Introduction
Pancreatic cancer is the most lethal of solid tumors
and is the fourth leading cause of cancer-related mortality in the
USA (1). At present, surgical
resection offers the only chance of a cure (2). Unfortunately, 80–85% of patients
present with advanced unresectable disease (2). Many patients with locally advanced or
metastatic pancreatic cancer rely on gemcitabine chemotherapy
(3). However, pancreatic cancer is
representative of the most highly vascularized and angiogenic solid
tumors, which responds poorly to most chemotherapeutic agents
(2,3). Therefore, the poor prognosis of
pancreatic cancer is related to angiogenesis. Thus, identification
of new treatment strategies possessing antiangiogenic capability is
urgently needed in clinical practice in order to inhibit the
metastasis of pancreatic cancer and improve its prognosis.
Traditional Chinese medicine has held an important
position in primary health care in China and has been recognized by
Western countries as a fertile source for revealing novel lead
molecules for modern drug discovery (4). Various active components of
traditional Chinese herbs have been reported to exhibit
antiproliferative effects on cancer cells and serve as potent
agents to enhance the therapeutic effects of chemotherapy in human
cancers (4–6). Oxymatrine is one of the main alkaloid
components in the traditional Chinese herbal medicine Sophora
japonica (Sophora flavescens Ait). It is commonly known
to have specific pharmacological properties for anti-hepatic
fibrosis, anti-inflammation and against hepatitis B viruses
(7,8). Oxymatrine also has been found to
exhibit antitumor effects including induction of apoptosis and cell
cycle arrest (9–11), downregulation of the activity of the
Wnt/β-catenin signaling pathway (12), upregulation of p53 (13,14)
and suppression of xenografted SGC-7901 human gastric cancer cell
growth in vivo(14).
However, the mechanisms of the antitumor properties of oxymatrine
in human pancreatic cancer are not well established to date.
Nuclear factor κB (NF-κB) activation has been
connected with multiple aspects of tumor development and
progression, including the control of apoptosis, the cell cycle,
differentiation and cell migration (15,16).
Accumulating evidence suggests that NF-κB transcriptional factors
are constitutively activated in the majority of pancreatic cancers
(17). It has been reported that
constitutive activation of NF-κB can regulate the expression of
genes associated with angiogenesis (18). Notably, one study also reported the
antitumor activity of oxymatrine against tumor cells by inhibition
of NF-κB activation (19). In the
present study, we aimed to investigate whether oxymatrine inhibits
the angiogenesis of pancreatic cancer through a possible mechanism
involving NF-κB. We found that treatment with oxymatrine inhibited
the growth of pancreatic cancer PANC-1 cells and decreased the
expression of angiogenesis-associated factors, including NF-κB and
vascular endothelial growth factor (VEGF). We further demonstrated
the tumor growth inhibition and antiangiogenic effects of
oxymatrine on pancreatic cancer in vivo.
Materials and methods
Reagents and antibodies
Oxymatrine and dimethyl sulfoxide (DMSO) were
obtained from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s
modified Eagle’s medium (DMEM), fetal bovine serum (FBS),
penicillin-streptomycin, trypsin-EDTA were obtained from Gibco-BRL
(Invitrogen Life Technologies, Grand Island, NY, USA). Antibodies
were obtained from the following commercial sources: rabbit NF-κB
p65 and VEGF antibody (Abcam, Cambridge, UK); rabbit vascular
endothelial growth factor receptor-2 (VEGFR-2) antibody (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA); rabbit β-tubulin
antibody (Cell Signaling Technology, Inc., Beverly, MA, USA); and
horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary
antibody (Beyotime Biotechnology, Haimen, China). The ultrasound
contrast agent SonoVue was obtained from Bracco (Switzerland). The
contrast agent was dissolved in 5 ml 0.9% sodium chloride solution
and the final concentration was 45 μg/ml.
Cell line and culture
The human pancreatic cancer cell line PANC-1 was
purchased from the Shanghai Cell Bank (Shanghai, China). Cells were
cultured in DMEM with 10% FBS, 100 units/ml penicillin and 100
μg/ml streptomycin at 37ºC under a humidified 5% CO2
atmosphere. The medium was replaced every 2–3 days, and the cells
were subcultured when confluency reached 70–80% by 0.25%
trypsin-EDTA at 37ºC.
Cell viability assay
The Cell Counting Kit-8 (CCK-8) (Dojindo Molecular
Technologies, Kunamoto, Japan) was used to assess the viability of
cells after oxymatrine treatment. PANC-1 cells were seeded into
96-well plates at a density of ~5×103 cells/well and
grown for 24 h. Cells were treated with 0.25, 0.5, 1, 2, 4 and 6
mg/ml oxymatrine or DMSO (control) for 12, 24 or 36 h, and then 10
μl CCK-8 reagent was added to 100 μl of media in each well, and the
incubation was continued for a further 3 h. The absorbance (A) of
each well was determined with an enzyme-linked immunosorbant assay
(ELISA) reader (ELx808; Bio-Tek Instruments, Inc., Winooski, VT,
USA) at a wavelength of 450 nm. The percentage of cell viability
was calculated using the following equation: Cell viability (%)=
(Asample − Ablank)/(Acontrol −
Ablank) ×100%.
Protein extraction and western blot
analysis
Approximately 5×105 PANC-1 cells/well
were seeded into 6-well plates, allowed to adhere overnight, and
treated with 0.5, 1, 2 mg/ml oxymatrine or DMSO (control)
respectively for 24 h. Treated cells were collected and total
proteins were extracted using cell lysis buffer (20 mmol/l Tris-HCl
pH 7.5, 150 mmol/l NaCl, 1 mmol/l Na2EDTA, 1 mmol/l
EGTA, 1% Triton, 2.5 mmol/l sodium pyrophosphate, 1 mmol/l
β-glycerophosphate, 1 mmol/l Na3VO4, 1 μg/ml
leupeptin, 1 mmol/l PMSF; Cell Signaling Technology, Inc.). After
centrifugation at 14,000 × g for 15 min at 4ºC, the supernatant was
collected and the protein concentration was detected using the BCA
Protein Assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA),
according to the manufacturer’s instructions. Equal amounts of
protein were separated on 8–12% SDS-PAGE and transferred onto a
polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA).
After blocking with 5% nonfat milk in TBST washing buffer, the
membrane was incubated with the specific primary antibodies at 4ºC
overnight. After being washed at room temperature with washing
buffer, the blots were labeled with peroxidase-conjugated secondary
antibodies. The formed immunocomplex was visualized using an
enhanced chemiluminescence kit (Pierce Biotechnology, Inc.)
according to the manufacturer’s instructions and exposed to X-ray
film.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
PANC-1 cells were seeded into 6-well plates at a
density of ~5×105 cells/well and grown for 24 h. Cells
were treated with 0.5, 1, 2 mg/ml oxymatrine or DMSO (control)
respectively for 24 h. Total RNA was isolated from the treated
cells using the TRIzol reagent (Invitrogen Life Technologies). For
reverse transcription (RT) analysis, 1 μg of total RNA was reverse
transcribed in a 20-μl volume, using the RevertAid™ First Strand
cDNA Synthesis kit (Fermentas, St. Leon-Rot, Germany). One
microliter of the RT reaction mixture was then PCR-amplified in a
PCR machine (Eppendorf, Hamburg, Germany). The PCR profile was as
follows: 5 min at 94ºC followed by 35 cycles of 30 sec at 94ºC, 30
sec at 54ºC, 30 sec at 72ºC; the final cycle was modified to allow
for a 5-min extension at 72ºC. The PCR primers were as follows:
NF-κB (300 bp) sense, 5′-AGCACAGATACCACCAAGACCC-3′ and antisense,
5′-CCCACGCTGCTCTTCTATAGGAAC-3′; VEGF (336 bp) sense,
5′-TGCCCACTGAGGAGTCCAAC-3′ and antisense,
5′-TGGTTCCCGAAACGCTGAG-3′; glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) (533 bp) sense, 5′-CGGAGTCAACGGATTTGGCC-3′ and antisense,
5′-GTGCAGAGATGGCATGGAC-3′. Sequences of the primers were designed
using the software Primer Premier 5. GAPDH was used for
quantification of the samples. PCR products were electrophoresed at
120 V for 45 min on a 1.2% agarose gel and imaged. The grey value
was analyzed using Quantity One version 4.5 software (Bio-Rad,
Hercules, CA, USA).
Animals and in vivo studies
BALB/C (nu/nu), 4 week-old, male mice were purchased
from Shanghai Laboratory Animal Center (Shanghai, China) and
maintained under specific pathogen-free conditions. Mice were
allowed to acclimate for 1 week before the start of the
experiments. All animal studies performed in this study were
reviewed and approved by the Animal Research and Ethics Committee
of Wenzhou Medical College. The orthotopic pancreatic cancer
xenograft tumor model was established as described by us previously
(20). The dose of oxymatrine was
determined by balancing the antitumor and side effects on the basis
of previous trials. Through numerous trials, we found that
oxymatrine at a dose of 100 mg/kg body weight by intraperitoneal
injection 3 days/week can effectively inhibit tumor growth without
significant side effects, such as noticeable weight loss and
decrease in overall activity. Therefore, oxymatrine treatment (100
mg/kg body weight) by intraperitoneal injection 3 days/week was
administered in this study. Briefly, nude mice were anesthetized
with pentobarbital sodium, a small left abdominal flank incision
was made, and PANC-1 cells (5×106) in 50 μl serum-free
media were injected into the subcapsular region of the pancreas.
All surgical procedures were conducted under sterilized conditions.
One week after cell implantation, a total of 32 nude mice were
randomized into 2 groups (control and oxymatrine group) with 16
mice/group. The control group mice were treated with 0.9% sodium
chloride and the treatment was continued for 4 weeks.
After the first treatment, 8 mice in each group were
used for a survival study which was carried out up to 60 days. When
mice died during the period of the survival study, the number of
living days were recorded. At the end of the survival study, the
living mice were sacrificed. One week after the last treatment, the
other 8 mice in each group were used for the study of tumor blood
flow detected by contrast enhanced ultrasonography (CEUS). Then the
mice were sacrificed, and the tumors were removed. The tumors were
weighed with an electronic balance, and tumor volumes were
calculated with a vernier caliper according to the following
formula: Volume = (4π/3) × (width/2)2 × (length/2).
CEUS for detecting tumor blood flow
One week after the last treatment, the mice were
used for the study of tumor blood flow as detected by CEUS. Mice
were anesthetized with sodium pentobarbital (50 mg/kg) and pronely
positioned on an operating table. Mice were injected with 20 μl
ultrasound SonoVue and 200 μl 0.9% sodium chloride solution per
mouse via the tail vein and then imaged. A 1-min data collection
was performed after the contrast agent injection. The images were
analyzed with the ultrasound contrast analysis software that
accompanies the ultrasonic imaging system (Siemens S2000, Germany).
A parameter, peak intensity (PI), is in direct proportion to blood
flow and was used here for quantification of the tumor blood
perfusion in the pancreatic cancer xenografts.
Statistical analysis
Data are represented as means ± SD for the absolute
values or percent of controls. SPSS13.0 software was used for
statistical analysis. Differences between the oxymatrine-treated
and DMSO-treated (control) groups were analyzed by the unpaired
Student’s t-test or ANOVA analysis. A value of P<0.05 was
considered to indicate a statistically significant result.
Results
Oxymatrine inhibited the viability of
human pancreatic cancer PANC-1 cells
The viability of human pancreatic cancer PANC-1
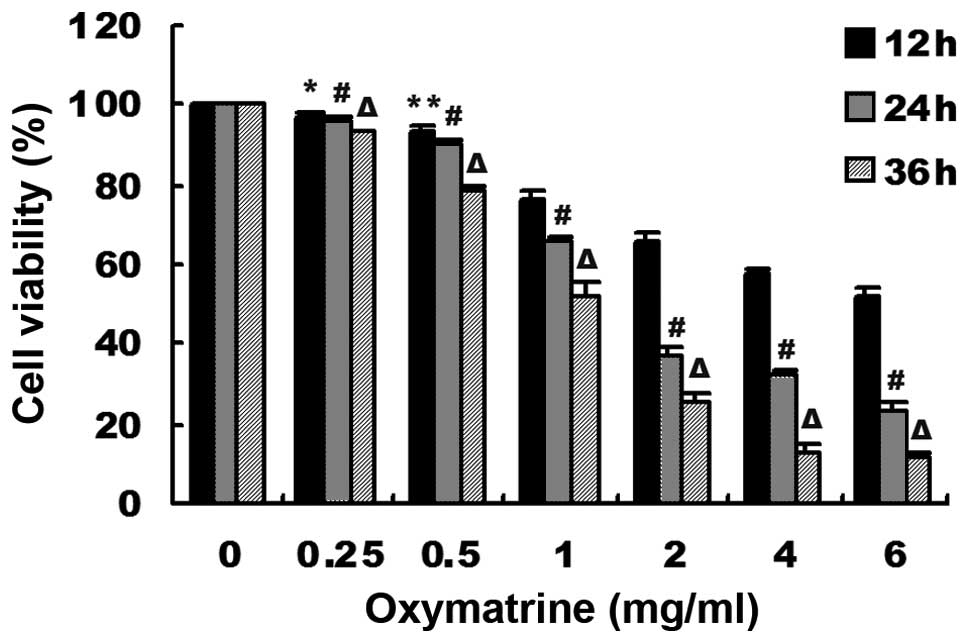
cells were measured by CCK-8 assay. As shown in Fig. 1, oxymatrine inhibited the cell
viability in a dose- and time-dependent manner. Notably, the cell
viability was drastically decreased at the range of concentrations
of 0.5–2 mg/ml of oxymatrine. However, at concentrations of
0.25–0.5 mg/ml oxymatrine, the cell viability was minimally
altered, and higher concentrations of oxymatrine (>2 mg/ml) had
a saturated inhibitory effect. Therefore, we chose the
concentrations of 0.5, 1 and 2 mg/ml for the following in
vitro studies. Meanwhile, we chose one time point (24 h) for
the further studies.
Effects of oxymatrine on the expression
of NF-κB in PANC-1 cells
We investigated whether NF-κB is involved in the
antiproliferative effects of oxymatrine on pancreatic cancer PANC-1
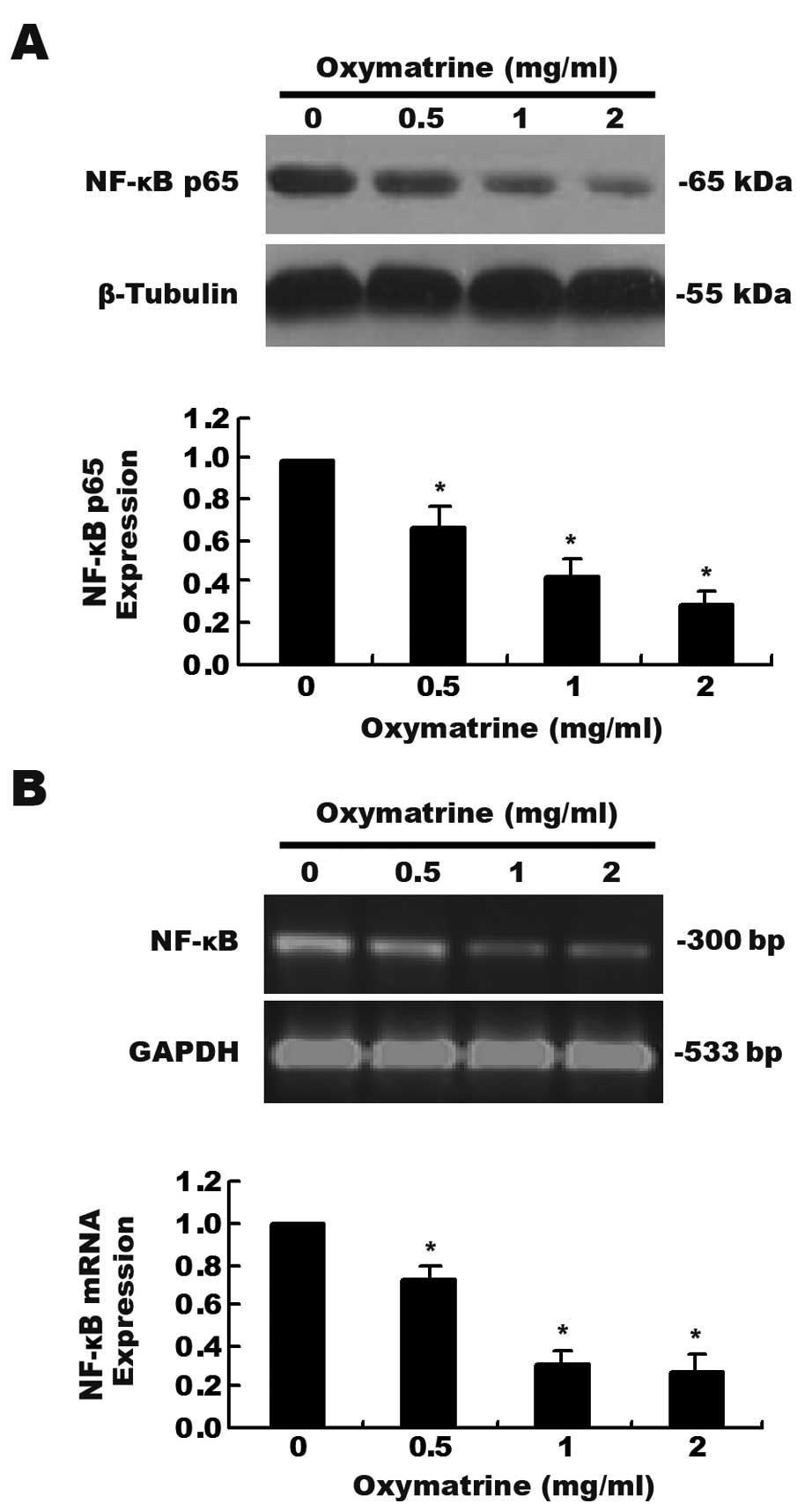
cells. As shown in Fig. 2A, a
dose-dependent decrease in NF-κB p65 expression as determined by
western blot assay was observed after the cells were exposed to
increasing concentrations of oxymatrine. The mRNA levels of NF-κB
as detected by RT-PCR analysis was significantly decreased when
compared with that in the control group (Fig. 2B, P<0.01).
Effects of oxymatrine on the expression
of VEGF and VEGFR-2 in PANC-1 cells
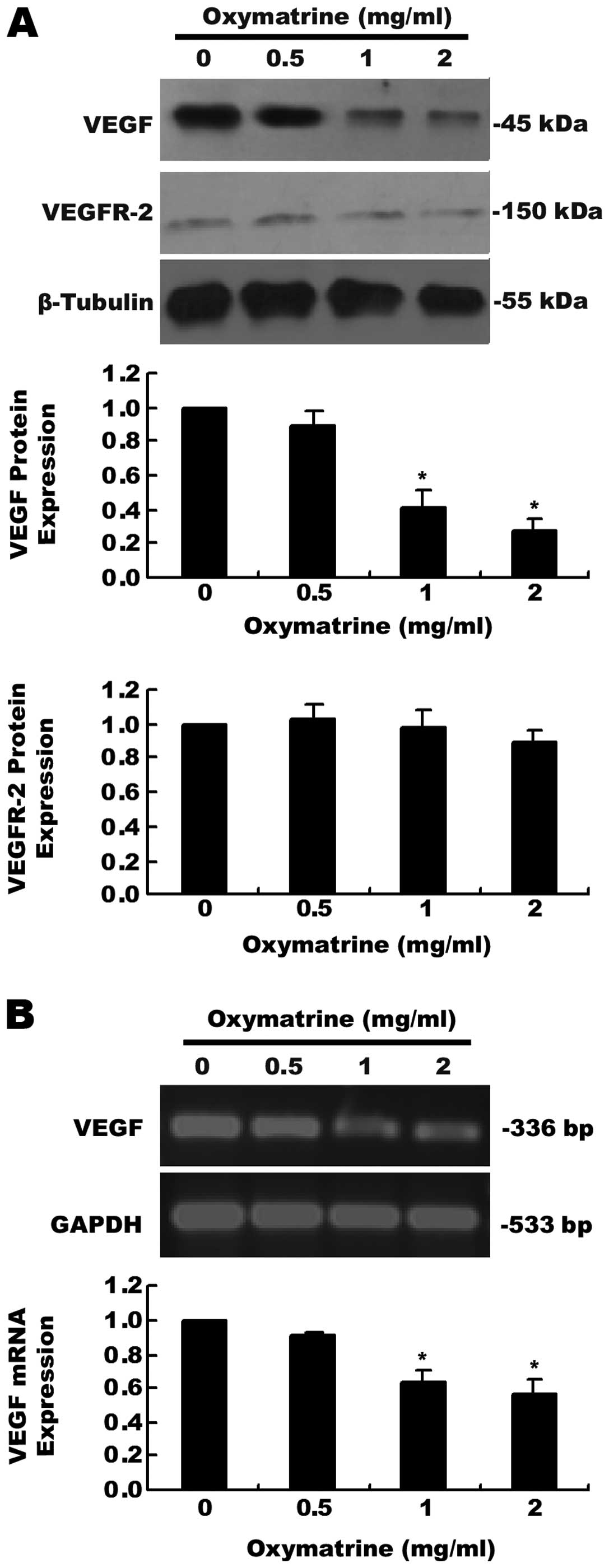
Our data showed that the protein (Fig. 3A) and mRNA (Fig. 3B) expression levels of VEGF were
significantly decreased after PANC-1 cells were treated with 1 or 2
mg/ml oxymatrine for 24 h. However, obvious inhibitory effects on
VEGF by oxymatrine were not observed in the 0.5 mg/ml oxymatrine
group. Moreover, we found that oxymatrine did not regulate the
expression of VEGFR-2 (a receptor of VEGF) as determined by western
blot assay in the PANC-1 cells (Fig.
3A).
Antiproliferative effects of oxymatrine
on pancreatic cancer xenograft tumors in nude mice
To further confirm the antiproliferative effects of
oxymatrine on pancreatic cancer, we established orthotopic
pancreatic cancer xenograft tumors in nude mice. One week after the
last oxymatrine treatment, the mice were sacrificed and tumors were
removed (Fig. 4A). The weight of
the tumors in the vehicle-treated mice was 1.54-fold higher than
that of the oxymatrine-treated mice (P<0.01, Fig. 4B). Tumor volumes in the
oxymatrine-treated mice and vehicle-treated mice were 438.23±123.40
and 702.73±101.00 mm3, respectively (P<0.01, Fig. 4C). The median survival time of mice
in the oxymatrine group (58 days) was significantly longer than
that in the control group (40 days) (Fig. 4D).
Antiangiogenic effects of oxymatrine on
pancreatic cancer xenograft tumors in nude mice
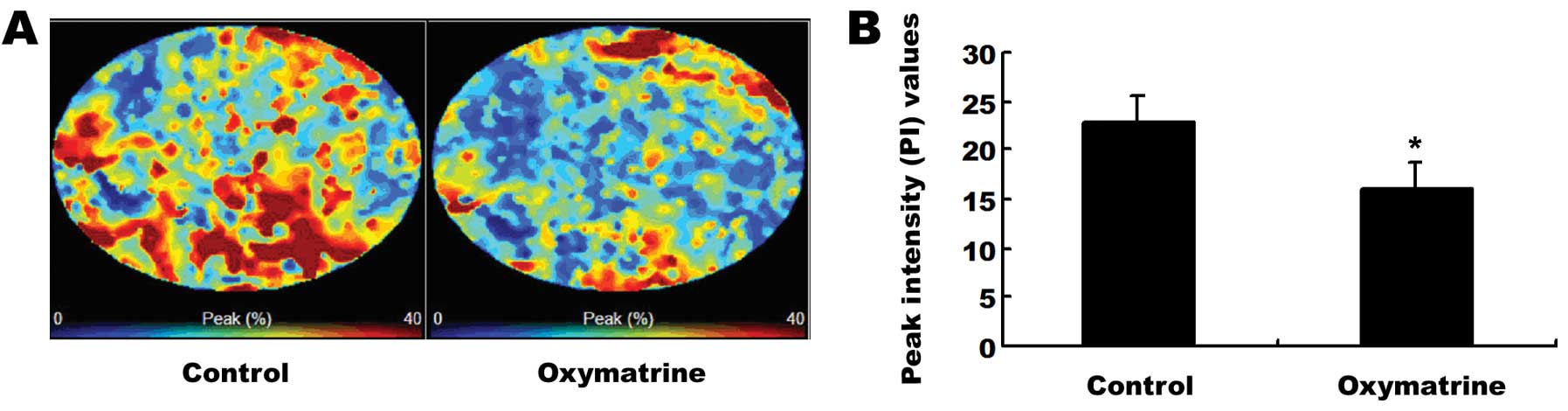
To further confirm the role of oxymatrine in the
inhibition of tumor angiogenesis in pancreatic cancer, CEUS was
employed to detect the tumor blood flow in pancreatic cancer
xenograft mouse tumors 1 week after the last oxymatrine treatment
(Fig. 5A). As shown in Fig. 5B, oxymatrine treatment markedly
reduced the PI value, the data was 15.93±2.77 (oxymatrine group) to
22.87±2.56 (control group) (P<0.05).
Discussion
Pancreatic cancer is a highly malignant tumor in the
alimentary system and is associated with a poor prognosis. It can
only be managed with surgical resection in limited cases, whereas
the majority of cases presenting with advanced tumors respond
poorly to currently available medical therapies (21). In this study, the viability of human
pancreatic cancer PANC-1 cells was largely inhibited by oxymatrine
through an NF-κB-mediated mechanism. In vivo studies, we
found that oxymatrine effectively inhibited the tumor growth and
angiogenesis in the pancreatic cancer xenograft mice tumors.
It was reported that NF-κB is constitutively active
in 9 of 11 pancreatic cancer cell lines (22), but not in immortalized,
non-tumorigenic pancreatic ductal epithelial cells (23). It was also found to play a crucial
role in predicting the efficacy of cetuximab and irinotecan in
advanced colorectal tumors (24).
Therefore, these findings suggest that NF-κB plays a major role in
the growth and chemoresistance of pancreatic cancer, and blocking
NF-κB activation could exert a growth inhibition effect on
pancreatic cancer. Presently, there are 5 known members of the
NF-κB family: p50/p105, p52/p100, p65, c-Rel, and RelB, and the
NF-κB complex is sequestered as an inactive form in the cytoplasm
by inhibitory IkB protein (15,25).
Upon stimulation, IkB is rapidly phosphorylated and degraded via
the ubiquitin proteosome pathway resulting in the liberation of
NF-κB, allowing NF-κB members to accumulate in the nucleus
(15,25). Moreover, Han et al(19) reported that oxymatrine exerted
antitumoral activity in H460 and Eca-109 tumor cells via inhibition
of NF-κB activation. In the present study, the result of western
blot analysis showed that oxymatrine decreased the levels of NF-κ’B
p65 in PANC-1 cells, and NF-κB mRNA levels detected by RT-PCR were
downregulated in oxymatrine-treated cells. These findings suggest
that inhibition of NF-κB is involved in the antiproliferative
activity of oxymatrine in pancreatic cancer.
Angiogenesis, is critical for normal and pathologic
processes and plays an important role in the growth and spread of
cancer. Upon formation of new blood vessels, cancer cells acquire
more oxygen and nutrients and invade nearby tissues, resulting in
the spread to other parts of the body. Previous studies have
suggested that NF-κB regulates the expression of several
angiogenesis-associated molecules such as VEGF, MMP-2, MMP-9 and
eNOS (26–28). Accumulating evidence had established
the fundamental role of VEGF as a key regulator of normal and
abnormal angiogenesis (29,30). VEGF strongly stimulates endothelial
migration and proliferation and the formation of new blood vessels.
VEGF withdrawal has been shown to result in regression of
vasculature in several physiological and pathological circumstances
(30). In the present in
vitro studies, the results of western blotting and RT-PCR
suggest that oxymatrine diminishes the expression of VEGF and thus
suppresses tumor angiogenesis. However, expression of VEGFR-2 (one
receptor of VEGF) shown by western blotting was minimally decreased
after oxymatrine treatment in PANC-1 cells, indicating that the
inhibition of angiogenesis by oxymatrine in pancreatic cancer is
not associated with the VEGF receptor.
To further confirm the role of oxymatrine in
inhibition of tumor growth and angiogenesis in pancreatic cancer,
we established orthotopic pancreatic cancer xenograft tumors in
nude mice. Our studies showed that oxymatrine effectively inhibited
tumor growth, and the median survival time of mice in the
oxymatrine-treated group was markedly longer than that in the
control group, which suggested that oxymatrine significantly
improves the survival time of mice bearing pancreatic cancer
xenograft tumors. The growth and metastasis of tumors are dependent
on new blood vessel formation, and the microvessel density is often
used as a quantified factor of tumor vasculature (31). It has been reported that detection
of microvessel density does not provide precise information
concerning antiangiogenic therapy (32). CEUS is a new diagnostic tool to
evaluate the early effects of antiangiogenic treatment and is being
increasingly employed in the clinic (33,34).
In this study, we employed CEUS to detect tumor blood flow in
pancreatic cancer xenograft mouse tumors aftrer oxymatrine
treatment. The results of CEUS showed that oxymatrine markedly
reduced PI values of tumors and thus inhibited the angiogenesis of
pancreatic cancer. The in vivo studies further confirmed the
antiangiogenic effects of oxymatrine on human pancreatic
cancer.
In the present study, we demonstrated the
antiproliferative and antiangiogenic effects of oxymatrine on
pancreatic cancer in vitro and in vivo. These results
suggest that the NF-κB pathway plays an important role in
oxymatrine-induced VEGF signaling inhibition and antiangiogenesis
in pancreatic cancer. Moreover, targeting the NF-κB and VEGF
pathway may reveal potential therapeutic options for pancreatic
cancer, and oxymatrine may be a promising antiangiogenic strategy
for pancreatic cancer.
Acknowledgements
The authors thank the entire staff of the Animal
Experimental Center in the scientific research platform of the
Second Affiliated Hospital of Wenzhou Medical College and the
Animal Experimental Center of Zhejiang University School of
Medicine for the helpful assistance. The authors are grateful for
the funding from the Zhejiang Provincial Science Fund for
Distinguished Young Scholars (grant no. LR12H280001) and the
National Natural Science Foundation of China (grant no. 81173606)
and Wenzhou Science and Technology Projects (grant no.
Y20110037).
References
|
1
|
Raimondi S, Maisonneuve P and Lowenfels
AB: Epidemiology of pancreatic cancer: an overview. Nat Rev
Gastroenterol Hepatol. 6:699–708. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar
|
|
3
|
Stathis A and Moore MJ: Advanced
pancreatic carcinoma: current treatment and future challenges. Nat
Rev Clin Oncol. 7:163–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li-Weber M: Targeting apoptosis pathways
in cancer by Chinese medicine. Cancer Lett. 332:304–312. 2013.
View Article : Google Scholar
|
|
5
|
Lin SZ, Wei WT, Chen H, et al: Antitumor
activity of emodin against pancreatic cancer depends on its dual
role: promotion of apoptosis and suppression of angiogenesis. PLoS
One. 7:e421462012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei WT, Chen H, Ni ZL, et al: Antitumor
and apoptosis-promoting properties of emodin, an anthraquinone
derivative from Rheum officinale Baill, against pancreatic
cancer in mice via inhibition of Akt activation. Int J Oncol.
39:1381–1390. 2011.PubMed/NCBI
|
|
7
|
Shi GF and Li Q: Effects of oxymatrine on
experimental hepatic fibrosis and its mechanism in vivo. World J
Gastroenterol. 11:268–271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu LG, Zeng MD, Mao YM, et al: Oxymatrine
therapy for chronic hepatitis B: a randomized double-blind and
placebo-controlled multi-center trial. World J Gastroenterol.
9:2480–2483. 2003.PubMed/NCBI
|
|
9
|
Ling Q, Xu X, Wei X, et al: Oxymatrine
induces human pancreatic cancer PANC-1 cells apoptosis via
regulating expression of Bcl-2 and IAP families, and releasing of
cytochrome c. J Exp Clin Cancer Res. 30:662011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Liu H, Jin J, Zhu X, Lu L and
Jiang H: The role of endogenous reactive oxygen species in
oxymatrine-induced caspase-3-dependent apoptosis in human melanoma
A375 cells. Anticancer Drugs. 21:494–501. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song G, Luo Q, Qin J, Wang L, Shi Y and
Sun C: Effects of oxymatrine on proliferation and apoptosis in
human hepatoma cells. Colloids Surf B Biointerfaces. 48:1–5. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Piao B, Zhang Y, et al:
Oxymatrine diminishes the side population and inhibits the
expression of β-catenin in MCF-7 breast cancer cells. Med Oncol.
28(Suppl 1): S99–S107. 2011.PubMed/NCBI
|
|
13
|
Zou J, Ran ZH, Xu Q and Xiao SD:
Experimental study of the killing effects of oxymatrine on human
colon cancer cell line SW1116. Chin J Dig Dis. 6:15–20. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song MQ, Zhu JS, Chen JL, et al:
Synergistic effect of oxymatrine and angiogenesis inhibitor NM-3 on
modulating apoptosis in human gastric cancer cells. World J
Gastroenterol. 13:1788–1793. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baldwin AS: Control of oncogenesis and
cancer therapy resistance by the transcription factor NF-κB. J Clin
Invest. 107:241–246. 2001.
|
|
16
|
Aggarwal BB: Nuclear factor-κB: the enemy
within. Cancer Cell. 6:203–208. 2004.
|
|
17
|
Carbone C and Melisi D: NF-κB as a target
for pancreatic cancer therapy. Expert Opin Ther Targets. 16(Supp
2): S1–S10. 2012.
|
|
18
|
North S, Moenner M and Bikfalvi A: Recent
developments in the regulation of the angiogenic switch by cellular
stress factors in tumors. Cancer Lett. 218:1–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han J, Sun M, Cui Y, et al: Kushen
flavonoids induce apoptosis in tumor cells by inhibition of NF-κB
activation and multiple receptor tyrosine kinase activities.
Phytother Res. 21:262–268. 2007.PubMed/NCBI
|
|
20
|
Wang ZH, Chen H, Guo HC, et al: Enhanced
antitumor efficacy by the combination of emodin and gemcitabine
against human pancreatic cancer cells via downregulation of the
expression of XIAP in vitro and in vivo. Int J Oncol.
39:1123–1131. 2011.PubMed/NCBI
|
|
21
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar
|
|
22
|
Liptay S, Weber CK, Ludwig L, Wagner M,
Adler G and Schmid RM: Mitogenic and antiapoptotic role of
constitutive NF-κB/Rel activity in pancreatic cancer. Int J Cancer.
105:735–746. 2003.PubMed/NCBI
|
|
23
|
Wang W, Abbruzzese JL, Evans DB, Larry L,
Cleary KR and Chiao PJ: The nuclear factor-κB RelA transcription
factor is constitutively activated in human pancreatic
adenocarcinoma cells. Clin Cancer Res. 5:119–127. 1999.
|
|
24
|
Scartozzi M, Bearzi I, Pierantoni C, et
al: Nuclear factor-κB tumor expression predicts response and
survival in irinotecan-refractory metastatic colorectal cancer
treated with cetuximab-irinotecan therapy. J Clin Oncol.
25:3930–3935. 2007.
|
|
25
|
Nishi T, Shimizu N, Hiramoto M, et al:
Spatial redox regulation of a critical cysteine residue of NF-κB in
vivo. J Biol Chem. 277:44548–44556. 2002.PubMed/NCBI
|
|
26
|
Gonzalez-Perez RR, Xu Y, Guo S, Watters A,
Zhou W and Leibovich SJ: Leptin upregulates VEGF in breast cancer
via canonic and non-canonical signalling pathways and NFκB/HIF-1α
activation. Cell Signal. 22:1350–1362. 2010.PubMed/NCBI
|
|
27
|
Suboj B, Priya PS, Nandini RJ, et al:
Nimbolide retards tumor cell migration, invasion, and angiogenesis
by downregulating MMP-2/9 expression via inhibiting ERK1/2 and
reducing DNA-binding activity of NF-κB in colon cancer cells. Mol
Carcinog. 51:475–490. 2012.
|
|
28
|
Ye Y, Martinez JD, Perez-Polo RJ, Lin Y,
Uretsky BF and Birnbaum Y: The role of eNOS, iNOS, and NF-κB in
upregulation and activation of cyclooxygenase-2 and infarct size
reduction by atorvastatin. Am J Physiol Heart Circ Physiol.
295:N343–N351. 2008.
|
|
29
|
Ferrara N and Alitalo K: Clinical
applications of angiogenic growth factors and their inhibitors. Nat
Med. 5:1359–1364. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ferrara N: Role of vascular endothelial
growth factor in regulation of physiological angiogenesis. Am J
Physiol Cell Physiol. 280:1358–1366. 2001.PubMed/NCBI
|
|
31
|
Brawer MK: Quantitative microvessel
density. A staging and prognostic marker for human prostatic
carcinoma. Cancer. 78:345–349. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hlatky L, Hahnfeldt P and Folkman J:
Clinical application of antiangiogenic therapy: microvessel
density, what it does and doesn’t tell us. J Natl Cancer Inst.
94:883–893. 2002.PubMed/NCBI
|
|
33
|
Lassau N, Chebil M, Chami L, Bidault S,
Girard E and Roche A: Dynamic contrast-enhanced ultrasonography
(DCE-US): a new tool for the early evaluation of antiangiogenic
treatment. Target Oncol. 5:53–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Claudon M, Cosgrove D, Albrecht T, et al:
Guidelines and good clinical practice recommendations for contrast
enhanced ultrasound (CEUS) - update 2008. Ultraschall Med.
29:28–44. 2008. View Article : Google Scholar : PubMed/NCBI
|