|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2
|
D'Addario G, Früh M, Reck M, Baumann P,
Klepetko W and Felip E: Metastatic non-small-cell lung cancer: ESMO
Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 21:v116–v119. 2010. View Article : Google Scholar
|
|
3
|
Dancey J and Sausville EA: Issues and
progress with protein kinase inhibitors for cancer treatment. Nat
Rev Drug Discov. 2:296–313. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cohen MH, Johnson JR, Chen YF, Sridhara R
and Pazdur R: FDA drug approval summary: erlotinib (Tarceva)
tablets. Oncologist. 10:461–466. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shepherd FA, Rodrigues PJ, Ciuleanu T, et
al: Erlotinib in previously treated non-small-cell lung cancer. N
Engl J Med. 353:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Herbst RS, Prager D, Hermann R, et al:
TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774)
combined with carboplatin and paclitaxel chemotherapy in advanced
non-small-cell lung cancer. J Clin Oncol. 23:5892–5899. 2005.
View Article : Google Scholar
|
|
7
|
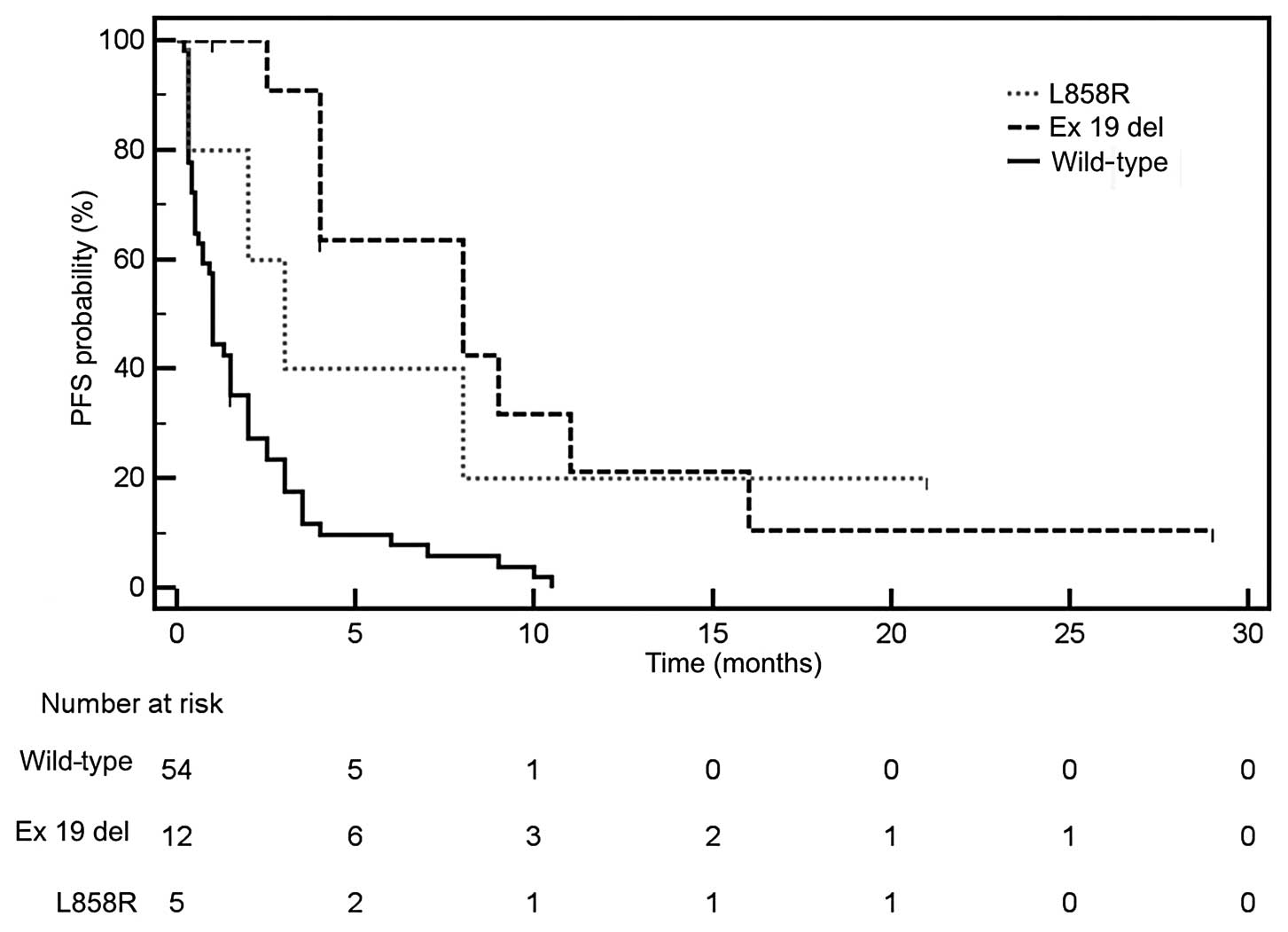
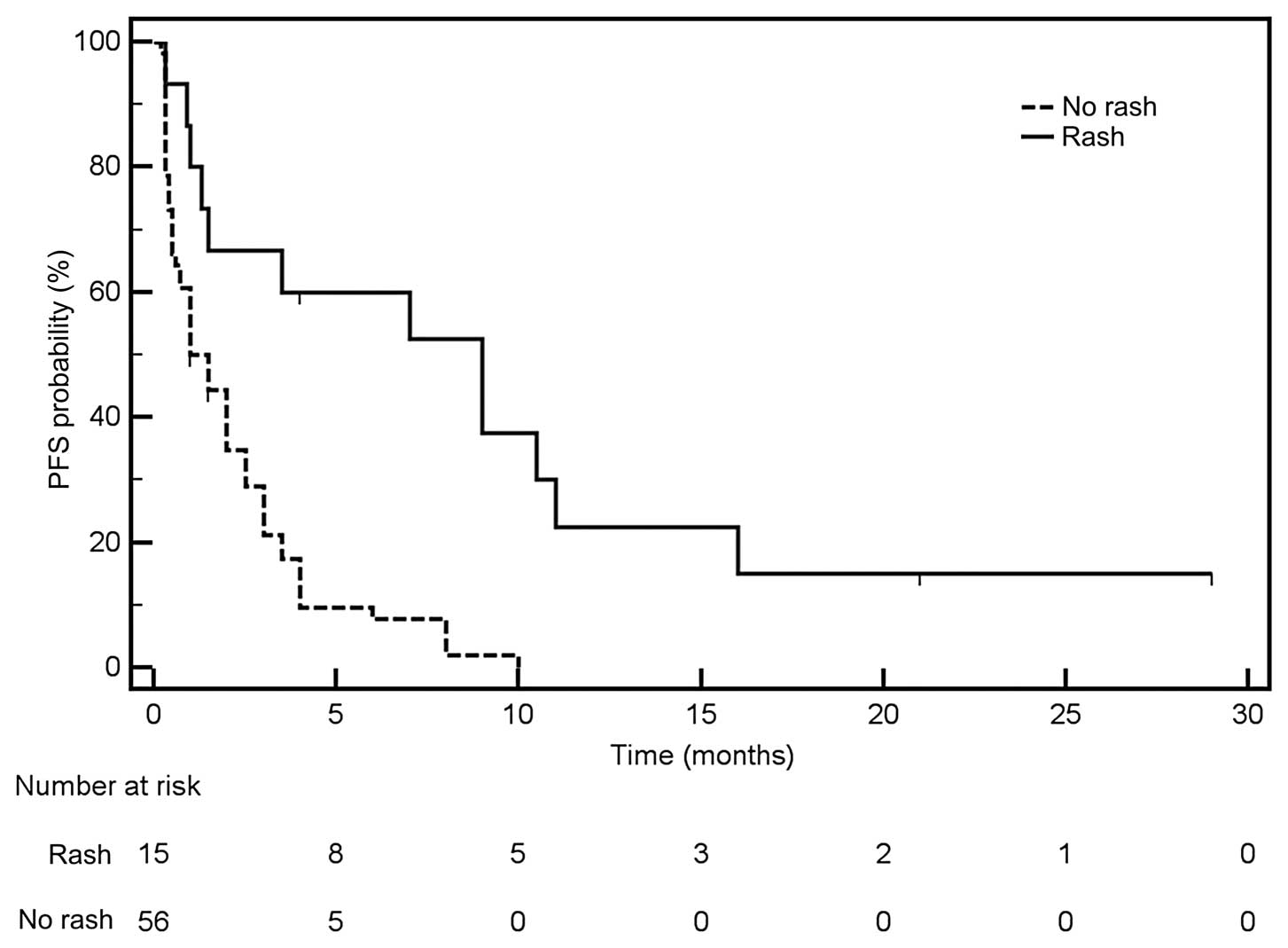
Petrelli F, Borgonovo K, Cabiddu M, Lonati
V and Barni S: Relationship between skin rash and outcome in
non-small-cell lung cancer patients treated with anti-EGFR tyrosine
kinase inhibitors: a literature-based meta-analysis of 24 trials.
Lung Cancer. 78:8–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsao MS, Sakurada A, Cutz JC, et al:
Erlotinib in lung cancer - molecular and clinical predictors of
outcome. N Engl J Med. 353:133–144. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takano T, Ohe Y, Sakamoto H, et al:
Epidermal growth factor receptor gene mutations and increased copy
numbers predict gefitinib sensitivity in patients with recurrent
non small- cell lung cancer. J Clin Oncol. 23:6829–6837. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukuoka M, Yano S, Giaccone G, et al:
Multi-institutional randomized phase II trial of gefitinib for
previously treated patients with advanced non-small-cell lung
cancer (the IDEAL 1 Trial). J Clin Oncol. 21:2237–2246. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kris MG, Natale RB, Herbst RS, et al: A
phase II trial of ZD 1839 (‘Iressa’) in advanced non-small-cell
lung cancer (NSCLC) patients who had failed platinum- and
docetaxel-based regimens (IDEAL 2). Proc Am Soc Clin Oncol.
21:292a2002.
|
|
12
|
Kim ES, Hirsh V, Mok T, et al: Gefitinib
versus docetaxel in previously treated non-small-cell lung cancer
(INTEREST): a randomised phase III trial. Lancet. 372:1809–1818.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thatcher N, Chang A, Parikh P, et al:
Gefitinib plus best supportive care in previously treated patients
with refractory advanced non-small-cell lung cancer: results from a
randomized, placebo-controlled, multicentre study (Iressa Survival
Evaluation in Lung Cancer). Lancet. 366:1527–1537. 2005. View Article : Google Scholar
|
|
14
|
Reck M, van Zandwijk N, Gridelli C, et al:
Erlotinib in advanced non-small cell lung cancer: efficacy and
safety findings of the global phase IV Tarceva Lung Cancer Survival
Treatment study. J Thorac Oncol. 5:1616–1622. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ciuleanu T, Stelmakh L, Cicenas S, et al:
Efficacy and safety of erlotinib versus chemotherapy in second-line
treatment of patients with advanced, non-small-cell lung cancer
with poor prognosis (TITAN): a randomised multicentre, open-label,
phase 3 study. Lancet Oncol. 13:300–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
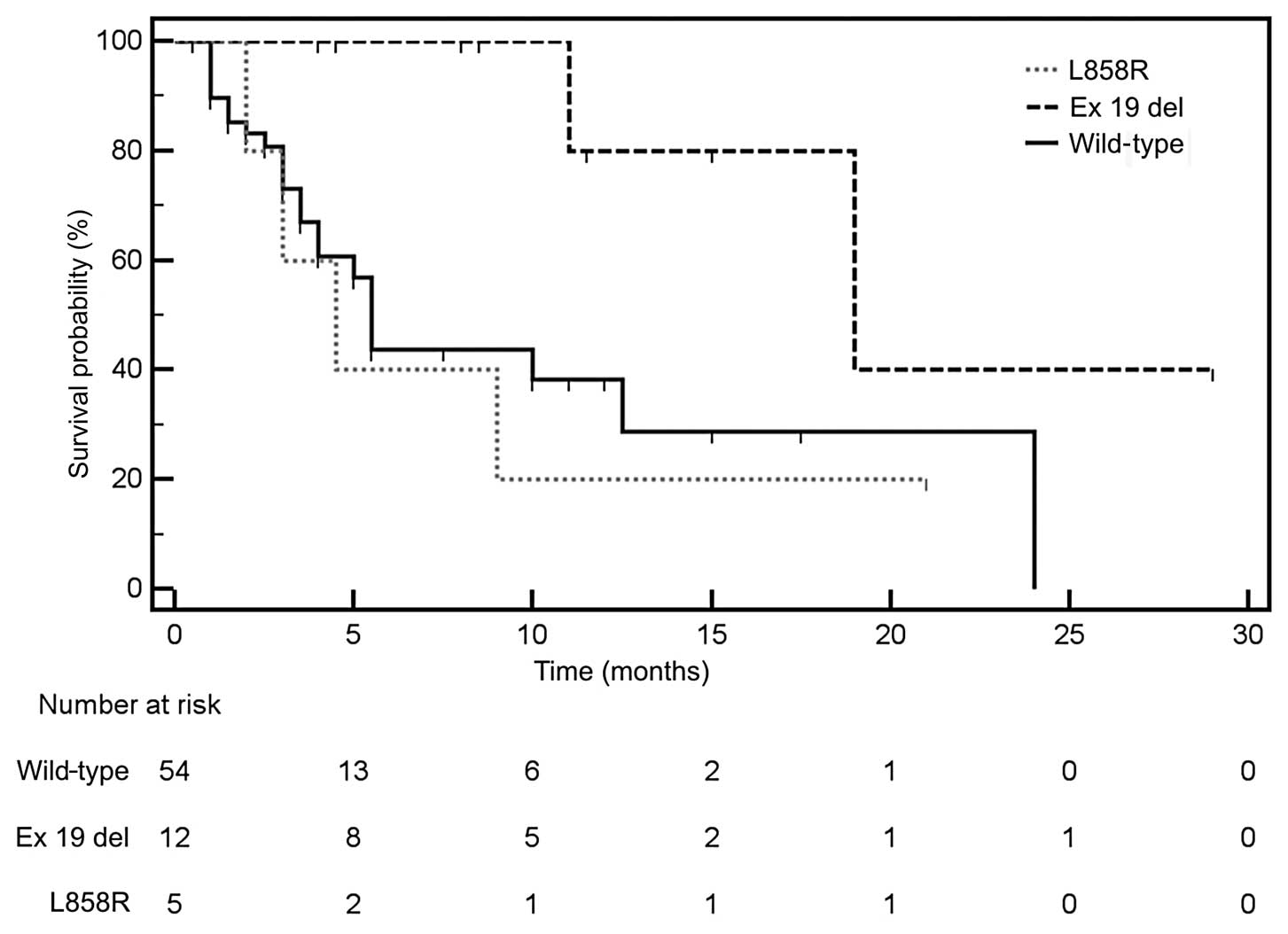
Taron M, Ichinose Y, Rosell R, et al:
Activating mutations in the tyrosine kinase domain of the epidermal
growth factor receptor are associated with improved survival in
gefitinib-treated chemorefractory lung adenocarcinomas. Clin Cancer
Res. 11:5878–5885. 2005. View Article : Google Scholar
|
|
17
|
Endo K, Konishi A, Sasaki H, et al:
Epidermal growth factor receptor gene mutation in non-small cell
lung cancer using highly sensitive and fast TaqMan PCR assay. Lung
Cancer. 50:375–384. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paez JG, Jänne PA, Lee JC, et al:
EGFR mutations in lung cancer: correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar
|
|
19
|
Fukuoka M, Wu YL, Thongprasert S, et al:
Biomarker analyses and final overall survival results from a phase
III, randomized, open-label, first-line study of gefitinib versus
carboplatin/paclitaxel in clinically selected patients with
advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol.
29:2866–2874. 2011. View Article : Google Scholar
|
|
20
|
Maemondo M, Inoue A, Kobayashi K, et al:
Gefitinib or chemotherapy for non-small-cell lung cancer with
mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mitsudomi T, Morita S, Yatabe Y, et al:
Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): an open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar
|
|
22
|
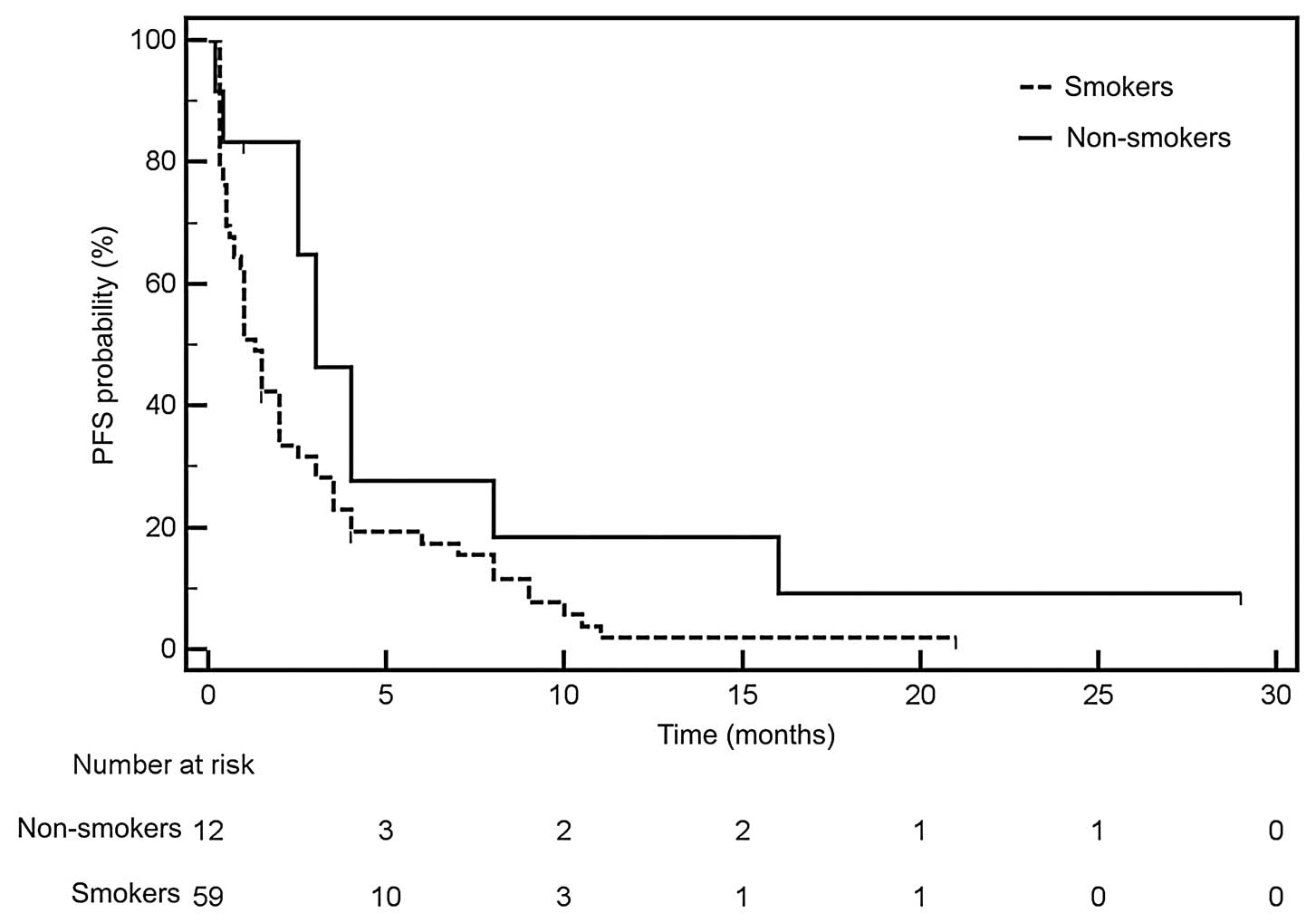
Han JY, Park K, Kim SW, et al:
First-SIGNAL: first-line single-agent iressa versus gemcitabine and
cisplatin trial in never-smokers with adenocarcinoma of the lung. J
Clin Oncol. 30:1122–1128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou C, Wu YL, Chen G, et al: Erlotinib
versus chemotherapy as first-line treatment for patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase
3 study. Lancet Oncol. 12:735–742. 2011.PubMed/NCBI
|
|
24
|
Rosell R, Carcereny E, Gervais R, et al:
Erlotinib versus standard chemotherapy as first-line treatment for
European patients with advanced EGFR mutation-positive
non-small-cell lung cancer (EURTAC): a multicentre, open-label,
randomised phace 3 trial. Lancet Oncol. 13:239–246. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rosell R, Moran T, Quertalt C, et al:
Screening for epidermal growth factor receptor mutations in lung
cancer. N Engl J Med. 361:958–967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao H, Ding X, Wei D, et al: Erlotinib in
patients with advanced non-small-cell lung cancer: a meta-analysis.
Trans Lung Cancer Res. 1:129–144. 2012.PubMed/NCBI
|