Introduction
Lung cancer is the most common malignancy in humans.
It is also the most common cause of cancer-related mortality among
both men and women in most developed countries. Approximately 85%
of cases involve non-small cell lung cancer (NSCLC), which
generally has the histological structure of squamous cell carcinoma
or adenocarcinoma (1,2). Surgery, chemotherapy and radiotherapy
remain the standard of treatment in NSCLC. Despite the development
of various methods of treatment, NSCLC is still associated with
very poor prognosis. Only 10–15% of patients survive 5 years from
diagnosis. Metastatic NSCLC is fatal in 100% of cases (2).
Progress in understanding the biology of cancer
leads to personalization of therapy and introduction of drugs aimed
at blocking defective metabolic pathways of cancer cells. Erlotinib
is an example of a drug with a molecular mechanism of action, which
is currently used in the treatment of patients with NSCLC. It is a
reversible small molecule tyrosine kinase inhibitor of epidermal
growth factor receptor (TKI EGFR). EGFR abnormalities such as
strong EGFR protein expression, high polysomy or amplification and
EGFR gene mutations are relatively common in NSCLC. Kinase
inhibition stops cancerous cells in the G1 phase of the cell cycle
and induces apoptosis, which leads to a decrease of proliferation
and reduces the capacity for invasion and metastasis (3).
The aim of the present study was to determine the
prognostic and predictive factors in second- and third-line
erlotinib therapy in NSCLC patients with known status of
EGFR gene mutation.
Materials and methods
Patient characteristics
This study presents results of research that was
conducted in a group of 71 patients (36 men and 35 women) with
inoperable, locally advanced and metastatic NSCLC treated with
second- or third-line erlotinib in the period between 2008 and
2011. The study group consisted of patients aged 42–84 years (mean
age 60.9±9.6), the majority (n=59, 83.1%) current or ex-smokers.
Adenocarcinoma was diagnosed in 57% of tumors. Qualification for
erlotinib was based on radiologically confirmed progression after
first-line chemotherapy in patients with documented first-line
response, without rapid deterioration of performance status and
weight loss. The clinical characteristics of the study group are
presented in Table I.
 | Table IClinical characteristics of the study
group. |
Table I
Clinical characteristics of the study
group.
| Factor | n (%) |
|---|
| Gender |
| Male | 36 (50.7) |
| Female | 35 (49.3) |
| Age (years) |
| Median | 61 |
| Mean ± SD | 60.9±9.6 |
| Range | 42–84 |
| Smoking status |
| Current and
ex-smokers | 59 (83.1) |
| Pack-years
(median; mean ± SD) | 31 31.3±22.4 |
| Non-smokers | 12 (16.9) |
| Histological
diagnosis |
|
Adenocarcinoma | 41 (57.7) |
| Squamous cell
carcinoma | 18 (25.3) |
| Adenosquamous
carcinoma | 2 (2.8) |
| Large cell
carcinoma | 7 (9.9) |
| NSCLC NOS | 3 (14.3) |
| Stage |
| IIIA | 11 (15.5) |
| IIIB | 20 (28.2) |
| IV | 40 (56.3) |
| First-line
chemotherapy |
| Cisplatin (or
carboplatin) + vinorelbine | 39 (54.9) |
| Cisplatin (or
carboplatin) + gemcitabine | 16 (22.6) |
| Cisplatin +
pemetrexet | 5 (7.1) |
| Cisplatin (or
carboplatin) + taxans (docetaxel or paclitaxel) | 3 (4.2) |
| Vinorelbine
monotherapy | 4 (5.6) |
| Other (including
carboplatin + etoposide) | 4 (5.6) |
| Radiotherapy |
| Yes | 31 (43.7) |
| No | 40 (56.3) |
| Prior surgical
treatment |
| Yes | 17 (23.9) |
| No | 54 (76.1) |
Criteria for assessing the effects of treatment
(treatment response, disease stabilization or progression) were
based mainly on the analysis of images from spiral computed
tomography (CT) of the chest and sites suspected of metastases and
were consistent with the RECIST 1.1 guidelines. The criteria for
anemia and non-hematological complications were based on the Common
Toxicity Criteria (CTC) v.6.0 guidelines. Patient performance
status was assessed according to the ECOG-WHO score.
Examination of EGFR gene
abnormalities
Patients were qualified for treatment without taking
molecular factors into consideration; molecular tests were carried
out retrospectively after completion of treatment. EGFR
expression was determined by immunohistochemistry (IHC) using
monoclonal antibody from Dako (Denmark). The fluorescence in
situ hybridization (FISH) method was used to assess
amplification of the EGFR gene using molecular probes for
Abbott Molecular (USA). The presence of exon 19 deletions was
examined using the PCR technique and the amplified PCR product
fragment length analysis. In order to evaluate the L858R
substitutions in exon 21, the ASP-PCR (allele-specific PCR)
technique was used along with two pairs of PCR primers. PCR primers
were marked with Cy5 fluorochrome. The results were analyzed on an
ALF Express II sequencer. The direct sequencing by the Sanger
method for EGFR gene mutation detection was also used.
Statistical analysis
For statistical analysis we used the Chi-square test
to compare the quantity of patients with different response to
treatment and survival, depending on the prevalence of selected
predictive and prognostic factors. This test was also used to
compare the number of patients with and without EGFR gene
mutation in different subgroups of age, gender, histopathological
diagnosis and smoking status. To compare the probability of
survival and progression between the groups with different clinical
and molecular factors, the Kaplan-Meier method was used. Cox
regression model with ‘step by step’ selection was used to
determine the influence of clinical and molecular factors on
overall survival (OS) of patients treated with erlotinib.
Results
Estimation of EGFR gene
abnormalities
Seventeen activating mutations were detected in the
EGFR gene (24%), including 12 deletions in exon 19 (70.6% of
all identified mutations including three rare deletions) and 5
L858R substitutions in exon 21 (19.4% of all identified mutations).
Amplification of the EGFR gene in the tumor cells was
observed in 29 patients (40.8%, a positive FISH test), and normal
number of copies of the EGFR gene in 10 patients (14.1%, a
negative FISH test). In 32 patients (45.1%) FISH testing either
gave unreliable results or could not be performed due to the
limited histological material. EGFR gene activating
mutations occurred with similar frequency in the groups of patients
with or without EGFR gene amplification detected by FISH,
and in groups of patients with both presence and absence of EGFR
expression on the cell surface detected by IHC.
The incidence of mutations was slightly higher among
women (n=12, 34.3%) than among men (n=5, 13.9%) and did not depend
on age. EGFR activating mutations were significantly more
frequent in non-smoking patients (n=8, 66.7%) than in former or
current smokers (n=9, 15.3%). The number of mutations in patients
with adenocarcinoma and non-adenocarcinomas did not differ
significantly. The incidence of grade 3–4 rash after erlotinib
treatment was not significantly associated with the presence of
EGFR gene activating mutations. It should be noted, however,
that rash as a result of TKI EGFR therapy occurred slightly more
often in patients with EGFR mutations (Table II), as well as in patients with
EGFR gene amplification.
 | Table IICharacteristics of patients in
relation to the status of EGFR gene. |
Table II
Characteristics of patients in
relation to the status of EGFR gene.
| Factor | Wild-type
EGFR gene n (%) | Mutation in
EGFR gene n (%) | P-value | χ2 | Deletion in exon 19
n (%) | L858R substitution
in exon 21 n (%) |
|---|
| Study group | 54 (76) | 17 (24) | | | 12 (16.9) | 5 (7.1) |
| Gender | | | 0.083 | 3.012 | | |
| Male | 31 (86.1) | 5 (13.9) | | | 4 (11.1) | 1 (2.8) |
| Female | 23 (65.7) | 12 (34.3) | | | 8 (22.9) | 4 (11.4) |
| Age (years) | | | 0.26 | 1.267 | | |
| <65 | 31 (70.4) | 13 (29.6) | | | 8 (18.2) | 5 (11.4) |
| ≥65 | 23 (85.2) | 4 (14.8) | | | 4 (14.8) | 0 (0) |
| Smoking status | | | 0.0006 | 11.788 | | |
| Smokers | 50 (84.7) | 9 (15.3) | | | 5 (8.5) | 4 (6.8) |
| Non-smokers | 4 (33.3) | 8 (66.7) | | | 7 (58.4) | 1 (8.3) |
| Histological
diagnosis | | | 0.299 | 4.88 | | |
|
Adenocarcinoma | 30 (73.2) | 11 (26.8) | | | 8 (19.5) | 3 (7.3) |
| Squamous-cell
carcinoma | 16 (88.9) | 2 (11.1) | | | 2 (11.1) | 0 (0) |
| Adenosquamous
carcinoma | 1 (50) | 1 (50) | | | 1 (50) | 0 (0) |
| Large-cell
carcinoma | 4 (57.1) | 3 (42.9) | | | 1 (14.3) | 2 (28.6) |
| NSCLC NOS | 3 (100) | 0 (0) | | | 0 (0) | 0 (0) |
| EGFR gene
amplification | | | 0.419 | 1.739 | | |
| Yes (positive FISH
result) | 24 (82.8) | 5 (17.2) | | | 4 (13.8) | 1 (3.4) |
| No (negative FISH
result) | 8 (80) | 2 (20) | | | 2 (20) | 0 (0) |
| No FISH result
available | 22 (68.75) | 10 (31.25) | | | 6 (18.75) | 4 (12.5) |
| Erlotinib
treatment-related rash | | | 0.193 | 1.69 | | |
| Yes | 9 (60) | 6 (40) | | | 5 (33.3) | 1 (6.7) |
| No | 45 (80.4) | 11 (19.6) | | | 7 (12.5) | 4 (7.1) |
| Response to
first-line treatment | | | 0.157 | 2.002 | 11 (21.6) | 4 (7.8) |
| CR, PR, SD | 36 (70.6) | 15 (29.4) | | | 1 (5) | 1 (5) |
| PD | 18 (90) | 2 (10) | | | | |
| Time of response to
first-line treatment (months) | | | 0.918 | 0.011 | | |
| ≤12 | 45 (75) | 15 (25) | | | 10 (16.7) | 5 (8.3) |
| >12 | 9 (81.8) | 2 (18.2) | | | 2 (18.2) | 0 (0) |
| Loss of body mass
in 3 months | | | 0.997 | 0 | | |
| ≤5% | 37 (77.1) | 11 (22.9) | | | 8 (16.65) | 3 (6.25) |
| >5% | 17 (73.9) | 6 (26.1) | | | 4 (17.4) | 2 (8.7) |
| Time from diagnosis
to erlotinib treatment (months) | | | 0.859 | 0.032 | | |
| ≤12 | 31 (75.6) | 10 (24.4) | | | 6 (14.6) | 4 (9.8) |
| >12 | 23 (76.7) | 7 (23.3) | | | 6 (20) | 1 (3.3) |
Although the response to first-line chemotherapy
with platinum compounds was slightly more frequent in patients with
EGFR mutations, the length of response and the time from
diagnosis to start of erlotinib did not depend on the presence of
the mutation. There was no significant relationship between the
presence of EGFR mutation and loss of body mass.
Estimation of response to erlotinib
treatment
An objective response to erlotinib treatment in the
form of partial response (PR) occurred in only 5 patients (7%),
including 3 second-line and 2 third-line patients. Duration of
remission in relation to clinical characteristics of patients were:
i) 4 months (observation cut-off) in a smoking (30 pack-years)
53-year-old woman with large-cell carcinoma and a deletion in exon
19 of the EGFR gene; ii) 8 months in a smoking (31
pack-years) 52-year-old man with large-cell carcinoma and mutation
in exon 21 of the EGFR gene; iii) 9 months in a smoking (10
pack-years) 46-year-old man with adenocarcinoma and a deletion in
exon 19 of the EGFR gene; iv) 16 months in a non-smoking
60-year-old woman with adenosquamous cell carcinoma and deletion in
exon 19 of the EGFR gene and EGFR gene amplification
(84% of tumor cells with abnormal number of EGFR gene
copies); v) 29 months (observation cut-off) in a non-smoking
74-year-old man with adenocarcinoma and a deletion in exon 19 of
the EGFR gene. It should be noted that in all cases of PR as
a result of erlotinib treatment activating mutation in the
EGFR gene was present in tumor cells, and that all patients
with PR suffered a rash of varying severity.
Stable disease (SD) during treatment with erlotinib
in second- or third-line occurred in 24 patients (33.8%), including
9 patients with activating EGFR mutations (37.5% of all
patients with SD). Three patients with rare deletions in exon 19 of
the EGFR gene achieved SD lasting 8 (ΔL747–P753), 8
(ΔE746–S752) and 4 months (ΔE746–P753).
Progressive disease (PD) during erlotinib treatment
occurred in 42 patients (59.2%), of which only 3 had EGFR
activating mutations (7.1% of all patients with PD); two cases with
L858R substitution and one patient with deletion in exon 19.
Absence of mutation in the EGFR gene was the
strongest independent factor increasing the percentage of early
progression in patients treated with erlotinib (P=0.0002,
χ2=13.76). Other factors that increased the incidence of
progression during erlotinib therapy were: short response to
first-line chemotherapy, high serum LDH level and the absence of a
rash associated with erlotinib treatment. Notably, the progression
or stabilization of disease did not depended on gender, age,
smoking status (there were only 12 non-smokers in the group),
pathological diagnosis and amplification of EGFR gene.
Patients with poorer performance status, weight loss and anemia had
only slightly decreased response rate compared to patients without
these adverse prognostic factors (Table III).
 | Table IIIClinical and molecular factors
influencing the risk of early progression in patients treated with
erlotinib. |
Table III
Clinical and molecular factors
influencing the risk of early progression in patients treated with
erlotinib.
| Factor | Total n (%) | PD n (%) | SD, PR n (%) | P-value | χ2 |
|---|
| Study group | 71 | 42 (59.2) | 29 (40.8) | | |
| Age (years) |
| ≤65 | 44 (62) | 24 (54.5) | 20 (45.5) | 0.447 | 0.578 |
| >65 | 27 (38) | 18 (66.7) | 9 (33.3) | | |
| Gender |
| Male | 36 (50.7) | 23 (63.9) | 13 (36.1) | 0.561 | 0.338 |
| Female | 35 (49.3) | 19 (54.3) | 16 (45.7) | | |
| Smoking status |
| Smoker | 59 (83.1) | 38 (64.4) | 21 (35.6) | 0.094 | 2.803 |
| Non-smoker | 12 (16.9) | 4 (33.3) | 8 (66.7) | | |
| Performance
status |
| PS = 0/1 | 33 (46.5) | 16 (48.5) | 17 (51.5) | 0.144 | 2.139 |
| PS = 2/3 | 38 (53.5) | 26 (68.4) | 12 (31.6) | | |
| Histological
types |
| Adenocarcinoma and
adenosquamous carcinoma | 43 (60.6) | 27 (62.8) | 16 (37.2) | 0.599 | 0.276 |
| Other | 28 (39.4) | 15 (53.6) | 13 (46.4) | | |
| Response to
first-line treatment |
| CR, PR, SD | 51 (71.8) | 29 (56.9) | 22 (43.1) | 0.344 | 0.896 |
| PD | 20 (28.2) | 13 (65) | 7 (35) | | |
| Time of response to
first-line chemotherapy (months) |
| ≤12 | 60 (84.5) | 39 (65) | 21 (35) | 0.045 | 4.026 |
| >12 | 11 (15.5) | 3 (27.3) | 8 (72.7) | | |
| Time from diagnosis
to erlotinib treatment (months) |
| ≤12 | 41 (57.8) | 27 (65.9) | 14 (34.1) | 0.272 | 1.206 |
| >12 | 30 (42.2) | 15 (50) | 15 (50) | | |
| Loss of body mass
in 3 months |
| ≤5% | 48 (67.6) | 25 (52.1) | 23 (47.9) | 0.135 | 2.230 |
| >5% | 23 (32.4) | 17 (73.9) | 6 (26.1) | | |
| Anemia |
| Yes | 54 (76.1) | 35 (64.8) | 19 (35.2) | 0.148 | 2.092 |
| No | 17 (23.9) | 7 (41.2) | 10 (58.8) | | |
| Clinical stage |
| IIIA, IIIB | 31 (43.7) | 18 (58.1) | 13 (41.9) | 0.937 | 0.006 |
| IV | 40 (56.3) | 24 (60) | 16 (40) | | |
| Previous
radiotherapy |
| Yes | 31 (43.7) | 19 (61.3) | 12 (38.7) | 0.937 | 0.006 |
| No | 40 (56.3) | 23 (57.5) | 17 (42.5) | | |
| Previous surgical
treatment |
| Yes | 17 (23.9) | 8 (47.1) | 9 (52.9) | 0.378 | 0.775 |
| No | 54 (76.1) | 34 (63) | 20 (37) | | |
| Line of erlotinib
therapy |
| Second | 53 (74.6) | 32 (60.4) | 21 (39.6) | 0.935 | 0.006 |
| Third | 18 (25.4) | 10 (55.6) | 8 (44.4) | | |
| Erlotinib
treatment-related rash |
| Yes | 15 (21.1) | 4 (26.7) | 11 (73.3) | 0.009 | 6.69 |
| No | 56 (78.9) | 38 (67.9) | 18 (32.1) | | |
| LDH serum
level |
| High | 8 (11.25) | 7 (87.5) | 1 (12.5) | 0.031 | 6.941 |
| Normal | 19 (26.75) | 7 (36.8) | 12 (63.2) | | |
| Unknown | 44 (62) | 28 (63.6) | 16 (36.4) | | |
| EGFR gene
amplification |
| Yes | 29 (40.8) | 17 (58.6) | 12 (41.4) | 0.582 | 1.081 |
| No | 11 (15.5) | 8 (72.7) | 3 (27.3) | | |
| Unknown | 31 (43.7) | 17 (54.8) | 14 (45.2) | | |
| EGFR gene
mutation |
| Yes | 17 (24) | 3 (17.6) | 14 (82.3) | 0.0002 | 13.76 |
| No | 54 (76) | 39 (72.2) | 15 (27.8) | | |
| Type of EGFR
gene mutation |
| Wild-type | 54 (76) | 39 (72.2) | 15 (27.8) | 0.0002 | 17.403 |
| Deletions in exon
19 | 12 (16.9) | 1 (8.3) | 11 (91.7) | | |
| L858R substitution
in exon 21 | 5 (7.1) | 2 (40) | 3 (60) | | |
The median progression-free survival (PFS) for the
entire group of patients treated with erlotinib was 1.5 months. The
study, however, had a considerable weakness resulting from the
large number of incomplete observations (n=41). Observations in
this group were carried out at least until the end of erlotinib
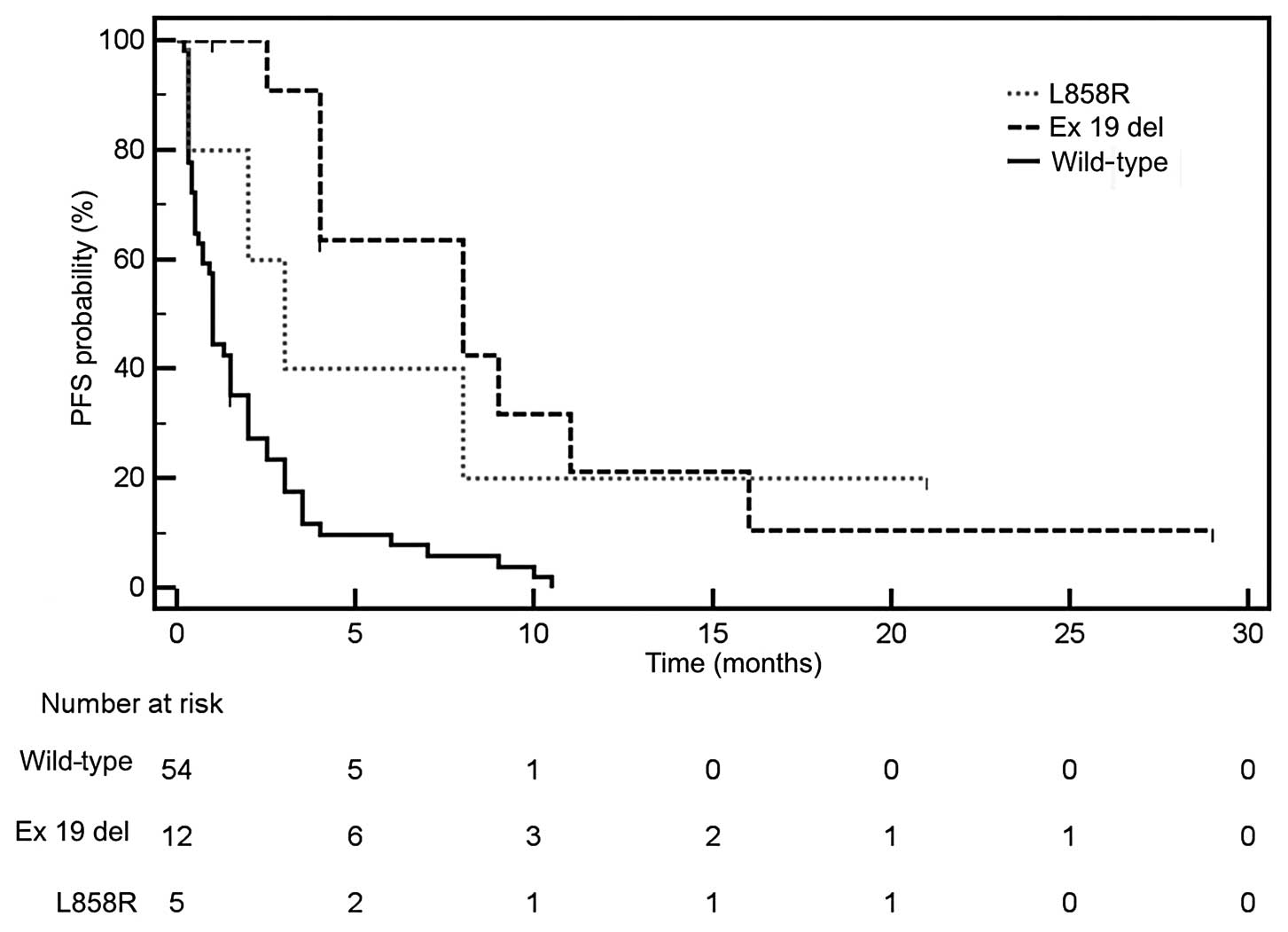
therapy. Median PFS was significantly longer in patients with
EGFR activating mutations (HR=0.309; 95% CI, 0.19–0.503)
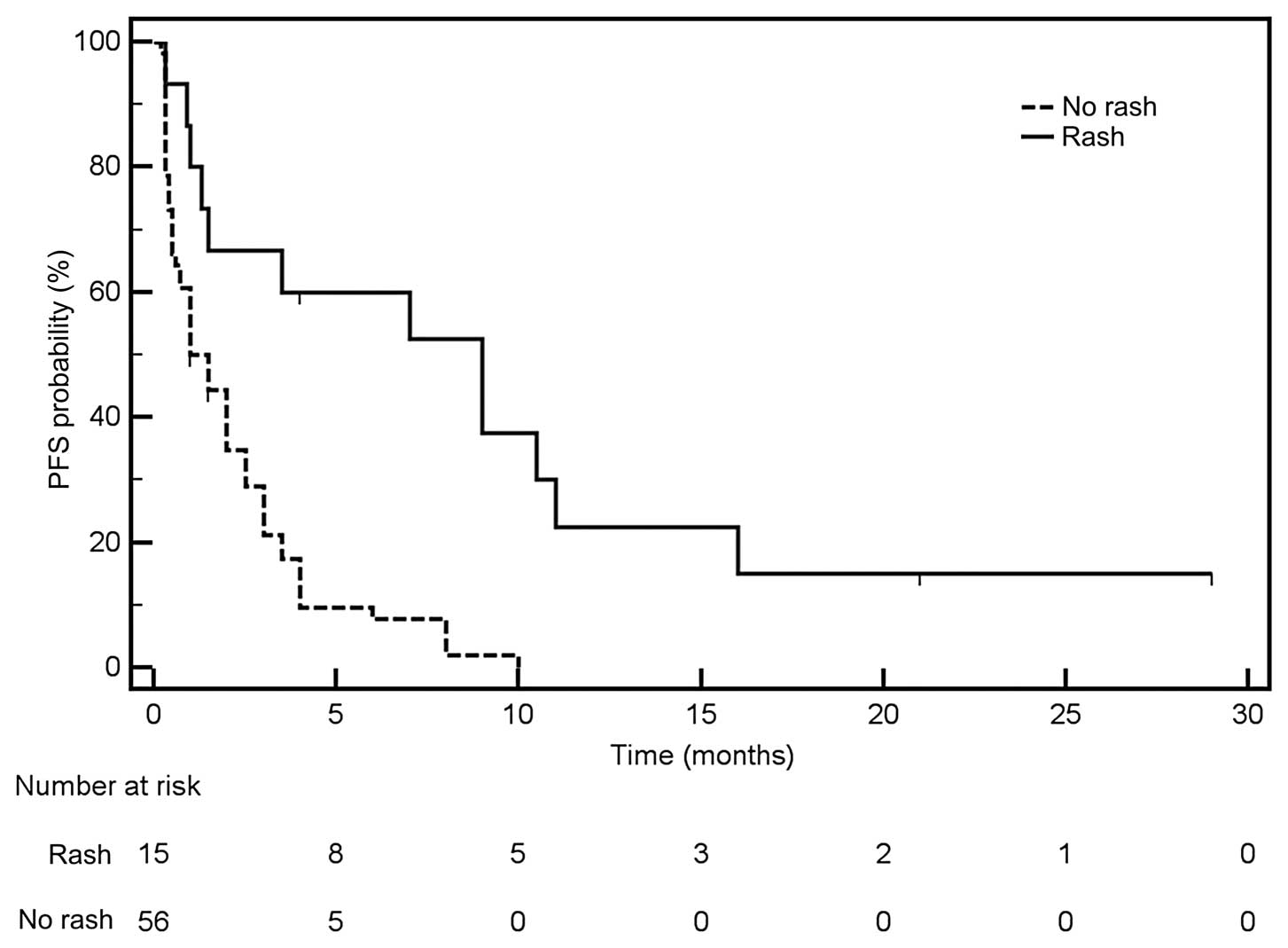
(Fig. 1) and in patients with rash
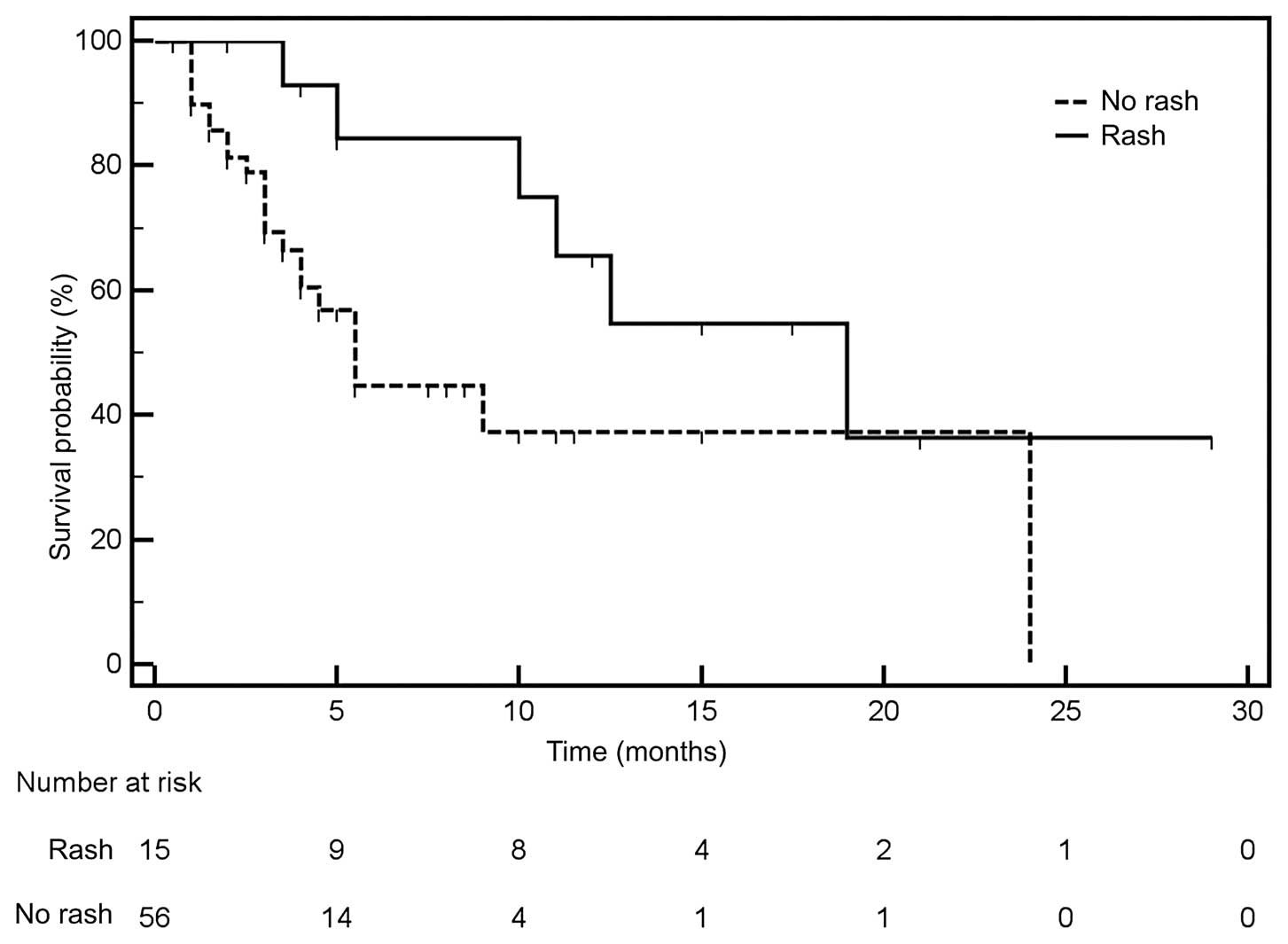
associated with erlotinib (Fig. 2)
as compared to patients without those factors. However, the median
PFS was not the same in all carriers of EGFR gene mutation
and was longer in patients with deletions in exon 19 compared to
patients with the L858R substitution in exon 21. Performance
status, weight loss and LDH levels had weaker but significant
effects on PFS in patients treated with erlotinib. Median PFS was
not dependent on the degree of amplification of the EGFR
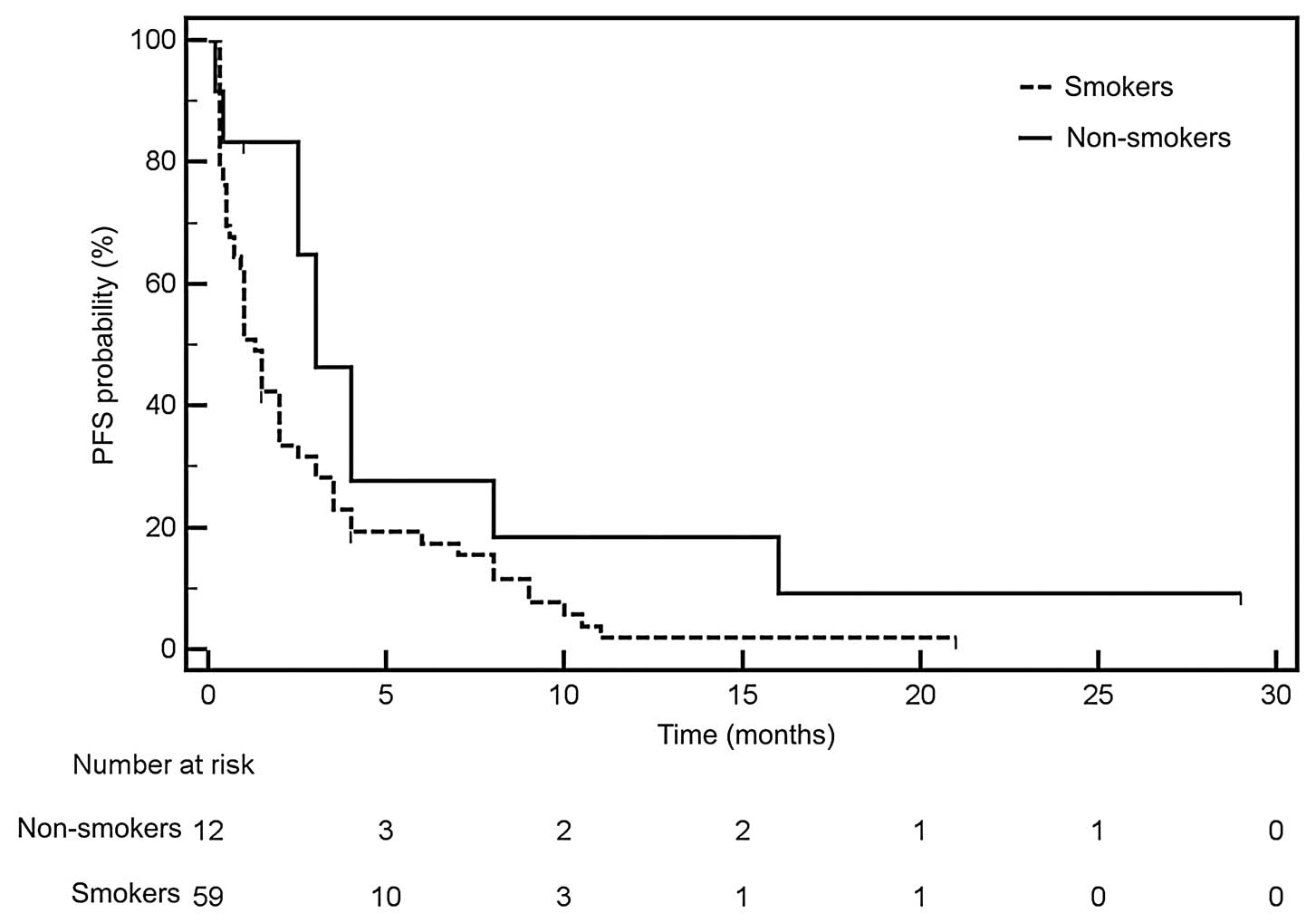
gene, gender, pathology and was only slightly longer in non-smokers
(Fig. 3).
Estimation of OS of erlotinib-treated
patients
Median OS was 10 months. OS in patients treated with
erlotinib was partially influenced by factors other than those
affecting the PFS. Median OS was only insignificantly longer in
patients with EGFR activating mutations than in patients
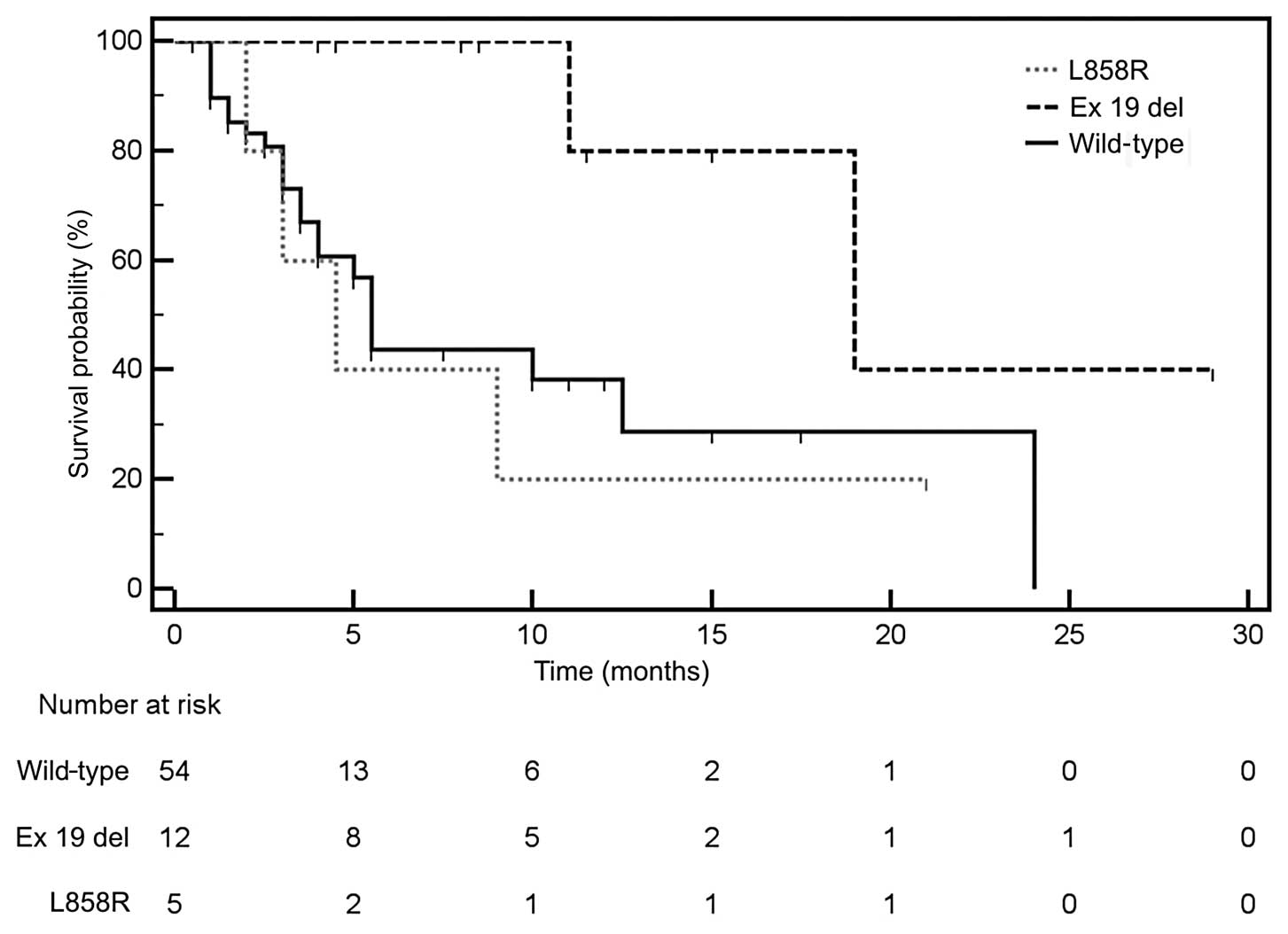
without such mutations. Prolonged median OS applied only to
patients with deletions in exon 19 of the EGFR gene, whereas
patients with L858R substitution had median OS similar to that of
patients without mutations in this gene (Fig. 4). Median OS was significantly longer
in patients with treatment-related rash compared to patients
without this adverse effect (Fig.
5). Median OS was not affected by smoking status, gender, age,
pathology and EGFR gene amplification. Significant
shortening of OS was associated with typical negative prognostic
factors, such as poor performance status, significant loss of body
mass, lack of response to first-line chemotherapy, short duration
of first-line response, short follow-up time since diagnosis and
LDH high serum levels.
OS and PFS are affected by multiple
factors in Cox regression model
Multivariate analysis using Cox logistic regression
confirmed that the strongest factors increasing risk of progression
in patients treated with erlotinib are the absence of EGFR
gene activating mutations (risk nearly 6-fold higher) and absence
of treatment-related rash (risk increase 4.5-fold). These factors
are closely related to the mechanism of action of TKI EGFR. The
value of other factors that affect the risk of progression is
associated with their traditional prognostic role, affecting the
course of the disease (Table
IV).
 | Table IVFactors affecting progression-free
survival of patients treated with erlotinib in multivariate Cox
logistic regression. |
Table IV
Factors affecting progression-free
survival of patients treated with erlotinib in multivariate Cox
logistic regression.
| Factor | β | P-value | Hazard ratio
(confidence interval) |
|---|
| Absence of
EGFR gene activating mutations | 1.7712 | >0.0001 | 5.878
(2.773–12.457) |
| Absence of
treatment-related rash | 1.5188 | 0.0002 | 4.567
(2.061–10.120) |
| Erlotinib in the
third-line therapy | 1.1965 | 0.0035 | 3.308
(1.489–7.349) |
| Short response to
first-line chemotherapy | 1.0511 | 0.0227 | 2.861
(1.164–7.031) |
| Short time from
diagnosis to the start of erlotinib treatment | 0.8365 | 0.0162 | 2.308
(1.171–4.548) |
| Poor performance
status (PS=2) | 0.8033 | 0.0078 | 2.232
(1.240–4.021) |
On the other hand, prognostic factors have a
decisive influence on the risk of early mortality in patients
treated with erlotinib. This risk is multiplied in patients with
poor performance status, short response to first-line chemotherapy
and short time from diagnosis to start of erlotinib. Predictive
factors affecting the effectiveness of TKI EGFR also affect the
risk of mortality. Although the absence of EGFR gene
mutation increases the risk by only 2.8-fold with low level of
significance (P=0.04), the absence of a rash associated with
erlotinib treatment has significant effect on the risk of mortality
(Table V).
 | Table VFactors affecting the overall
survival in patients treated with erlotinib in multivariate Cox
logistic regression. |
Table V
Factors affecting the overall
survival in patients treated with erlotinib in multivariate Cox
logistic regression.
| Factor | β | P-value | Hazard ratio
(confidence interval) |
|---|
| Poor performance
status (PS=2) | 3.62 | >0.0001 | 37.344
(7.912–176.256) |
| Absence of
erlotinib-related rash | 2.7037 | 0.0002 | 14.9348
(3.543–62.954) |
| Short response to
first line chemotherapy | 2.253 | 0.0445 | 9.519
(1.069–84.774) |
| Short period from
diagnosis to start of erlotinib treatment | 2.167 | 0.0015 | 8.735
(2.299–33.194) |
| Third line
erlotinib treatment | 1.49 | 0.0135 | 4.439
(1.369–14.387) |
| Absence of
EGFR gene activating mutation | 1.032 | 0.04 | 2.805
(1.051–7.487) |
Discussion
Erlotinib was introduced for the treatment of
patients with NSCLC, according to the recommendations of the FDA,
on November 18, 2004 (4). The
efficacy and safety of the drug was confirmed in a randomized
clinical double-blind, placebo-controlled trial (BR.21). The study
included 731 patients with locally advanced or metastatic NSCLC in
whom standard chemotherapy had failed. Median OS in patients
treated with erlotinib was 6.7 months compared to 4.7 months in the
placebo group. Regardless of the presence of molecular factors,
erlotinib prolonged PFS and improved quality of life compared to
the best supportive care (BSC), but the objective response occurred
in only 8.9% of patients (5).
Our study does not indicate that the median OS and
PFS depend significantly on gender, age, tumor pathology or smoking
status. Results differ from those commonly found in literature but
our group was dominated by smokers or ex-smokers. Shepherd et
al(5) in the BR.21 study
demonstrated that the median OS is higher in non-smokers vs. past
or active smokers (12.3 vs. 5.5 months, P<0.006), women vs. men
(8.4 vs. 5.7 months, P=0.76) and in patients diagnosed with
adenocarcinoma vs. squamous (7.8 vs. 5.6 months, P=0.97). The
TRIBUTE study demonstrated results of treatment in non-smoking
patients. OS was significantly longer (by 12.4 months) in patients
who were treated with erlotinib compared to placebo (22.5 vs. 10.1
months; HR, 0.49; 95% CI, 0.28–0.85; P=0.01). It should be noted
that the above mentioned group of patients was dominated by younger
people (average age 58 vs. 64 years), women (60 vs. 37%), and
patients diagnosed with adenocarcinoma (82 vs. 58%) (6).
There is a clinically confirmed correlation between
the occurrence of rash and response to erlotinib treatment, which
is also demonstrated in this study. Petrelli et al compared
the relationship between the occurrence of a rash and the results
of TKI treatment based on 24 trials. They found that the presence
of a rash is an important independent predictive factor for
treatment with EGFR TKI, affecting OS (HR, 0.30; P<0.00001) and
the risk of disease progression (HR, 0.50; P<0.00001). In
addition, objective response to treatment was significantly higher
in patients who experienced cutaneous adverse effects of treatment
(42% compared to 7% in patients without rash) (7). Tendency for cutaneous adverse effect
complications in the case of erlotinib is most likely associated
with EGFR gene polymorphism.
With the new data on intracellular signaling
pathways and their role in cancer development, molecular targets
for small molecule TKI EGFR, including erlotinib, have been
identified. Biomarkers which were initially analyzed included
expression score of the EGFR extracellular domain and the gene
number copies in tumor cells.
The increased number of EGFR gene copies was
the first molecular predictive marker for TKI. There are no clear
data on the impact of gene amplification on the effectiveness of
gefitinib or erlotinib. Results of previous studies are
controversial. Conclusions from the BR.21 study showed that
patients with amplification or high polisomy of EGFR gene
benefit more from treatment with erlotinib (8). Similar conclusions were presented by a
group of Japanese researchers evaluating the efficacy of gefitinib
in patients with EGFR gene amplification; they achieved
statistically longer survival, higher objective response rate (ORR)
and longer PFS compared to patients without gene amplification
(P=0.014) (9). A number of phase
III studies have been performed concerning erlotinib and gefitinib
effectiveness in second- and third-line therapy of unselected NSCLC
patients. However, molecular analysis in these studies was
conducted retrospectively and on small groups of patients (Table VI).
 | Table VIResults of clinical trials concerning
erlotinib and gefitinib effectiveness in second- and third-line
therapy examined at molecular level in NSCLC patients. |
Table VI
Results of clinical trials concerning
erlotinib and gefitinib effectiveness in second- and third-line
therapy examined at molecular level in NSCLC patients.
| Clinical trial | No. of patients and
type of treatment | Subgroups (only
patients treated with TKI EGFR) | ORRa (%) | Median PFSa (months) | Median OSa (months) |
|---|
| IDEAL1 (10) | N=210 (50% Asian
patients) - gefitinib 250 or 500 mg/day | 250 mg/day | 18.4 | 2.7 | 7.6 |
| 500 mg/day | 19 | 2.8 | 8.0 |
| IDEAL2 (11) | N=216 (Caucasian
patients) - gefitinib 250 or 500 mg/day after platinium compounds
and docetaxel | 250 mg/day | 11.8 | 1.9 | 6.5 |
| 500 mg/day | 8.8 | 1.9 | 6.0 |
| Both IDEAL1
(10) | FISH performer on
90 samples and EGFR mutation analysis on 79 samples | FISH+ (n=7) | 29 | 2.9 | BD |
| IDEAL2 (11)b | M+ (n=14) | 46 | 3.9 | BD |
| INTEREST (12) | N=729 - gefitinib
250 mg/day, N=715 - docetaxel 75 mg/m2 | All patients | 9.1 | 2.2 | 7.6 |
| FISH+
(n=77/157) | 13 | 2.5 | 8.4 |
| M+ (n=19/125) | 42.1 | 7.0 | 14.2 |
| ISEL (13) | N=1129 - gefitinib
250 mg/day, N=563 - placebo | All patients | 8 | 3c | 5.6 |
| FISH+
(n=37/114) | 16.4 | 4.5 | 8.3 |
| M+ (n=16/132) | 37.5 | 10.8 | BD |
| BR.21 (5) | N=488 - erlotinib
150 mg/day, N= 243 - placebo (<13% Asian patients) | All patients | 8.9 | 2.2 | 6.7 |
| FISH+
(n=61/159) | 21 | BD | 10.5 |
| M+ (n=37/204) | 27 | BD | 10.9 |
| TRUST (14) | N=4002 (Caucasian)
- erlotinib 150 mg/day | All patient | 9.2 | 3 | 6.7 |
| FISH+
(n=49/208) | 17 | 4 | 8.6 |
| M+ (n=6/86) | 33.3 | 12.7 | 16.7 |
| TITAN (15) | N=203 - erlotinib
150 mg/day
N=221- docetaxel or pemetrexed | All patients | 7.9 | 1.5 | 5.3 |
| FISH+
(n=121/132) | BD | BD | 6.4 |
| M+ (n=8/83) | BD | 8.8 | 19.3 |
The results of our analysis do not indicate
EGFR gene amplification as a predictive marker for therapy.
Median OS and PFS do not depend on the presence of gene
amplification. Moreover, there was no correlation between
EGFR gene amplification and the presence of activating
mutation of EGFR gene. This may be associated with FISH
analysis limitations, challenges with obtaining results of
diagnostic value.
The results of first clinical trials, of low
molecular weight, proved that there are populations of patients who
are significantly more responsive to TKI EGFR (erlotinib and
gefitinib) treatment. These are patients of predominantly Asian
origin, diagnosed with adenocarcinoma, with therapy associated
rash, women and never smokers. Subsequently, activating mutations
in the EGFR gene responsible for this relationship were
described (16–18). The presence of the mutation is
currently the most important molecular factor for predicting
response to TKI EGFR treatment.
The results of several phase III clinical trials
with gefitinib (IPASS, JP 0056 - NEJ 002, WJTOG3405, First-Signal)
in the group of patients with confirmed mutation in the EGFR
gene, showed a significantly higher efficacy of gefitinib compared
to chemotherapy (19–22). In the OPTIMAL study conducted among
Asian patients with EGFR mutations, erlotinib therapy
achieved a significant increase in response rate (83 vs. 36%) and
PFS (13.1 vs. 4.6 months) (23). In
the EURTAC study, which included Caucasian patients with
EGFR gene mutations the results were: ORR = 58 vs. 15%; PFS
= 9.7 vs. 5.2 months (24).
The presence of activating mutations in the
EGFR gene was an important predictive factor for erlotinib
therapy in our study group as well. Rosell et al(25) evaluated the incidence of activating
mutations in the EGFR gene in 2105 Caucasian patients, of
whom 350 (16.6%) showed a mutation. Erlotinib was used as a
first-line therapy in 113 patients, and as a second- or third-line
therapy in 104 patients. PFS and median OS in patients with
EGFR activating mutations treated with TKI EGFR were similar
regardless of line of therapy (PFS, 14 months; OS, 28 months in
first-line therapy and 27 months in second- and third-line
therapy). Patients who had a deletion in exon 19 achieved a higher
ORR compared to patients with L858R substitution (P=0.001).
Although, there is a clear need for the use of TKI
EGFR for second- and third-line therapy in patients with
EGFR gene mutations, the use of these drugs in patients with
undetected EGFR mutation remains problematic (26). In the present study, disease control
was observed in 27.8% of patients with no detected EGFR
mutation. On the other hand, early progression was described in
17.6% of patients with activated EGFR gene mutation. The
reason for this may be underestimation of EGFR mutation or
appearance of genetic abnormalities responsible for resistance to
TKI EGFR. Therefore, careful analysis of clinical factors for
qualification to molecular examination and TKI EGFR therapy as well
as appropriate selection of molecular tests are required.
In conclusion, this study presents the results of
second- and third-line erlotinib treatment in a group of 71
patients with advanced NSCLC. Objective response occurred in only 5
patients (7%); all had activating mutations of EGFR and
developed rash during therapy. The influence of a number of
clinical and molecular factors on the efficacy of erlotinib was
assessed. We concluded that detection of EGFR gene mutation
is not the only factor determining the effectiveness of erlotinib
in second- or third-line therapy in advanced NSCLC patients.
Evaluation of molecular and clinical predictive factors in
individual patients is important both from a clinical and an
economic point of view, assuming similar efficacy and favorable
toxicity profile of erlotinib compared to docetaxel or pemetrexed
in patients with relapsed NSCLC.
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2
|
D'Addario G, Früh M, Reck M, Baumann P,
Klepetko W and Felip E: Metastatic non-small-cell lung cancer: ESMO
Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 21:v116–v119. 2010. View Article : Google Scholar
|
|
3
|
Dancey J and Sausville EA: Issues and
progress with protein kinase inhibitors for cancer treatment. Nat
Rev Drug Discov. 2:296–313. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohen MH, Johnson JR, Chen YF, Sridhara R
and Pazdur R: FDA drug approval summary: erlotinib (Tarceva)
tablets. Oncologist. 10:461–466. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shepherd FA, Rodrigues PJ, Ciuleanu T, et
al: Erlotinib in previously treated non-small-cell lung cancer. N
Engl J Med. 353:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Herbst RS, Prager D, Hermann R, et al:
TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774)
combined with carboplatin and paclitaxel chemotherapy in advanced
non-small-cell lung cancer. J Clin Oncol. 23:5892–5899. 2005.
View Article : Google Scholar
|
|
7
|
Petrelli F, Borgonovo K, Cabiddu M, Lonati
V and Barni S: Relationship between skin rash and outcome in
non-small-cell lung cancer patients treated with anti-EGFR tyrosine
kinase inhibitors: a literature-based meta-analysis of 24 trials.
Lung Cancer. 78:8–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsao MS, Sakurada A, Cutz JC, et al:
Erlotinib in lung cancer - molecular and clinical predictors of
outcome. N Engl J Med. 353:133–144. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takano T, Ohe Y, Sakamoto H, et al:
Epidermal growth factor receptor gene mutations and increased copy
numbers predict gefitinib sensitivity in patients with recurrent
non small- cell lung cancer. J Clin Oncol. 23:6829–6837. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukuoka M, Yano S, Giaccone G, et al:
Multi-institutional randomized phase II trial of gefitinib for
previously treated patients with advanced non-small-cell lung
cancer (the IDEAL 1 Trial). J Clin Oncol. 21:2237–2246. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kris MG, Natale RB, Herbst RS, et al: A
phase II trial of ZD 1839 (‘Iressa’) in advanced non-small-cell
lung cancer (NSCLC) patients who had failed platinum- and
docetaxel-based regimens (IDEAL 2). Proc Am Soc Clin Oncol.
21:292a2002.
|
|
12
|
Kim ES, Hirsh V, Mok T, et al: Gefitinib
versus docetaxel in previously treated non-small-cell lung cancer
(INTEREST): a randomised phase III trial. Lancet. 372:1809–1818.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thatcher N, Chang A, Parikh P, et al:
Gefitinib plus best supportive care in previously treated patients
with refractory advanced non-small-cell lung cancer: results from a
randomized, placebo-controlled, multicentre study (Iressa Survival
Evaluation in Lung Cancer). Lancet. 366:1527–1537. 2005. View Article : Google Scholar
|
|
14
|
Reck M, van Zandwijk N, Gridelli C, et al:
Erlotinib in advanced non-small cell lung cancer: efficacy and
safety findings of the global phase IV Tarceva Lung Cancer Survival
Treatment study. J Thorac Oncol. 5:1616–1622. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ciuleanu T, Stelmakh L, Cicenas S, et al:
Efficacy and safety of erlotinib versus chemotherapy in second-line
treatment of patients with advanced, non-small-cell lung cancer
with poor prognosis (TITAN): a randomised multicentre, open-label,
phase 3 study. Lancet Oncol. 13:300–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taron M, Ichinose Y, Rosell R, et al:
Activating mutations in the tyrosine kinase domain of the epidermal
growth factor receptor are associated with improved survival in
gefitinib-treated chemorefractory lung adenocarcinomas. Clin Cancer
Res. 11:5878–5885. 2005. View Article : Google Scholar
|
|
17
|
Endo K, Konishi A, Sasaki H, et al:
Epidermal growth factor receptor gene mutation in non-small cell
lung cancer using highly sensitive and fast TaqMan PCR assay. Lung
Cancer. 50:375–384. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paez JG, Jänne PA, Lee JC, et al:
EGFR mutations in lung cancer: correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar
|
|
19
|
Fukuoka M, Wu YL, Thongprasert S, et al:
Biomarker analyses and final overall survival results from a phase
III, randomized, open-label, first-line study of gefitinib versus
carboplatin/paclitaxel in clinically selected patients with
advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol.
29:2866–2874. 2011. View Article : Google Scholar
|
|
20
|
Maemondo M, Inoue A, Kobayashi K, et al:
Gefitinib or chemotherapy for non-small-cell lung cancer with
mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mitsudomi T, Morita S, Yatabe Y, et al:
Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): an open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar
|
|
22
|
Han JY, Park K, Kim SW, et al:
First-SIGNAL: first-line single-agent iressa versus gemcitabine and
cisplatin trial in never-smokers with adenocarcinoma of the lung. J
Clin Oncol. 30:1122–1128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou C, Wu YL, Chen G, et al: Erlotinib
versus chemotherapy as first-line treatment for patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase
3 study. Lancet Oncol. 12:735–742. 2011.PubMed/NCBI
|
|
24
|
Rosell R, Carcereny E, Gervais R, et al:
Erlotinib versus standard chemotherapy as first-line treatment for
European patients with advanced EGFR mutation-positive
non-small-cell lung cancer (EURTAC): a multicentre, open-label,
randomised phace 3 trial. Lancet Oncol. 13:239–246. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rosell R, Moran T, Quertalt C, et al:
Screening for epidermal growth factor receptor mutations in lung
cancer. N Engl J Med. 361:958–967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao H, Ding X, Wei D, et al: Erlotinib in
patients with advanced non-small-cell lung cancer: a meta-analysis.
Trans Lung Cancer Res. 1:129–144. 2012.PubMed/NCBI
|