Introduction
Gastric cancer is the second most common cancer
worldwide and the fourth leading cause of cancer-related mortality
(1,2). The incidence, diagnostic studies and
therapeutic options have undergone major changes in recent years,
but the prognosis for gastric cancer remains poor, particularly in
more advanced stages (3).
Currently, the delivery of chemotherapeutic agents following
surgical resection defines the standard treatment for this
malignancy. However, one clinically significant problem that often
results in the failure of chemotherapy is multidrug resistance
(MDR), which severely limits the effectiveness of chemotherapy in
gastric cancer and is responsible for the overall poor efficacy of
cancer therapy (4).
MDR is generally used to describe a resistance
phenotype where resistance, either inherent or acquired, develops
not only to a single cytotoxic agent but also to a whole range of
drugs with different structures and cellular targets (4,5). The
mechanisms of drug resistance that can act individually or
synergistically are complicated, and have been described and
extensively studied over the past decades. These include reduction
of intracellular drugs by increasing drug efflux and/or decreasing
drug uptake (6,7), increased repair of drug-induced DNA
damage (8,9), evasion of drug-induced apoptosis
(10,11), disruptions in signaling pathways and
some other changes in the factors that regulate the resistance
mechanisms. Chemotherapeutic drugs induce a series of cellular
responses that have an impact on tumor cell proliferation and
survival. Furthermore, several studies have suggested a direct
correlation between alterations in survival pathways and
chemoresistance, and certain components of these pathways have been
identified as critical targets for cancer intervention (12). Of these survival pathways, the
phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway plays a
major role not only in tumor development but also in the potential
response of the tumor to treatment.
The PI3K/Akt signal transduction pathway plays a
crucial role in tumorigenesis and tumor progression by promoting
cell proliferation and inhibiting apoptosis. The PI3Ks are lipid
kinases, which can be activated downstream of receptor tyrosine
kinases, including epidermal growth factor receptor (EGFR),
vascular endothelial growth factor receptor (VEGFR) and
insulin-like growth factor receptor (IGFR) (13,14).
Following PI3K activation, phosphoinositol-4,5-bisphosphate (PIP2)
is converted into the second messenger
phosphoinositol-3,4,5-trisphosphate (PIP3) on the inner side of the
plasma membrane (15). Then, the
lipid product of PI3K and PIP3 recruits a subset of signaling
proteins with pleckstrin homology (PH) domains to the membrane,
including PDK1 and Akt/PKB (15).
Once activated, Akt mediates the activation and inhibition of
several targets, resulting in cellular survival, growth and
proliferation through various mechanisms. Thus, inhibition of PI3K
or molecules involved in the PI3K signaling pathway is a promising
approach to treat tumors. Moreover, several studies have indicated
that LY294002, a commonly used pharmacological inhibitor of PI3K,
could decrease tumor growth, inhibit tumor invasion and migration,
and sensitize various tumors to chemotherapy (16–19).
Since PI3K/Akt is found to be highly activated in gastric cancer
and is positively correlated to progression and chemoresistance of
gastric cancer, we hypothesized that treatment with LY294002 may
inhibit proliferation and increase the sensitivity of gastric
cancer to chemotherapy.
In the present study, we evaluated the effect of
inhibition of the PI3K/Akt pathway on the chemosensitivity of
gastric cancer cells in vitro and in vivo and
investigated the possible underlying cellular mechanisms. Here, we
present evidence that LY294002 can enhance chemosensitivity of
human gastric cancer cells to vincristine (VCR) by inactivation of
the PI3K/Akt signaling pathway. Our findings suggest that
modulation of the PI3K/Akt pathway may present a new strategy to
improve current therapeutic regimens and provide a molecular basis
for the novel design of combination treatments for gastric
cancer.
Materials and methods
Ethics statement
The experiments involving the use of laboratory
animals were carried out in accordance with the Animal Welfare Act
and the recommendations of the Institutional Animal Care and Use
Committee of the Third Military Medical University. All animal
studies were approved by the Ethics Committee of Xinqiao Hospital
of the Third Military Medical University (Chongqing, China). The
animals were humanely sacrificed and all efforts were made to
ameliorate suffering.
Cell lines and animals
A moderately differentiated stomach adenocarcinoma
cell line, SGC-7901, was purchased from the cell bank of the
Chinese Academy of Sciences (Shanghai, China). SGC-7901/VCR, a
VCR-resistant cell line, was established and generously gifted by
Dr Daimin Fan (Institute of Gastroenterology, Xijing Hospital, The
Fourth Military Medical University, China). The SGC-7901 cells were
maintained in RPMI-1640 medium supplemented with 10% fetal bovine
serum (both from Gibco-BRL, Carlsbad, CA, USA), SGC-7901/VCR cells
were cultured in the same medium with an additional 1 mg/l VCR. All
cells were cultured at 37°C in a humidified atmosphere containing
5% CO2.
Twenty-four nude male mice (four weeks old) were
purchased from the Shanghai Experimental Animal Center (Shanghai,
China) and were maintained in a specific pathogen-free environment,
fed with a regular diet.
Western blot analysis
A western blot analysis was used to confirm the
inhibition of PI3K/Akt activity in SGC-7901 and SGC-7901/VCR cells.
SGC-7901 and SGC-7901/VCR cells were collected by centrifugation
and washed twice with PBS. Proteins from these cell extracts were
denatured by boiling for 10 min. Protein concentration was
determined using the bicinchoninic acid protein assay (Pierce
Biochemicals, Rockford, IL, USA) and equal amounts of protein were
loaded on a 10% SDS-PAGE gel and transferred onto a nitrocellulose
membrane (Millipore, Bedford, MA, USA) by electroblotting.
Subsequently, the membranes were blocked with 5% non-fat milk in
PBS, and incubated with primary antibodies for anti-Akt (Upstate,
Billerica, MA, USA), anti-phospho-Akt (Cell Signaling Technology,
Danvers, MA, USA) or anti-β-actin mAb (Sigma, St. Louis, MO, USA)
at 4°C overnight. The membranes were washed and incubated with an
alkaline phosphatase-conjugated goat anti-mouse IgG antibody
(Amersham Biosciences, Buckinghamshire, UK) for 1 h at room
temperature. Immunoreactive bands were detected using the ECL
western blot analysis system (Amersham Biosciences).
Cell viability assay
Cell proliferation was determined by the MTT assay
as previously described (19).
Briefly, SGC-7901 and SGC-7901/VCR cells were divided into three
groups: VCR, LY294002, and VCR + LY294002, respectively. Cells were
digested and plated in 96-well plates at a density of
5×103 cells/well. The cells in different groups were
then treated with varying concentrations of VCR and/or LY294002.
Untreated cells were considered as negative control. After 72 h of
incubation, the medium was removed and replaced with 20 μl MTT (5
mg/ml), and incubated for 4 h at 37°C. Then, the supernatant was
carefully removed and DMSO was added to each well to dissolve the
crystals by gentle agitation for 10 min. The absorbance at 570 nm
(A570) of each well was read on a Microplate Reader
(Bio-Tek ELX-800; BioTek, Winooski, VT, USA). The survival rate of
cells in each well was calculated according to the following
formula: survival rate = (A570 of treated
wells/A570 of untreated wells) × 100%. Finally,
IC50 values were determined. Each experiment was
performed in quadruplicates.
High performance liquid chromatography
(HPLC)
The cells were plated at a density of
2×107 cells/10 cm dish. On the following day, the cells
were treated with VCR combined with LY294002 or not for 3 and 6 h,
respectively. After the incubation, the cells were washed three
times with cold PBS, collected by digestion and centrifugation, and
then resuspended in saline in 2 ml Eppendorf tubes. Cells were
broken down by repeated freezing and thawing and then centrifuged
for 15 min at 16,000 rpm. Subsequently, 20% perchloric acid (50
μl/500 μl of PBS) was added to denature the protein and 1 ml of
diethyl ether was added to each tube and mixed by vortexing for 1
min. Following centrifugation for 15 min at 16,000 rpm, the organic
phase (500 μl) was collected and evaporated to dryness. The dried
residue was reconstituted with 500 μl of the mobile phase (93%
methanol, 7% water and 0.18% triethylamine) and used for HPLC.
Samples were analyzed using a C-18 reverse phase column and eluted
isocratically with a mobile phase of water and triethylamine in
methanol. The flow rate was set at 0.5 ml/min and column
temperature was 40°C.
TUNEL assay
SGC7901 and SGC7901/VCR cells were digested and
plated on glass coverslips in 24-well plates. Subsequently, the
drug was added to the corresponding wells of each group with the
final volume of 2.2 ml. Cells were cultured at 37°C in a humidified
atmosphere containing 5% CO2 for 72 h and then fixed
with 4% paraformaldehyde for 1 h. Endogenous peroxidase was blocked
by 3% H2O2 in methanol for 10 min.
Subsequently, cells were rinsed with PBS and incubated with
permeabilization solution for 2 min on ice. The coverslips were
washed twice with PBS and then 50 μl of the TUNEL reaction mixture
(Roche, Boulder, CO, USA) were added to the cells. Finally, the
coverslips were stained with DAB for 10 min, washed twice with PBS
and were observed under a microscope. Labeled nuclei and the total
number of cells were counted in at least five different fields.
RT-PCR
RT-PCR was performed for semiquantitative detection
of the mRNA expression of MDR1, Bax, Bcl-2, XIAP and
caspase-3 in SGC-7901 and SGC-7901/VCR cells using the Access
RT-PCR system (Promega Co., Madison, WI, USA). The primers for the
above genes were synthesized (Table
I). Total RNA was isolated using Tripure reagent (Roche
Diagnostics, Mannheim, Germany) using standard methods. The total
volume of the RT-PCR reaction system was 25 μl, including 0.2
mmol/l dNTP, 1 mmol/l of each primer, 1 mmol/l MgSO4,
0.1 U/ml AMV transcriptase, 0.1 U/ml Tf1 DNA polymerase, 1X
reaction buffer and 100 ng of RNA template. The amplification
conditions were 48°C for 45 min for cDNA synthesis and 40 cycles
(94°C, 30 sec; 60°C, 30 sec; 72°C, 1 min) for PCR (Perkin-Elmer
Cetus, Norwalk, CA, USA). The amplified products were
electrophoresed on a 2% agarose gel and visualized after ethidium
bromide staining of the gel. β-actin was used as an internal
control.
 | Table IPrimers for RT-PCR analysis. |
Table I
Primers for RT-PCR analysis.
| Genes | Primers |
|---|
| MDR1 |
| Sense |
5′-CTCGAGGAATCAGCATTCAG-3′ |
| Antisense |
5′-AGATCTCTTTGAGCTTGGAAGAGC-3′ |
| Bax |
| Sense |
5′-CTGACATGTTTTCTGACGGC-3′ |
| Antisense |
5′-TCAGCCCATCTTCTTCCAGA-3′ |
| Bcl-2 |
| Sense |
5′-ACACTGTTAAGCATGTGCCG-3′ |
| Antisense |
5′-CCAGCTCATCTCACCTCACA-3′ |
| XIAP |
| Sense |
5′-TGGCACGAGCAGGGTTTCTTT-3′ |
| Antisense |
5′-TGGCACGAGCAGGGTTTCTTT-3′ |
| Caspase-3 |
| Sense |
5′-AAGCGAATCAATGGACTCTG-3′ |
| Antisense |
5′-GACTTCTACAACGATCCCCTC-3′ |
| β-actin |
| Sense |
5′-CCACGAAACTACCTTCAACTCC-3′ |
| Antisense |
5′-ACTCGTCATACTCCTGCTTGCT-3′ |
Immunohistochemical staining
The protein expression of P-glycoprotein (P-gp),
Bax, Bcl-2, caspase-3 and XIAP in SGC-7901 and SGC-7901/VCR cells
was detected by immunohistochemical assay. Briefly, cells were
plated in 24-well plates (3×104 cells/well) and cultured
for 72 h. The cells were washed and fixed with 70% ethanol and then
0.3% H2O2-methanol solution was added to each
well, and the plates were placed in a 25°C incubator for 30 min to
inhibit the activity of endogenous peroxidase. Subsequently, the
cells were incubated with the primary antibodies for P-gp, Bax,
Bcl-2, caspase-3 or XIAP (Beijing ZhongShan Biotechnology Co.,
Beijing, China) at 4°C overnight. After a thorough wash with PBS
containing 0.1% Triton X-100, the cells were incubated with a
secondary antibody (Beijing ZhongShan Biotechnology Co.) for 30 min
at 25°C. Finally, cells were incubated for 15 min with an
avidin-biotin enzyme reagent (Beijing ZhongShan Biotechnology Co.).
The staining was performed by adding a
3,3-diaminobenzidine/H2O2 solution in each
well under an inverted microscope. The staining reaction was
terminated by adding PBS to each well. The figure was captured and
analyzed by IPP5.0 (Media Cybernetics, Rockville, MD, USA).
Tumor xenografts in nude mice
Twenty-four nude mice at four weeks of age were
maintained in a pathogen-free animal facility in accordance with
the local Ethics Committee of The Third Military Medical
University. Mice were divided into four groups: the VCR group, the
LY294002 group, the VCR + LY294002 group, and the control group.
SGC7901 and SGC7901/VCR cells were digested and injected
subcutaneously into nude mice to induce gastric tumor xenografts.
After 10 days, mice were treated with VCR (40–50 mg/kg body weight)
and/or LY294002 (15–20 mg/kg body weight) once per day
intraperitoneally for one week. The mice were sacrificed when
established tumors in the control group reached ~1 cm3.
Then, the tumor was stripped out and blood, fat, necrotic tissues
and non-tumor ingredients were removed. Tumor was weighed (tumor
weight in grams) and tumor inhibition rate was calculated. Tumor
inhibition rate = (average tumor weight of control group - the
average tumor weight of experimental group)/average tumor weight of
control group × 100%.
Statistical analysis
The data are expressed as the means ± standard
deviation (SD). Statistical analysis of the data was performed
using SPSS 17.0 (SPSS, Chicago, IL, USA). Statistical significance
was determined using ANOVA followed by Student’s t-test. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
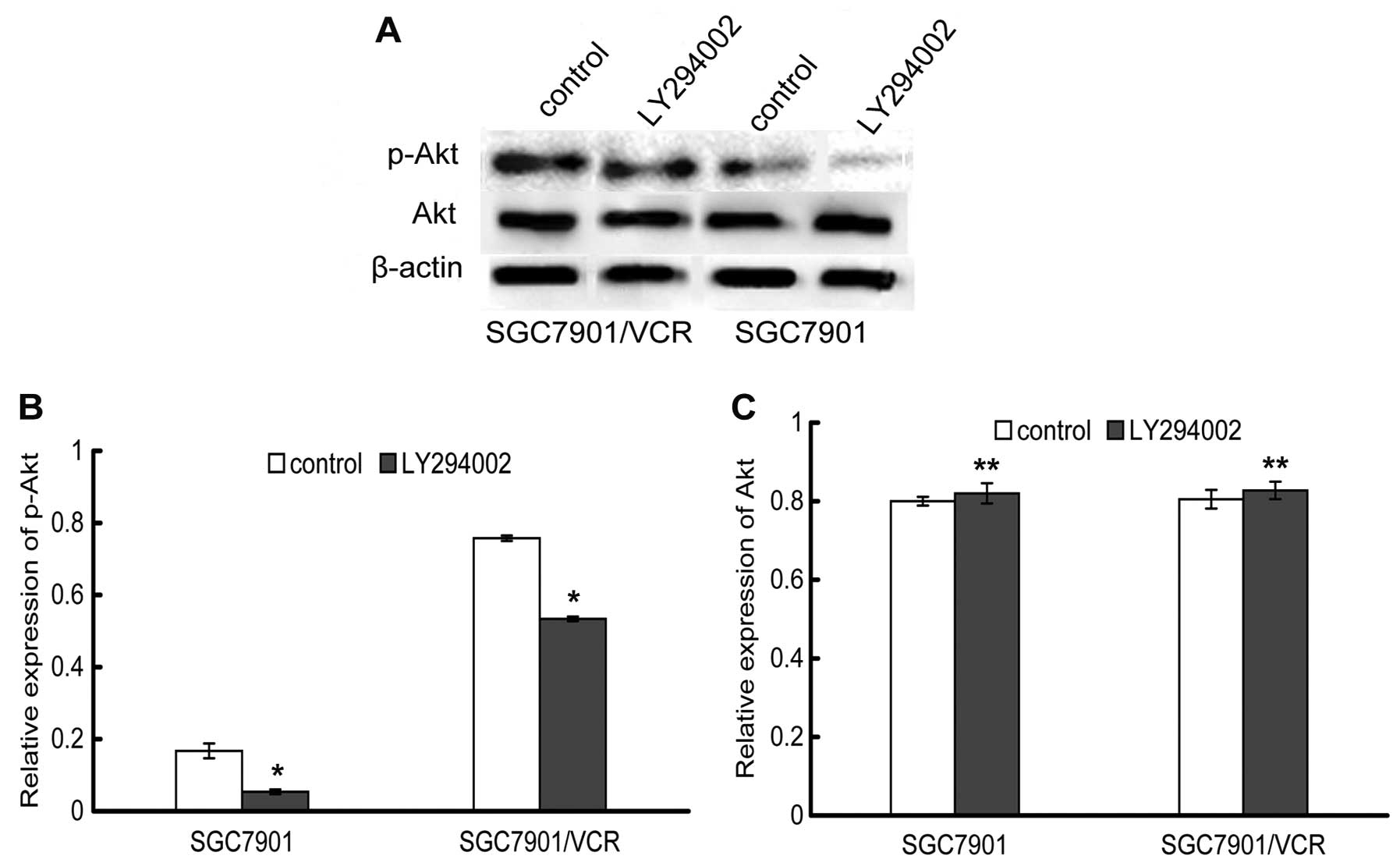
Inhibition of the PI3K/Akt signaling
pathway by LY294002
To explore the possible role of the PI3K/Akt pathway
in SGC7901 and SGC7901/VCR cells and to evaluate the influence of
LY294002 on PI3K activity, phosphorylated Akt and total Akt were
examined by western blot analysis. As expected, the phospho-Akt
level in SGC7901/VCR cells was higher than that in SGC7901 cells,
suggesting a higher PI3K/Akt activity in the resistant SGC7901/VCR
cells. Treatment of these two cell lines with 20 μmol/l of LY294002
caused inactivation of Akt, as seen by a significant decrease in
Akt phosphorylation. However, LY294002 did not inhibit the
expression of total Akt, thereby demonstrating that LY294002
inhibits the activity of Akt and not its expression (Fig. 1).
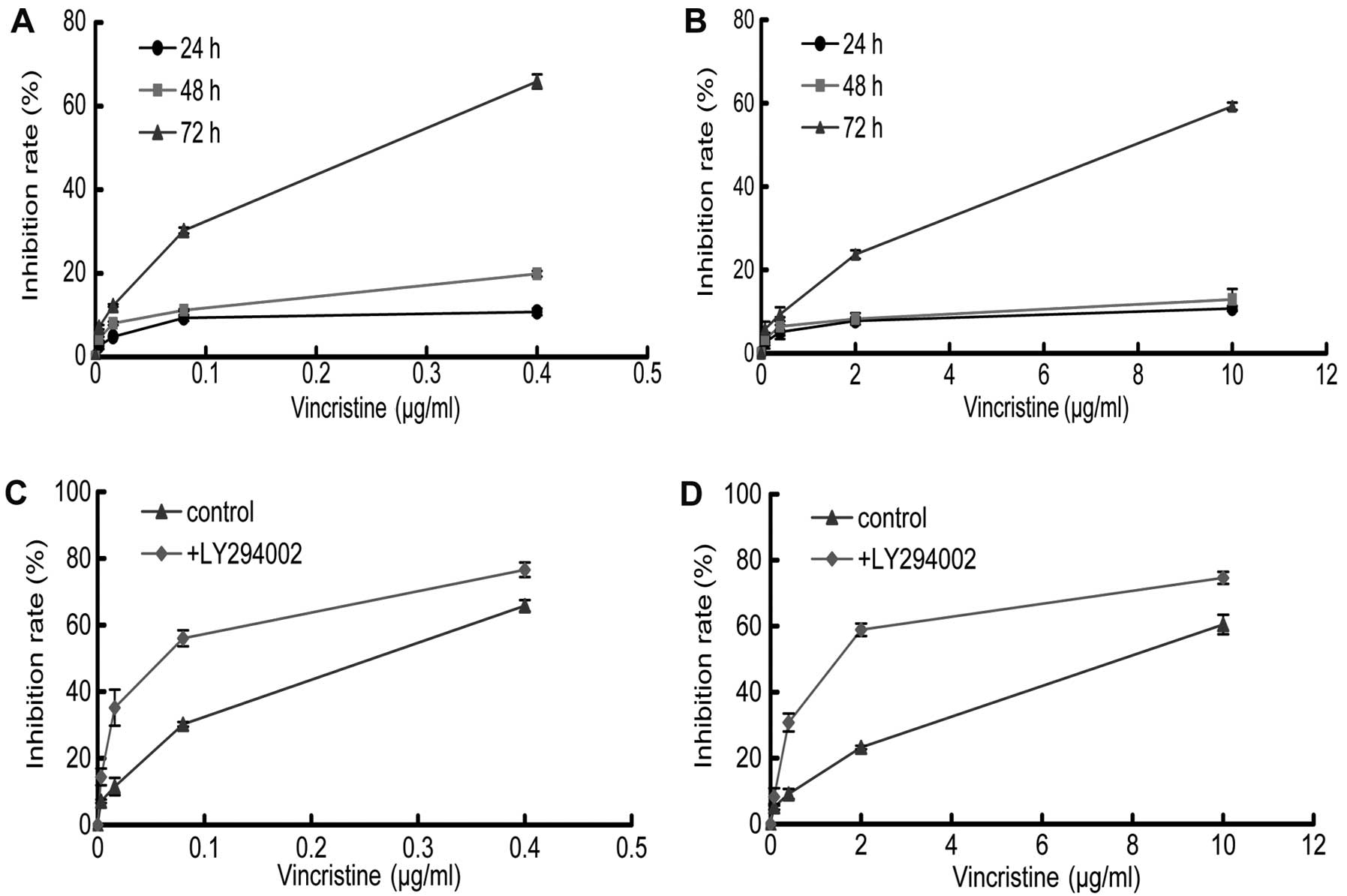
LY294002 enhances the sensitivity of
gastric cancer cells to VCR
The MTT assay was performed to evaluate the effect
of LY294002 and/or VCR on the proliferation of SGC7901 and
SGC7901/VCR cells. The results showed that VCR was cytotoxic to the
two cell lines in a dose and time-dependent manner at 72 h, with an
IC50 of 0.2±0.03 μg/ml and 8.09±0.6 μg/ml for the
SGC7901 and SGC7901/VCR cells, respectively (Fig. 2). The resistance index (ratio of
IC50 for resistant and parental cells) was ~40.45,
demonstrating that the SGC7901/VCR cells were highly resistant to
VCR. The growth inhibitory effect of VCR was significantly reduced
in SGC7901/VCR cells compared with their parental cells.
Subsequently, we combined LY294002 (20 μmol/l) with varying
concentrations of VCR to test whether this combination could
further inhibit growth of gastric cancer cells. The addition of
LY294002 significantly increased the potency of VCR in these two
cell lines, and the IC50 decreased further to 0.05±0.006
μg/ml and 1.70±0.20 μg/ml, respectively (Fig. 2). We then used the combination index
method to determine the exact interactions between LY294002 and
VCR. The combination index for the two drugs was <1, indicating
their synergistic effect.
LY294002 increases the intracellular
concentrations of VCR in gastric cells
To investigate whether the inhibition of the
PI3K/Akt pathway by LY294002 could result in increased accumulation
of VCR in gastric cancer cell lines, we performed HPLC to determine
the intracellular concentrations of VCR in these cells, either
pretreated with LY294002 or not. The results showed that the VCR
concentration in SGC7901 cells treated with LY294002 was twice as
much as that in SGC7901 cells without treatment with LY294002
(Table II). In SGC7901/VCR cells,
the concentration of VCR was undetectable without treatment with
LY294002, thereby demonstrating a high resistance of the cells to
VCR. However, the concentration of VCR was detected 6 h after
LY294002 treatment, suggesting that LY294002 could increase the
accumulation of VCR in resistant gastric cancer cells (Table II).
 | Table IIIntracellular concentrations of VCR
in cells. |
Table II
Intracellular concentrations of VCR
in cells.
| Groups | SGC7901 | SGC7901/VCR |
|---|
|
|
|
|---|
| Time point | 3 h | 6 h | 3 h | 6 h |
|---|
| VCR | 6.87±0.06 | 7.58±0.12 | 0.00±0.00 | 0.00±0.00 |
| LY294002 + VCR | 13.47±0.14a | 15.56±0.14a | 0.00±0.00 | 0.17±0.01a |
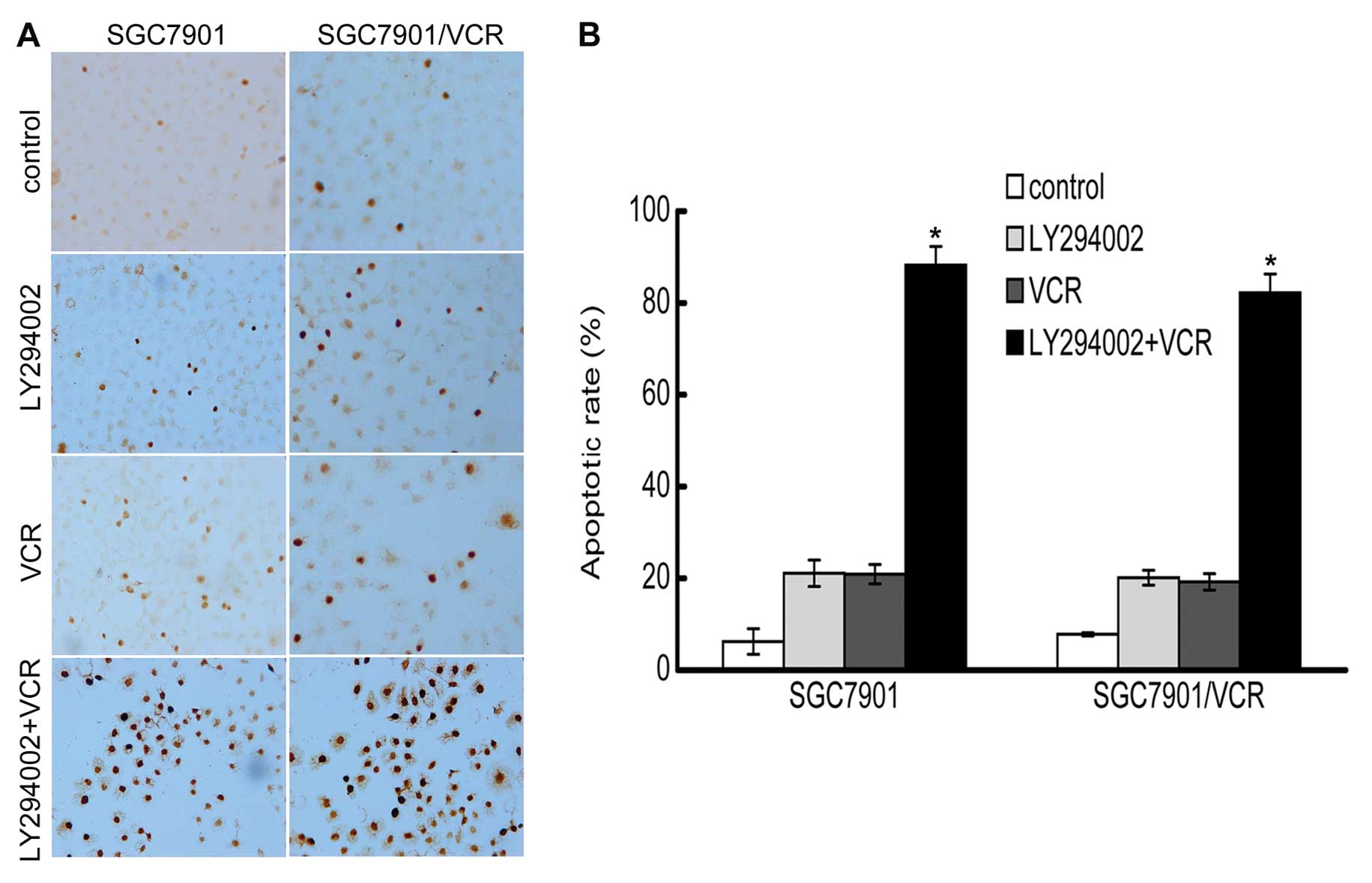
LY294002 enhances VCR-induced
apoptosis
Apoptosis was determined by the detection of DNA
strand breaks using TUNEL staining. SGC7901 and SGC7901/VCR cells
that were treated with either LY294002 or VCR alone showed fewer
apoptotic cells compared with the control. However, following
treatment with LY294002 and VCR, a significant increase in the
apoptotic cells was observed, suggesting that LY294002 could
markedly enhance VCR-induced apoptosis. The results from the cell
counts were expressed as the percentage of apoptotic cells among
the total cells observed in each microscopic field, and the
resulting apoptotic indices showed a significant increase in cells
treated with the combination of LY294002 and VCR (P<0.05)
(Fig. 3).
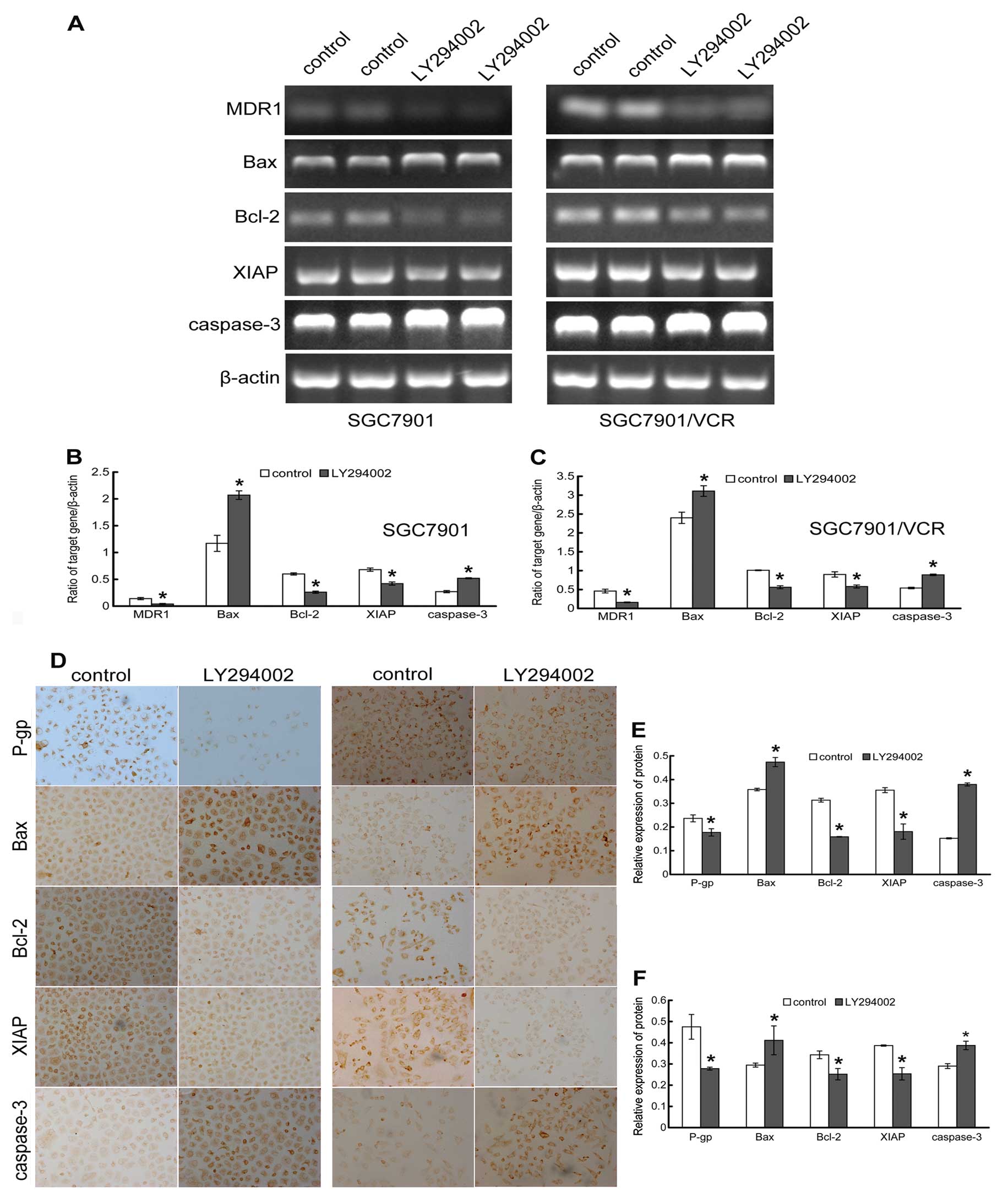
Effect of LY294002 on the expression of
MDR1/P-gp and apoptosis-related factors
To further illustrate the mechanism by which
inhibition of PI3K/Akt enhances the chemosensitivity of resistant
gastric cancer cells to VCR, the mRNA [semiquantitative reverse
transcription PCR (RT-PCR)] and protein (immunohistochemical
staining) expression of the MDR1/P-gp and apoptosis-related
factors was analyzed. Our results showed that the expression levels
of MDR1 were higher in SGC7901/VCR cells than that in
SGC7901 cells, suggesting an increased activity of the drug pump in
these resistant cells (Fig. 4),
which was in accordance with the lower concentration of VCR in
SGC7901/VCR cells. Inhibition of the PI3K/Akt pathway by LY294002
significantly decreased the expression of MDR1 at both the
mRNA and protein levels, suggesting transcriptional regulation of
MDR1 by PI3K/Akt in gastric cancer cells (Fig. 4).
Our previous results showed that inhibition of
PI3K/Akt by LY294002 could significantly enhance VCR-induced
apoptosis. Given that the PI3K/Akt activity was actively involved
in the apoptotic process, we hypothesized that the PI3K activity
may modulate the expression patterns of one or more
apoptosis-related factors. As anticipated, LY294002 treatment
resulted in the downregulation of anti-apoptotic factors Bcl-2 and
XIAP, and upregulation of pro-apoptotic factors Bax and caspase-3
at both the mRNA and protein levels in the cells (Fig. 4). Therefore, inhibition of the
PI3K/Akt pathway may enhance VCR-induced apoptosis, partly by
regulating the transcription of these apoptosis-related
factors.
In vivo effect of LY294002 on tumor
growth
Next, we evaluated the effects of LY294002, VCR and
the combination of these two drugs on the growth of SGC7901 and
SGC7901/VCR cells in vivo, which were injected
subcutaneously in nude mice. In both cell lines, treatment with
either LY294002 or VCR alone resulted in a slight decrease in tumor
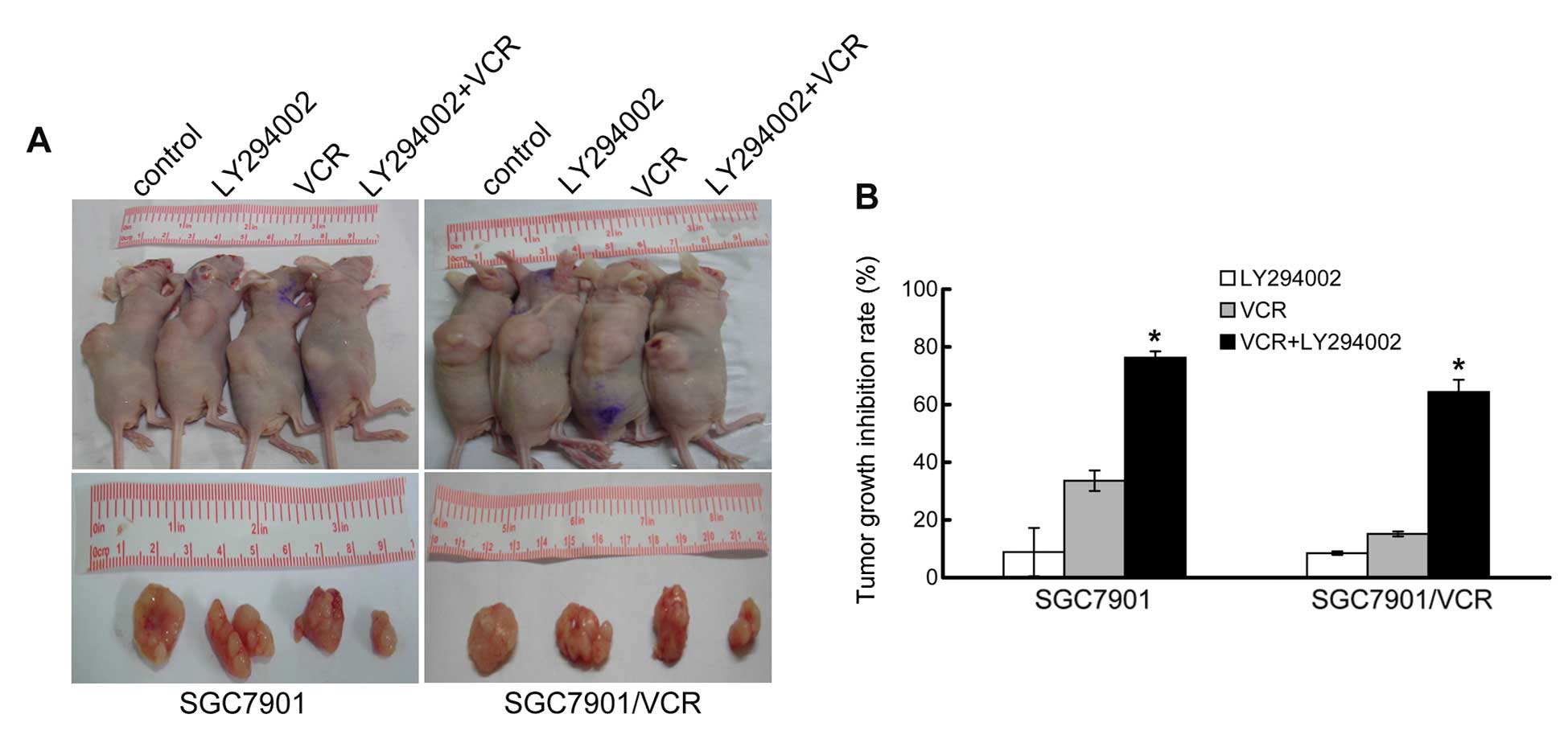
growth compared to control untreated mice (Fig. 5). The results showed that LY294002
had almost no effect on the inhibition of tumor growth in
vivo, consistent with the in vitro results. The tumor
growth inhibition following VCR treatment in SGC7901/VCR cells was
less than that in SGC7901 cells, indicating significant resistance
to VCR. However, treatment with LY294002 and VCR together caused a
significant inhibition of tumor growth of SGC7901 and SGC7901/VCR
cells in vivo compared to LY294002 or VCR alone (P<0.05)
(Fig. 5, Table III).
 | Table IIIAverage weight of tumor and
inhibition rate of tumor in each group. |
Table III
Average weight of tumor and
inhibition rate of tumor in each group.
| Cells | Groups | No. | Tumor size (g) | Inhibition rate
(%) |
|---|
| SGC7901 | Control | 3 | 1.219±0.041 | - |
| LY294002 | 3 | 1.111±0.102 | 8.862±8.361 |
| VCR | 3 | 0.809±0.043 | 33.590±3.544 |
| LY294002 + VCR | 3 | 0.289±0.027a |
76.279±2.194a |
| SGC7901/VCR | Control | 3 | 0.795±0.061 | - |
| LY294002 | 3 | 0.727±0.076 | 8.489±0.768 |
| VCR | 3 | 0.674±0.009 | 15.160±1.082 |
| LY294002 + VCR | 3 | 0.283±0.042a |
64.348±5.308a |
Discussion
PI3K and its downstream signaling protein Akt have
been implicated in the regulation of major responses to
extracellular growth stimulation, including cell proliferation,
development, differentiation, cell cycle and apoptosis (19,20).
Constitutive activation of the PI3K signaling pathway is commonly
observed in various types of cancer (13,21)
and is considered to contribute to tumorigenesis by simultaneously
promoting proliferation and inhibiting apoptosis (22). Excessive activation of PI3K/Akt is
found in gastric cancer and is positively correlated to tumor stage
(23); it is also associated with
chemoresistance in multiple cancer cells including gastric cancer
(24), suggesting a close
association with carcinogenesis, progression and MDR in gastric
cancer. Therefore, inhibition of PI3K/Akt signaling can serve as a
potentially useful approach for the treatment of gastric
cancer.
In the present study, we investigated whether
LY294002, by inhibition of PI3K/Akt, could increase the
chemosensitivity of human gastric cancer cells SGC7901 and the
related vincristine (VCR)-resistant cells SGC7901/VCR, to VCR. When
the two cell lines were exposed to VCR, cell proliferation was
inhibited in a dose- and time-dependent manner. The IC50
of SGC7901/VCR cells exposed to VCR was clearly higher than that of
SGC7901 cells, with a resistance index of approximately 40.45,
demonstrating that SGC7901/VCR cells are chemoresistant to VCR.
Subsequently, when cells were treated with 20 μM LY294002 in
combination with various concentrations of VCR, the rate of growth
inhibition increased significantly in both SGC7901 and SGC7901/VCR
cells, with an approximately 4- and 4.76-fold reduction in
IC50, respectively. However, the IC50 value
of LY294002 in both SGC7901 and SGC7901/VCR cells was similar,
therefore LY294002 alone would be expected to have similar effects
in these two cell lines. LY294002 not only enhanced
chemosensitivity to VCR but also exerted synergetic inhibition of
proliferation in SGC7901 and SGC7901/VCR cells. Furthermore, we
investigated the effects of LY294002 on tumor growth in
tumor-bearing nude mice. As seen in vitro, LY294002 combined
with VCR could significantly inhibit the growth of tumors in
vivo, and the inhibition rate was significantly higher than
LY294002 or VCR alone. Akt is a key regulator of cell survival and
apoptosis, and increased Akt phosphorylation is a surrogate for
high PI3K/Akt activity. The results showed that the resistant
SGC7901/VCR cells presented higher PI3K/Akt activity than SGC7901
cells using western blotting, which is in accordance with the close
relationship between PI3K signaling and drug resistance phenotype.
Following treatment with LY294002, the phospho-Akt expression level
in these two cell lines decreased significantly, suggesting that
the drug is highly potent in inhibiting the PI3K/Akt signaling
pathway. In addition, we found that there was no alteration of
total Akt in response to LY294002, indicating that downregulation
of Akt phosphorylation was independent of downregulation of the
total Akt in these cells. These results indicated that PI3K/Akt
inhibition by LY294002 could suppress tumor cell proliferation and
enhance chemosensitivity to VCR in human gastric cancer cells.
Several mechanisms may be implicated in MDR, an
important one being the prevention of intracellular accumulation of
anti-tumor drugs by the expression of transport proteins that pump
drugs out of cancer cells. The MDR1 gene product P-gp (also
known as ABCB1) is one of the most thoroughly studied transport
proteins (25). Previous studies
indicated that the activation of the PI3K/Akt signaling pathway was
closely associated with upregulation of MDR1 expression (26,27).
Similarly, our study found that high PI3K/Akt activity resulted in
increased expression of MDR1 or P-gp in the resistant SGC7901/VCR
cells when compared to SGC7901 cells, leading to a decline in VCR
accumulation in SGC7901/VCR cells in the absence of LY294002.
Therefore, we inferred that LY294002 treatment may interfere with
the intracellular trafficking of VCR by interrupting with P-gp
activity. Our results showed that an increase in VCR accumulation
was observed in SGC7901/VCR cells treated with LY294002, most
likely due to a significant downregulation of P-gp after treatment
with LY294002 in these cells. However, a great deal remains to be
understood about how P-gp expression is regulated. Previous studies
had demonstrated that MDR1 expression was driven by the
NF-κB pathway, using a common agonist known as the phorbol ester
12-O-tetradecanoyl-13-acetate (TPA) (28,29).
Crosstalk between the PI3K/Akt pathway and NF-κB has been
demonstrated; NF-κB expression can be downregulated by treatment
with LY294002 (26,30), indicating that downregulation of
MDR1 expression is mediated by inhibition of the PI3K/Akt
pathway. Even so, the relationship between signal transduction
pathways and P-gp expression is a complex process involving more
than a single pathway, of which we have highlighted regulation by
the PI3K/Akt pathway. Additionally, regulation of P-gp
phosphorylation by the Akt kinase or direct interaction of P-gp
with LY294002 cannot be excluded (27). Of note, the fact that the presence
of both LY294002 and VCR in SGC7901/VCR cells induced detectable
drug accumulation only after 6 h indicates that the effect of
LY294002 was relatively delayed. Thus, we can infer that the
mechanism of LY294002 action involves downregulation of P-gp
expression rather than direct interaction with the drug binding
site of P-gp.
The susceptibility of cancer cells to apoptosis
induced by chemotherapeutic drugs depends on the balance between
pro-apoptotic and anti-apoptotic signals. Altered cellular
responses to apoptosis and induction of anti-apoptotic proteins are
thought to play an important role in drug resistance in cancer
cells. PI3K/Akt plays a vital role in mediating survival signals,
contributing to the inhibition of apoptosis and therapeutic
resistance through multiple mechanisms (31,32).
Therefore, the inhibition of the PI3K/Akt pathway may promote
chemotherapeutic drug-induced apoptosis and consequently enhance
the chemosensitivity of various types of cancer. Our results
further confirmed that LY294002 combined with VCR could
significantly induce higher apoptosis levels in SGC7901 and
SGC7901/VCR cells than the effects of LY294002 or VCR alone. There
are two major pathways that initiate apoptosis and eventually lead
to activation of caspases: the death receptor (extrinsic) pathway
and the mitochondrial (intrinsic) pathway (33,34).
The anti-cancer drugs mainly initiate the intrinsic pathway, which
is engaged by the release of apoptogenic factors such as cytochrome
c into the cytosol that further triggers caspase-3
activation (35,36). The mitochondria-mediated apoptosis
is mainly regulated by the Bcl-2 family of proteins, which consists
of anti-apoptotic proteins Bcl-2 and Bcl-xL, as well as a number of
pro-apoptotic proteins such as Bax, Bid and Bim (36–38).
In addition, XIAP is a member of the inhibitor of apoptosis protein
(IAP) family, the only member capable of blocking active caspases,
which can inhibit apoptosis by binding and thereby inactivating
certain caspases including initiator caspase-9 and the effector
caspase-3 (39). In the present
study, we showed that in SGC7901 and SGC7901/VCR cells, the
PI3K/Akt inhibitor LY294002 could downregulate the expression of
Bcl-2 and XIAP, and upregulate the expression of Bax and caspase-3.
Downregulation of Bcl-2 in turn allows conformational changes in
the Bax protein and subsequent translocation to mitochondria,
leading to the release of cytochrome c into the cytoplasm.
Downregulation of XIAP along with the release of cytochrome
c results in a sequential activation of caspase-9 and
caspase-3, and initiates the apoptotic cascade (40). Our results indicated that the
expression levels of these apoptotic factors that are regulated by
inhibition of PI3K/Akt are not merely dependent on caspase activity
or direct interactions with some regulatory factors; there is also
a significant regulation of their transcriptional levels. It is
known that among the various functions of the PI3K/Akt pathway, Akt
inhibits apoptosis either directly by phosphorylating
apoptosis-signaling molecules or indirectly by modulating the
activity of transcription factors (41). The cross-talk between the PI3K/Akt
pathway and the NF-κB pathway results in Akt-mediated activation of
the transcription factors CREB (c-AMP response element binding
protein) or IκB kinases (IKKs) that further activate NF-κB to exert
an anti-apoptotic effect (24,40,42,43).
Inhibition of NF-κB may lead to a reduced transcription of target
genes such as Bcl-2 and XIAP (30,40),
as was shown in our present results. Since caspase-3 is at the
crossroad of extrinsic and intrinsic apoptosis pathways (40), inhibition of PI3K/Akt by LY294002
can simultaneously affect these two pathways, consistent with a
previous report implicating the PI3K/Akt pathway in apoptosis
mediated by both the death receptor and the mitochondrial pathway
(44). A previous report indicated
that P-gp may counteract apoptosis by transporting a key caspase
out of the cell or inhibiting caspase activity, and in turn be
cleaved in a caspase-dependent manner during apoptosis (45); this demonstrates the complexity of
the apoptosis and drug resistance signaling network. There
certainly may be other pathways or regulatory factors involved in
the complicated mechanisms of MDR, and this will be the direction
of our future research focus. Presently, we have demonstrated that
inhibition of the PI3K/Akt pathway by LY294002 could significantly
enhance VCR-induced apoptosis in gastric cancer cells,
predominantly through downregulation of anti-apoptotic factors and
upregulation of apoptotic factors.
A recent study provided key evidence that the use of
LY294002 in cancer therapy can be detrimental when the PI3K
signaling pathway is inhibited prior to anticancer drug
administration (46). This may be
because the inhibition of PI3K/Akt decreases the proliferation of
various cancer cell lines by preventing cell cycle progression,
particularly at the G1-phase; since several chemotherapeutic agents
depend mainly on cell proliferation for their cytotoxic activities,
inhibition of PI3K may lead to decreased susceptibility to cell
cycle-dependent chemotherapy (46,47).
Therefore, the timing of administering LY294002 is critical in the
context of chemotherapy, and using LY294002 after chemotherapeutic
agents may act in a synergistic manner to enhance chemosensitivity
of cancer cells. Moreover, the inhibition of PI3K/Akt in different
cancer cell lines in relation to diverse chemotherapeutic agents,
may exert different effects (24,32).
Therefore, it is necessary to illustrate the complicated
interactions between signaling pathways and MDR, in order to
conduct a rational combination of various chemotherapeutic agents
in the treatment of cancer.
In conclusion, our results demonstrate that the
PI3K/Akt inhibitor LY294002 can enhance chemosensitivity of VCR in
human gastric cancer cells by inactivation of the PI3K/Akt
signaling pathway. Therefore, our results indicate that modulation
of the PI3K/Akt pathway may provide a new strategy to improve
current therapeutic regimens, and this preclinical evaluation of a
rational combination of LY294002 and VCR in relevant in
vitro and in vivo models of gastric cancer also provides
a molecular basis for the new design of combination treatments for
gastric cancer.
Acknowledgements
The authors thank Professor Shiming Yang (Department
of Gastroenterology, Xinqiao Hospital, Third Military Medical
University) and the technician Yuyun Wu (Department of
Gastroenterology, Xinqiao Hospital, Third Military Medical
University) for their excellent technical support.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Plummer M, Franceschi S and Muñoz N:
Epidemiology of gastric cancer. IARC Sci Publ. 157:311–326.
2004.
|
|
3
|
Catalano V, Labianca R, Beretta GD, Gatta
G, de Braud F and Van Cutsem E: Gastric cancer. Crit Rev Oncol
Hematol. 71:127–164. 2009. View Article : Google Scholar
|
|
4
|
Ozeben T: Mechanisms and strategies to
overcome multiple drug resistance in cancer. FEBS Lett.
580:2903–2909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gillet JP and Gottesman MM: Mechanisms of
multidrug resistance in cancer. Methods Mol Biol. 596:47–76. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gillet JP, Efferth T and Remacle J:
Chemotherapy-induced resistance by ATP-binding cassette transporter
genes. Biochim Biophys Acta. 1775:237–262. 2007.PubMed/NCBI
|
|
7
|
Callaghan R, Crowley E, Potter S and Kerr
ID: P-glycoprotein: so many ways to turn it on. J Clin Pharmacol.
48:365–378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martin LP, Hamilton TC and Schilder RJ:
Platinum resistance: the role of DNA repair pathways. Clin Cancer
Res. 14:1291–1295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sarkaria JN, Kitange GJ, James CD, Plummer
R, Calvert H, Weller M and Wick W: Mechanisms of chemoresistance to
alkylating agents in malignant glioma. Clin Cancer Res.
14:2900–2908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodriguez-Nieto S and Zhivotovsky B: Role
of alterations in the apoptotic machinery in sensitivity of cancer
cells to treatment. Curr Pharm Des. 12:4411–4425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Viktorsson K, Lewensohn R and Zhivotovsky
B: Apoptotic pathways and therapy resistance in human malignancies.
Adv Cancer Res. 94:143–196. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim D, Dan HC, Park S, Yang L, Liu Q,
Kaneko S, Ning J, He L, Yang H, Sun M, Nicosia SV and Cheng JQ:
AKT/PKB signaling mechanisms in cancer and chemoresistance. Front
Biosci. 10:975–987. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu P, ChenG H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Courtney KD, Corcoran RB and Engelman JA:
The PI3K pathway as drug target in human cancer. J Clin Oncol.
28:1075–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004.PubMed/NCBI
|
|
16
|
Kunnimalaiyaan M, Ndiaye M and Chen H:
Apoptosis-mediated medullary thyroid cancer growth suppression by
the PI3K inhibitor LY294002. Surgery. 140:1009–1014. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu P, Xu B, Li J and Lu H: LY294002
inhibits leukemia cell invasion and migration through early growth
response gene 1 induction independent of phosphatidylinositol
3-kinase-Akt pathway. Biochem Biophys Res Commun. 377:187–190.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Imai Y, Yoshimori M, Fukuda K, Yamagishi H
and Ueda Y: The PI3K/Akt inhibitor LY294002 reverses BCRP-mediated
drug resistance without affecting BCRP translocation. Oncol Rep.
27:1703–1709. 2012.PubMed/NCBI
|
|
19
|
Wu D, Tao J, Xu B, Qing W, Li P, Lu Q and
Zhang W: Phosphatidylinositol 3-kinase inhibitor LY294002
suppresses proliferation and sensitizes doxorubicin chemotherapy in
bladder cancer cells. Urol Int. 87:105–113. 2011. View Article : Google Scholar
|
|
20
|
Gelman AE, LaRosa DF, Zhang J, Walsh PT,
Choi Y, Sunyer JO and Turka LA: The adaptor molecule MyD88
activates PI-3 kinase signaling in CD4+ T cells and
enables CpG oligodeoxynucleotide-mediated costimulation. Immunity.
25:783–793. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Crowell JA, Steele VE and Fay JR:
Targeting the AKT protein kinase for cancer chemoprevention. Mol
Cancer Ther. 6:2139–2148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hennessy BT, Smith DL, Ram PT, Lu Y and
Mills GB: Exploiting the PI3K/AKT pathway for cancer drug
discovery. Nat Rev Drug Discov. 4:988–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paweletz CP, Charboneau L, Bichsel VE,
Simone NL, Chen T, Gillespie JW, Emmert-Buck MR, Roth MJ, Petricoin
EF III and Liotta LA: Reverse phase protein microarrays which
capture disease progression show activation of pro-survival
pathways at the cancer invasion front. Oncogene. 20:1981–1989.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
García MG, Alaniz LD, Cordo Russo RI,
Alvarez E and Hajos SE: PI3K/Akt inhibition modulates multidrug
resistance and activates NF-κB in murine lymphoma cell lines. Leuk
Res. 33:288–296. 2009.PubMed/NCBI
|
|
25
|
Filipits M: Mechanisms of cancer:
multidrug resistance. Drug Discov Today Dis Mech. 1:229–234. 2004.
View Article : Google Scholar
|
|
26
|
Kuo MT, Liu Z, Wei Y, Lin-Lee YC, Tatebe
S, Mills GB and Unate H: Induction of human MDR1 gene expression by
2-acetylaminofluorene is mediated by effectors of the
phosphoinositide 3-kinase pathway that activate NF-κB signaling.
Oncogene. 21:1945–1954. 2002.PubMed/NCBI
|
|
27
|
Barancík M, Bohácová V, Sedlák J, Sulová Z
and Breier A: LY294,002, a specific inhibitor of PI3K/Akt kinase
pathway, antagonizes P-glycoprotein-mediated multidrug resistance.
Eur J Pharm Sci. 29:426–434. 2006.PubMed/NCBI
|
|
28
|
Gill PK, Gescher A and Gant TW: Regulation
of MDR1 promoter activity in human breast carcinoma cells by
protein kinase C isozymes α and θ. Eur J Biochem. 268:4151–4157.
2001.PubMed/NCBI
|
|
29
|
Vertegaal AC, Kuiperij HB, Yamaoka S,
Courtois G, van der Eb AJ and Zantema A: Protein kinase C-α is an
upstream activator of the IκB kinase complex in the TPA signal
transduction pathway to NF-κB in U2OS cells. Cell Signal.
12:759–768. 2000.
|
|
30
|
Chao X, Zao J, Xiao-Yi G, Li-Jun M and Tao
S: Blocking of PI3K/AKT induces apoptosis by its effect on NF-κB
activity in gastric carcinoma cell line SGC7901. Biomed
Pharmacother. 64:600–604. 2010.
|
|
31
|
Engelman JA: Targeting PI3K signalling in
cancer: opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nuutinen U, Postila V, Mättö M, Eeva J,
Ropponen A, Eray M, Riikonen P and Pelkonen J: Inhibition of
PI3-kinase-Akt pathway enhances dexamethasone-induced apoptosis in
a human follicular lymphoma cell line. Exp Cell Res. 312:322–330.
2006.PubMed/NCBI
|
|
33
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Riedl SJ and Salvesen GS: The apoptosome:
signalling platform of cell death. Nat Rev Mol Cell Biol.
8:405–413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Elumalai P, Gunadharini DN, Senthilkumar
K, Banudevi S, Arunkumar R, Benson CS, Sharmila G and Arunakaran J:
Induction of apoptosis in human breast cancer cells by nimbolide
through extrinsic and intrinsic pathway. Toxicol Lett. 215:131–142.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gillings AS, Balmanno K, Wiggins CM,
Johnson M and Cook SJ: Apoptosis and autophagy: BIM as a mediator
of tumour cell death in response to oncogene-targeted therapeutics.
FEBS J. 276:6050–6062. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tait SW and Green DR: Mitochondria and
cell death: outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kaufmann T, Strasser A and Jost PJ: Fas
death receptor signalling: roles of Bid and XIAP. Cell Death
Differ. 19:42–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hussain AR, Ahmed SO, Ahmed M, Khan OS, Al
Abdulmohsen S, Platanias LC, Al-Kuraya KS and Uddin S: Cross-talk
between NFκB and the PI3-kinase/AKT pathway can be targeted in
primary effusion lymphoma (PEL) cell lines for efficient apoptosis.
PLoS One. 7:e399452012.
|
|
41
|
Shaw RJ and Cantley LC: Ras, PI(3)K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nicholson KM and Anderson NG: The protein
kinase B/Akt signalling pathway in human malignancy. Cell Signal.
14:381–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shant J, Cheng K, Marasa BS, Wang JY and
Raufman JP: Akt-dependent NF-κB activation is required for bile
acids to rescue colon cancer cells from stress-induced apoptosis.
Exp Cell Res. 315:432–450. 2009.
|
|
44
|
Uchida M, Iwase M, Takaoka S, Yoshiba S,
Kondo G, Watanabe H, Ohashi M, Nagumo M and Shintani S: Enhanced
susceptibility to tumor necrosis factor-related apoptosis-inducing
ligand-mediated apoptosis in oral squamous cell carcinoma cells
treated with phosphatidylinositol 3-kinase inhibitors. Int J Oncol.
30:1163–1171. 2007.
|
|
45
|
Mantovani I, Cappellini A, Tazzari PL,
Papa V, Cocco L and Martelli AM: Caspase-dependent cleavage of
170-kDa P-glycoprotein during apoptosis of human T-lymphoblastoid
CEM cells. J Cell Physiol. 207:836–844. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
McDonald GT, Sullivan R, Paré GC and
Graham CH: Inhibition of phosphatidylinositol 3-kinase promotes
tumor cell resistance to chemotherapeutic agents via a mechanism
involving delay in cell cycle progression. Exp Cell Res.
316:3197–3206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shingu T, Yamada K, Hara N, Moritake K,
Osago H, Terashima M, Uemura T, Yamasaki T and Tsuchiya M: Growth
inhibition of human malignant glioma cells induced by the
PI3-K-specific inhibitor. J Neurosurg. 98:154–161. 2003. View Article : Google Scholar : PubMed/NCBI
|