiamine for 24 h. The cells were harvested,
washed with PBS, and fixed in ice-cold 70% ethanol at 4°C
overnight. The cells were washed with ice-cold PBS and stained with
PI (50 μg/ml) in the dark for 30 min. Cell cycle distribution was
analyzed using Mcycle software (Beckman Coulter, Fullerton, CA,
USA).
Annexin V-FITC binding assay
The quantification of apoptotic cells was performed
by flow cytometric analysis using an Annexin V-FITC/PI Apoptosis
kit (Beckman Coulter, Miami, FL, USA). MDA-MB-231 cells were
treated with different concentrations of evodiamine for 48 h.
Annexin V and PI staining was performed according to the
manufacturer’s instructions. After staining, the quantification of
apoptotic cells was measured with a flow cytometer (Beckman
Coulter). The percentage of early apoptosis was detected by Annexin
V-positivity and PI-negativity, while the percentage of advanced
apoptosis was detected by Annexin V-positivity and
PI-positivity.
Acridine orange (AO) fluorescence
staining
Apoptotic morphological analysis was performed using
AO fluorescence staining. MDA-MB-231 cells were seeded on
coverslips in 6-well plates and treated with evodiamine for 48 h.
The coverslips were fixed with 95% ethanol for 15 min, acidified
with 1% acetic acid for 30 sec, dyed with AO (0.1 mg/ml) for 10
min, differentiated with CaCl2 (0.1 mol/l) for 2 min,
and observed under a fluorescence microscope (x400
magnification).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay
Apoptotic cells in sections of tumor tissues were
detected using a KeyGene TUNEL apoptosis detection kit (Kai-ji,
Nanjing, China) according to the manufacturer’s protocol. Briefly,
tumor histological sections were permeabilized with 20 μg/ml
protease K. Apoptosis was detected by labeling the 3′-OH ends of
the fragmented DNA with Biotin-11-dUTP under TdT enzyme at 37°C for
60 min. The tumor slides were then incubated with streptavidin
horseradish peroxidase (streptavidin-HRP) conjugate at 37°C for 30
min, followed by incubation with DAB and after-stain with
hematoxylin. The TUNEL-positive cells (dark brown nuclei) were
counted in 6 randomly selected fields under a microscope (x200
magnification). The apoptotic index (AI) was calculated as follows:
AI (%) = number of apoptotic cells/total number of cells ×
100%.
In-cell western analysis
In-cell western assay (19) was used to determine the level of
protein expression in cells. MDA-MB-231 cells
(1.5×105/ml) were plated in 96-well plates. The next
day, the cells were treated with 60 μM evodiamine for 24 or 48 h.
The cells were fixed with 4% formaldehyde for 20 min and washed
with 0.1% Triton, and finally blocked with 10% non-fat milk
overnight at 4°C. The cells were then incubated with the primary
antibodies: Bcl-2, Bax, cyclin D1, p27Kip1, CDK6, MMP-2,
MMP-9, uPA, uPAR, p38 MAPK, p-p38 MAPK, ERK, p-ERK, SAPK/JNK and
p-SAPK/JNK overnight at 4°C, respectively. The housekeeping
protein, GAPDH, was added to each well at the same time as the
control. The wells were then incubated with the corresponding
secondary IRDye™ 680 DX (red fluorescence) or IRDye™ 800 DX (green
fluorescence) fluorescence antibody recommended by the manufacturer
(Rockland, Gilbertsville, PA, USA) in the dark. Images were
obtained using the Odyssey Infrared Imaging System (LI-COR
Biosciences). The levels of protein expression were calculated as
the ratio of the intensity of the target protein to that of GAPDH.
The experiments were carried out in triplicate.
Animal tumor xenograft model and
treatment with drugs
Six-week-old female BALB/c athymic mice were
obtained from the Laboratory Animal Center at Shanghai University
of Traditional Chinese Medicine. The mice were housed in barrier
facilities with rodent chow and water under a
specified-pathogen-free (SPF) condition throughout the experimental
duration. MDA-MB-231 cancer cells (3×106, suspended in
100 μl of serum-free DMEM) were injected into the mammary fat pad
of the mice. One day after tumor cell transplantation, 10 mg/kg
evodiamine (17) was
intraperitoneally (i.p.) injected once every 2 days. The body
weight of each mouse was measured each week after treatment. All
mice were sacrificed after drug administration for 2 months, and
the tumors were removed and weighed. The lung and tumor tissues
were fixed with formalin. Thin sections were stained with
hematoxylin and eosin (H&E) and metastatic nodules present in
the lungs were counted. Representative fields (x100 magnification)
for each group were photographed. All procedures conformed to the
consideration of animal welfare and were approved by the Ethics
Committee of Shanghai University of Traditional Chinese
Medicine.
Statistical analysis
Experimental results are presented as the means ±
standard deviation (SD). Differences were evaluated by the
Student’s t-test or one-way analysis of variance (ANOVA). P<0.05
was considered to indicate a statistically significant result.
Results
Anti-proliferative effects of evodiamine
in MDA-MB-231 cells
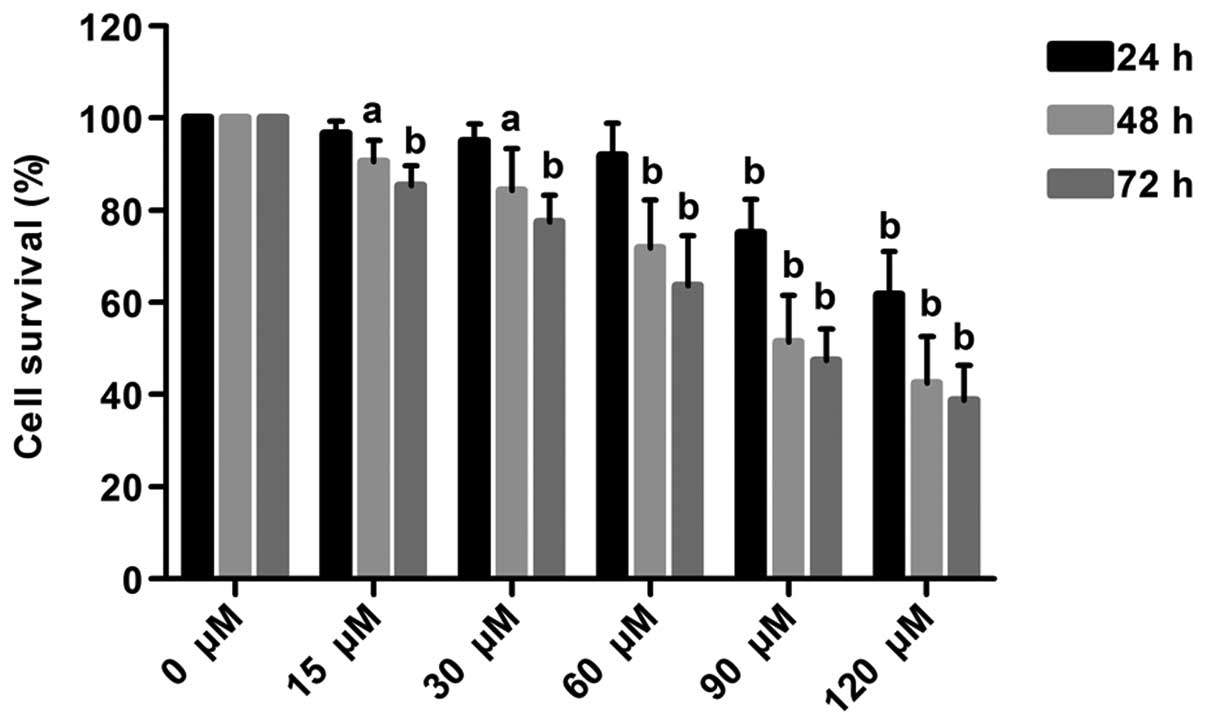
The growth of MDA-MB-231 cells was inhibited by
evodiamine in a dose- and time-dependent manner. Treatment at doses
of 15, 30 and 60 μM did not cause cytotoxicity in MDA-MB-231 cells
at 24 h (P>0.05); however, the survival rates of MDA-MB-231
cells were significantly decreased to 75.06 and 61.76% following
treatment with 90 and 120 μM, respectively (P<0.01). Following
treatment at doses of 15, 30, 60, 90 and 120 μM for 48 h, the
survival rates were decreased to 90.50, 84.40, 71.70, 51.44 and
42.47%, respectively (P<0.01). The half maximal inhibitory
concentration (IC50) at 48 h was ~90 μM. Following
treatment at doses of 15, 30, 60, 90 and 120 μM for 72 h, the
survival rates were decreased to 85.44, 77.45, 63.69, 47.49 and
38.77%, respectively (P<0.01) (Fig.
1).
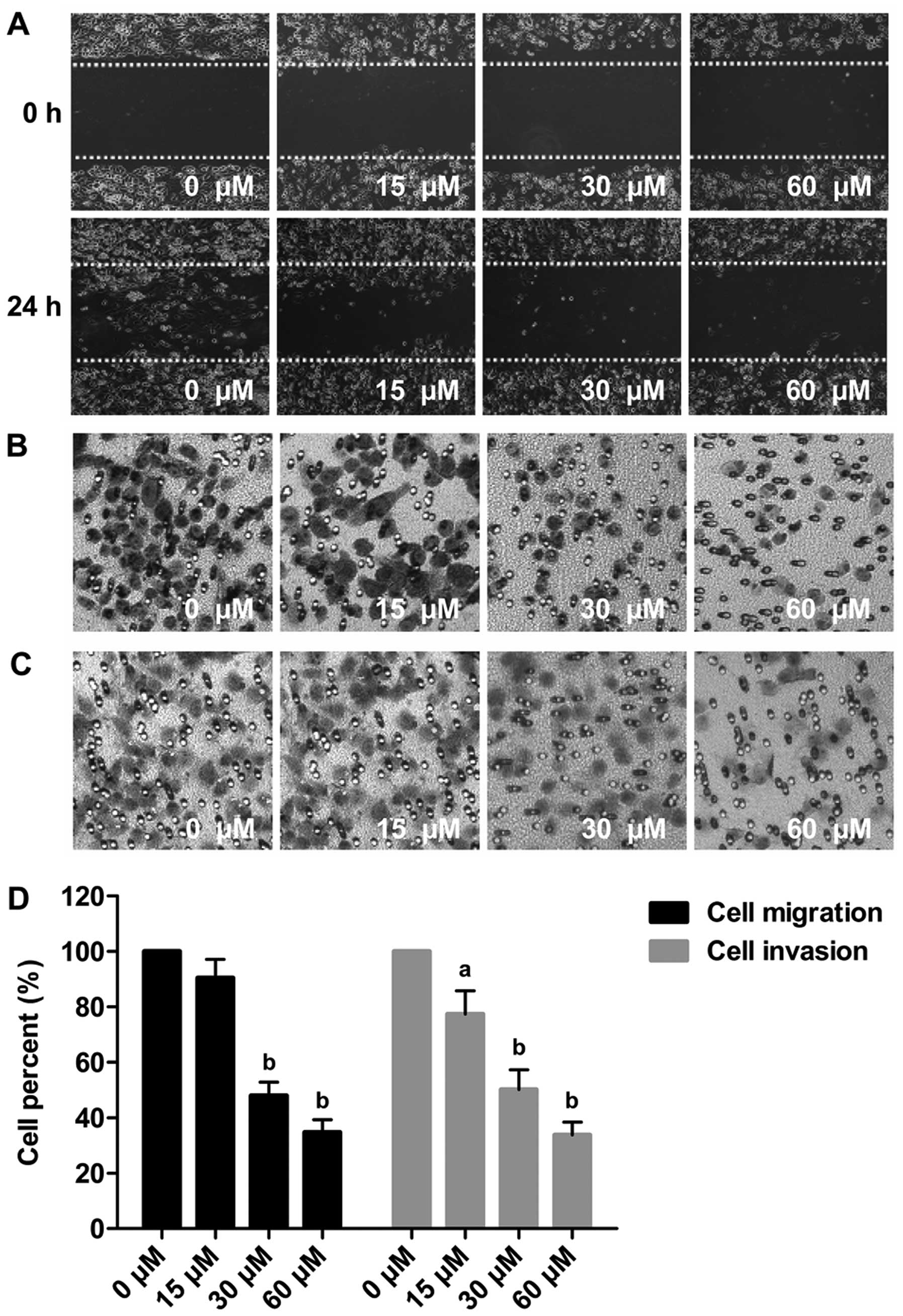
Evodiamine inhibits the migration and
invasion ability of MDA-MB-231 cells
In the wound healing assay, the gaps in the cells
treated with 15, 30 and 60 μM evodiamine were wider than that in
the untreated group (Fig. 2A). In
the Transwell migration assay, the cells that had migrated to the
lower chambers was reduced by evodiamine in a
concentration-dependent manner. The number of migrated cells
following treatment with 15, 30 and 60 μM evodiamine were reduced
to ~90.41, 47.93 and 34.72%, respectively (P<0.01) (Fig. 2B and D). In the Transwell invasion
assay, the number of cells invaded through the Matrigel-coated
filter was reduced by evodiamine dose-dependently. Compared with
the untreated cells, the number of invaded cells following
treatment 15, 30 and 60 μM evodiamine was reduced to ~77.31, 50.14
and 33.89%, respectively (P<0.05 or P<0.01) (Fig. 2C and D).
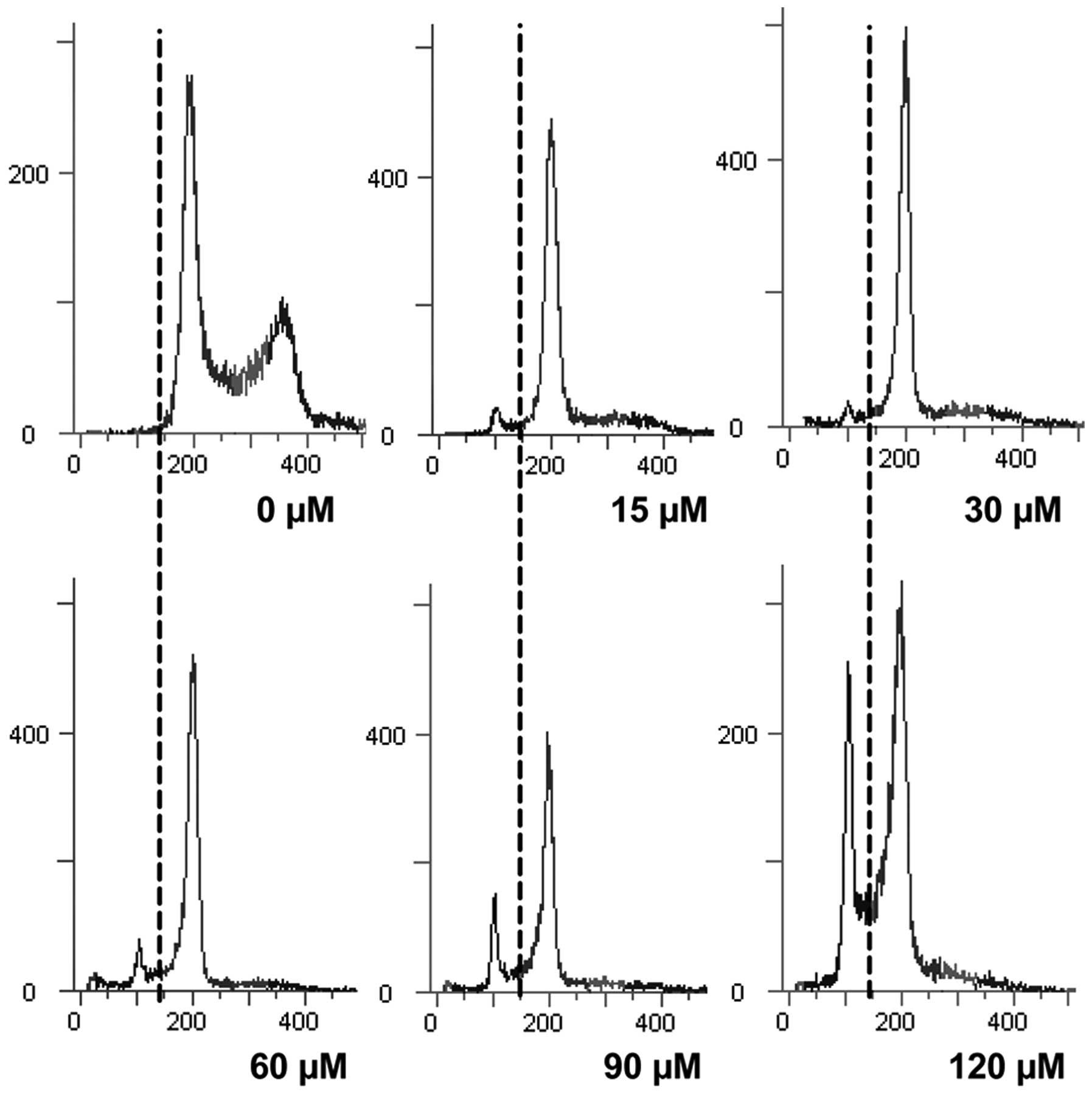
Evodiamine induces G0/G1 cell cycle
arrest of MDA-MB-231 cells
Exposure to different concentrations of evodiamine
(0, 15, 30, 60, 90 and 120 μM) caused G0/G1 arrest of MDA-MB-231
cells and a dose-dependent accumulation in the sub-G1 phase.
Concomitant with this result, there was a dose-dependent decrease
in the S and G2/M phases, when compared with the untreated cells
(Fig. 3 and Table I).
 | Table ICell cycle distribution in MDA-MB-231
cells exposed to different concentrations of evodiamine for 24
h. |
Table I
Cell cycle distribution in MDA-MB-231
cells exposed to different concentrations of evodiamine for 24
h.
| Evodiamine
(μM) | Sub-G1 (%) | G0/G1 (%) | S (%) | G2/M (%) |
|---|
| 0 | 0.13±0.03 | 53.29±2.73 | 15.50±0.96 | 27.01±0.56 |
| 15 | 7.79±1.88b | 74.92±2.35b | 6.48±1.30 | 8.04±2.75 |
| 30 | 10.69±3.89b | 74.20±2.02b | 5.27±2.29 | 6.13±3.46 |
| 60 | 15.93±1.89b | 72.94±0.29b | 3.38±0.68 | 3.85±1.46 |
| 90 | 24.28±3.46b | 65.30±2.69b | 3.99±1.47 | 3.90±2.82 |
| 120 | 32.19±1.84b | 60.12±1.26a | 3.71±0.13 | 2.46±1.17 |
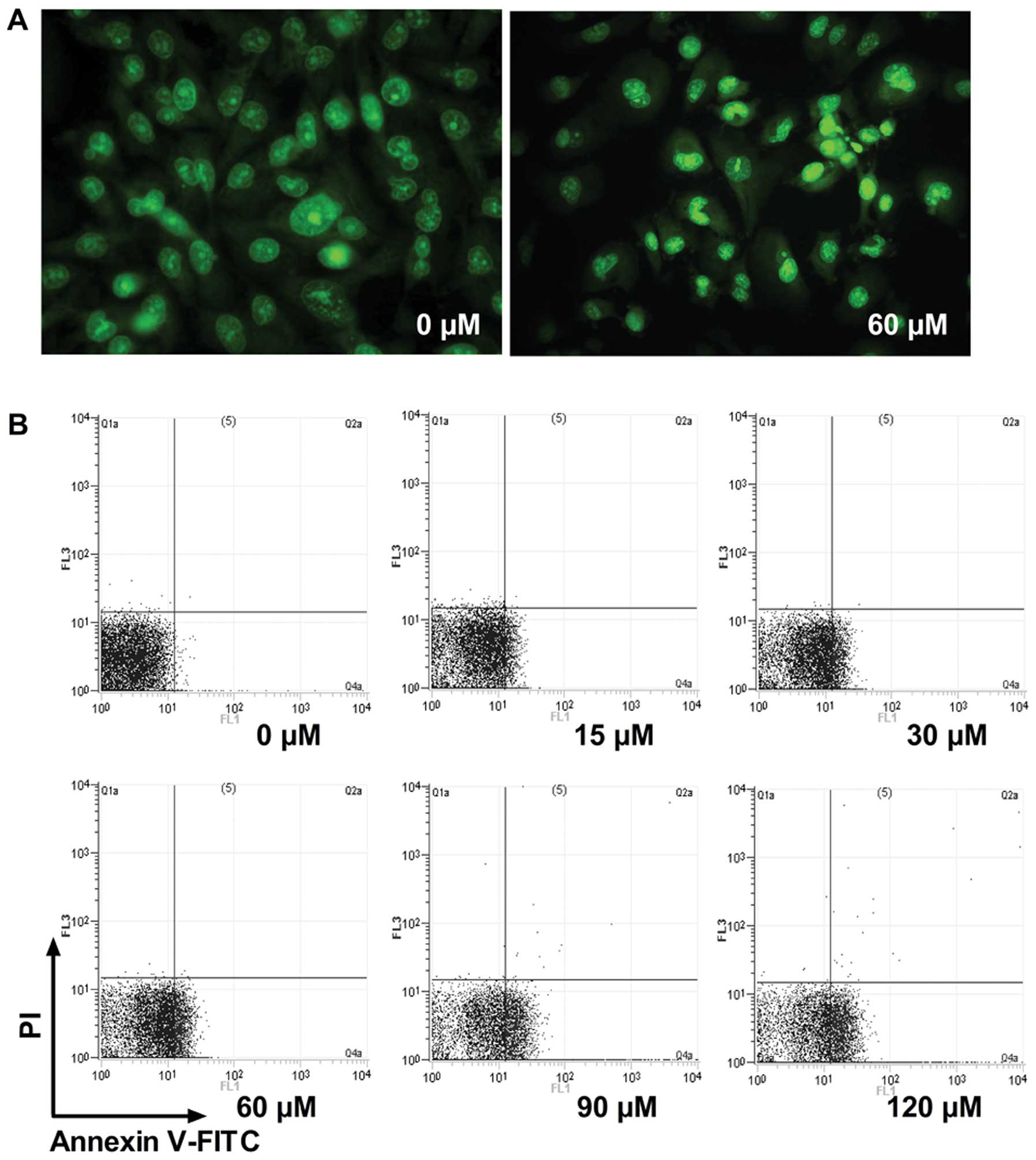
Evodiamine induces apoptosis in
MDA-MB-231 cells
To confirm whether evodiamine inhibits MDA-MB-231
cell viability due to apoptosis, morphological observation and
Annexin V-FITC binding assay of MDA-MB-231 cells were conducted.
Chromatin condensation, nuclear fragmentation and apoptotic bodies
were found in the evodiamine-treated cells (Fig. 4A). This result indicated that
MDA-MB-231 cells underwent the typical morphological changes of
apoptosis when exposed to 60 μM evodiamine for 48 h. To quantify
apoptosis, the Annexin V-FITC apoptosis assay was further carried
out by FACS. Treatment of MDA-MB-231 cells with different
concentrations of evodiamine resulted in a dose-dependent increase
in apoptotic cells (Fig. 4B). The
detailed percentages of early apoptosis, late apoptosis and total
apoptosis in MDA-MB-231 cells were calculated (Table II).
 | Table IIPercentage of apoptosis in the
MDA-MB-231 cells exposed to different concentrations of evodiamine
for 48 h. |
Table II
Percentage of apoptosis in the
MDA-MB-231 cells exposed to different concentrations of evodiamine
for 48 h.
| Early apoptosis
(%) | Late apoptosis
(%) | Total apoptosis
(%) |
|---|
|
|
| |
|---|
| Evodiamine
(μM) | Annexin
V+, PI− | Annexin
V+, PI+ | |
|---|
| 0 | 1.24±0.52 | 0.00±0.01 | 1.24±0.52 |
| 15 | 11.89±0.73 | 0.04±0.01 | 11.93±0.74b |
| 30 | 18.98±6.06 | 0.02±0.01 | 19.00±6.05b |
| 60 | 31.44±1.78 | 0.05±0.06 | 31.48±1.83b |
| 90 | 50.55±1.98 | 0.08±0.06 | 50.63±1.96b |
| 120 | 57.98±2.60 | 0.17±0.07 | 58.15±2.55b |
Evodiamine regulates the expression of
proteins related to apoptosis, cell cycle, cell migration and
invasion in the MDA-MB-231 cells
To investigate the underlying mechanism of the
induction of apoptosis and cell cycle arrest and inhibition of cell
migration and invasion by evodiamine, expression of proteins
related to apoptosis, cell cycle and cell migration and invasion
was determined by in-cell western analysis. Compared to untreated
cells, evodiamine significantly decreased the level of Bcl-2
(P<0.01) and increased the level of Bax (P<0.05), resulting
in upregulation of the Bax/Bcl-2 ratio. Evodiamine treatment
decreased the levels of cyclin D1 (P<0.05) and CDK6 (P<0.01)
and increased the level of p27Kip1 (P<0.01); as well
as decreased the levels of MMP-9, uPA and uPAR (P<0.05), whereas
evodiamine treatment did not affect MMP-2 expression (P>0.05)
(Fig. 5).
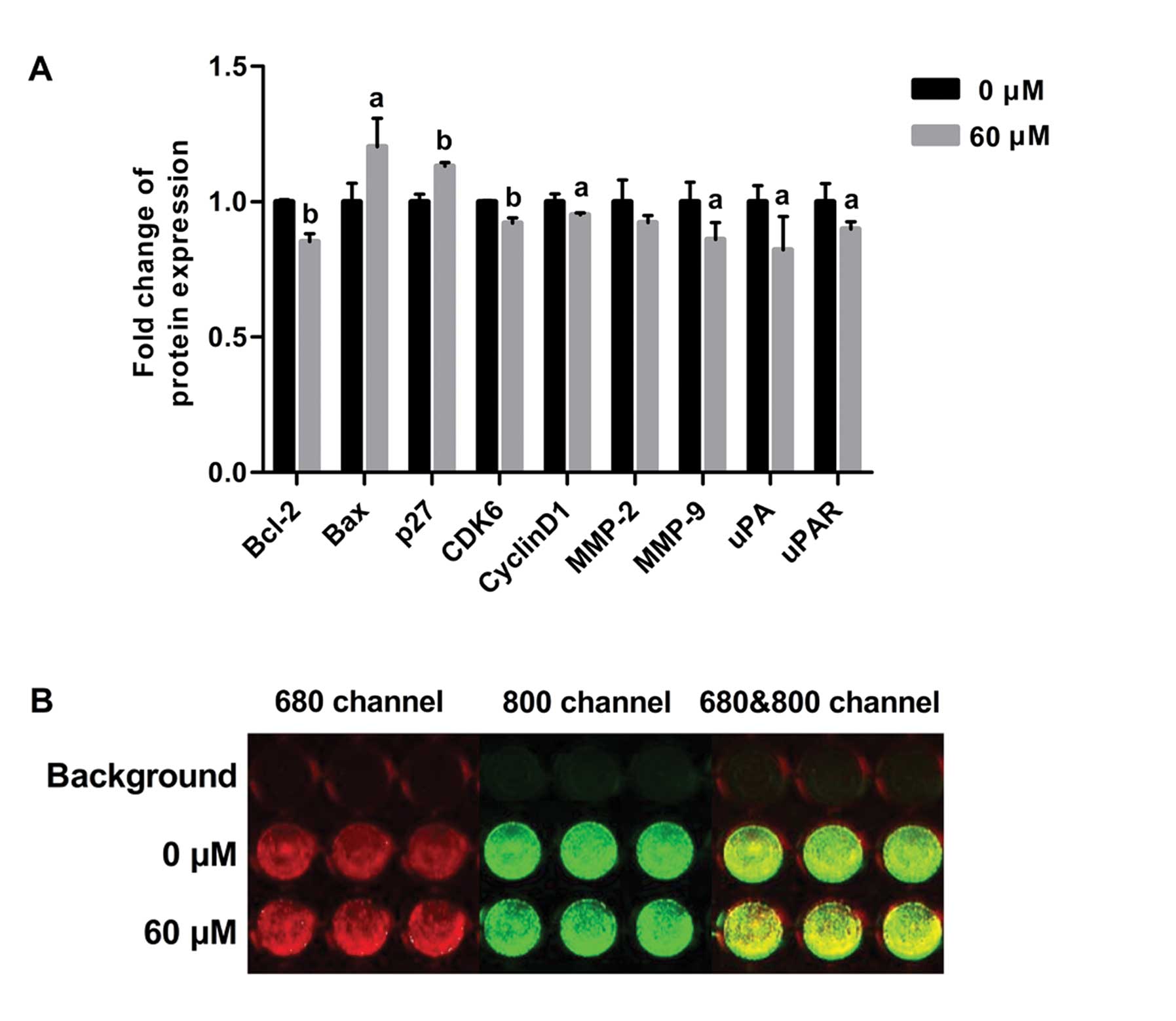 | Figure 5Effect of evodiamine on the
expression of Bcl-2, Bax, p27Kip1, cyclin-dependent
kinase 6 (CDK6), cyclin D1, MMP-2, MMP-9, urokinase-type
plasminogen activator (uPA) and uPAR in MDA-MB-231 cells. The
expression of p27Kip1, CDK6, cyclin D1, MMP-2, MMP-9,
uPA and uPAR was determined after treatment with 60 μM evodiamine
for 24 h. The expression of Bcl-2 and Bax was determined after
treatment with 60 μM evodiamine for 48 h. (A) The fluorescence
intensity ratio of proteins to GAPDH is shown as relative
expression. Values are represented as the means ± SD.
aP<0.05, bP<0.01, vs. untreated cells.
(B) A representative image is shown. Red fluorescence indicates the
expression of GAPDH in 680 channel and green fluorescence indicates
the expression of MMP-9 in 800 channel. Experiments were repeated
with similar results. |
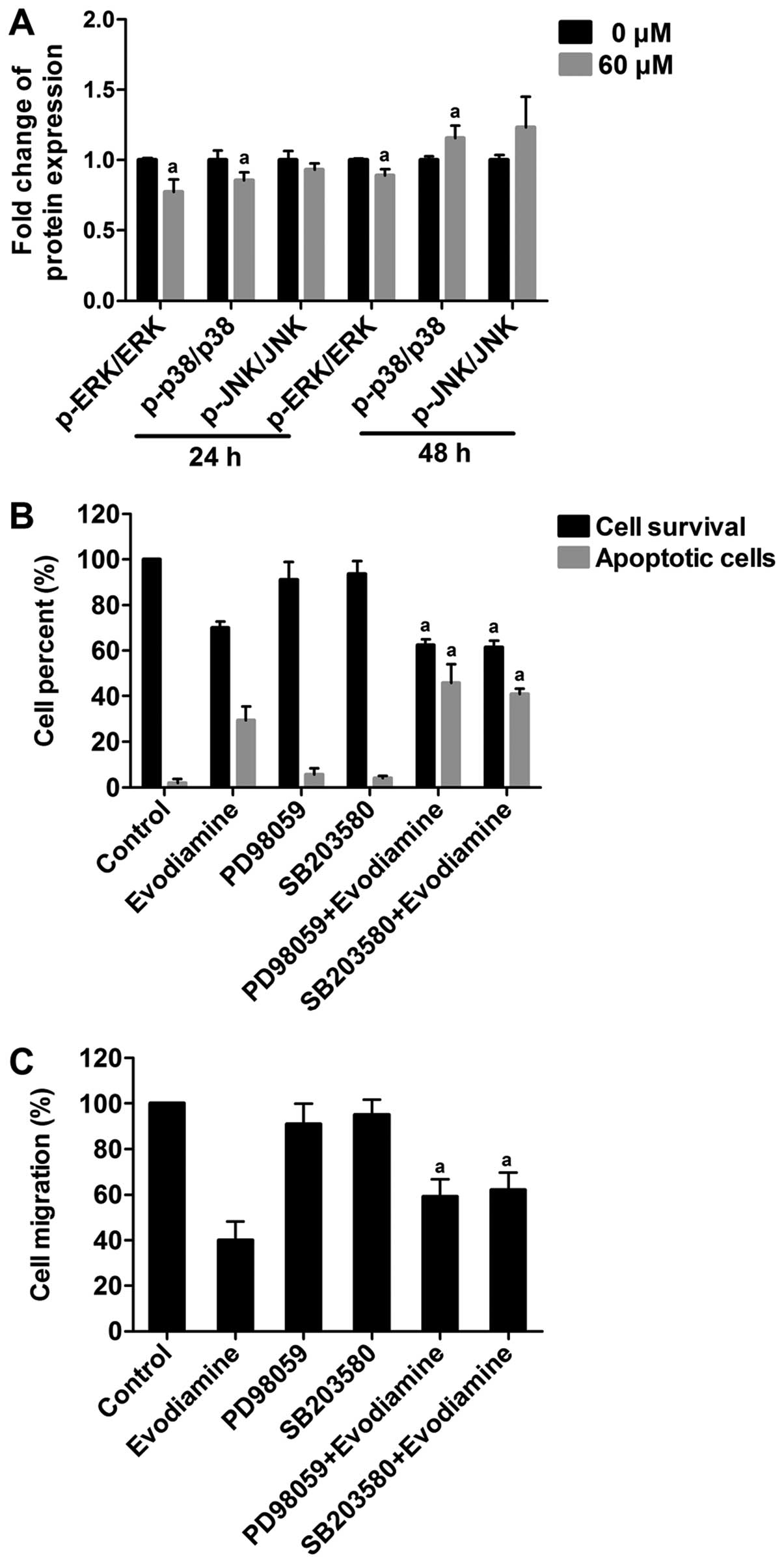
Evodiamine regulates the levels of p-ERK
and p-p38 MAPK in MDA-MB-231 cells
The expression levels of p-ERK, p-p38 MAPK, p-JNK
and each non-phosphorylated form were detected by in-cell western
analysis. The levels of p-ERK, p-p38 MAPK and p-JNK expression were
normalized to non-phosphorylated ERK, p38 MAPK and JNK,
respectively. As shown in Fig. 6A,
compared to the untreated cells, the levels of p-ERK and p-p38 MAPK
were significantly decreased following exposure to evodiamine for
24 h (P<0.05). Moreover, compared to the untreated cells, the
level of p-ERK was significantly decreased (P<0.05), while the
level of p-p38 MAPK was increased following exposure to evodiamine
for 48 h (P<0.05). The level of p-JNK remained constant
following evodiamine treatment for 24 or 48 h (P>0.05). These
results indicate that evodiamine regulates the activities of the
ERK and p38 MAPK pathways.
The role of ERK and p38 MAPK in the
induction of apoptosis and inhibition of migration
To address the role of ERK and p38 MAPK in
evodiamine-induced apoptosis, MDA-MB-231 cells were treated with 20
μM PD98059 (ERK inhibitor), or 30 μM SB203580 (p38 MAPK inhibitor)
alone and co-treated with 60 μM evodiamine for 48 h. Co-treatment
of 20 μM PD98059 or 30 μM SB203580 with 60 μM evodiamine reduced
the cell viability to 37.52 or 38.50%, respectively. Cell survival
rate was decreased ~10%, compared to that following treatment with
60 μM evodiamine alone (P<0.05). FACS revealed that the
percentage of apoptotic cells was significantly higher following
treatment with the two inhibitors in combination with 60 μM
evodiamine, compared to the cultures exposed to 60 μM evodiamine
alone (P<0.05) (Fig. 6B).
Furthermore, to address the role of ERK and p38 MAPK
in evodiamine-inhibited migration, MDA-MB-231 cells were treated
with 20 μM PD98059 or 10 μM SB203580 alone or co-treated with 60 μM
evodiamine for 24 h. As shown in Fig.
6C, co-treatment of 20 μM PD98059 or 10 μM SB203580 with 60 μM
evodiamine increased the cell migration rate ~20%, compared to that
following treatment with 60 μM evodiamine alone (P<0.05)
(Fig. 6C). These results suggest
that the ERK and p38 MAPK pathways may play an important role in
evodiamine-induced apoptosis and inhibition of migration in
MDA-MB-231 cells.
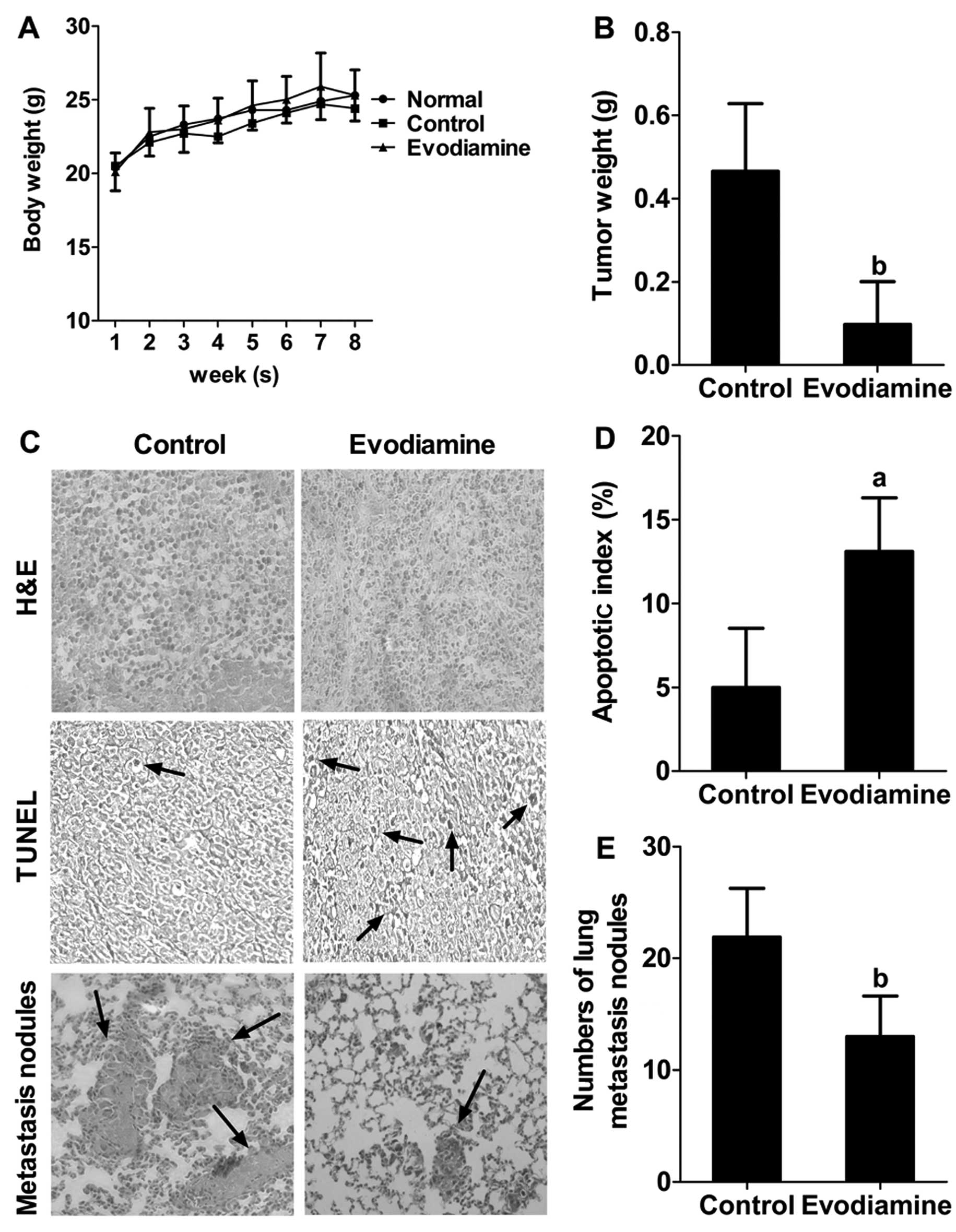
Evodiamine administration retards tumor
growth, induces apoptosis and inhibits the pulmonary metastasis of
MDA-MB-231 cells in nude mice
The average body weights of the control
(saline-treated) and evodiamine-treated mice did not differ
significantly throughout the study (Fig. 7A). Movement, digestion and swelling
seemed normal in the evodiamine-treated mice. Moreover, the tumor
weight in the evodiamine-treated group was lower than that of the
control group (P<0.01) (Fig.
7B). H&E staining revealed a relatively higher nuclear to
cytoplasmic ratio in the tumors from the control group when
compared with the ratio in the evodiamine-treated group. TUNEL
staining demonstrated that the number of apoptotic-positive cells
in the evodiamine-treated group was significantly higher than that
in the control group (Fig. 7C). The
AI was subsequently calculated. The average AI was 13% in the
evodiamine-treated group (Fig. 7D).
Furthermore, the lungs in the evodiamine-treated mice contained
less metastasized MDA-MB-231 cells morphologically, when compared
to the control group (Fig. 7C). The
average number of tumor nodules in the control group was 22, while
that in the evodiamine-treated group was 13, indicating evodiamine
treatment significantly decreased lung metastasis (P<0.01)
(Fig. 7E). Collectively, these
results suggest that evodiamine administration significantly
retards tumor growth, induces apoptosis and inhibits pulmonary
metastasis, without an abnormal response in the MDA-MB-231
xenograft model.
Discussion
Evodiamine was found to possess antitumor potential
in different cancer cell lines, however, there is no available
information on the effects of evodiamine on highly metastasis human
breast cancer MDA-MB-231 cells. Moreover, the mechanism involved in
the inhibition of growth and metastasis of MDA-MB-231 cells by
evodiamine requires further investigation. In the present study,
for the first time, we investigated the effects of evodiamine on
the growth, migration and invasion of MDA-MB-231 cells and
primarily focused on its molecular mechanism.
We found that evodiamine inhibited cell motility,
migration and invasion of MDA-MB-231 cells (Fig. 2) and exerts an antimetastasis effect
by inhibiting pulmonary metastatic nodules in xenograft BALB/c nude
mice (Fig. 7). During the
progression of cancer cell metastasis, matrix metalloproteinases
(MMPs) play a crucial role in tumor invasion and metastasis
formation due to their ability to degrade extracellular matrix
proteins (20). In our study, the
level of MMP-9 was downregulated by evodiamine (Fig. 5), and this result was consistent
with the evidence of evodiamine blocking the expression of
TNF-induced NF-κB-regulated gene products including MMP-9 in KBM-5
cells (18). uPA and its receptor
uPAR are usually overexpressed in breast cancer cells. uPA binding
to uPAR directly contributes to the degradation of the
extracellular matrix, and also mediates activation of MMPs, thereby
promoting cancer cell invasion and migration (21,22).
The levels of uPA and uPAR were downregulated by evodiamine
(Fig. 5). Therefore, inhibition of
the levels of MMP-9, uPA and uPAR may be, at least in part,
responsible for the antimetastatic potential of evodiamine against
MDA-MB-231 cells.
Treatment of MDA-MB-231 cells with evodiamine
resulted in significant G0/G1 phase arrest (Fig. 3) and apoptosis induction (Figs. 4 and 7). Control of cell cycle progression in
cancer cells is regarded to be an effective strategy for inhibition
of tumor cell proliferation. Cyclin Dl and cyclin-dependent kinase
6 (CDK6) are overexpressed in breast cancer and play a role in Gl
progression and oncogenesis. One of the CDK inhibitors,
p27Kip1, inhibits a wide variety of cyclin-CDK complexes
in vitro, and its overexpression blocks the progression of
cells through the Gl phase (23,24).
In our study, evodiamine treatment caused G0/G1 phase arrest
accompanied by downregulation of the expression of cyclin D1 and
CDK6 and upregulation of the expression of p27Kip1
(Fig. 5). Previous studies have
reported that evodiamine blocks G0/G1 phase in L929 cells (25), induces S phase arrest in LoVo cells
(11) and causes blockage of the
G2/M phase in LNCaP cells (12).
These studies showed that evodiamine arrests the cancer cell cycle
at different phases. This may provide explanations concerning the
types of cell lines and their specific response to evodiamine.
In our study, we found that evodiamine plays an
anti-proliferative role via inducing MDA-MB-231 cell apoptosis.
Apoptosis is tightly regulated by anti-apoptotic and pro-apoptotic
molecules, including proteins of the Bcl-2 family. Among them,
Bcl-2 and its dominant inhibitor Bax are key regulators of several
survival and apoptosis pathways. Over-expression of Bcl-2 enhances
cell survival by suppressing apoptosis, but overexpression of Bax
accelerates cell death, and the ratio of Bax/Bcl-2 plays a critical
role in determining whether cells will undergo apoptosis (26). Here, evodiamine increased
pro-apoptotic Bax expression and decreased anti-apoptotic Bcl-2
expression, leading to upregulation of the ratio of Bax/Bcl-2
(Fig. 5). Thus, this finding
confirmed that MDA-MB-231 cells underwent apoptosis induced by
evodiamine through the Bax/Bal-2 pathway.
Recent studies have demonstrated that
mitogen-activated protein kinases (MAPKs), including Jun N-terminus
kinase (JNK), p38 mitogen-activated protein kinase (p38 MAPK) and
extracellular signal-regulated kinase (ERK), play crucial roles in
cell survival, apoptosis and migration in tumor development and
progression (27–29). The aberrant activation of ERK and
p38 MAPK is associated with the promotion of migration. Activated
ERK1/2 was found to result in high uPA expression, rapid cell
proliferation and the migration of breast cancer cells (30,31).
Daintain/AIF-1 activated p38 MAPK signaling pathway contributed to
the upregulation of TNF-α and led to enhanced migration of
MDA-MB-231 and MCF-7 cells (32).
In our study, the expression levels of p-ERK and p-p38 MAPK were
both downregulated following exposure to evodiamine for 24 h in
MDA-MB-231 cells (Fig. 6A). These
findings may account for the inhibition of MDA-MB-231 cell
migration and invasion by evodiamine, and such anti-metastatic
activities of evodiamine may partly involve the suppression of ERK
and p38 MAPK signaling pathways.
In general, the activation of JNK/SAPK and p38 MAPK
is associated with promotion of apoptosis, while ERK activity
inhibits apoptosis (33). In the
present study, the expression of p-ERK was downregulated while the
expression of p-p38 MAPK was upregulated following exposure to
evodiamine for 48 h in MDA-MB-231 cells (Fig. 6A). This result was consistent with
the inhibitory effect of evodiamine on proliferation of A375-S2
cells. Evodiamine blocked the protective role of ERK in A375-S2
cells through the downregulation of p-ERK expression (34). Thus, evodiamine induces cell
apoptosis through ERK inactivation and p38 MAPK activation.
According to the above findings, indicating that
evodiamine regulates p-ERK and p-p38 MAPK, it was hypothesized that
ERK and p38 MAPK may have some effects on evodiamine-mediated cell
metastasis and apoptosis. In the present study, the
anti-metastastic effect of evodiamine was partly blocked by PD98059
and SB203580 (Fig. 6C). Therefore,
we may conclude that evodiamine-inhibited migration of MDA-MB-231
cells may partly occur through suppression of the ERK or p38 MAPK
pathway. When ERK or p38 MAPK was blocked, the anti-migration
potential of evodiamine was reduced.
Zelivianski et al(35) found that the ERK inhibitor PD98059
enhanced docetaxel-induced apoptosis of androgen-independent human
prostate cancer cells. In the present study, PD98059 or SB203580
enhanced the potential of evodiamine-induced growth suppression in
breast cancer MDA-MB-231 cells (Fig.
6B). The mechanism of PD98059 involved in evodiamine-mediated
cell apoptosis was previously associated with decreased expression
of c-Myc and cyclin D1 which are key targets of the MAPK pathway
and activate the caspase cascade (36). Although activation of p38, to some
extent, accounted for the growth inhibition by evodiamine, p38
inhibition also synergized evodiamine-induced cell apoptosis. p38
had dual effects during the regulation of cell growth. It
phosphorylated p53, was involved in Fas/FasL-mediated apoptosis
pathway and induced Bax translocation to induce apoptosis,
otherwise, it initiated the cell survival pathway, and inhibited
apoptosis signal generation (37,38).
In addition, many of the signals from other pathways may converge
with that of the MAPK cascade and downstream factors may be
involved in this response. For these reasons, the detailed
mechanisms involved in the synergistic induction of apoptosis
generating by co-treatment of PD98059 and SB203580 with evodiamine
require further investigation.
In conclusion, the results of the present study
indicate that evodiamine not only inhibits proliferation, induces
G0/G1 phase arrest and apoptosis, but also inhibits migration and
invasion of human breast cancer MDA-MB-231 cells. Moreover,
evodiamine regulated ERK and p38 MAPK activities, and its
pro-apoptotic and anti-metastastic effects were enhanced or
weakened by an ERK inhibitor and a p38 MAPK inhibitor,
respectively. Although further investigations are needed to verify
the anticancer mechanism of evodiamine, this study emphasizes the
promising application of evodiamine in breast cancer treatment.
Acknowledgements
The present study was supported by the Leading
Academic Discipline Project of Shanghai Municipal Education
Commission (no. J50301) and E-institutes of Shanghai Municipal
Education Commission (no. E03008).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Weinberg RA: How cancer arises. Sci Am.
275:62–70. 1996. View Article : Google Scholar
|
|
3
|
Weigelt B, Peterse JL and van’t Veer LJ:
Breast cancer metastasis: markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paridaens R, Biganzoli L, Bruning P, Klijn
JG, Gamucci T, Houston S, Coleman R, Schachter J, Van Vreckem A,
Sylvester R, Awada A, Wildiers J and Piccart M: Paclitaxel versus
doxorubicin as first-line single-agent chemotherapy for metastatic
breast cancer: a European Organization for Research and Treatment
of cancer randomized study with cross-over. J Clin Oncol.
18:724–733. 2000.PubMed/NCBI
|
|
5
|
Garcia-Carbonero R and Supko JG: Current
perspectives on the clinical experience, pharmacology, and
continued development of the camptothecins. Clin Cancer Res.
8:641–661. 2002.PubMed/NCBI
|
|
6
|
Galano G, Caputo M, Tecce MF and Capasso
A: Efficacy and tolerability of vinorelbine in the cancer therapy.
Curr Drug Saf. 6:185–193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kobayashi Y, Nakano Y, Kizaki M, Hoshikuma
K, Yokoo Y and Kamiya T: Capsaicin-like anti-obese activities of
evodiamine from fruits of Evodia rutaecarpa, a vanilloid
receptor agonist. Planta Med. 67:628–633. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dai JP, Li WZ, Zhao XF, Wang GF, Yang JC,
Zhang L, Chen XX, Xu YX and Li KS: A drug screening method based on
the autophagy pathway and studies of the mechanism of evodiamine
against influenza A virus. PLoS One. 7:e427062012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rang WQ, Du YH, Hu CP, Ye F, Xu KP, Peng
J, Deng HW and Li YJ: Protective effects of evodiamine on
myocardial ischemia-reperfusion injury in rats. Planta Med.
70:1140–1143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin H, Tsai SC, Chen JJ, Chiao YC, Wang
SW, Wang GJ, Chen CF and Wang PS: Effects of evodiamine on the
secretion of testosterone in rat testicular interstitial cells.
Metabolism. 48:1532–1535. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang C, Fan X, Xu X, Yang X, Wang X and
Liang HP: Evodiamine induces caspase-dependent apoptosis and S
phase arrest in human colon lovo cells. Anticancer Drugs.
21:766–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kan SF, Huang WJ, Lin LC and Wang PS:
Inhibitory effects of evodiamine on the growth of human prostate
cancer cell line LNCaP. Int J Cancer. 110:641–651. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen MC, Yu CH, Wang SW, Pu HF, Kan SF,
Lin LC, Chi CW, Ho LL, Lee CH and Wang PS: Anti-proliferative
effects of evodiamine on human thyroid cancer cell line ARO. J Cell
Biochem. 110:1495–1503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang ZG, Chen AQ and Liu B:
Antiproliferation and apoptosis induced by evodiamine in human
colorectal carcinoma cells (COLO-205). Chem Biodivers. 6:924–933.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ogasawara M, Matsubara T and Suzuki H:
Screening of natural compounds for Inhibitory activity on colon
cancer cell migration. Biol Pharm Bull. 24:720–723. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ogasawara M, Matsunaga T, Takahashi S,
Saiki I and Suzuki H: Anti-invasive and metastatic activities of
evodiamine. Biol Pharm Bull. 25:1491–1493. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ogasawara M, Matsubara T and Suzuki H:
Inhibitory effects of evodiamine on in vitro invasion and
experimental lung metastasis of murine colon cancer cells. Biol
Pharm Bull. 24:917–920. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takada Y, Kobayashi Y and Aggarwal BB:
Evodiamine abolishes constitutive and inducible NF-κB activation by
inhibiting IκBα kinase activation, thereby suppressing
NF-κB-regulated antiapoptotic and metastatic gene expression,
up-regulating apoptosis, and inhibiting invasion. J Biol Chem.
280:17203–17212. 2005.PubMed/NCBI
|
|
19
|
Zhou WH, Du MR, Dong L, Zhu XY, Yang JY,
He YY and Li DJ: Cyclosporin A increases expression of matrix
metalloproteinase 9 and 2 and invasiveness in vitro of the
first-trimester human trophoblast cells via the mitogen-activated
protein kinase pathway. Hum Reprod. 22:2743–2750. 2007. View Article : Google Scholar
|
|
20
|
Köhrmann A, Kammerer U, Kapp M, Dietl J
and Anacker J: Expression of matrix metalloproteinases (MMPs) in
primary human breast cancer and breast cancer cell lines: new
findings and review of the literature. BMC Cancer.
9:1882009.PubMed/NCBI
|
|
21
|
Ulisse S, Baldini E, Sorrenti S and
D’Armiento M: The urokinase plasminogen activator system: a target
for anti-cancer therapy. Curr Cancer Drug Targets. 9:32–71. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han B, Nakamura M, Mori I, Nakamura Y and
Kakudo K: Urokinase-type plasminogen activator system and breast
cancer (Review). Oncol Rep. 14:105–112. 2005.PubMed/NCBI
|
|
23
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: a changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hunter T and Pines J: Cyclins and cancer.
II: cyclin D and CDK inhibitors come of age. Cell. 79:573–582.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Zhang QH, Wu LJ, Tashiro S,
Onodera S and Ikejima T: Atypical apoptosis in L929 cells induced
by evodiamine isolated from Evodia rutaecarpa. J Asian Nat
Prod Res. 6:19–27. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Korsmeyer SJ, Shutter JR, Veis DJ, Merry
DE and Oltvai ZN: Bcl-2/Bax: a rheostat that regulates an
anti-oxidant pathway and cell death. Semin Cancer Biol. 4:327–332.
1993.PubMed/NCBI
|
|
27
|
Wu GS: Role of mitogen-activated protein
kinase phosphatases (MKPs) in cancer. Cancer Metastasis Rev.
26:579–585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reddy KB, Nabha SM and Atanaskova N: Role
of MAP kinase in tumor progression and invasion. Cancer Metastasis
Rev. 22:395–403. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Seddighzadeh M, Zhou JN, Kronenwett U,
Shoshan MC, Auer G, Sten-Linder M, Wiman B and Linder S: ERK
signalling in metastatic human MDA-MB-231 breast carcinoma cells is
adapted to obtain high urokinase expression and rapid cell
proliferation. Clin Exp Metastasis. 17:649–654. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
You J, Mi D, Zhou X, Qiao L, Zhang H,
Zhang X and Ye L: A positive feedback between activated
extracellularly regulated kinase and cyclooxygenase/lipoxygenase
maintains proliferation and migration of breast cancer cells.
Endocrinology. 150:1607–1617. 2009. View Article : Google Scholar
|
|
32
|
Li T, Feng Z, Jia S, Wang W, Du Z, Chen N
and Chen Z: Daintain/AIF-1 promotes breast cancer cell migration by
up-regulated TNF-α via activate p38 MAPK signaling pathway. Breast
Cancer Res Treat. 131:891–898. 2012.PubMed/NCBI
|
|
33
|
Xia Z, Dickens M, Raingeaud J, Davis RJ
and Greenberg ME: Opposing effects of ERK and JNK-p38 MAP kinases
on apoptosis. Science. 270:1326–1331. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Y, Zhang QH, Wu LJ, Shin-Ichi
Tashiro, Satoshi O and Takashi I: Regulation of ERK MAPK in
evodiamine-induced A375-S2 cell death. Chin J Pathophysiol.
20:2175–2179. 2004.
|
|
35
|
Zelivianski S, Spellman M, Kellerman M,
Kakitelashvilli V, Zhou XW, Lugo E, Lee MS, Taylor R, Davis TL,
Hauke R and Lin MF: ERK inhibitor PD98059 enhances
docetaxel-induced apoptosis of androgen-independent human prostate
cancer cells. Int J Cancer. 107:478–485. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fiddes RJ, Janes PW, Sivertsen SP,
Sutherland RL, Musgrove EA and Daly RJ: Inhibition of the MAP
kinase cascade blocks heregulin-induced cell cycle progression in
T-47D human breast cancer cells. Oncogene. 16:2803–2813. 1998.
View Article : Google Scholar
|
|
37
|
Boldt S, Weidle UH and Kolch W: The role
of MAPK pathways in the action of chemotherapeutic drugs.
Carcinogenesis. 23:1831–1838. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Porras A, Zuluaga S, Black E, Valladares
A, Alvarez AM, Ambrosino C, Benito M and Nebreda AR: P38α
mitogen-activated protein kinase sensitizes cells to apoptosis
induced by different stimuli. Mol Biol Cell. 15:922–933. 2004.
|