Introduction
Human telomerase, a ribonucleoprotein enzyme
complex, is composed of catalytic component human telomerase
reverse transcriptase (hTERT), human telomerase RNA component (RNA
template hTERC) and human telomerase associated protein 1 (hTEP1)
(1–3). hTERT can catalyze the synthesis of the
repeating sequence (TTAGGG)n and maintain telomere length at
chromosomal ends using hTERC as a template (4). It counteracts telomere shortening due
to each round of cell division and therefore prevents senescence
and cellular aging. In line with these conclusions, telomerase
activity has been detected in 80–90% of human cancer cases, but it
is rare in normal somatic cells. Exceptions include stem cells,
reproductive cells and activated lymphocytes. (5–11). It
has been shown in numerous studies that activation of telomerase is
strongly correlated with tumorigenesis and metastasis; therefore,
it may serve as an indicator of prognosis (12–21).
Previous studies have shown that the majority of
hepatocellular carcinomas (HCCs) exhibit telomerase activity and
that the level of telomerase activity is related to upregulated
hTERT expression (22). Early
recurrence after hepatectomy is one of the most important factors
affecting the prognosis of patients with HCC. Quantitative analyses
of telomerase activity suggested that HCC resection patients
positive for telomerase activity in non-cancerous liver tissue have
a higher rate of recurrence. The relative telomerase activity (RTA)
of early recurrent patients is significantly higher than in
patients who do not experience recurrence (23). Peripheral blood telomerase activity
can be used as a molecular marker for the detection of circulating
hepatoma cells in the blood of HCC patients; it also indicates
hematogenous micrometastasis (24).
Previous studies have consistently shown that telomerase activity
may serve as an independent predictor of recurrence after HCC
resection (25). These observations
led to the hypothesis that telomerase activity may be correlated to
invasiveness and metastasis of cancer. To support this hypothesis,
recent research data was collected and showed that telomerase can
promote the invasion and metastasis of telomerase-negative tumor
cells (26–28). By contrast, tumor cell growth,
proliferation, invasion and metastasis can be reduced through
inhibition of telomerase activity of tumor cells by various methods
(29,30). However, to date, few research
studies have focused on the mechanisms underlying this process. The
issue of whether the motility and invasiveness of
telomerase-positive tumor cells can be further promoted by
overexpression of hTERT has yet to be addressed.
In the present study, we observed enhanced motility
and invasive capacity in telomerase-positive human hepatoma cell
line (HepG2) cells through exogenous expression of hTERT gene using
a retroviral vector. Our data suggested that changes in motility
and invasive capacity may be caused by upregulation of the
metastasis-related genes, matrix metalloproteinase 9 (MMP9) and Ras
homolog gene family member C (RhoC).
Materials and methods
Cell lines and retroviral vectors
Human hepatoma cell line (HepG2) (ATCC, Manassas,
VA, USA) and GP2-293 packaging cell line (Clontech Laboratories,
Mountain View, CA, USA) were cultured in Dulbecco’s modified
Eagle’s medium (DMEM; Invitrogen Life Technologies, Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum (FBS; Gibco-BRL,
Carlsbad, CA, USA) at 37°C in a humidified incubator containing 5%
CO2. The retroviral expression vector xlox(gfp)TERT
containing coding sequences for hTERT and green fluorescent protein
(GFP) was provided by Dr David Ott (Frederick National Laboratory
for Cancer Research, Frederick, MD, USA). The control vector
xlox-GFP was constructed on the basis of xlox(gfp)TERT in our
laboratory. Briefly, the portion of hTERT in xlox(gfp)TERT was
removed using EcoRI followed by religation using T4 DNA
ligase.
Cell transduction
Retroviral vector stocks were produced by
cotransfecting the plasmid encoding VSV-G protein and
xlox(gfp)TERT/xlox-GFP vector into the packaging cell line GP2-293
and harvesting virus-containing culture medium 48 h after
transfection. These actions were performed as described in the
Retroviral Gene Transfer and Expression user manual and
instructions from VigoFect (transfection reagent; Vigorous
Biotechnology, Beijing, China). A 2:1 ratio of retroviral
vector/VSV-G DNA mixture was used in these experiments. The
resulting vector stocks (Virus-hTERT and Virus-GFP) were then used
to infect HepG2 cells in the presence of polybrene (8 μg/ml). Two
groups of transduced cells were identified and sorted 72 h after
transduction using flow cytometry for GFP expression, termed
hTERT/HepG2 and GFP/HepG2. Both untransduced HepG2 and GFP/HepG2
were used as the controls and cultured at the same time as the
experimental groups.
Real-time PCR for hTERT mRNA
Total RNA was isolated from cells using TRIzol
reagent (Invitrogen Life Technologies) according to the
manufacturer’s instructions. RNA was then converted to cDNA using
oligo(dT) primers and SuperScript III Reverse Transcriptase
(Invitrogen Life Technologies). Real-time PCR analysis was
performed using the SYBR® Green Real-time PCR Master mix
(Toyobo, Japan) and Bio-Rad (Hercules, CA, USA) MiniOpticon
real-time PCR system. Primers for human β-actin and hTERT were
designed with Primer Express Software Version 3.0 and synthesized
by Sangon Biotech Co. (Shanghai, China), and were: β-actin sense,
5′-TGG ACTTCGAGCAAGAGATG-3′ and antisense,
5′-GAAGGAAGGCTGGAAGAGTG-3′ (137 bp); hTERT sense,
5′-TGTCAAGGTGGATGTGACGGGC-3′ and antisense, 5′-GGCATACC
GACGCACGCAGT-3′ (112 bp).
hTERT protein detection
Expression of the hTERT protein was detected using
western blot analysis. Total proteins were isolated from cells of
each group. Forty micrograms of protein were separated using 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and then transferred onto a nitrocellulose membrane
(Bio-Rad). The membranes were first incubated with anti-hTERT mAb
overnight at 4°C and then with HRP-labeled secondary antibody
(Zhong-Shan Golden Bridge BioTechnology, China) for 1 h at RT. The
membranes were washed again, treated with chemiluminescence reagent
(Amersham Pharmacia Biotech, Amersham, UK), exposed to
autoradiography film (Kodak) and developed.
TRAP assay for telomerase activity
Telomerase activity of cells was detected using the
telomeric repeat amplification protocol (TRAP) assay with a TRAP
kit (Tiandz, Beijing, China). The procedure was performed according
to the manufacturer’s instructions. Briefly, the cell pellet was
resuspended in 200 μl of 1X lysis buffer/106 cells,
incubated on ice for 30 min and then centrifuged at 12,000 rpm for
20 min at 4°C. The supernatant was collected and the protein
concentration was determined using standard procedures (BCA protein
assay). The reaction system containing 25 μl TRAP reagent was
incubated for 30 min at 30°C and then for 3 min at 93°C, and 1.5 μl
TRAP primer mixture and 0.5 μl of Taq polymerase were added to the
PCR tube followed by 30 cycles of 94°C for 30 sec, 59°C for 30 sec
and 72°C for 60 sec. PCR product (25 μl) was loaded onto a 10%
non-denaturing PAGE in 0.5X TBE buffer. Following electrophoresis,
the gel was stained using a Rapid Silver Stain kit for Nucleic Acid
(Tiandz). Lysis buffer (provided in the TRAP kit) was used as a
negative control.
FISH assay for telomere
hTERT/HepG2 and GFP/HepG2 cells ~10 PDs after
transduction and untransduced cells were cultured in a 6-well plate
to the logarithmic phase. Then, colcemid was added and the cells
were incubated for 1 h. Cells were then treated with hypotonic KCl
for 60 min at 37°C and fixed in methanol/acetic-acid. Fluorescence
in situ hybridization (FISH) was performed on metaphase
chromosomes with Cy-3 labeled (CCCTAA)3 PNA probe
(Panagen, South Korea) according to the manufacturer’s
instructions. Digital images were captured for 4,6
diamidino-2-phenylindole (DAPI) and Cy-3. The subsequent
quantitative analysis of telomere fluorescence was detected using
Leica QWin Pro. version 2.6 (Germany).
In vitro motility assay and invasiveness
assay
Cell motility was analyzed using a wound healing
assay. Cells (5×105 cells/well) were seeded into a
6-well plate. A scratch wound in the monolayer was created using a
sterile 200 μl pipette tip following incubation for 24 h. The
detached cells were gently washed with PBS. The cells were
incubated with new medium containing 1% FBS and then the distances
between the wounds were measured by microscope at 0 and 48 h. These
distances were measured using ImageJ. Cell motility was evaluated
using the following formula: Cell motility =(distance48
h - distance0 h)/distance0 h. For
invasiveness assays, cells (5×104 cells/well) were
suspended in DMEM with no FBS and seeded onto the inner compartment
of Matrigel-coated 24-well chambers with a filter membrane
containing 8-μm pores (Corning Incorporated, Corning, NY, USA). The
outer chamber contained the same medium with 10% FBS. Following
incubation for 24 h at 37°C, cells that did not migrate through the
pores were removed by gentle scraping of the membrane with a cotton
swab. Cells that transversed the membrane were fixed in 70% ethanol
and stained with 0.1% crystal violet. The number of cells that
invaded the undersurface of the membrane was determined using 5
randomly selected microscopic fields per sample. Data represent the
average of 3 wells.
Cell growth curves
Cell proliferation was determined using Cell
Counting Kit-8 (CCK-8, Dojindo, Japan) according to the
manufacturer’s instructions. One hundred microliters of cell
suspension (2,000 cells/well) were seeded into the 96-well plate
and incubated for 24 h (37°C, 5% CO2). CCK-8 solution
(10 μl) was added to each well and the cultures were incubated at
37°C for 90 min. Absorbance at 450 nm was measured using an
automatic microplate reader (Tecan Sunrise, Switzerland). The
results were plotted as means ± SD of 3 separate experiments having
6 determinations per experiment for each experimental
condition.
Real-time PCR for MMP9 and RhoC mRNA
The expression levels of mRNA for MMP9 and RhoC were
analyzed using real-time PCR as previously described. Primers for
MMP9 and RhoC were designed with Primer Express Software version
3.0 and synthesized by Sangon Biotech Co., and were: MMP9 sense,
5′-GGCGGTGATTGACGACGCCT-3′ and antisense,
5′-CCGTGCTCCGCGACACCAAA-3′ (126 bp); RhoC sense,
5′-CGGAGCGGAAGCCCCACCAT-3′ and antisense,
5′-AGGGACGTAGACCTCCGGAAACT-3′ (126 bp).
MMP9 and RhoC protein detection
The expression levels of proteins for MMP9 and RhoC
were detected using western blot analysis, as previously
described.
Statistical analysis
All experiments were performed in triplicate and
data are presented as means ± SD of 3 separate determinations.
Statistical analysis was performed using one-way analysis of
variance (ANOVA). P<0.05 was considered to indicate
statistically significant differences. All statistical analyses
were performed using SPSS 16.0 software.
Results
Control vector construction and cell
transduction
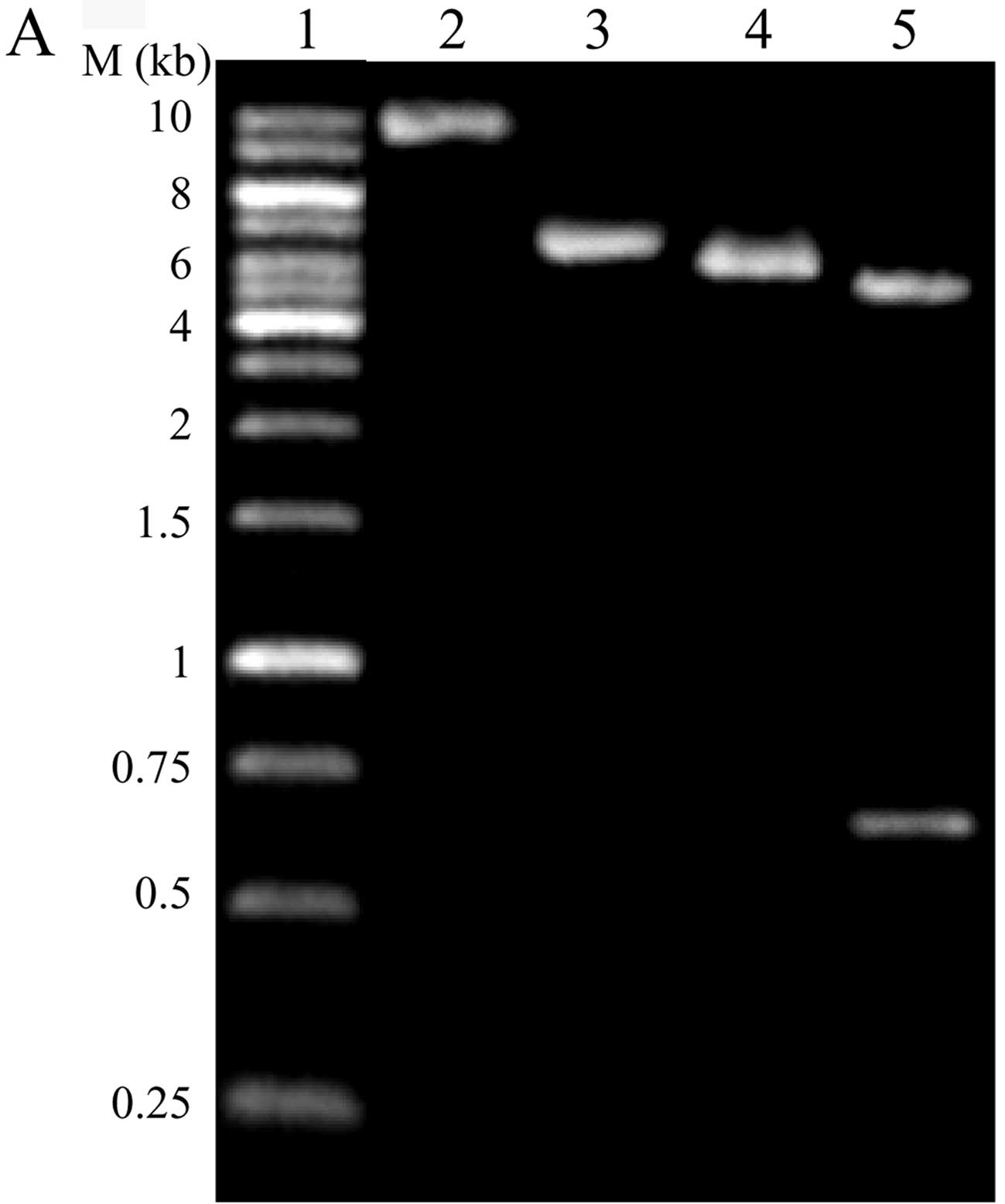
The control vector xlox-GFP was constructed on the
basis of xlox(gfp)TERT by removing part of the hTERT in
xlox(gfp)TERT with EcoRI and linking it using T4 DNA ligase.
The vector was confirmed by digestion with EcoRI and
NotI (Fig. 1A). Virus-hTERT
and Virus-GFP were produced and used to infect HepG2 cells. Two
groups of transduced cells were identified and sorted 72 h after
transduction using flow cytometry for GFP expression, hTERT/HepG2
and GFP/HepG2 (Fig. 1B). The
following experiments were performed using hTERT/HepG2, GFP/HepG2
and HepG2 cells.
Overexpression of hTERT mRNA and protein
in hTERT/HepG2
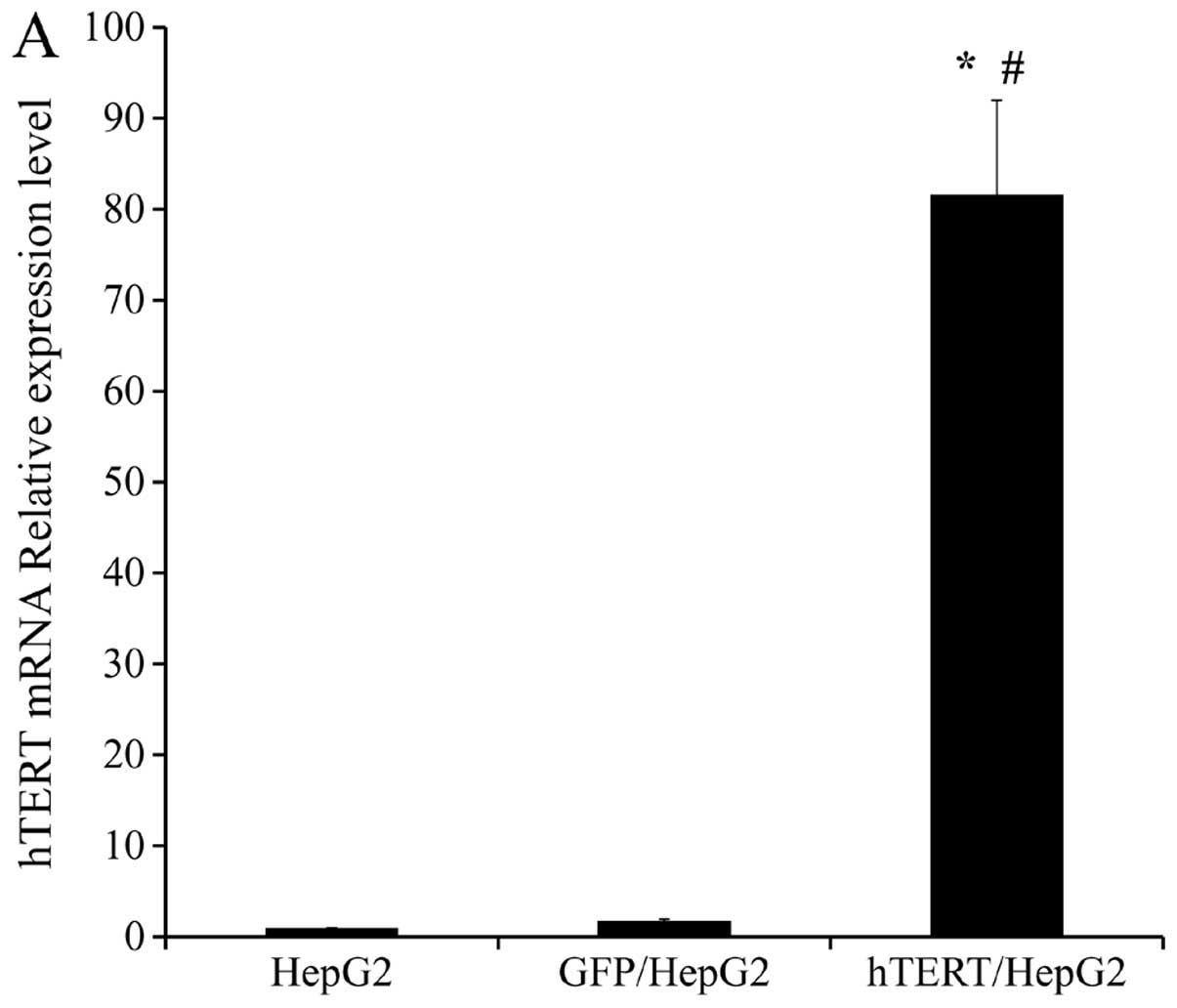
The expression of hTERT in all 3 groups of cells was
examined using real-time PCR. β-actin served as a loading control
for both mRNA and protein analyses. We found hTERT mRNA expression
to be markedly higher in hTERT/HepG2 cells (up to 80-fold) than in
HepG2 and GFP/HepG2 control cells (Fig.
2A). Elevated hTERT protein levels were also detected in
hTERT/HepG2 cells relative to HepG2 and GFP/HepG2 cells (Fig. 2B). There was no significant
difference between HepG2 and GFP/HepG2 in either mRNA or protein
expression. The relative expression levels of hTERT mRNA in HepG2,
GFP/HepG2 and hTERT/HepG2 were 1.00, 1.76 and 81.65 (Fig. 2A). We used this information to
generate a stable cell line with high hTERT expression. This line
was used for subsequent studies.
hTERT overexpression enhances telomerase
activity and telomere length in HepG2 cells
To further examine the effect of hTERT
overexpression, TRAP assay was performed to assess telomerase
activity in HepG2, GFP/HepG2 and hTERT/HepG2. Our results showed
telomerase activity in the hTERT/HepG2 cells to be significantly
more pronounced than in the 2 control cells (Fig. 3A). The average length of telomere
repeats (indicated by the intensity of telomere fluorescence) at
chromosome ends in HepG2, GFP/HepG2 and hTERT/HepG2 was determined
using semiquantitative FISH. As in our experiments on elevated
telomerase activity, telomere length, here indicated by
fluorescence intensity, was significantly longer in hTERT/HepG2
cells than in HepG2 and GFP/HepG2 control cells (Fig. 3B).
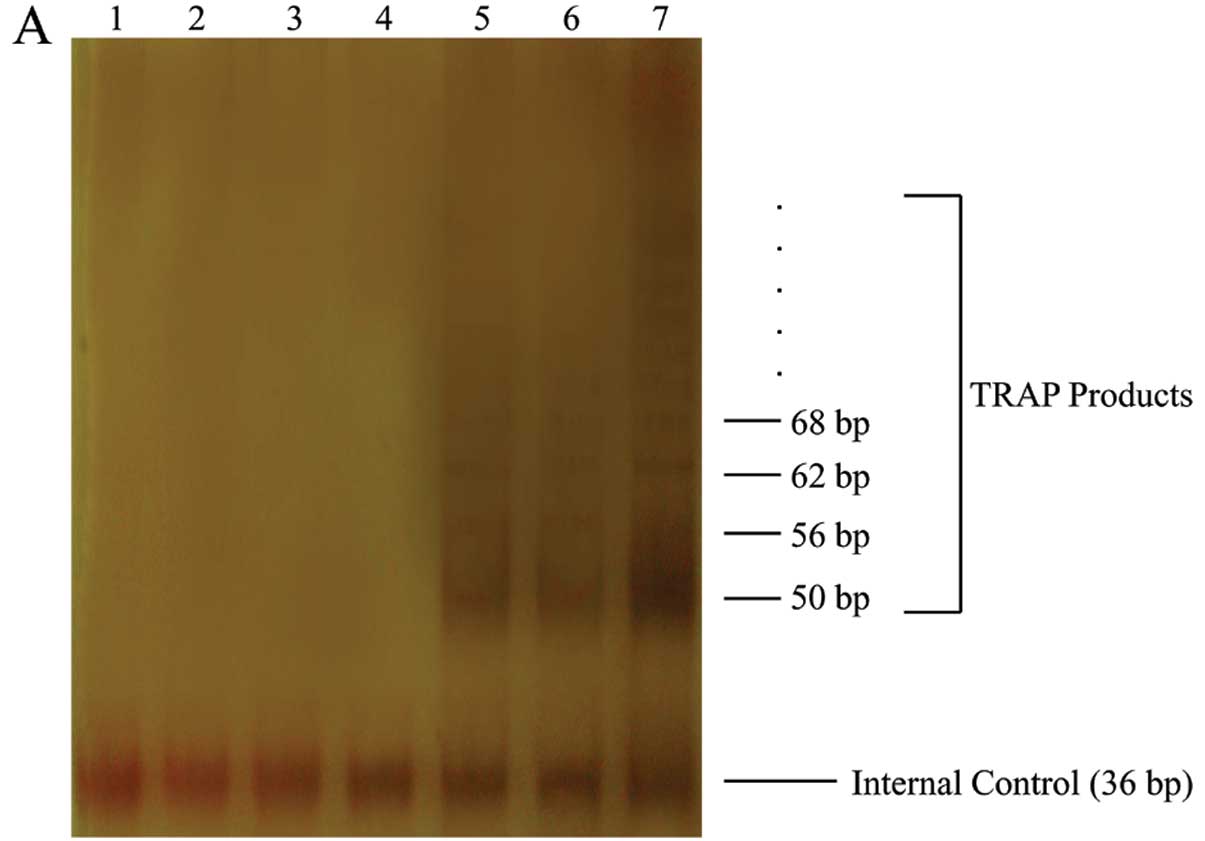 | Figure 3Telomerase activity and telomere
fluorescence intensity assay. (A) Telomerase activity (TRAP assay).
Lane 1, negative control (1X TRAP lysis buffer); lanes 2–4,
heat-treated control cell extracts (HepG2, GFP/HepG2, hTERT/HepG2);
lanes 5–7, cell extracts (HepG2, GFP/HepG2, hTERT/HepG2). (B) FISH
assay of HepG2, GFP/HepG2, hTERT/HepG2. (C) Quantitative analysis
of telomere fluorescence intensity. Data are means ± SD
(*P<0.05, compared with HepG2 mean values;
#P<0.05, relative to GFP/HepG2 mean values,
ANOVA). |
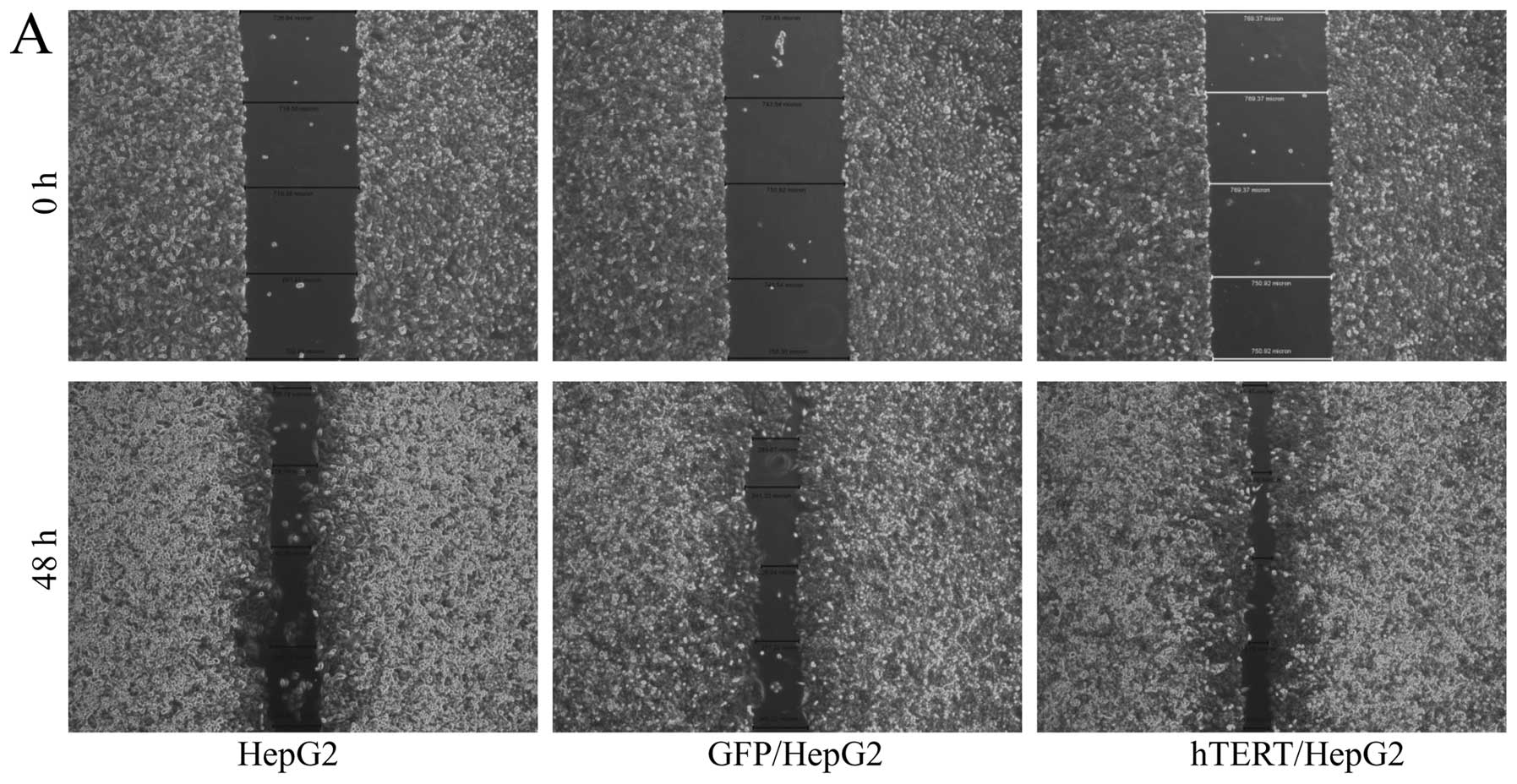
hTERT overexpression leads to increased
motility and invasiveness of HepG2 cells
Previous studies conducted using tissue samples from
certain cancer patients indicated that levels of high telomerase
expression and activity are correlated with increased motility and
invasiveness, suggesting that telomerase expression plays a
critical role in tumor progression. To assess the motility and
invasiveness of infected hTERT/HepG2 cells, we carried out wound
healing assay and invasive experiment using Matrigel-coated
transwell chambers. As in previous studies, overexpression of hTERT
in HepG2 cells led to increased cell motility and invasiveness
relative to 2 sets of control cells (Fig. 4). Taken together, our data suggested
that increased telomerase expression enhanced cell motility and
invasiveness in vitro and facilitated tumor progression
towards metastasis stage in vivo.
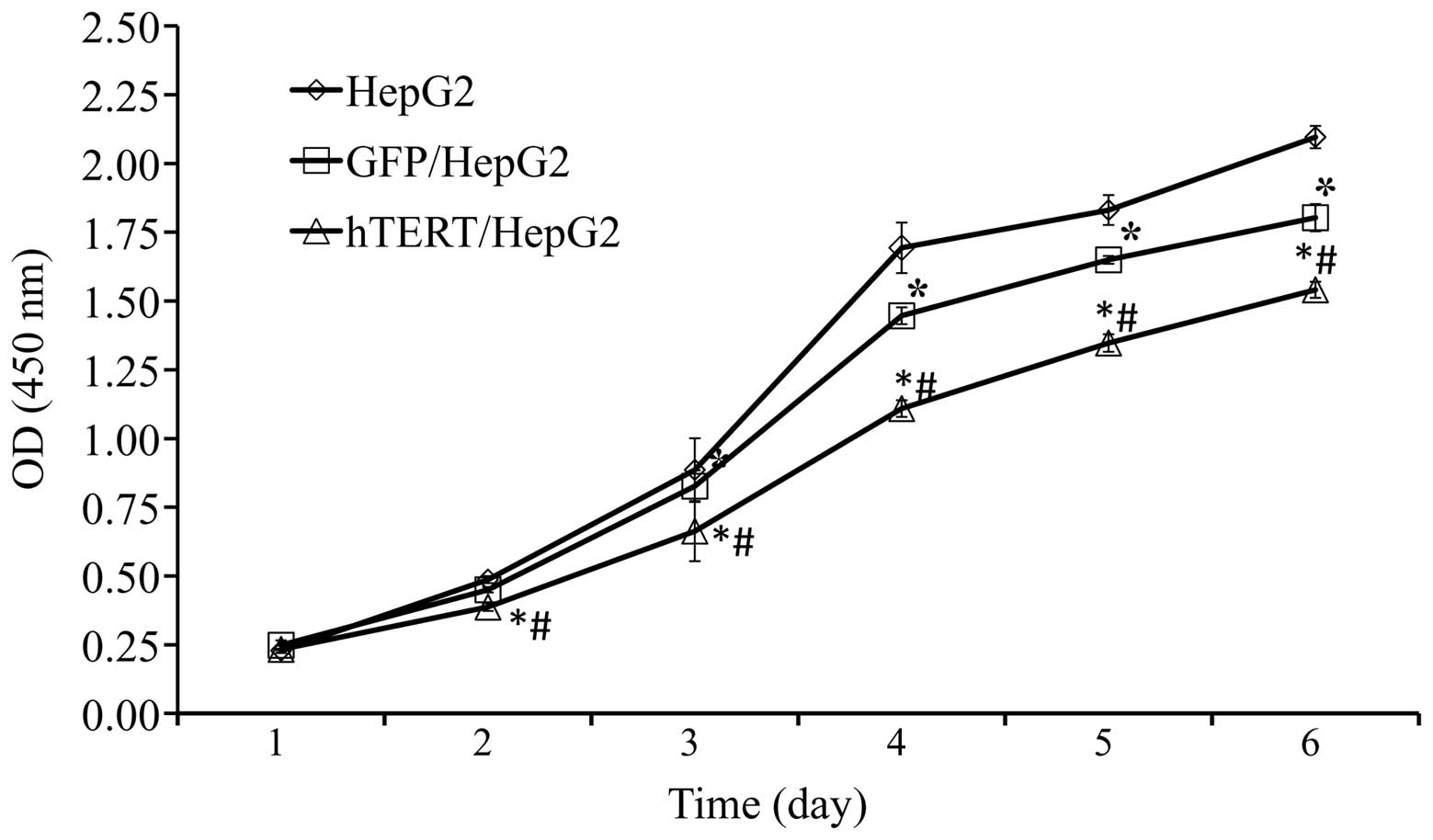
Overexpression in telomerase-positive
HepG2 slows down cell proliferation
It has been suggested that telomerase expression may
promote cell proliferation and maintain homeostasis in adult
tissue. To determine whether this is the case in HCCs
overexpressing hTERT, a CCK-8 assay was performed to assess the
cell proliferation rate. The speed of cell proliferation among
hTERT/HepG2 was significantly slower than among GFP/HepG2 and HepG2
control cells (Fig. 5). However,
further studies are required to fully clarify this.
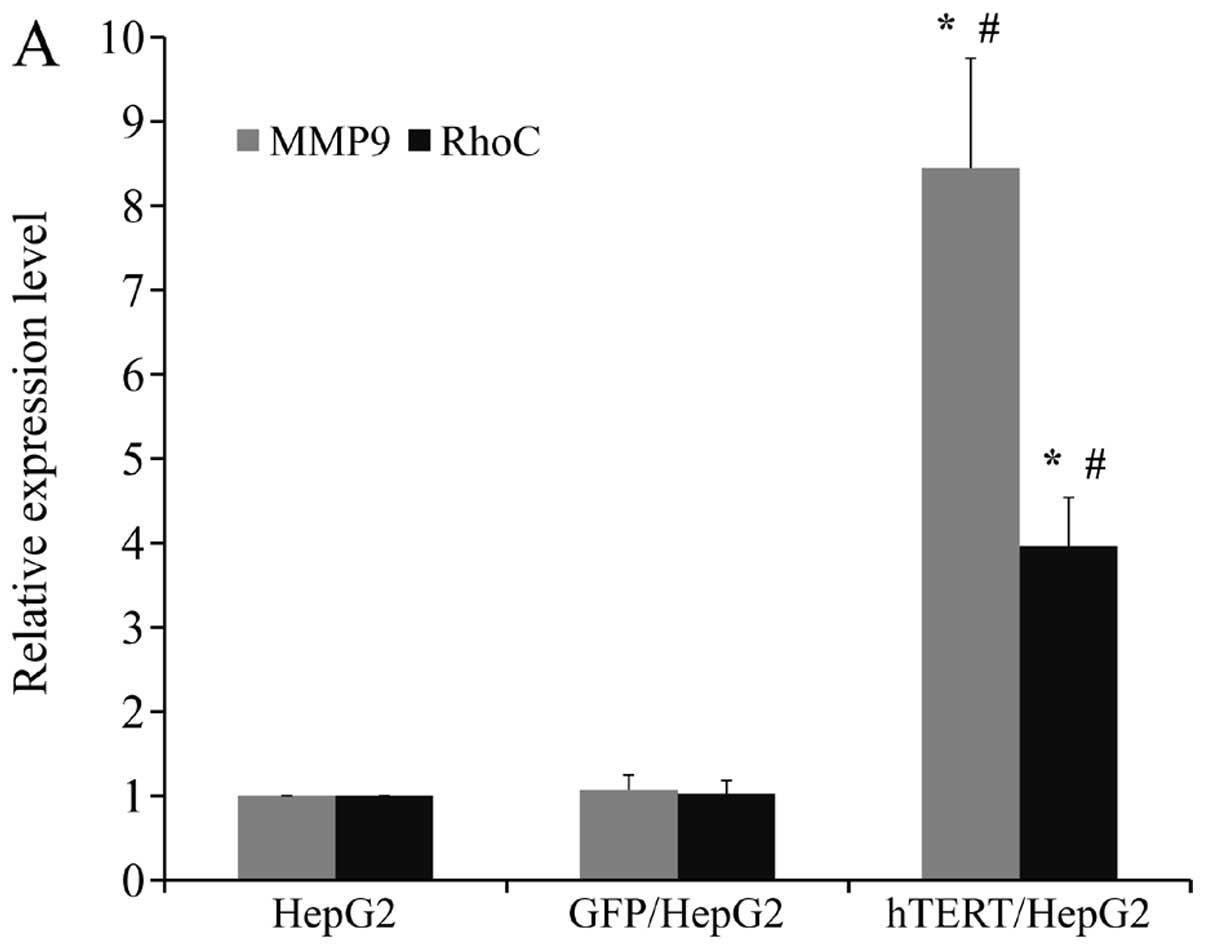
Upregulation of MMP9 and RhoC may be
responsible for enhanced motility and invasiveness in HepG2 when
hTERT expression is forced
In order to determine the molecular mechanism by
which hTERT promotes the motility and invasiveness of HepG2 cells,
we assessed the mRNA and protein levels of 2 important molecules
involved in cell migration and invasion. We observed marked
upregulation of RhoC and MMP9 on both the mRNA and protein levels
in hTERT/HepG2 (Fig. 6A and B). Our
findings suggested that enhanced cell motility and invasiveness
induced by high levels of hTERT in HepG2 cells may have been caused
by upregulation of MMP9 and RhoC expression.
Discussion
Aggressiveness is a significant biological
characteristic of malignant tumor cells and a leading cause of
mortality in patients with cancer. Several studies have
demonstrated that telomerase plays an important role in elongating
telomeres and maintaining the infinite proliferation ability of
telomerase-positive tumor cells. Clinical findings have indicated
that pronounced telomerase activity may be a prognostic marker of
various human malignant tumors, including HCC (23). Sato et al(31) found relative telomerase levels to be
closely correlated with both motility and invasiveness in 13
pancreatic carcinoma cell lines and concluded that the magnitude of
telomerase activation may reflect the potential for aggressive
behavior within cancer cells. Previous experimental studies
regarding the link between telomerase and tumor aggressiveness have
focused on the following two phenomena; first, the transfection or
transduction of hTERT can promote the motility and invasiveness of
telomerase-negative tumor cells. The invasive and metastatic
potential of RasG12V/SV40 Large T antigen-transduced
bovine cells can be restored by transduction of hTERT (26). The proliferation, adhesion and
invasion of telomerase-negative osteosarcoma cell line U2OS was
found to be significantly promoted by hTERT transfection (28). Second, cell proliferation, migration
and invasion of telomerase-positive tumor cells can be inhibited by
inhibition of either hTERT expression or telomerase activity.
Anti-TER ribozyme-mediated suppression of mouse telomerase RNA
reduced telomerase RNA expression, telomerase activity and telomere
length, which significantly reduced the invasiveness and metastatic
potential of melanoma cells (32).
Knockdown of hTERT siRNA and concurrent treatment with IFN-γ
effectively inhibited cell proliferation, migration, and invasion
in glioblastoma cells (30).
However, to our knowledge, there are no experimental studies on the
link between hTERT overexpression and aggressiveness in
telomerase-positive tumor cells. In the present study, we found
that hTERT overexpression can also significantly promote the
motility and invasive potential of cancer cells, at least in
telomerase-positive HepG2 cell lines. This provides further
evidence of the relationship between telomerase activity and
metastatic potential of tumor cells.
Tumor cell invasion and metastasis are multi-step,
complex processes. They involve proteolytic degradation of basement
membranes and extracellular matrix (ECM), cell adhesion, migration
and angiogenesis. Both MMPs and RhoC have been shown to play
important roles in some of these processes. MMPs are a family of
zinc-dependent endoproteinases responsible for degradation of the
components of basement membranes and ECM. Overexpression of MMPs
has been observed in most malignant tumors. MMP9 expression has
been found to be strongly correlated to metastasis of HCC (33,34).
Analysis of cell phenotypes expressing dominant-negative Rho or
RhoC indicates that RhoC is important in tumor cell invasion
(35). RhoC plays a critical role
in the movement of tumor cells through regulation of actin
cytoskeleton (36,37). RhoC has been shown to be upregulated
in various types of tumors, including HCC (38–42).
Studies have indicated that RhoC significantly promotes metastasis
by augmenting motility and invasion of tumor cells via activation
of MMP2 and MMP9 (43,44). In this study, we chose these two key
molecules for invasion of tumor cells for our preliminary
investigation of the molecular mechanisms by which hTERT promotes
motility and invasiveness. We found expression levels of MMP9 and
RhoC to be markedly upregulated in hTERT/HepG2 cells at both the
mRNA and protein levels. We speculate that upregulation of RhoC and
MMP9 may be one of the mechanisms through which hTERT enhances the
motility and invasiveness of tumor cells. In this process, there
may be complicated regulating networks that control tumor
aggressiveness. However, the details of these mechanisms require
further study in subsequent experiments.
hTERT is generally considered to be closely related
to tumor cell growth and proliferation (45) and it is believed to promote cell
growth and proliferation (46–48).
However, the few studies that have been performed have shown no
significant association between telomerase activity and
proliferative index in various tumor tissues (14,15,31,49).
Presumably, malignancy and poor outcome among cancer patients may
not necessarily be strictly correlated with tumor size or
proliferation rate. Other characteristics, such as the degree of
differentiation, may be more suitable indicators of malignancy and
poor outcome in these patients. In the current study, we showed
that cell proliferation was not increased by overexpression of
hTERT. Our results also show that cell proliferation is slower in
the two transduced groups than in untransduced cells. One possible
reason for this may be that forced expression of increased amounts
of TERT may cause telomerase-positive cells to acquire some
properties and capacities, such as those involving
de-differentiation, motility, and invasiveness, but not an
increased growth rate. Whatever the reason, the motility and
invasiveness of tumor cells were found to have been significantly
promoted by overexpression of hTERT, and these were considered
indicators of enhanced metastatic potential. In this sense, our
results support previous observations that hTERT and telomerase
play important roles in the promotion of motility and invasiveness
in tumor cells.
The overexpression of hTERT was found to increase
the motility and invasiveness of telomerase-positive HepG2 cells.
However, whether the depletion of hTERT in these cells can lead to
the opposite effects requires further studies. Additional efforts
should be made to investigate the universality of our findings with
respect to other cancer cell lines.
In conclusion, our findings demonstrate that hTERT
overexpression can significantly promote cell migration and
invasiveness among human telomerase-positive HepG2 cell lines but
does not promote proliferation. Upregulation of
metastasis-associated molecules, MMP9 and RhoC, may be one of the
most important mechanisms underlying this process. Our study
provides experimental evidence of the relationship between
telomerase activity and metastatic potential of telomerase-positive
tumor cells and also aids in the research into the mechanisms
underlying this process. These findings provide a possible
explanation of the clinical observation that high telomerase
activity predicts poor patient outcome in HCC, particularly in
cases of recurrence after HCC resection.
Acknowledgements
The authors thank Dr David Ott (NCI-Frederick, USA)
and Dr Eugene Barsov for providing the plasmid retroviral
expression vector xlox(gfp)TERT.
References
|
1
|
Morin GB: The human telomere terminal
transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG
repeats. Cell. 59:521–529. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Greider CW and Blackburn EH: The telomere
terminal transferase of Tetrahymena is a ribonucleoprotein enzyme
with two kinds of primer specificity. Cell. 51:887–898. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Greider CW and Blackburn EH:
Identification of a specific telomere terminal transferase activity
in Tetrahymena extracts. Cell. 43:405–413. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feng J, Funk WD, Wang SS, et al: The RNA
component of human telomerase. Science. 269:1236–1241. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Toshikuni N, Nouso K, Higashi T, et al:
Expression of telomerase-associated protein 1 and telomerase
reverse transcriptase in hepatocellular carcinoma. Br J Cancer.
82:833–837. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakayama J, Tahara H, Tahara E, et al:
Telomerase activation by hTRT in human normal fibroblasts and
hepatocellular carcinomas. Nat Genet. 18:65–68. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohta K, Kanamaru T, Morita Y, Hayashi Y,
Ito H and Yamamoto M: Telomerase activity in hepatocellular
carcinoma as a predictor of postoperative recurrence. J
Gastroenterol. 32:791–796. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakashio R, Kitamoto M, Tahara H,
Nakanishi T, Ide T and Kajiyama G: Significance of telomerase
activity in the diagnosis of small differentiated hepatocellular
carcinoma. Int J Cancer. 74:141–147. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kojima H, Yokosuka O, Imazeki F, Saisho H
and Omata M: Telomerase activity and telomere length in
hepatocellular carcinoma and chronic liver disease.
Gastroenterology. 112:493–500. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohta K, Kanamaru T, Yamamoto M and Saitoh
Y: Clinical significance of telomerase activity in hepatocellular
carcinoma. Kobe J Med Sci. 42:207–217. 1996.PubMed/NCBI
|
|
11
|
Ide T, Tahara H, Nakashio R, Kitamoto M,
Nakanishi T and Kajiyama G: Telomerase in hepatocellular
carcinogenesis. Hum Cell. 9:283–286. 1996.
|
|
12
|
Taga S, Osaki T, Ohgami A, Imoto H and
Yasumoto K: Prognostic impact of telomerase activity in non-small
cell lung cancers. Ann Surg. 230:715–720. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marchetti A, Bertacca G, Buttitta F, et
al: Telomerase activity as a prognostic indicator in stage I
non-small cell lung cancer. Clin Cancer Res. 5:2077–2081.
1999.PubMed/NCBI
|
|
14
|
Dome JS, Chung S, Bergemann T, et al: High
telomerase reverse transcriptase (hTERT) messenger RNA level
correlates with tumor recurrence in patients with favorable
histology Wilms’ tumor. Cancer Res. 59:4301–4307. 1999.PubMed/NCBI
|
|
15
|
Okusa Y, Shinomiya N, Ichikura T and
Mochizuki H: Correlation between telomerase activity and DNA ploidy
in gastric cancer. Oncology. 55:258–264. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bechter OE, Eisterer W, Pall G, Hilbe W,
Kühr T and Thaler J: Telomere length and telomerase activity
predict survival in patients with B cell chronic lymphocytic
leukemia. Cancer Res. 58:4918–4922. 1998.PubMed/NCBI
|
|
17
|
Langford LA, Piatyszek MA, Xu R, Schold SC
Jr, Wright WE and Shay JW: Telomerase activity in ordinary
meningiomas predicts poor outcome. Hum Pathol. 28:416–420. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hiyama E, Hiyama K, Ohtsu K, et al:
Telomerase activity in neuroblastoma: is it a prognostic indicator
of clinical behaviour? Eur J Cancer. 33:1932–1936. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clark GM, Osborne CK, Levitt D, Wu F and
Kim NW: Telomerase activity and survival of patients with
node-positive breast cancer. J Natl Cancer Inst. 89:1874–1881.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hiyama E, Yokoyama T, Tatsumoto N, et al:
Telomerase activity in gastric cancer. Cancer Res. 55:3258–3262.
1995.PubMed/NCBI
|
|
21
|
Hiyama E, Hiyama K, Yokoyama T, Matsuura
Y, Piatyszek MA and Shay JW: Correlating telomerase activity levels
with human neuroblastoma outcomes. Nat Med. 1:249–255. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saini N, Srinivasan R, Chawla Y, Sharma S,
Chakraborti A and Rajwanshi A: Telomerase activity, telomere length
and human telomerase reverse transcriptase expression in
hepatocellular carcinoma is independent of hepatitis virus status.
Liver Int. 29:1162–1170. 2009. View Article : Google Scholar
|
|
23
|
Suda T, Isokawa O, Aoyagi Y, et al:
Quantitation of telomerase activity in hepatocellular carcinoma: a
possible aid for a prediction of recurrent diseases in the remnant
liver. Hepatology. 27:402–406. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tatsuma T, Goto S, Kitano S, Lin YC, Lee
CM and Chen CL: Telomerase activity in peripheral blood for
diagnosis of hepatoma. J Gastroenterol Hepatol. 15:1064–1070. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kobayashi T, Kubota K, Takayama T and
Makuuchi M: Telomerase activity as a predictive marker for
recurrence of hepatocellular carcinoma after hepatectomy. Am J
Surg. 181:284–288. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun B, Huang Q, Liu S, et al: Progressive
loss of malignant behavior in telomerase-negative tumorigenic
adrenocortical cells and restoration of tumorigenicity by human
telomerase reverse transcriptase. Cancer Res. 64:6144–6151. 2004.
View Article : Google Scholar
|
|
27
|
Brachner A, Sasgary S, Pirker C, et al:
Telomerase- and alternative telomere lengthening-independent
telomere stabilization in a metastasis-derived human non-small cell
lung cancer cell line: effect of ectopic hTERT. Cancer Res.
66:3584–3592. 2006. View Article : Google Scholar
|
|
28
|
Yu ST, Chen L, Wang HJ, Tang XD, Fang DC
and Yang SM: hTERT promotes the invasion of telomerase-negative
tumor cells in vitro. Int J Oncol. 35:329–336.
2009.PubMed/NCBI
|
|
29
|
Yao X, Wang X, Zhang S, Yan L and Zhu H:
Inhibitory effect of silencing hTERT gene on growth of human
squamous cell carcinoma xenograft in nude mice. Lin Chung Er Bi Yan
Hou Tou Jing Wai Ke Za Zhi. 25:939–943. 2011.(In Chinese).
|
|
30
|
George J, Banik NL and Ray SK: Knockdown
of hTERT and concurrent treatment with interferon-gamma inhibited
proliferation and invasion of human glioblastoma cell lines. Int J
Biochem Cell Biol. 42:1164–1173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sato N, Maehara N, Mizumoto K, et al:
Telomerase activity of cultured human pancreatic carcinoma cell
lines correlates with their potential for migration and invasion.
Cancer. 91:496–504. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bagheri S, Nosrati M, Li S, et al: Genes
and pathways downstream of telomerase in melanoma metastasis. Proc
Natl Acad Sci USA. 103:11306–11311. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nart D, Yaman B, Yilmaz F, Zeytunlu M,
Karasu Z and Kiliç M: Expression of matrix metalloproteinase-9 in
predicting prognosis of hepatocellular carcinoma after liver
transplantation. Liver Transpl. 16:621–630. 2010.PubMed/NCBI
|
|
34
|
Arii S, Mise M, Harada T, et al:
Overexpression of matrix metalloproteinase 9 gene in hepatocellular
carcinoma with invasive potential. Hepatology. 24:316–322. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Clark EA, Golub TR, Lander ES and Hynes
RO: Genomic analysis of metastasis reveals an essential role for
RhoC. Nature. 406:532–535. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ridley AJ: Rho GTPases and cell migration.
J Cell Sci. 114:2713–2722. 2001.PubMed/NCBI
|
|
37
|
Van Aelst L and D’Souza-Schorey C: Rho
GTPases and signaling networks. Genes Dev. 11:2295–2322.
1997.PubMed/NCBI
|
|
38
|
Wang W, Yang LY, Huang GW, et al: Genomic
analysis reveals RhoC as a potential marker in hepatocellular
carcinoma with poor prognosis. Br J Cancer. 90:2349–2355.
2004.PubMed/NCBI
|
|
39
|
Wang W, Yang LY, Yang ZL, Huang GW and Lu
WQ: Expression and significance of RhoC gene in hepatocellular
carcinoma. World J Gastroenterol. 9:1950–1953. 2003.PubMed/NCBI
|
|
40
|
Okabe H, Satoh S, Kato T, et al:
Genome-wide analysis of gene expression in human hepatocellular
carcinomas using cDNA microarray: identification of genes involved
in viral carcinogenesis and tumor progression. Cancer Res.
61:2129–2137. 2001.
|
|
41
|
Kleer CG, Griffith KA, Sabel MS, et al:
RhoC-GTPase is a novel tissue biomarker associated with
biologically aggressive carcinomas of the breast. Breast Cancer Res
Treat. 93:101–110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shikada Y, Yoshino I, Okamoto T, Fukuyama
S, Kameyama T and Maehara Y: Higher expression of RhoC is related
to invasiveness in non-small cell lung carcinoma. Clin Cancer Res.
9:5282–5286. 2003.PubMed/NCBI
|
|
43
|
Xue F, Takahara T, Yata Y, et al: Blockade
of Rho/Rho-associated coiled coil-forming kinase signaling can
prevent progression of hepatocellular carcinoma in matrix
metalloproteinase-dependent manner. Hepatol Res. 38:810–817. 2008.
View Article : Google Scholar
|
|
44
|
Iiizumi M, Bandyopadhyay S, Pai SK, et al:
RhoC promotes metastasis via activation of the Pyk2 pathway in
prostate cancer. Cancer Res. 68:7613–7620. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Belair CD, Yeager TR, Lopez PM and
Reznikoff CA: Telomerase activity: a biomarker of cell
proliferation, not malignant transformation. Proc Natl Acad Sci
USA. 94:13677–13682. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Smith LL, Coller HA and Roberts JM:
Telomerase modulates expression of growth-controlling genes and
enhances cell proliferation. Nat Cell Biol. 5:474–479. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gronthos S, Chen S, Wang CY, Robey PG and
Shi S: Telomerase accelerates osteogenesis of bone marrow stromal
stem cells by upregulation of CBFA1, osterix, and osteocalcin. J
Bone Miner Res. 18:716–722. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xiang H, Wang J, Mao Y, Liu M, Reddy VN
and Li DW: Human telomerase accelerates growth of lens epithelial
cells through regulation of the genes mediating RB/E2F pathway.
Oncogene. 21:3784–3791. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bednarek AK, Sahin A, Brenner AJ, Johnston
DA and Aldaz CM: Analysis of telomerase activity levels in breast
cancer: positive detection at the in situ breast carcinoma stage.
Clin Cancer Res. 3:11–16. 1997.PubMed/NCBI
|