Introduction
Metastasis is a characteristic event in cancer
progression and a critical determinant in the prognosis of patients
with malignant disease. The process involved in the initiation of
metastasis from malignant tumors, such as in colorectal carcinoma,
consists of multiple steps. During metastasis, tumor cells first
migrate through the stroma, invade a vessel to enter the
circulation, then adhere to the microvascular endothelium, before
finally extravasating and proliferating in the target organ
(1–4). Each step has been investigated for the
purpose of establishing targeted antimetastatic agents.
Metastasis suppressor genes represent a new gene
family involved in the pathogenesis of malignant progression in
various types of cancer (5). These
genes have been suggested to contribute to key steps during
metastasis.
KiSS1 was first described as a metastasis suppressor
in human melanoma and breast cancer (6–8). KiSS1
encodes a 145 amino-acid protein, which is processed into
kisspeptin (also known as a metastin) of several sizes (9–11). The
KiSS1 gene product was isolated from human placenta and an
endogenous ligand was used to bind a G-protein-coupled receptor
known as GPR54 (10). A correlation
has been shown between the loss of KiSS1 expression and cancer
progression as well as poor prognosis in esophageal squamous cell
carcinoma (12), gastric (13), pancreatic (14), bladder (15), ovarian (15) and breast cancer (16).
Although a growing number of studies have
demonstrated the function of KiSS1 in experimental systems, no
reports have shown any clinical relationship between KiSS1
expression and cancer progression in colorectal cancer. The aim of
this study was to clarify the clinical significance of KiSS1
expression in colorectal cancer.
Materials and methods
Patients and sample collection
A total of 175 patients (99 men, 76 women) with a
mean age of 67 years (range 12–91 years) who underwent surgery for
colorectal cancer from November 2000 to October 2006 at Mie
University Hospital, Japan, were enrolled in the study. Fresh
frozen surgical cancer samples, for which complete clinical data
and isolated RNA of sufficient quality for real-time PCR were
available, were obtained from the patients. No patients had
received anticancer therapy prior to surgery and there were no
perioperative mortalities among these patients.
The locations of tumors and distant metastases were
determined by barium enema, colonoscopy, computerized tomography
(CT) and magnetic resonance imaging (MRI). The location of the
primary lesion was in the rectum in 57 patients, the sigmoid colon
in 63 patients, the ascending colon in 38 patients, the transverse
colon in 13 patients and the descending colon in 4 patients.
Forty-one of the 175 patients had synchronous distant metastasis
(liver, lung and peritoneum) at the time of surgery. Resection of
the primary tumor was performed in all patients and simultaneous
partial hepatectomy for liver metastasis was performed in 9 of
these 27 patients. Only 16 patients had poorly differentiated
adenocarcinoma, whereas 159 patients had either well or moderately
differentiated adenocarcinoma. All patients were classified
according to UICC staging of resected specimens. Overall, 39
patients had UICC stage I (T1–2N0M0) disease, 51 patients had UICC
stage II (T3–4N0M0) disease, 44 patients had UICC stage III
(TXN1–2M0) disease and 41 patients had UICC stage IV (TXNXM1)
disease. Stage III and IV patients received fluorouracil-based
chemotherapy, whereas no adjuvant therapy was given to stage I or
II patients. Patients were observed at 3-month intervals for 24
months after surgery, then every 6 months for 3 years and then on
an annual basis. A history was obtained and a physical examination
was performed at each visit. Chest X-rays, colonoscopies and CTs
were performed annually. The median follow-up time was 43.2 months
(mean, 43.2±29.8 months).
Among the 175 patients evaluated, 40 patients died
due to primary or recurrent disease. Matched control samples were
acquired from adjacent normal mucosa located far from the tumor
site. These samples were frozen in liquid nitrogen immediately
after surgical resection and were stored at −80ºC until RNA
extraction. The purpose of this study was to analyze KiSS1
expression made post hoc using previously and prospectively
collected tissue samples. Written informed consent was obtained
from all patients. The diagnosis of colorectal cancer was confirmed
in all 175 patients on the basis of clinicopathological
findings.
Total RNA extraction and cDNA
synthesis
Tumor specimens were homogenized with a Mixer Mill
MM 300 homogenizer (Qiagen, Chatsworth, CA, USA). Total RNA was
isolated using an RNeasy Mini kit (Qiagen), used according to the
manufacturer’s instructions. cDNA was synthesized from 5.0 mg of
RNA with random hexamer primers and Superscript™ III reverse
transcriptase (Invitrogen, Carlsbad, CA, USA) used according to the
manufacturer’s instructions.
Real-time quantitative RT-PCR
Quantitative PCR (qPCR) analysis was performed using
the TaqMan® Universal PCR Master Mix (Applied
Biosystems, Foster City, CA, USA). The relative abundance of target
transcripts, measured using TaqMan® probes for
KiSS1 (Hs00158486_m1, TaqMan® Gene Expression
Assays; Applied Biosystems) was normalized to the expression level
of β-actin (Hs99999903_m1, TaqMan® Gene
Expression Assays; Applied Biosystems) and measured using Applied
Biosystems StepOne™ Software v2.1. All reactions for standard
samples and for patient samples were performed in triplicate. The
data were averaged from the values obtained in each reaction.
Immunohistochemical analysis
Immunohistochemical analysis of KiSS1 protein
expression was performed on colorectal cancer surgical specimens
using avidin-biotin peroxidase methods (DakoCytomation,
Carpinteria, CA, USA) on formalin-fixed, paraffin-embedded tissues.
All sections were counterstained with hematoxylin. A primary rabbit
polyclonal antibody against KiSS1 (sc101246; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) was used at a dilution of
1:50.
Statistical analysis
Statistical analysis was performed using StatView
software (version 5; Abacus Concepts, Inc., Berkeley, CA, USA).
Results are expressed as the means ± standard deviation (SD) and
differences were evaluated by the Wilcoxon rank correlations test.
Mann-Whitney U tests were used to evaluate differences between
unpaired observations. Analyses of nonparametric receiver operating
characteristics (ROCs) were performed to calculate the cut-off
values according to the most accurate value obtained using MedCalc
7.2 for Windows (MedCalc, Mariakerke, Belgium). Actuarial survival
curves were obtained using the Kaplan-Meier method and comparisons
were made using log-rank tests. The Cox proportional hazards
regression model was used for multivariate analysis after the
relevant prognostic variables had been defined by univariate
analysis. Logistic regression analysis was used to evaluate the
independent influence of factors on peritoneal dissemination as the
final outcome. Two-sided P-values <0.05 were considered to
indicate statistically significant differences.
Results
Overexpression of the KiSS1 gene in
colorectal cancer
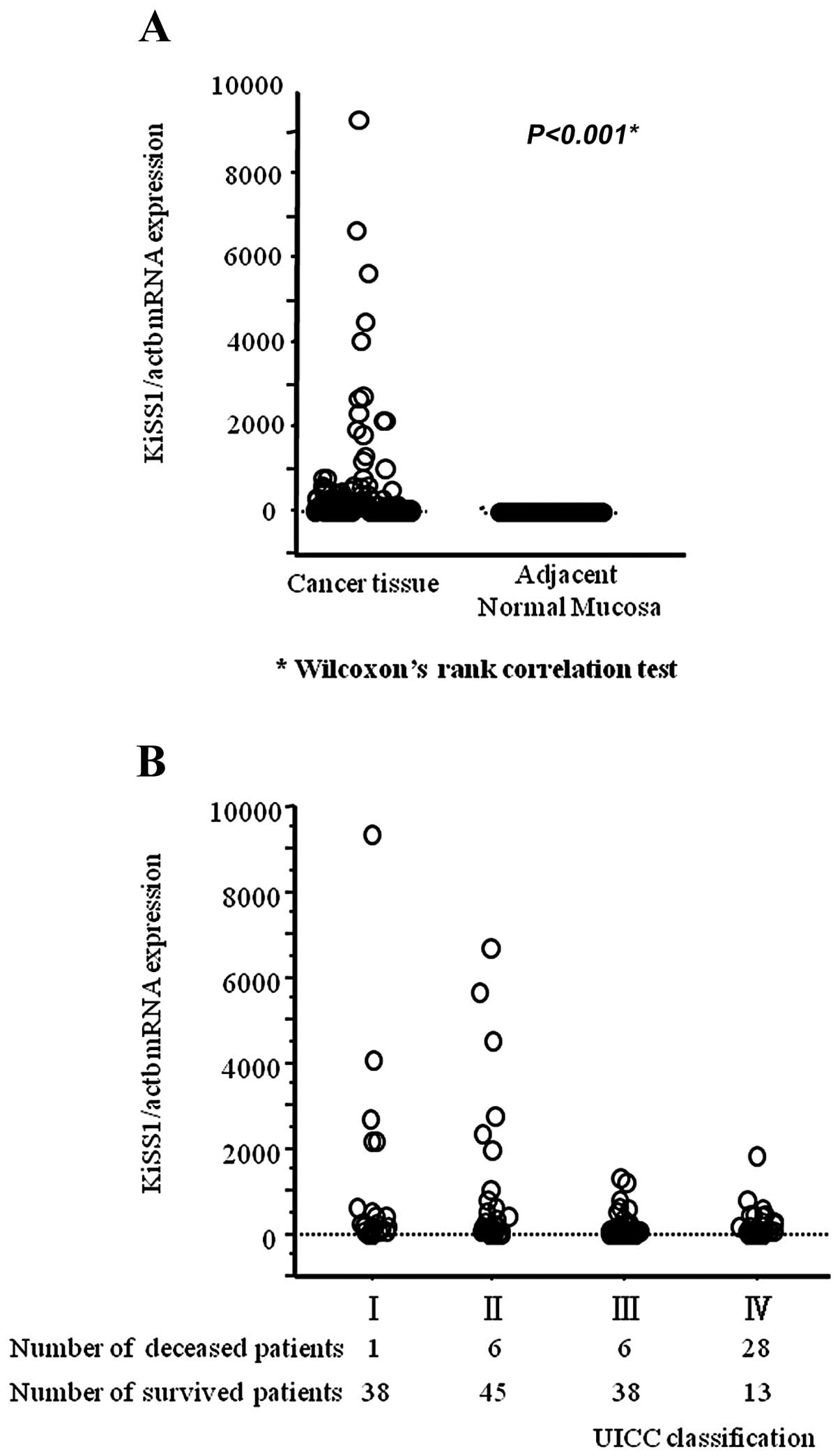
Expression values (relative mRNA levels) of KiSS1
were expressed as ratios between the gene of interest (KiSS1) and
an internal reference gene (β-actin), which provided a
normalization factor for the amount of RNA. In clinical samples
obtained from patients with colorectal cancer, quantitative
real-time RT-PCR revealed that the mean KiSS1 mRNA expression level
in cancerous tissues was significantly higher than that in
corresponding adjacent normal mucosa (cancerous tissue,
373.2±1129.6; adjacent normal mucosa, 0.06±0.12; P<0.001)
(Fig. 1A). In addition, the mean
level of KiSS1 expression was likely to decrease in a
stage-dependent manner (Fig.
1B).
Clinical significance of KiSS1 expression
in colorectal cancer
Table I shows
clinicopathological variables and KiSS1 mRNA expression levels in
tumor specimens from colorectal cancer patients. The KiSS1
expression level was significantly associated with lymph node
metastasis. In the entire colorectal cancer population, the best
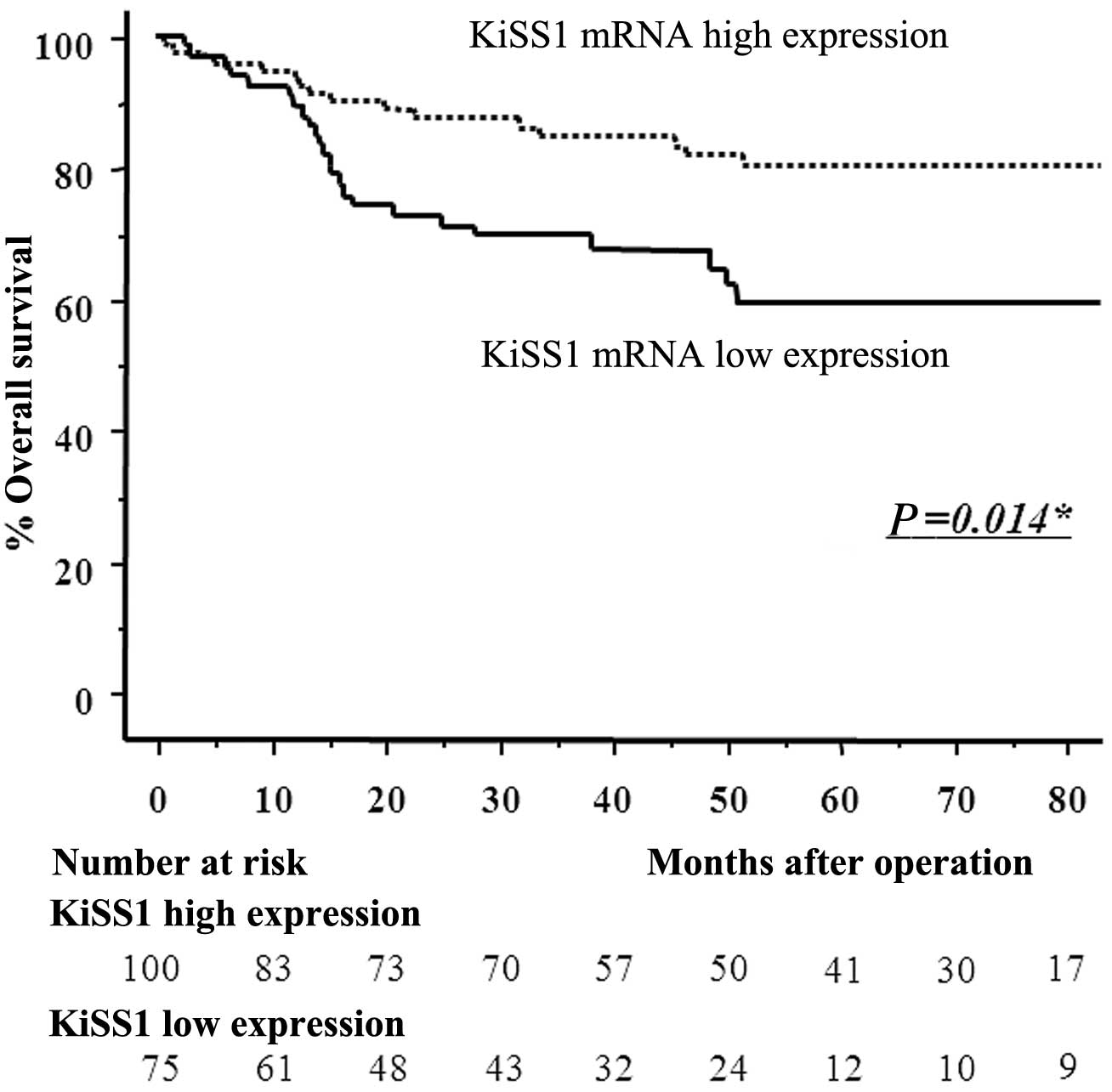
pair of values for highest sensitivity (58.5%) and highest
specificity (61.9%) was found using a peak KiSS1 mRNA expression
cut-off point of 9.276. Patients with KiSS1 expression levels
<9.276 in cancerous tissues were assigned to the low-expression
group (n=75), whereas those with values ≥9.276 were assigned to the
high-expression group (n=100). Patients in the low-expression group
had significantly poorer prognoses than those in the
high-expression group (log-rank test, P=0.014) (Fig. 2).
 | Table IClinicopathological variables and
KiSS1 mRNA expression in 175 colorectal cancer patients. |
Table I
Clinicopathological variables and
KiSS1 mRNA expression in 175 colorectal cancer patients.
| | KiSS1 mRNA
expression | |
|---|
| |
| |
|---|
| Variable | n | High (n=100) | Low (n=75) | P-value |
|---|
| Gender |
| Male | 99 | 60 | 39 | 0.291 |
| Female | 76 | 40 | 36 | |
| Age (years) |
| <67 (median) | 76 | 40 | 36 | 0.291 |
| ≥67 | 99 | 60 | 39 | |
| Tumor location |
| Colon | 118 | 64 | 54 | 0.264 |
| Rectum | 57 | 36 | 21 | |
| Tumor size (cm) |
| ≥4.5 (median) | 86 | 50 | 36 | 0.793 |
| <4.5 | 89 | 50 | 39 | |
| Histological
type |
| Differentiated | 159 | 93 | 66 | 0.256 |
|
Undifferentiated | 16 | 7 | 9 | |
| Pathological T
category |
| T1 | 21 | 15 | 6 | 0.508 |
| T2 | 25 | 14 | 11 | |
| T3 | 97 | 52 | 45 | |
| T4 | 32 | 19 | 13 | |
| Venous
invasion |
| + | 138 | 78 | 60 | 0.749 |
| − | 37 | 22 | 15 | |
| Lymphatic
invasion |
| + | 156 | 88 | 68 | 0.575 |
| − | 19 | 12 | 7 | |
| Lymph node
metastasis |
| − | 106 | 68 | 38 | 0.020a |
| + | 69 | 32 | 37 | |
| Hepatic
metastasis |
| − | 148 | 87 | 61 | 0.304 |
| + | 27 | 13 | 14 | |
| Pulmonary
metastasis |
| − | 156 | 91 | 65 | 0.362 |
| + | 19 | 9 | 10 | |
| Peritoneal
dissemination |
| − | 171 | 98 | 73 | 0.771 |
| + | 4 | 2 | 2 | |
Loss of KiSS1 expression is correlated
with lymph node metastasis and a poorer prognosis in colorectal
cancer
The association between KiSS1 expression and
prognosis in colorectal cancer patients was evaluated by
multivariate analysis. Table II
shows the resulting risk ratios and 95% confidence intervals (CIs)
calculated using the Cox proportional hazards model. Based on a Cox
univariate proportional hazards analysis, advanced T classification
(T3, T4), venous invasion, lymph node metastasis and low KiSS1
expression were associated with poor prognosis. Our multivariate
analysis revealed that advanced T classification, lymph node
metastasis and low KiSS1 expression were independent prognostic
factors for colorectal cancer patients. Factors that correlated
with lymph node metastasis were analyzed by logistic regression
analysis (Table III). Notably, T
classification and low KiSS1 expression were independent factors
for lymph node metastasis in colorectal cancer patients.
 | Table IIMultivariate analysis for predictors
of survival. |
Table II
Multivariate analysis for predictors
of survival.
| Univariate | Multivariate |
|---|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Tumor size [≥4.4 cm
(median)] | 1.79 | 0.95–3.35 | 0.07 | | | |
| T classification
(pT3/4) | 18.5 | 2.53–142.8 | 0.004 a | 14.93 | 1.89–125 | 0.011 a |
| Histological type
(differentiated type) | 0.77 | 0.30–1.95 | 0.576 | | | |
| Lymphatic invasion
(present) | 5.75 | 0.79–41.7 | 0.084 | | | |
| Venous invasion
(present) | 3.58 | 1.11–116.3 | 0.033 a | 0.96 | 0.28–3.29 | 0.951 |
| Node involvement
(present) | 3.10 | 1.65–5.81 | <0.001 a | 1.94 | 1.02–3.69 | 0.044 a |
| Low KiSS1
expression | 2.15 | 1.15–4.02 | 0.016 a | 1.91 | 1.01–3.58 | 0.045 a |
 | Table IIIMultivariate analysis for lymph node
metastasis. |
Table III
Multivariate analysis for lymph node
metastasis.
| Univariate | Mutivariate |
|---|
|
|
|
|---|
| Variable | Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value |
|---|
| Tumor Size [≥4.4 cm
(median)] | 1.80 | 0.97–3.32 | 0.061 | | | |
| T classification
(pT3/4) | 6.36 | 2.52–16.1 | <0.001 a | 4.55 | 1.57–13.2 | 0.005 a |
| Histological type
(differentiated type) | 0.47 | 0.17–1.33 | 0.154 | | | |
| Lymphatic invasion
(present) | 6.40 | 1.43–28.7 | 0.015 a | 1.02 | 0.15–6.88 | 0.983 |
| Venous invasion
(present) | 4.34 | 1.70–11.1 | <0.001 a | 2.32 | 0.74–7.29 | 0.149 |
| Low KiSS1
expression | 2.07 | 1.12–3.84 | 0.021 a | 2.07 | 1.07–3.99 | 0.031 a |
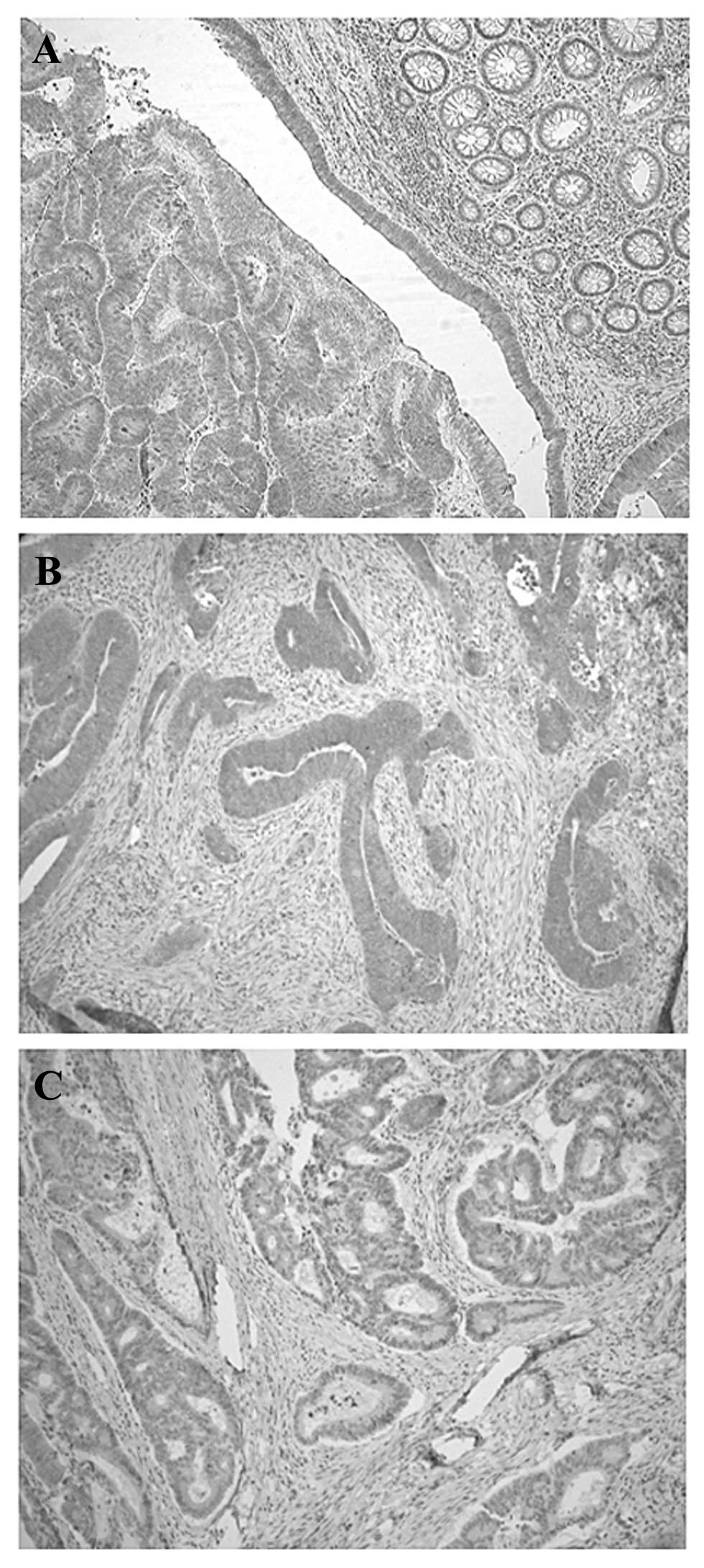
Immunohistochemical staining of KiSS1 in
the early and advanced stages of colorectal cancer
Immunohistochemical analysis revealed that the KiSS1
protein was predominantly expressed in the cytoplasm of primary
colorectal cancer cells without expression in normal mucosa
(Fig. 3A). Although KiSS1 protein
was highly expressed in the early stage of colorectal cancer cells
(Fig. 3B), KiSS1 expression was
decreased in advanced-stage colorectal cancer tissues (Fig. 3C).
Discussion
To the best of our knowledge, this is the first
study to show the relationship between KiSS1 expression and cancer
progression, particularly lymph node metastasis, in colorectal
cancer patients.
Metastasis suppressors regulate one or more
processes in the metastatic cascade without blocking orthotopic
tumor growth (17–20). Since Nm23-H1 was identified as a
metastasis suppressor gene in 1988, the number of metastasis
suppressor families identified has increased beyond 25 (21,22).
Studying the dysregulation of metastasis suppressor genes may help
discern important cellular pathways in the metastatic behavior of
various tumors and potentially suggest a new strategy for the
treatment of locally advanced and metastatic cancer.
KiSS1 is one of the inhibitors identified as a human
melanoma metastasis suppressor gene using subtractive hybridization
between highly metastatic and nonmetastatic cell lines and
respective cell line variants (6,8).
Transfection of full-length KiSS1 cDNA into metastatic cancer cell
lines suppressed metastasis in athymic mice and supported a defined
role of KiSS1 in the metastasis process (6–9).
Kisspeptin is considered to possess potent antimetastatic
properties and that GPR54 (KiSS-1R, hOT7T175 and AXOR12) has been
detected as an orphan receptor for this protein. KiSS1/GPR54
signaling has been shown to inhibit cell motility, proliferation,
invasion, chemotaxis and metastasis in various types of cancer
(7,9,10,23–26).
Although a growing number of studies have demonstrated the function
of KiSS1 in various epithelial tumors, no reports have shown any
clinical significance associated with KiSS1 expression in
colorectal cancer.
The major finding of this study is that KiSS1 is
expressed at a statistically significantly higher level in
colorectal cancer tissue than in corresponding adjacent normal
mucosa. Although KiSS1 expression was not clearly associated with
any clinicopathological factors (tumor size, histological type,
pathological T category, or hepatic metastasis) decreased KiSS1
expression was shown to correlate significantly with lymph node
metastasis and poor prognosis. Multivariate analysis revealed that
decreased KiSS1 expression is a significant independent indicator
of survival. Reduced KiSS1 expression was shown to be a strong
prognostic marker in patients with urinary bladder cancer and
gastric cancer (13,15). Our results also suggest that KiSS1
expression may be a useful tool for classification of colorectal
cancer at its early stage and that loss of KiSS1 expression could
be a powerful prognostic indicator in colorectal cancer
patients.
This study also revealed a significant relationship
between KiSS1 expression and lymph node metastasis. Metastasis to
regional lymph nodes mainly affects the prognosis of non stage IV
colon cancer patients (27,28). The presence of lymph node metastasis
in colorectal cancer is an indicator for which adjuvant
chemotherapy confers significant survival benefit (29). Of note, multivariate analysis for
lymph node metastasis in our study revealed that the loss of KiSS1
expression is a suitable predictor of lymph node metastasis in
colorectal cancer patients. Recent reports suggest that KiSS1 may
cause cancer dormancy in disseminated tumor cells at secondary
sites without the colonization of ectopic tissues (30,31).
KiSS1 has been shown to suppress matrix
metalloproteinase-9 (MMP-9) activity (24,32).
The extracellular matrix (ECM) structure is dynamic and can be
degraded by the family of enzymes known as MMP. The crosstalk
between dormant tumor cells and the ECM, which is regulated by
stromal and tumor cells, may control the entry and exit of the cell
to the dormant state. Nash and Welch (33) reported that kisspeptins bind to
GPR54 and regulate events downstream of cell-matrix adhesion,
perhaps involving cytoskeletal reorganization in order to block
metastases through the induction of dormancy in solitary cells. In
addition, Ikeguchi et al(12) also demonstrated that the loss of
KiSS1 or GRP54 gene expression was a significant predictor of lymph
node metastasis in esophageal squamous cell carcinoma. These
reports and our results suggest that the loss of KiSS1 may be
significantly involved in the pathogenesis, including the
colonization steps of lymph node metastasis, in colorectal cancer.
Furthermore, the analysis of KiSS1 expression using biopsy
specimens may provide a more accurate evaluation to detect the
absence of lymph node metastasis, which may then translate to
minimally invasive treatments, such as endoscopic resection or
laparoscopic assisted colectomy for early colorectal cancer.
In conclusion, we have demonstrated the clinical
significance of KiSS1 expression in colorectal cancer. The loss of
KiSS1 appears to play an important role in the progression of lymph
node metastasis. The assessment of KiSS1 expression may assist in
the accurate colorectal cancer diagnosis and may contribute to the
prediction of clinical outcomes.
Acknowledgements
The authors thank Motoko Ueeda and Chihiro Hibi for
their technical assistance. This study was supported in part by a
Grant in Aid for Scientific Research (B: 23890083) from the
Ministry of Education, Culture, Sports, Science and Technology,
Japan.
References
|
1
|
Fidler IJ: Critical determinants of
metastasis. Semin Cancer Biol. 12:89–96. 2002. View Article : Google Scholar
|
|
2
|
Chan DA and Giaccia AJ: Hypoxia, gene
expression, and metastasis. Cancer Metastasis Rev. 26:333–339.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
ten Kate M, Hofland LJ, van Grevenstein
WM, van Koetsveld PV, Jeekel J and van Eijck CH: Influence of
proinflammatory cytokines on the adhesion of human colon carcinoma
cells to lung microvascular endothelium. Int J Cancer. 112:943–950.
2004.PubMed/NCBI
|
|
4
|
Basoglu M, Yildirgan MI, Taysi S, et al:
Levels of soluble intercellular adhesion molecule-1 and total
sialic acid in serum of patients with colorectal cancer. J Surg
Oncol. 83:180–184. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Steeg PS, Ouatas T, Halverson D, Palmieri
D and Salerno M: Metastasis suppressor genes: basic biology and
potential clinical use. Clin Breast Cancer. 4:51–62. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JH, Miele ME, Hicks DJ, et al: KiSS-1,
a novel human malignant melanoma metastasis-suppressor gene. J Natl
Cancer Inst. 88:1731–1737. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee JH and Welch DR: Suppression of
metastasis in human breast carcinoma MDA-MB-435 cells after
transfection with the metastasis suppressor gene, KiSS-1. Cancer
Res. 57:2384–2387. 1997.PubMed/NCBI
|
|
8
|
Lee JH and Welch DR: Identification of
highly expressed genes in metastasis-suppressed chromosome 6/human
malignant melanoma hybrid cells using subtractive hybridization and
differential display. Int J Cancer. 71:1035–1044. 1997. View Article : Google Scholar
|
|
9
|
Ohtaki T, Shintani Y, Honda S, et al:
Metastasis suppressor gene KiSS-1 encodes peptide ligand of a
G-protein-coupled receptor. Nature. 411:613–617. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kotani M, Detheux M, Vandenbogaerde A, et
al: The metastasis suppressor gene KiSS-1 encodes kisspeptins, the
natural ligands of the orphan G protein-coupled receptor GPR54. J
Biol Chem. 276:34631–34636. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muir AI, Chamberlain L, Elshourbagy NA, et
al: AXOR12, a novel human G protein-coupled receptor, activated by
the peptide KiSS-1. J Biol Chem. 276:28969–28975. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ikeguchi M, Yamaguchi K and Kaibara N:
Clinical significance of the loss of KiSS-1 and orphan
G-protein-coupled receptor (hOT7T175) gene expression in esophageal
squamous cell carcinoma. Clin Cancer Res. 10:1379–1383. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dhar DK, Naora H, Kubota H, et al:
Downregulation of KiSS-1 expression is responsible for tumor
invasion and worse prognosis in gastric carcinoma. Int J Cancer.
111:868–872. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Masui T, Doi R, Mori T, et al: Metastin
and its variant forms suppress migration of pancreatic cancer
cells. Biochem Biophys Res Commun. 315:85–92. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sanchez-Carbayo M, Capodieci P and
Cordon-Cardo C: Tumor suppressor role of KiSS-1 in bladder cancer:
loss of KiSS-1 expression is associated with bladder cancer
progression and clinical outcome. Am J Pathol. 162:609–617. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stark AM, Tongers K, Maass N, Mehdorn HM
and Held-Feindt J: Reduced metastasis-suppressor gene
mRNA-expression in breast cancer brain metastases. J Cancer Res
Clin Oncol. 131:191–198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chirco R, Liu XW, Jung KK and Kim HR:
Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev.
25:99–113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Freije JM, MacDonald NJ and Steeg PS: Nm23
and tumour metastasis: basic and translational advances. Biochem
Soc Symp. 63:261–271. 1998.PubMed/NCBI
|
|
19
|
Stafford LJ, Vaidya KS and Welch DR:
Metastasis suppressors genes in cancer. Int J Biochem Cell Biol.
40:874–891. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rinker-Schaeffer CW, O’Keefe JP, Welch DR
and Theodorescu D: Metastasis suppressor proteins: discovery,
molecular mechanisms, and clinical application. Clin Cancer Res.
12:3882–3889. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vaidya KS and Welch DR: Metastasis
suppressors and their roles in breast carcinoma. J Mammary Gland
Biol Neoplasia. 12:175–190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bodenstine TM and Welch DR: Metastasis
suppressors and the tumor microenvironment. Cancer Microenviron.
1:1–11. 2008. View Article : Google Scholar
|
|
23
|
Hori A, Honda S, Asada M, et al: Metastin
suppresses the motility and growth of CHO cells transfected with
its receptor. Biochem Biophys Res Commun. 286:958–963. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan C, Wang H and Boyd DD: KiSS-1
represses 92-kDa type IV collagenase expression by down-regulating
NF-κB binding to the promoter as a consequence of IκBα-induced
block of p65/p50 nuclear translocation. J Biol Chem. 276:1164–1172.
2001.PubMed/NCBI
|
|
25
|
Kang HS, Baba T, Mandai M, et al: GPR54 is
a target for suppression of metastasis in endometrial cancer. Mol
Cancer Ther. 10:580–590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Olbrich T, Ziegler E, Türk G, Schubert A,
Emons G and Grundker C: Kisspeptin-10 inhibits bone-directed
migration of GPR54-positive breast cancer cells: Evidence for a
dose-window effect. Gynecol Oncol. 119:571–578. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sasaki H, Miura K, Horii A, et al:
Orthotopic implantation mouse model and cDNA microarray analysis
indicates several genes potentially involved in lymph node
metastasis of colorectal cancer. Cancer Sci. 99:711–719. 2008.
View Article : Google Scholar
|
|
28
|
Schumacher P, Dineen S, Barnett C Jr,
Fleming J and Anthony T: The metastatic lymph node ratio predicts
survival in colon cancer. Am J Surg. 194:827–831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moertel CG: Chemotherapy for colorectal
cancer. N Engl J Med. 330:1136–1142. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nash KT, Phadke PA, Navenot JM, et al:
Requirement of KISS1 secretion for multiple organ metastasis
suppression and maintenance of tumor dormancy. J Natl Cancer Inst.
99:309–321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Beck BH and Welch DR: The KISS1 metastasis
suppressor: a good night kiss for disseminated cancer cells. Eur J
Cancer. 46:1283–1289. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hou YK, Wang Y, Cong WM and Wu MC:
Expression of tumor metastasis-suppressor gene KiSS-1 and matrix
metalloproteinase-9 in portal vein tumor thrombus of hepatocellular
carcinoma. Ai Zheng. 26:591–595. 2007.(In Chinese).
|
|
33
|
Nash KT and Welch DR: The KISS1 metastasis
suppressor: mechanistic insights and clinical utility. Front
Biosci. 11:647–659. 2006.PubMed/NCBI
|