Introduction
Macrophages perform a variety of functions from
phagocytosis and the release of pro-inflammatory cytokines, to the
repair and remodeling of damaged tissues. Two phenotypes have been
proposed to identify their diverse roles: types M1 and M2 (1). The M1 macrophage refers to the cell
which aggressively destroys foreign invaders and tumor cells, and
releases pro-inflammatory cytokines. The M2 macrophage acts as a
support cell by promoting the healing of damaged cells by
angiogenesis and tissue remodeling. The transition from M1 to M2
might be facilitated by tumor environments (1). Tumors produce vascular endothelial
growth factor (VEGF) and macrophage colony stimulating factor
(M-CSF), both of which have the ability to interact with tyrosine
kinase receptors and induce macrophage migration (2,3). The
hypoxic center of the tumor releases factors that attract an M1
type, but as the macrophage enters the tumor microenvironment, it
is exposed to a variety of cytokines. These cytokines may possibly
convert type M1 macrophages to M2. In this microenvironment,
Interleukin-10, (IL-10), IL-6, IL-4, transforming growth factor β
(TGF-β1), and prostaglandin F2 (PGF2) are present. These factors
inhibit the cytotoxic activity of macrophages (4). In addition; IL-10, IL-4 and IL-13 do
more than just inhibit macrophage activation. IL-10 induces a
specific activation program and leads to alternatively activated
macrophages. This exposure to IL-10, and possibly IL-4 and IL-13
has been suggested to convert M1 macrophages to an M2 phenotype
(1). The possibility that
aggressive macrophages (M1), programmed to destroy cancerous cells,
might become converted to ones (M2) whose functions aid and promote
tumor survival is alarming, and would be a promising target for
cancer therapy.
Tumor-associated macrophages (TAMs) are thought to
behave and function as M2 macrophages in their abilities to aid
tumor cell survival and growth. In certain cancers, such as breast
cancer, TAMs can comprise as much as 50% of the tumor mass
(5). High numbers of TAM
infiltrates in tumor masses correlate with poor prognosis in
breast, prostate, ovarian, cervical and lung cancer (6). This poor prognosis could be due to a
variety of cytokines expressed by TAMs that stimulate tumor cell
proliferation and survival. TAMs have been shown to release a
number of pro-angiogenic cytokines and growth factors, including,
epidermal growth factor (EGF), platelet-derived growth factor
(PDGF), TGF-β1, hepatocyte growth factor (HGF), basic fibroblast
growth factor (BFGF), vascular endothelial growth factor (VEGF),
tumor necrosis factor α (TNF-α), and IL-8 (6). TAMs also contain various angiogenesis
modulating enzymes, such as matrix metalloproteinases (MMP) MMP-2,
MMP-9, MMP-7, MMP-12 and cyclooxygenase 2 (COX-2) (4). The many pro-angiogenic functions of
TAMs explain the correlation found between increased TAM numbers
and high vascular grades of many tumor types (7). By stimulating the growth and
proliferation of tumor cells, TAMs have been shown to promote tumor
development. An important question still remains, could TAMs help
in metastatic progression as well?
Studies of the migration of mammary carcinoma cells
in primary tumors indicated the migration to be regulated by
macrophages (8). In vitro
fusion of weakly metastatic mouse Cloudman S91 (6neo) melanoma
cells with peritoneal macrophages from DBA/2J mice produced
hybrids, a majority of which displayed enhanced metastatic
potential in vivo(9). It has
also been shown that co-culturing tumor cells with macrophages
increases the tumor cell’s invasive properties (10). Further studies have indicated that
TAMs are associated with tumor cells that move away from the main
body of the tumor (11). The
movement of tumor cells into blood vessels frequently occurs at
sites containing clusters of macrophages (12). All of these observations indicate a
metastatic association between TAMs and cancerous cells. According
to Pollard, macrophages are educated by the tumor microenvironment,
so that they adopt a trophic role that facilitates angiogenesis,
matrix breakdown and tumor-cell motility: all of which are elements
of the metastatic process (13).
To further investigate the possible relationship
between TAMs and metastasis, we used the Macrophage Fas-Induced
Apoptosis (MaFIA) mouse. In the transgenic MaFIA mouse, the cells
of the myeloid lineage have a transgenic receptor that when exposed
to the drug AP20187 (Ariad Pharmaceuticals), induces selective cell
death by apoptosis. These mice have been developed with a special
cellular receptor gene under the control of a macrophage specific
promoter, the cfms promoter. Because of this promoter,
expression of the cellular receptors only occurs in cells of the
myeloid lineage. The receptor is part of the FAS apoptosis pathway,
and normally binds to a ligand that induces apoptosis. The drug
AP20187 causes a trimerization of these FAS receptors and leads to
apoptosis through activation of the caspase 8 pathway (14). Enhanced green fluorescent protein
(EGFP) was fused to the suicide gene to allow for easy
identification of transgene-expressing cells. Some dendritic cells
are also depleted upon addition of the drug AP20187; however,
because of reduced expression of the MaFIA transgene, a smaller
population of dendritic cells is depleted compared to macrophages.
Neutrophils increase as a result of macrophage and dendritic cell
depletion, but these cells are immature and their role is limited
(15). The original MaFIA mice were
created with wild-type C57Bl/6J mice purchased from Charles River
Laboratories (Boston, MA, USA).
The object of the proposed research was to
investigate the metastatic process in the presence and absence of
macrophages. Since MaFIA mice can undergo the selective depletion
of macrophages we examined the metastatic potential of tumors in
the presence and absence of macrophages. With subdermal and
intravenous injections of B16-F10 ATCC # CRL-6475 melanoma cells,
we compared induced metastasis from mice with depleted macrophages
to control mice with macrophages. The subdermal injections were
designed to create primary tumors to observe spontaneous
metastasis. The intravenous injections were designed to simulate
circulating tumor cells from the primary tumor, or experimental
metastasis.
Materials and methods
Cell culture
A mouse melanoma cell line, B16 F10 ATCC # CRL-6475,
was grown in Dulbecco’s modified Eagle’s medium, (DMEM)
supplemented with 10% bovine calf serum (Hyclone, Logan, UT, USA).
Cells were kept in a 37ºC incubator supplied with 5% CO2
and sub-cultured every 2 days. To prepare cells for injection, the
medium was changed 24 h before adding trypsin. Cells were
trypsinized using 1% Trypsin (Gibco) dissolved in Hank’s solution
(Hyclone). A cell count was performed and cells were re-suspended
at a concentration of 5×106 cells/ml.
Mice
Mice were cared for and used under proper
Institutional Animal Care and Use Committee (IACUC) procedures and
practices. Breeding pairs were established between two MaFIA
positive mice. Not all mice were transgene positive, so pups were
tested for transgene expression using a tail snip. Blood samples
from the tail were examined using flow cytometry. Mice expressing
the transgene also expressed GFP, while mice without the transgene
did not. For the spontaneous and experimental metastasis
experiments, mice were divided into two experimental groups. The
control groups received injections of melanoma cells. The
macrophage-depleted groups received depletion treatment and then
the injection of melanoma cells. Subdermal and intravenous
injections consisted of 1×106 cells in 200 μl of growth
medium. The mice were anesthetized using Avertin. Subdermal and
intravenous injections were performed using a 27-gauge needle and a
1cc syringe. The subdermal injections were placed on the back of
the mouse between the shoulders, while the intravenous injections
were administered through tail veins.
Depletion
Depletion of the positive mice was achieved using
the drug AP20187. The drug was dissolved in 100% ethanol at a
concentration of 62.5 mg/ml. Injection volumes were adjusted to
administer 10 mg of the drug AP20187 for every kg of mouse body
weight. Drug formulation included sterile phosphate buffered saline
(PBS), 2% Tween in PBS, and polyethylene glycol (PEG) 400. Mice
were weighed each day before injections. Intra-peritoneal
injections were given using a 27-gauge needle and a 1cc syringe.
Mice were given the five-day depletion regimen as previously
described (15). After the five-day
period, mice were given injections three times a week to maintain
depletion.
Tissue collection and staining
Peritoneal lavages were utilized to ensure that the
depletion protocol functioned properly. A lavage was performed by
filling the peritoneal cavity with 5 ml of Hank’s balanced salt
solution (HBSS), injected with a 27-gauge needle and a 5-ml
syringe. The cavity was then rubbed with a wet needle cap to shake
the macrophages free from the tissues. The HBSS was then retrieved
from the cavity using a 21-gauge needle and a 5-ml syringe. The
cells were centrifuged at 650 × g for 5 min and then re-suspended
in 500 μl of HBSS and analyzed using flow cytometry for the
quantification of GFP-positive cells.
The tumors, from subdermal and intravenous
injections, were allowed to grow for two weeks after which the mice
were sacrificed by CO2 asphyxiation. The lungs, kidneys
and tumors of each mouse were removed for analysis of melanoma cell
content. The organs were placed into a 24-well plate, and each well
was filled with 1.5 ml of HBSS. The organs were then macerated with
scissors and transferred into a stomacher bag filled with 1.5 ml of
HBSS to be homogenized. Lungs were incubated with 15 μl of DNASE
collagenase solution at 37ºC for 1 h. Once the organs were
homogenized, the sample was divided in half and filtered. The
samples were centrifuged at 900 × g for 5 min and the supernatant
was decanted. A red blood cell lysis was performed by adding 2 ml
of Tris ammonium chloride lysis buffer, pH 7.5 at 50 nM, and
incubating for 2-5 min. After 5 min, 2 ml of HBSS was added and
samples were again centrifuged and the supernatant decanted. An Fc
block, (Mouse BD Fc Block™), was added to samples containing
macrophages and allowed to incubate for 10 min on ice and then the
samples were washed twice with HBSS. An aliquot of 50 μl of a 1:100
dilution of the combined melanoma antibodies HMB45ab732, DT101ab732
and BC199ab732 (Abcam Inc., Cambridge, MA, USA) was added. These
antibodies are specific for melanoma cells. The antibodies were
allowed to incubate for 1 h on ice in the dark. The samples were
then washed twice with HBSS and stained with a secondary antibody
conjugated with Alexa Fluor 633, on ice for 1 h. The samples were
washed twice and then analyzed using flow cytometry.
Flow cytometry
Flow cytometric analysis was performed on a BD
FACSCanto. Cell sorting was performed on the FACSVantage machine.
Cells were sorted into tubes containing 1 ml of bovine calf serum.
Data were collected and analyzed using FACSDIVA software.
Slide preparation and analysis
Samples from the cell sort and organ samples were
placed onto slides using a Cytospin. The slides were stained using
May Grunwald stain, 4% Geimsa, and then rinsed briefly in distilled
water. Some slides were not stained with the May Grunwald and
Geimsa, but analyzed for fluorescence. A cover slip was placed on
top of each slide with a drop of Cytoseal. Slides were analyzed
using a Zeiss Axioscop fluorescent microscope.
Results
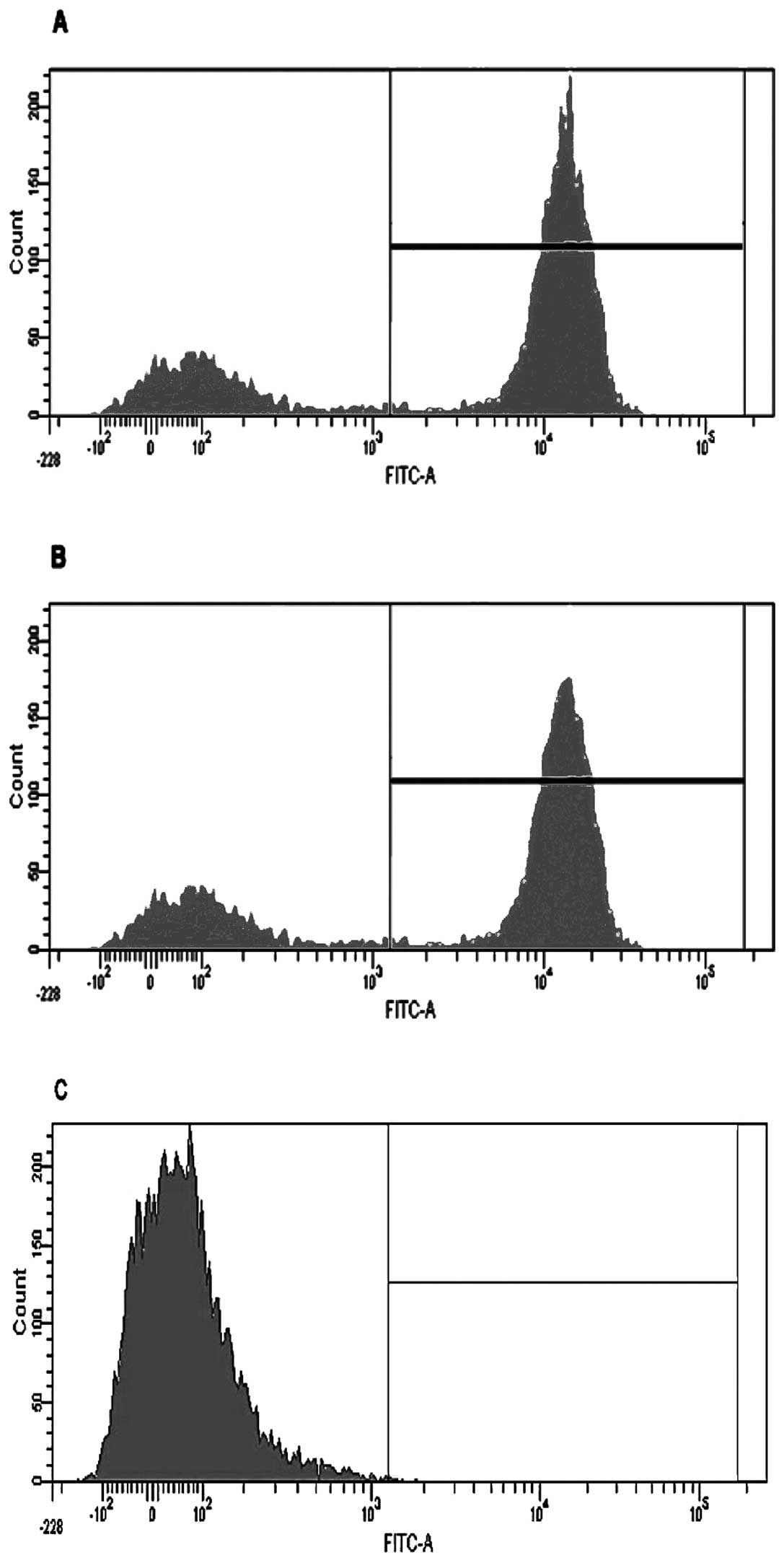
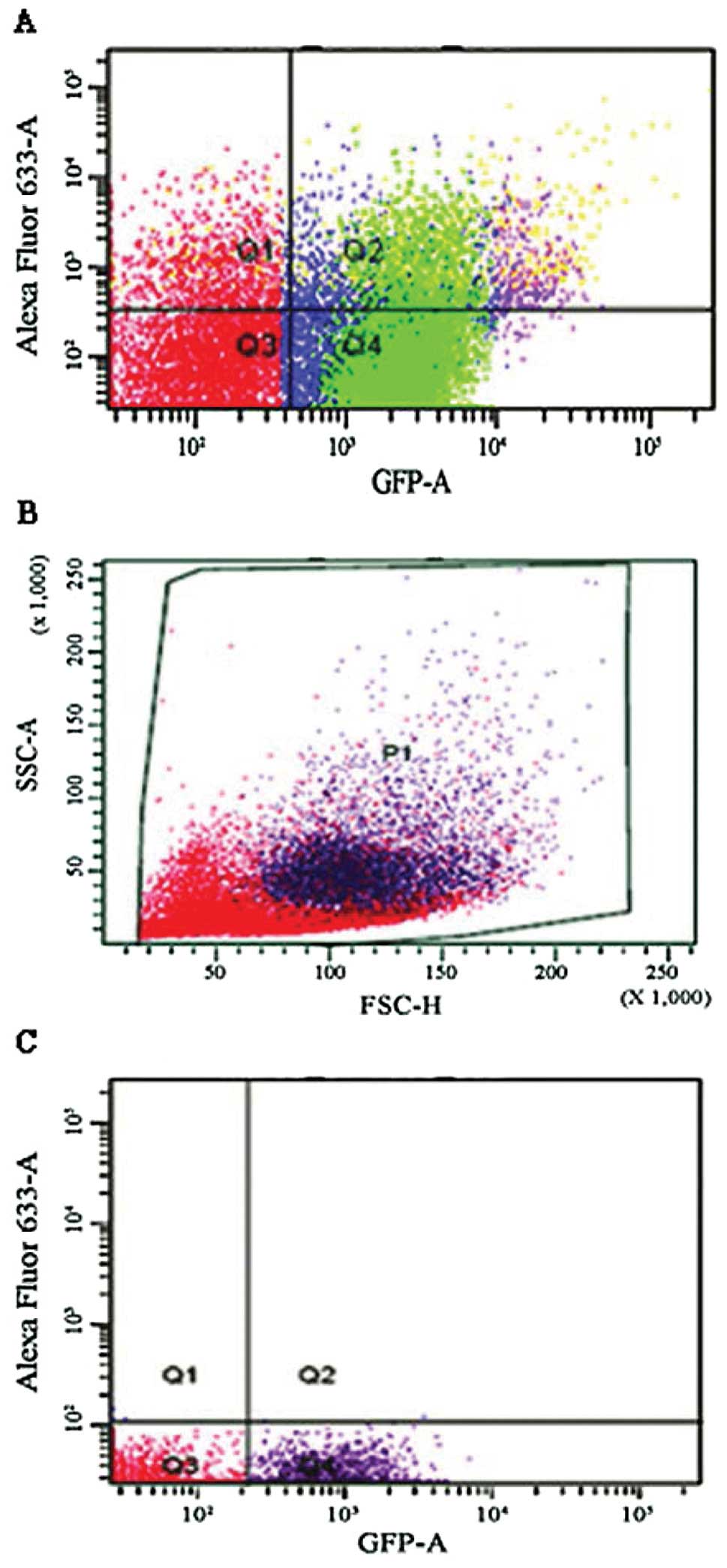
Initially macrophage depletion was confirmed by
conducting a depletion study with three cohorts (10 mice each) of
MaFIA positive mice. One cohort designated as a control was left
untreated, another was mock-treated using vehicle only, and the
third was treated with AP20187. Mice were injected for five
consecutive days as previously described (15) and then sacrificed 24 h following the
last injection. Peritoneal lavages were performed on all three
cohorts of mice and analyzed by flow cytometry (a representative
graph of one mouse from each cohort is shown in Fig. 1). Once inserted, the transgene
expression of GFP could be visualized in the macrophages using a
fluorescent microscope. The macrophages from AP20187-treated mice
showed a GFP level of 2%, mock-treated mice showed 61%, and the
positive control showed 73%, indicating that the depletion protocol
was effective. Lungs and kidneys were also tested and showed a
similar trend (data not shown).
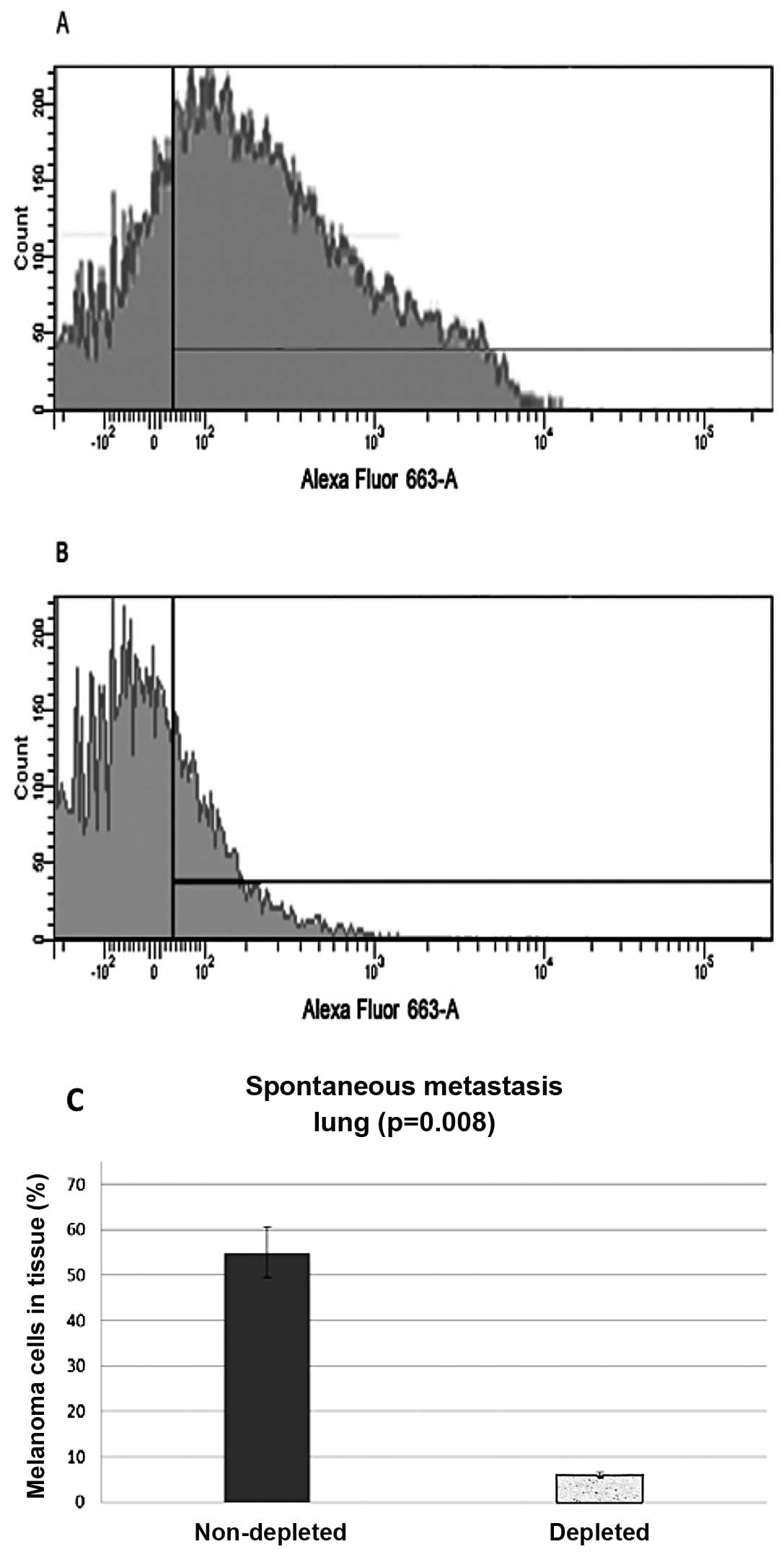
Spontaneous metastasis
Subdermal injections were administered and the lungs
and kidneys were harvested and analyzed for migrated melanoma
cells. Samples were stained with a cocktail of melanoma specific
antibodies (namely HMB45ab732, DT101ab732 and BC199ab732) (Abcam
Inc.), and then a secondary antibody, conjugated with Alexa Fluor
633. Flow cytometry analysis from the control group of the
harvested lungs showed that 55% (N=20, P=0.008) of the harvested
cells stained positive, while the lung samples from the
macrophage-depleted mice showed only 6% (N=20, P=0.008) of the
cells stained positive or the melanoma markers (Fig. 2).
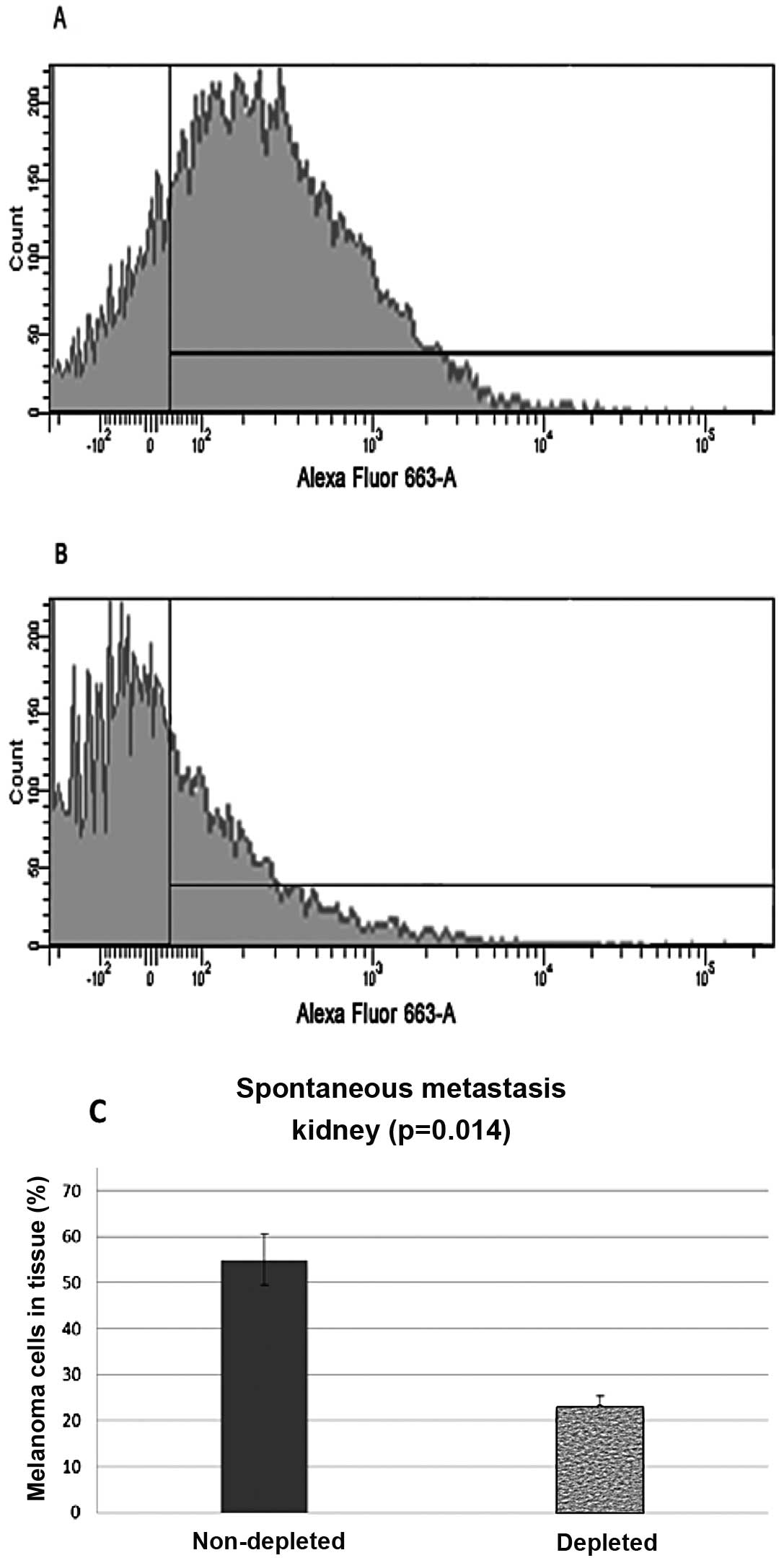
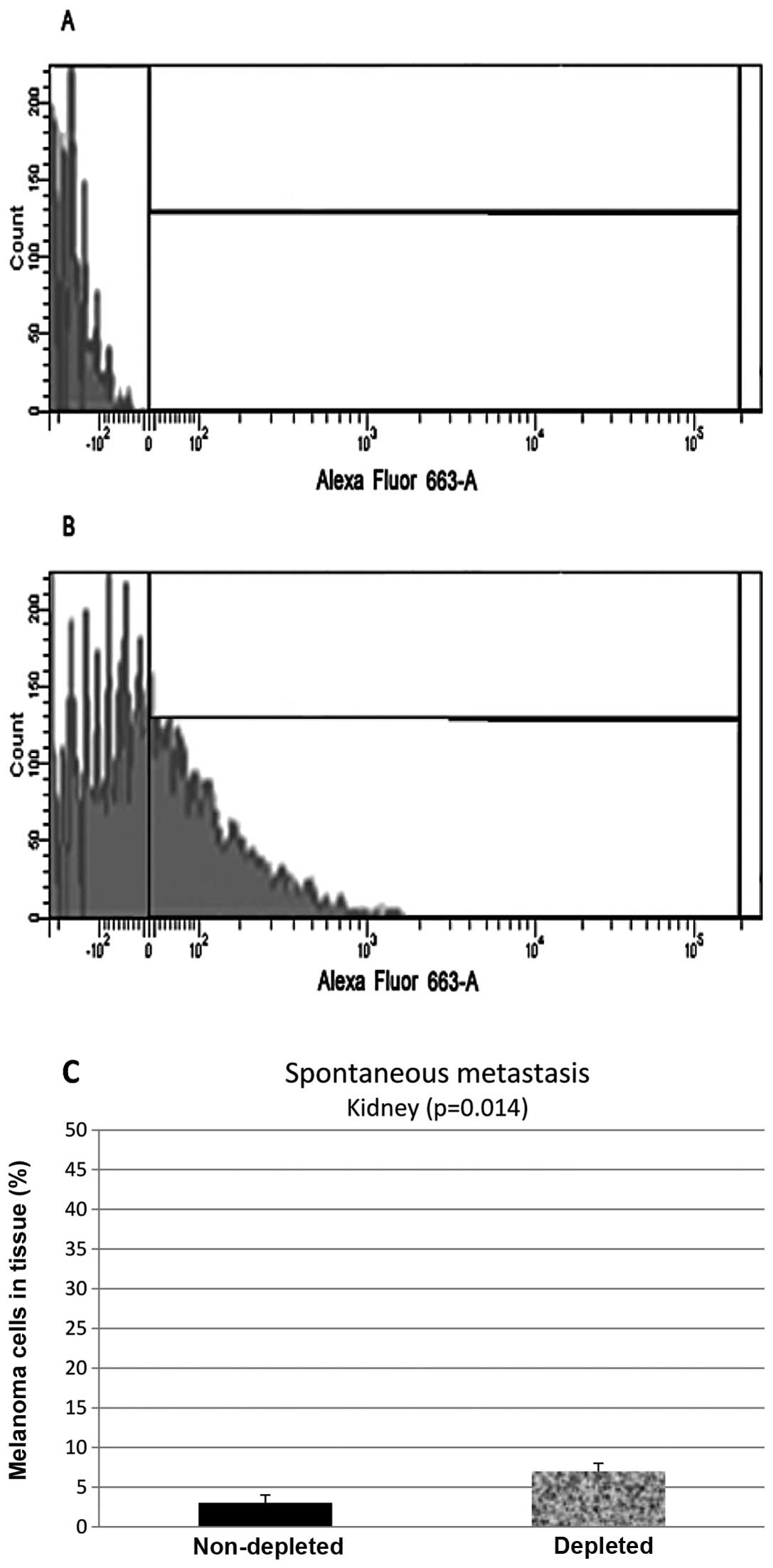
Flow cytometry analysis of the samples from the
kidney control group showed that 55% (N=20, P=0.014) of the
harvested cells stained positive, while samples from the depleted
mice showed 23% (N=20, P=0.014) of the cells stained positive
(Fig. 3).
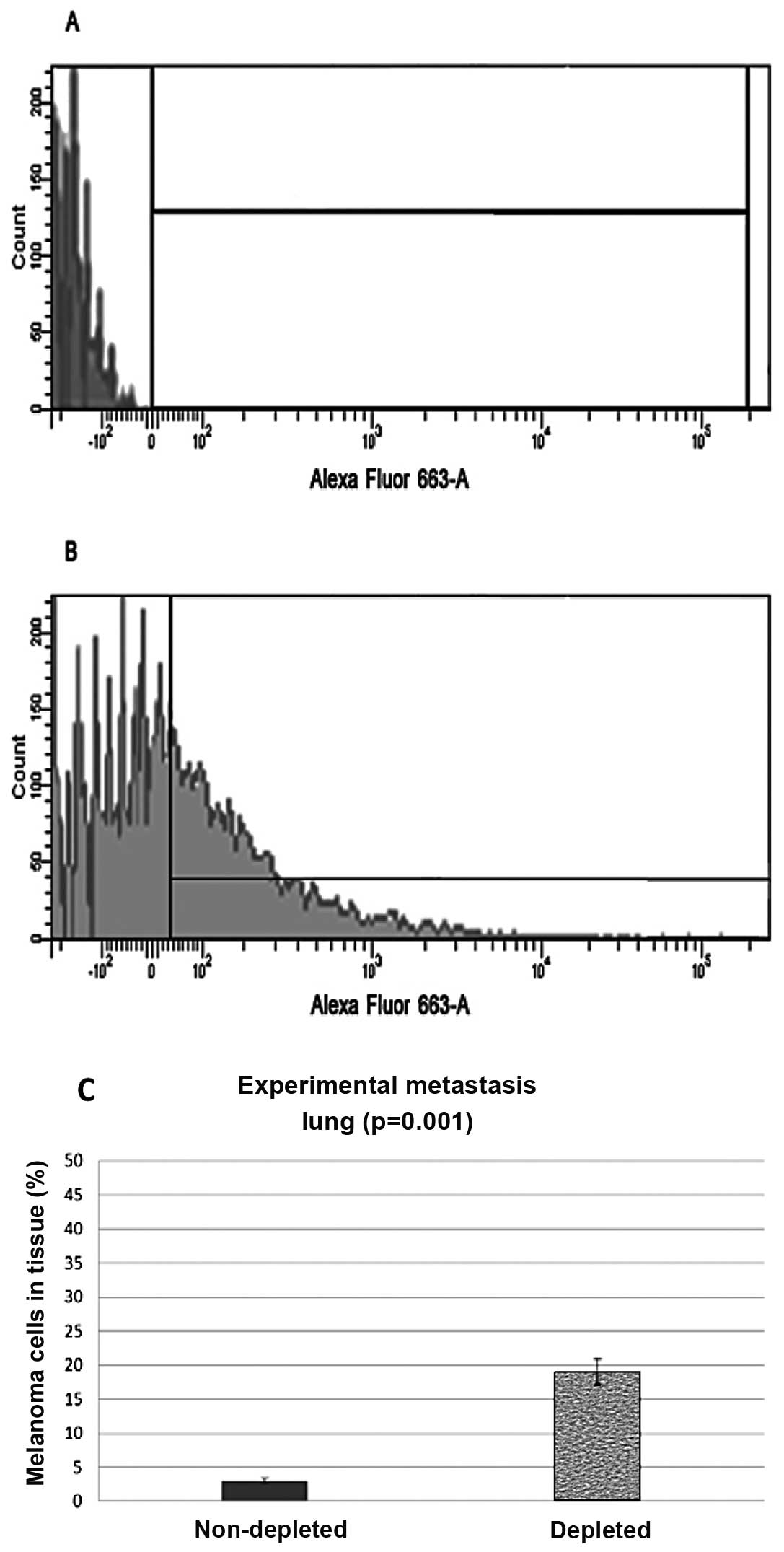
Experimental metastasis
Intravenous injections of melanoma cells through the
tail vein of the mice were also administered and the lungs and
kidneys were harvested and analyzed in the same manner as the
subdermal injected mice. Experimental metastasis flow cytometry
data from lung control samples showed that 3% (N=8, P=0.001) of the
harvested cells stained positive with the anti-melanoma antibodies.
The lung samples from the depleted mice showed that 19% (N=8,
P=0.001) of the cells stained positive for the melanoma markers
(Fig. 4).
Flow cytometry data from kidney control samples
showed that 2.5%, (N=8, P>0.01), of the harvested cells stained
positive with the melanoma markers. The kidney samples from the
depleted mice showed that 8% (N=8, P>0.01) of the cells stained
positive for the melanoma markers (Fig.
5).
Double-positive cells
From the spontaneous metastasis experiment, we
observed double-positive cells in the tumors, lungs and kidneys of
the non-depleted mice. These double- positive cells expressed both
GFP and stained positive for the anti-melanoma antibodies. This was
a possible indication of macrophage/melanoma fusion. By means of
both immunohistochemistry and immunofluorescence we further
examined the double-positive cells. We exposed macrophages taken
from a lavage of a MaFIA mouse to melanoma cell debris to see if
the macrophage MHC presentation yields false double-positives.
Based on the results (Figs. 6 and
7), it was clear that MHC
presentation did not yield any double-positive cells.
Discussion
Many sources indicate that the presence of TAMs
increases the metastatic potential of cancerous cells, or that TAMs
are directly involved in metastasis (8-13). Our
preliminary data are supportive evidence that TAMs play a role in
facilitating metastasis. In mice that received subdermal
injections, we noted a reduction in migrated melanoma cells when
the macrophages were selectively depleted. This suggests that
macrophages facilitate the migration of melanoma cells from the
primary tumor site to the lungs and kidneys. There was a
significant difference between the control (untreated group) and
the macrophage-depleted group with regards to the number of
melanoma cells found in both the lungs (P=0.008) and the kidneys
(P=0.014).
The results from the experimental metastasis
experiment indicated that a decrease in macrophages enables
melanoma cells, already in the blood stream, to have a greater
metastatic potential. The lungs in macrophage-depleted mice had a
significantly increased level of metastasis when compared to the
control (untreated) mice. From the results obtained, it could be
suggested that the presence of macrophages in the lungs helps to
prevent metastatic spread. The same trend was also seen in the
experimentally-induced metastases in the kidneys. There was also a
significant difference in the amount of metastasis in the kidneys
when comparing the control and experimental groups, suggesting that
the kidney metastasis is also influenced by the presence of
macrophages in the kidneys.
Since dendritic cells were also depleted it is
possible that they are also involved in metastasis. The likelihood
of their involvement is minimal because they have a reduced
expression of the MaFIA transgene, and therefore a smaller
population of dendritic cells is depleted compared to macrophages.
Second, the depletion of dendritic cells is delayed. Seven days
after the 5 day depletion regimen, the percentage of depleted cells
increased from 48 to 98%. Since mice were sacrificed 2 weeks after
the 5 day depletion regimen, the effects of reduced dendritic cells
would be reduced compared to macrophages. This leads us to believe
that the mice were sacrificed before any significant effects from
the loss of dendritic cells occurred. Neutrophils increase as a
result of macrophage and dendritic cell depletion, and it is also
possible that their increase could be involved in the decrease in
metastasis. The likelihood of this occurring is limited since the
neutrophils produced in response to deficient macrophages and
dendritic cells are immature (14,15).
By comparing both the spontaneous and experimental
metastasis systems, we can see further evidence for TAM involvement
in metastasis. The data are suggestive of TAMs serving as an aid to
cancer metastasis. The involvement of TAMs in metastasis is further
supported by the number of double-positive cells (Fig. 7). These are cells that stain
positive for melanoma markers and are also positive for GFP
expression. GFP is expressed only in the myeloid cell line;
therefore the double-positive cells indicate possible cell fusion.
Since EGFP was placed in the suicide gene of transgene-expressing
cells, macrophages express GFP. Fig.
7D-F indicate that cells that migrated from target organs can
express both GFP and melanoma surface markers as indicated by the
binding of the anti-melanoma antibodies, which leads us to believe
that this is evidence of TAM/melanoma fusion. It is possible that
the tumors convert naturally programmed M1 macrophages into type M2
cells. These M2 TAMs can then be utilized as carrier cells which
facilitate metastasis. The disruption of a tumor’s ability to
convert macrophages from M1 to M2, or inhibiting macrophages from
fusing with cancerous cells could be potential targets for cancer
therapies. Further research needs to be conducted to confirm that
macrophages facilitate metastasis and that tumors provide the means
for the conversion of macrophage phenotype.
Acknowledgements
Funding was provided by a Mentoring Environment
Grant (MEG) from Brigham Young University. MaFIA mice were provided
by Dr Sandra Burnett, originally developed by the University of
Kentucky, Division of Laboratory Animal Resources (Lexington, KY,
USA). A. Clement and K. Wells are also acknowledged for their help
with the mouse procedures.
References
|
1
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 11:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eubank TD, Galloway M, Montague CM,
Waldman WJ and Marsh CB: M-CSF induces vascular endothelial growth
factor production and angiogenic activity from human monocytes. J
Immunol. 171:2637–2643. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Elgert KD, Alleva DG and Mullins DW:
Tumor-induced immune dysfunction: the macrophage connection. J
Leukocyte Biol. 64:275–290. 1998.PubMed/NCBI
|
|
4
|
Lewis CE and Pollard JW: Distinct role of
macrophages in different tumor microenvironments. Cancer Res.
66:605–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nardin A and Abastado JP: Macrophages and
cancer. Front Biosci. 13:3494–3505. 2008. View Article : Google Scholar
|
|
6
|
Lewis C and Murdoch C: Macrophages
responses to hypoxia: implications for tumor progression and
anti-cancer therapies. Am J Pathol. 167:627–635. 2005.PubMed/NCBI
|
|
7
|
Polverini PJ and Leibovich SJ: Effect of
macrophage depletion on growth and neovascularization of hamster
buccal pouch carcinomas. J Oral Pathol. 16:436–444. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamaguchi H, Wyckoff J and Condeelis J:
Cell migration in tumors. Curr Opin Cell Biol. 17:559–564. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rachkovsky M, Sodi S, Chakraborty A,
Avissar Y, Bolognia J, McNiff JM, Platt J, Bermudes D and Pawelek
J: Melanoma x macrophage hybrids with enhanced metastatic
potential. Clin Exp Metastasis. 16:299–312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hagemann T, Robinson SC, Schulz M, Trümper
L, Balkwill FR and Binder C: Enhanced invasiveness of breast cancer
cell lines upon co-cultivation with macrophages is due to TNF-alpha
dependent up-regulation of matrix metalloproteases. Carcinogenesis.
25:1543–1549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goswami S, Sahai E, Wyckoff JB, Cammer M,
Cox D, Pixley FJ, Stanley ER, Segall JE and Condeelis JS:
Macrophages promote the invasion of breast carcinoma cells via a
colony-stimulating factor-1/epidermal growth factor paracrine loop.
Cancer Res. 65:5278–5283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wyckoff J, Wang W, Lin EY, Wang Y, Pixley
F, Stanley ER, Graf T, Pollard JW, Segall J and Condeelis J: A
paracrine loop between tumor cells and macrophages is required for
tumor cell migration in mammary tumors. Cancer Res. 64:7022–7029.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pollard JW: Tumor-educated macrophages
promote tumor progression and metastasis. Nat Rev Cancer. 4:71–78.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burnett SH, Kershen EJ, Zhang J, Zeng L,
Straley SC, Kaplan AM and Cohen DA: Conditional macrophage ablation
in transgenic mice expressing a Fas-based suicide gene. J Leukoc
Biol. 75:612–623. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Allende ML, Bektas M, Lee BG, Bonifacino
E, Kang J, Tuymetova G, Chen W, Saba JD and Proia RL:
Sphingosine-1-phosphate lyase deficiency produces a
pro-inflammatory response while impairing neutrophil trafficking. J
Biol Chem. 286:7348–7358. 2011. View Article : Google Scholar : PubMed/NCBI
|