Introduction
Thymidine phosphorylase (TP; EC 2.4.2.4) catalyzes
the reversible conversion of thymidine, deoxyuridine and their
analogs to their respective bases and 2-deoxy-D-ribose-1-phosphate
(1). TP is identical to the
angiogenic factor, platelet-derived endothelial cell growth factor
(PD-ECGF) (2,3). TP stimulates chemotaxis and
[3H]thymidine incorporation by endothelial cells in
vitro and has an angiogenic activity in vivo(4–7). We
previously demonstrated that TP enzymatic activity is indispensable
for its angiogenic activity (4,7). Among
the degradation products generated by TP enzymatic activity,
2-deoxy-D-ribose, a dephosphorylated product derived from
2-deoxy-D-ribose-1-phosphate, also displays chemotactic activity
in vitro and angiogenic activity in vivo. TP is
expressed at higher levels in a wide variety of tumors when
compared to that in the adjacent non-neoplastic tissues (8–10).
Under hypoxic conditions, TP can also enhance the growth of tumor
cells and confer resistance to apoptosis induced by hypoxia
(11). 2-Deoxy-D-ribose was able to
partially prevent hypoxia-induced apoptosis in human leukemia HL-60
cells (12,13). TP also inhibited upregulation of
HIF-1α, BNIP3 and caspase-3 under hypoxic conditions (14). These findings indicated that TP has
another function apart from angiogenesis.
To elucidate the mechanism by which 2-deoxy-D-ribose
suppresses HIF-1α levels under hypoxic conditions, we examined the
effect of 2-deoxy-D-ribose on key regulators of HIF-1α: von
Hippel-Lindau (VHL) and prolyl hydroxylase (PHD)2.
Materials and methods
Reagents and antibodies
The monoclonal anti-HIF-1α antibody was purchased
from Transduction Laboratories (Lexington, KY, USA). The rabbit
anti-VHL polyclonal antibody (FL-181) was obtained from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). A rabbit anti-PHD2 polyclonal
antibody was from Bethyl Laboratories (Montgomery, TX, USA). A
rabbit anti-hydroxy-HIF-1α (Pro564) was from Cell Signaling
Technology (Boston, MA, USA).
Cell lines and induction of hypoxia
Human leukemia HL-60 cells were maintained in
RPMI-1640 containing 10% fetal calf serum. 2-Deoxy-D-ribose was
added to the culture medium and then hypoxia (1% O2) was
induced in a Personal Multigas Incubator (Astec).
Immunoblotting analysis
Samples were resolved by sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE) according to the
method of Laemmli. Proteins in the gel were electrophoretically
transferred onto polyvinylidene difluoride membranes (Immobilon-P
transfer membrane; Millipore, Bedford, MA, USA) using Bio-Rad
Trans-Blot SD apparatus. The membrane was treated with buffer A
[350 mM NaCl, 10 mM Tris-HCl (pH 8.0), 0.05% Tween 20] containing
3% skimmed milk for 1 h and incubated with the indicated antibody
(1:1,000) in buffer A containing 3% skimmed milk for 1 h. Following
four washes with buffer A (10 min each), the membrane was incubated
with peroxidase-conjugated horse anti-mouse IgG diluted 1:1,000 in
buffer A containing 3% skimmed milk for 1 h. Following washing with
buffer A, the membrane was developed using the enhanced
chemiluminescence western blotting detection system (Amersham
Pharmacia, Buckinghamshire, UK).
RT-PCR method
Total cellular RNA was extracted using TRIzol
reagent according to the manufacturer’s instructions (Invitrogen,
Carlsbad, CA, USA). RT-PCR was performed with the SuperScript
One-Step RT-PCR system and gene-specific primers according to the
manufacturer’s instructions (Invitrogen). Reaction mixtures
containing total RNA (500 ng of each), 0.2 mM dNTPs, 0.2 mM of each
primer, 2 units of enzyme mixture including SuperScript II RT,
Platinum Taq DNA polymerase, and 1X buffer with 1.2 mM
MgSO4 were maintained at 50°C for 20 min, and then at
94°C for 2 min, and PCR was performed as follows. The PCR profile
consisted of 30 cycles at 94°C for 15 sec, 55°C for 30 sec, and
70°C for 30 sec. The primers for RT-PCRs were designed based on
human sequences in GenBank. These sequences used the following
primers: PHD1, 5′-gccagtgctgggctgatggagg-3′ and
5′-cgcagtggcggatgacggcgtc-3′; PHD2, 5′-tgcatgaacaa gcacggcatct-3′
and 5′-atatacatgtcacacatcttcc-3′; PHD3, 5′-ga
ctgcgtcctggagcgcgtca-3′ and 5′-atatccgcaggatcccaccatg-3′; VHL,
5′-atgccccggagggcggagaactggg-3′ and 5′-tcaatctcccatcc
gttgatgtgca-3′; HIF-1α, 5′-tcgaggcctctgtgatgagg-3′ and 5′-ggc
ctctgtgatgaggcttt-3′; GAPDH, 5′-agaacatcatccctgcctctactgg-3′ and
5′-aaaggtggaggagtgggtgtcgctg-3′.
Immunoprecipitation
For immunoprecipitation experiments, FLAG tagged VHL
or PHD2 and HIF-1α cDNA (1 μg each) were transiently transfected
into COS or HL-60 cells plated in 6-cm diameter dishes. Twenty four
hours following transfection, the cells were treated for 5 h under
hypoxic conditions and cells were harvested. The cells were
suspended in 200 μg of whole cell extract buffer [10 mM HEPES (pH
7.9), 400 mM NaCl, 0.1 mM EDTA, 5% (vol/vol) glycerol, 1 mM DTT, 1
mM APMSF], and centrifuged at 12,500 rpm for 30 min at 4°C.
Subsequent to protein extraction, 300 μg of total proteins was
incubated with anti-FLAG or anti-PHD2 antibodies at 4°C for 1 h.
Thirty microliters of a 50% slurry of protein G-Sepharose 4B in TEG
buffer [20 mM Tris-HCl (pH 7.9), 1 mM EDTA, 10% glycerol, 1 mM
DTT], containing 150 mM NaCl and 0.1% Triton X-100, were then added
to reaction mixtures and incubated for 12 h at 4°C with rotation.
Following a rapid centrifugation, the resulting pellets were washed
three times with TEG buffer, and the immunoprecipitated proteins
were analyzed by immunoblotting using an anti-HIF-1α antibody.
Results
Effect of 2-deoxy-D-ribose on the
expression of HIF-1α under hypoxic conditions
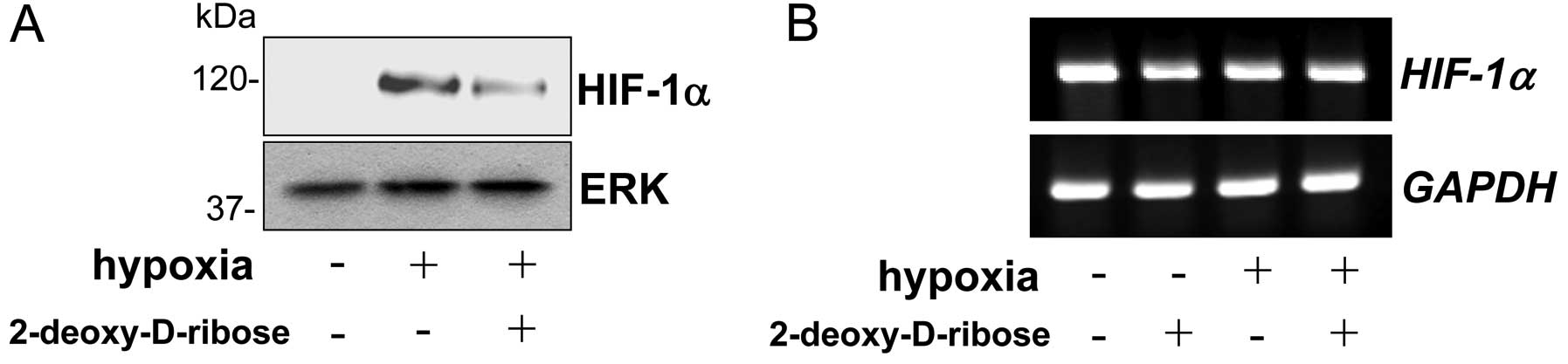
We determined the effect of 2-deoxy-D-ribose on the
HIF-1a protein level under hypoxic conditions. As shown in Fig. 1A, we could not detect HIF-1α in
HL-60 cells under a normoxic condition. When HL-60 cells were
incubated under hypoxic conditions, the HIF-1α protein level was
markedly increased (Fig. 1A).
Treatment of the cells with 2-deoxy-D-ribose substantially
suppressed the level of HIF-1α in the cells under hypoxic
conditions. We next determined the effect of 2-deoxy-D-ribose on
the expression of HIF-1α mRNA levels by RT-PCR.
HIF-1α mRNA levels were not affected by hypoxia, and
treatment of the cells under hypoxic conditions by 2-deoxy-D-ribose
did not alter the levels of HIF-1α mRNA (Fig. 1B).
Effect of 2-deoxy-D-ribose on the
interaction of HIF-1α and VHL
The HIF-1α protein is continuously synthesized and
degraded under a normoxic condition, while it accumulates rapidly
following exposure to hypoxic conditions. HIF-1α interacts with VHL
and is degraded via the ubiquitin-proteasome pathway under a
normoxic condition. As the oxygen concentration decreases, PHDs
become inactive and the HIF-1α protein is consequently stabilized.
One possible mechanism for the attenuation of HIF-1α caused by
2-deoxy-D-ribose under hypoxic conditions might be the enhanced
interactions of HIF-1α with VHL and consequent degradation of
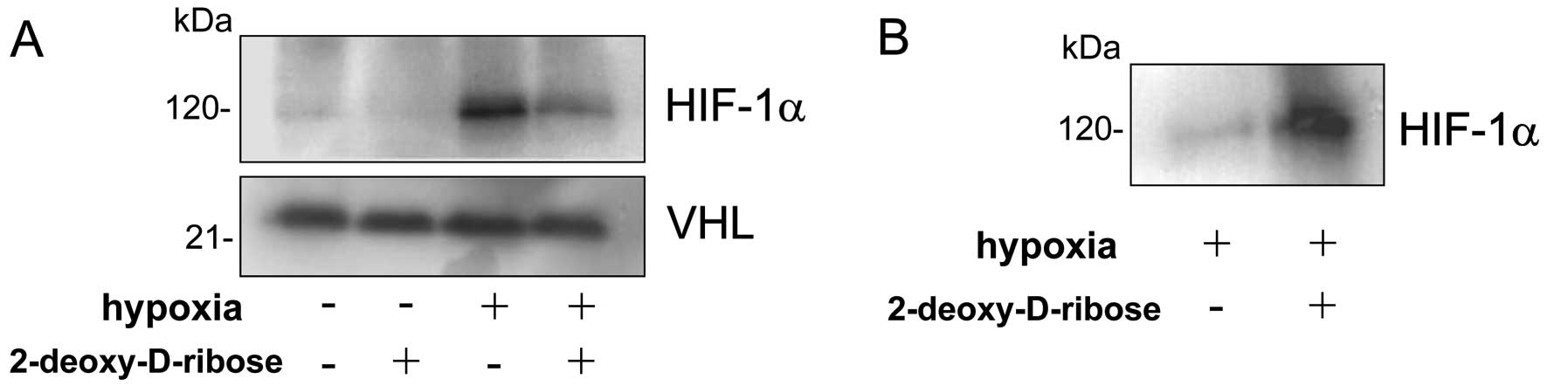
HIF-1α. To assess the interactions between HIF-1α and VHL in the
presence or absence of 2-deoxy-D-ribose under hypoxic conditions,
COS cells were cotransfected with HIF-1α and VHL-expressing
plasmids. After 24 h following transfection, the cells were exposed
for 5 h to hypoxic conditions in the presence or absence of
2-deoxy-D-ribose. As shown in Fig.
2A, incubation of cells under hypoxic conditions resulted in a
marked increase in HIF-1α protein levels, and treatment with
2-deoxy-D-ribose substantially suppressed HIF-1α in the cells under
hypoxic conditions. To examine the effect of 2-deoxy-D-ribose on
the interaction between VHL and HIF-1α, the cell lysates were
immunoprecipitated with an anti-FLAG antibody and the
coprecipitated HIF-1α was detected with an anti-HIF-1α antibody.
Treatment of COS cells with 2-deoxy-D-ribose enhanced interaction
of HIF-1α with VHL under hypoxic conditions (Fig. 2B).
Effect of 2-deoxy-D-ribose on the
expression of PHD1/2/3 and VHL
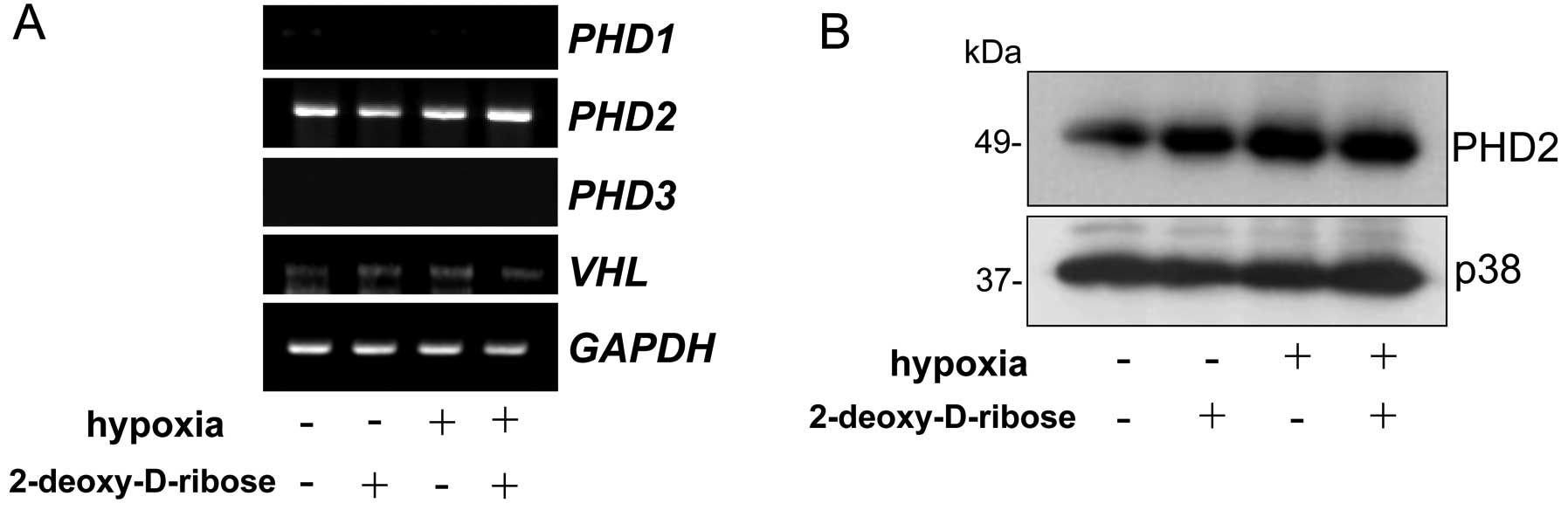
To determine the effect of 2-deoxy-D-ribose on the
expression of PHD1/2/3 and VHL in HL-60 cells, the
cells were cultured under normoxic or hypoxic conditions in the
presence or absence of 2-deoxy-D-ribose. As shown in Fig. 3A, the expression of PHD2 was
detected but those of PHD1 and PHD3 mRNA were not
detected by RT-PCR. 2-Deoxy-D-ribose did not affect the expression
of PDH2 mRNA and protein levels under normoxic and hypoxic
conditions in HL-60 cells (Fig.
3).
Effect of 2-deoxy-D-ribose on the
interaction between HIF-1a and PHD2
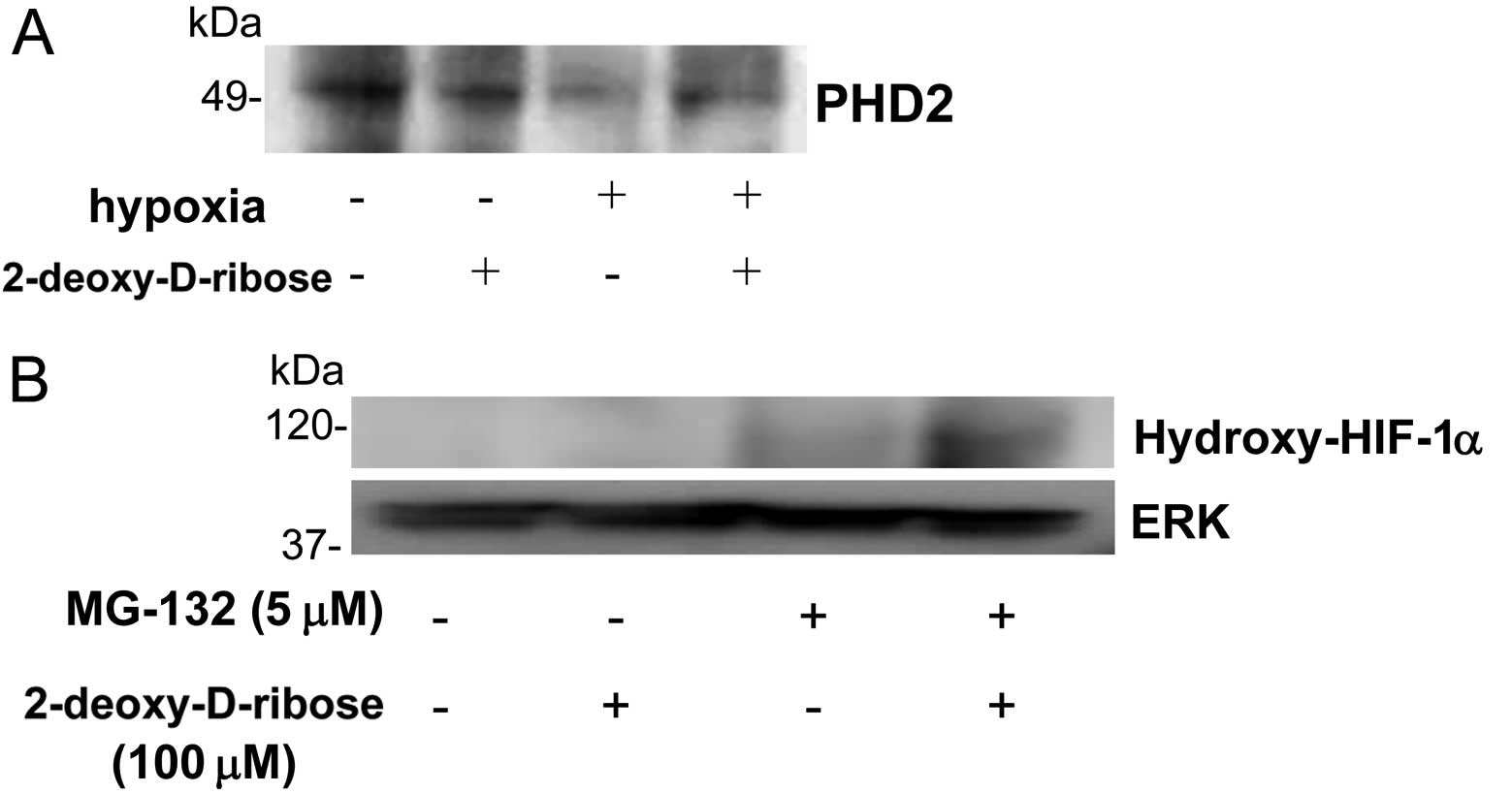
2-Deoxy-D-ribose may decrease HIF-1α protein levels
by affecting the interaction between HIF-1α and PHD2 and
consequently modulating its degradation. To assess the effect of
2-deoxy-D-ribose on the interaction between HIF-1α and PHD2, HL-60
cells were cultured under normoxic or hypoxic conditions in the
presence or absence of 2-deoxy-D-ribose. Whole cell extracts were
prepared and immunoprecipitated with an anti-HIF-1α antibody and
coprecipitated PHD2 was detected with an anti-PHD2 antibody.
Hypoxia attenuated the interaction between HIF-1α and PHD2.
Treatment of the cells with 2-deoxy-D-ribose augmented the
interaction between HIF-1α and PHD2 under hypoxic conditions
(Fig. 4A).
Effect of 2-deoxy-D-ribose on the levels
of hydroxy-HIF-1a
HIF-1α can be hydroxylated by proline hydroxylase
under normoxic conditions. Hydroxylation of HIF-1α leads to its
binding to VHL and ubiquitin-mediated degradation (30,31).
To further characterize the mechanisms leading to decreased HIF-1α
levels by 2-deoxy-D-ribose, we analyzed the levels of
hydroxy-HIF-1α. Direct analysis of the hydroxylation state of
HIF-1α was carried out using an antibody specifically directed
against hydroxy-HIF-1α. To visualize hydroxy-HIF-1α, the cells were
treated with the proteasome inhibitor MG-132 to prevent the
proteasomal degradation of HIF-1α. As shown in Fig. 4B, 2-deoxy-D-ribose increased the
amounts of hydroxy-HIF-1α in the presence of MG-132. This finding
suggests that 2-deoxy-D-ribose enhances proline hydroxylase
activity.
Discussion
Previous studies have demonstrated that TP confers
resistance to apoptosis induced by hypoxia and that the enzymatic
activity of TP is required for this effect. 2-Deoxy-D-ribose, a
degradation product of thymidine generated by TP activity, can also
prevent hypoxia-induced apoptosis in human KB epidermoid carcinoma
cells (11,13) suggesting that it may be a downstream
mediator of the TP function. 2-Deoxy-D-ribose prevented the
hypoxia-induced activation of caspase-3 and -9 in HL-60 cells
(12). TP-overexpressing Jurkat
cells were also resistant to hypoxia-induced apoptosis. The
induction of caspase-3 activity and the expression of HIF-1α and
BNIP3 were found to be suppressed under hypoxic conditions in
TP-expressing Jurkat cells (14).
HIF-1α protein is rapidly accumulated under hypoxia
and degraded under a normoxic condition (15–17).
The accumulated HIF-1α under hypoxia dimerizes with HIF-1β and
translocates into the nucleus. HIF-1 binds to the hypoxia response
element (HRE) on nuclear DNA, recruits coactivators p300/CBP, and
promotes gene transcription of various genes (18,19).
Many tumors contain a hypoxic microenvironment, a condition
associated with resistance to anticancer agents and poor prognosis.
HIF-1α is upregulated in a broad range of tumors and is involved in
angiogenesis, invasion and altered energy metabolism (20). There appears to be a delicate
balance between the pro- and anti-tumorigenic effects of HIF-1α.
Several gene products such as BNIP3, RTP801 and Noxa were
identified as HIF-1α-responsive pro-apoptotic proteins.
Hypoxia-mediated BNIP3 expression is regulated by HIF-1α that
directly binds to a consensus HRE in the BNIP3 promoter
(21). Overexpression of BNIP3 has
been shown to be cytotoxic in a number of tumor cell lines
(22–25). Mitochondria may be a direct target
of death signals mediated via BNIP3 under hypoxic conditions. BNIP3
induced by HIF-1α binds to mitochondria and opens the mitochondrial
permeability transition pore (26).
BNIP3 is a key regulator of mitochondrial function and cell death
of ventricular myocytes during hypoxia (26). We previously demonstrated that TP
suppressed the level of BNIP3 under hypoxic conditions, and
2-deoxy-D-ribose, a downstream mediator of the TP function,
accelerated the proteasome-mediated degradation of HIF-1α by
enhancing the ubiquitination under hypoxic conditions (12,14).
O2-dependent regulation of the HIF-1α
protein is mediated by a functional domain of 200 amino acids
located on the carboxy terminal to the PAS domain, which was named
the oxygen-dependent degradation (ODD) domain (27). HIF-1α prolyl hydroxylases utilize
O2 and α-ketoglutarate as a substrate to generate
4-hydroxyproline at residues 402 and/or 546 of human HIF-1α
(28,29). Three such prolyl hydroxylases 1–3
(PHD1–3) were identified in mammalian cells. Under normoxic
conditions, the hydroxylation of specific proline residues of
HIF-1α by PHD2 promotes the interaction of HIF-1α with the VHL
protein and consequently ubiquitination and proteasomal degradation
of HIF-1α (30,31). Although PHD1 and PHD3 can
hydroxylate HIF-1α in vitro, HIF does not appear to be their
physiological target in the cell (32). During hypoxia, the reduced levels of
proline hydroxylation lead to HIF-1α degradation, causing an
increase in its levels.
2-Deoxy-D-ribose had no effect on HIF-1α mRNA
expression levels indicating that 2-deoxy-D-ribose affected the
level of HIF-1α at the protein level. In a previous study, we also
demonstrated that 2-deoxy-D-ribose accelerated proteasome-mediated
degradation of HIF-1α by enhancing the ubiquitination of HIF-1α
(12). In this study, we
demonstrated that 2-deoxy-D-ribose increased the interaction of
HIF-1α with VHL and PHD2 under hypoxic conditions, and levels of
proline hydroxy-HIF-1α (Figs. 2 and
4). A potential mechanism of the
enhanced levels of proline hydroxy-HIF-1α by 2-deoxy-D-ribose might
be the increased level of α-ketoglutarate. The cosubstrates oxygen
and α-ketoglutarate as well as the cofactors Fe2+ and
ascorbate are required for PHD activity. 2-Deoxy-D-ribose is
phosphorylated to 2-deoxy-D-ribose 5-phosphate which is then
cleaved by deoxy-D-ribose phosphate aldolase to acetoaldehyde and
glyceraldehyde-3-phosphate, which then produces pyruvate.
α-ketoglutarate and alanine are produced by enzymatic
transamination reaction between glutamate and pyruvate (33,34).
α-Ketoglutarate produced by 2-deoxy-D-ribose may have increased PHD
activity.
2-Deoxy-D-ribose is produced by the catalytic action
of TP and is the downstream mediator of TP function. TP is
expressed at higher levels in a wide variety of solid tumors when
compared to adjacent non-neoplastic tissues. TP may thus play an
important role in the progression of tumors by producing
2-deoxy-D-ribose from thymidine and decreasing the level of
HIF-1α.
References
|
1
|
Iltzsch MH, Kouni MH and Cha S: Kinetic
studies of thymidine phosphorylase from mouse liver. Biochemistry.
24:6799–6807. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Furukawa T, Yoshimura A, Sumizawa T,
Haraguchi M, Akiyama S, Fukui K, Ishizawa M and Yamada Y:
Angiogenic factor. Nature. 356:6681992. View Article : Google Scholar
|
|
3
|
Sumizawa T, Furukawa T, Haraguchi M,
Yoshimura A, Takeyasu A, Ishizawa M, Yamada Y and Akiyama S:
Thymidine phosphorylase activity associated with platelet-derived
endothelial cell growth factor. J Biochem. 114:9–14.
1993.PubMed/NCBI
|
|
4
|
Haraguchi M, Miyadera K, Uemura K,
Sumizawa T, Furukawa T, Yamada K, Akiyama S and Yamada Y:
Angiogenic activity of enzymes. Nature. 368:1981994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ishikawa F, Miyazono K, Hellman U, Drexler
H, Wernstedt C, Hagiwara K, Usuki K, Takaku F, Risau W and Heldin
CH: Identification of angiogenic activity and the cloning and
expression of platelet-derived endothelial cell growth factor.
Nature. 338:557–562. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyazono K, Okabe T, Urabe A, Takaku F and
Heldin CH: Purification and properties of an endothelial cell
growth factor from human platelets. J Biol Chem. 262:4098–4103.
1987.PubMed/NCBI
|
|
7
|
Miyadera K, Sumizawa T, Haraguchi M,
Yoshida H, Konstanty W, Yamada Y and Akiyama S: Role of thymidine
phosphorylase activity in the angiogenic effect of platelet-derived
endothelial cell growth factor/thymidine phosphorylase. Cancer Res.
55:1687–1690. 1995.PubMed/NCBI
|
|
8
|
Takebayashi Y, Akiyama S, Akiba S, Yamada
K, Miyadera K, Sumizawa T, Yamada Y, Murata F and Aikou T:
Clinicopathologic and prognostic significance of an angiogenic
factor, thymidine phosphorylase, in human colorectal carcinoma. J
Natl Cancer Inst. 88:1110–1117. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takebayashi Y, Yamada K, Miyadera K,
Sumizawa T, Furukawa T, Kinoshita F, Aoki D, Okumura H, Yamada Y,
Akiyama S and Aikou T: The activity and expression of thymidine
phosphorylase in human solid tumours. Eur J Cancer. 32A:1227–1232.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takebayashi Y, Miyadera K, Akiyama S,
Hokita S, Yamada K, Akiba S, Yamada Y, Sumizawa T and Aikou T:
Expression of thymidine phosphorylase in human gastric carcinoma.
Jpn J Cancer Res. 87:288–295. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kitazono M, Takebayashi Y, Ishitsuka K,
Takao S, Tani A, Furukawa T, Miyadera K, Yamada Y, Aikou T and
Akiyama S: Prevention of hypoxia-induced apoptosis by the
angiogenic factor thymidine phosphorylase. Biochem Biophys Res
Commun. 253:797–803. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ikeda R, Furukawa T, Kitazono M, Ishitsuka
K, Okumura H, Tani A, Sumizawa T, Haraguchi M, Komatsu M, Uchimiya
H, Ren XQ, Motoya T, Yamada K and Akiyama S: Molecular basis for
the inhibition of hypoxia-induced apoptosis by 2-deoxy-D-ribose.
Biochem Biophys Res Commun. 291:806–812. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ikeda R, Che XF, Ushiyama M, Yamaguchi T,
Okumura H, Nakajima Y, Takeda Y, Shibayama Y, Furukawa T, Yamamoto
M, Haraguchi M, Sumizawa T, Yamada K and Akiyama S:
2-Deoxy-D-ribose inhibits hypoxia-induced apoptosis by suppressing
the phosphorylation of p38 MAPK. Biochem Biophys Res Commun.
342:280–285. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ikeda R, Tajitsu Y, Iwashita K, Che XF,
Yoshida K, Ushiyama M, Furukawa T, Komatsu M, Yamaguchi T,
Shibayama Y, Yamamoto M, Zhao HY, Arima J, Takeda Y, Akiyama S and
Yamada K: Thymidine phosphorylase inhibits the expression of
proapoptotic protein BNIP3. Biochem Biophys Res Commun.
370:220–224. 2008.PubMed/NCBI
|
|
15
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl
Acad Sci USA. 92:5510–5514. 1995.PubMed/NCBI
|
|
16
|
Jiang BH, Semenza GL, Bauer C and Marti
HH: Hypoxia-inducible factor 1 levels vary exponentially over a
physiologically relevant range of O2 tension. Am J
Physiol. 271:C1172–C1180. 1996.PubMed/NCBI
|
|
17
|
Salceda S and Caro J: Hypoxia-inducible
factor 1α (HIF1α) protein is rapidly degraded by the
ubiquitin-proteasome system under normoxic conditions. Its
stabilization by hypoxia depends on redox-induced changes. J Biol
Chem. 272:22642–22647. 1997.
|
|
18
|
Semenza GL, Jiang BH, Leung SW, Passantino
R, Concordet JP, Maire P and Giallongo A: Hypoxia response elements
in the aldolase A, enolase 1, and lactate dehydrogenase A gene
promoters contain essential binding sites for hypoxia-inducible
factor 1. J Biol Chem. 271:32529–32537. 1996.
|
|
19
|
Ebert BL and Bunn HF: Regulation of
transcription by hypoxia requires a multiprotein complex that
includes hypoxia-inducible factor 1, an adjacent transcription
factor, and p300/CREB binding protein. Mol Cell Biol. 18:4089–4096.
1998.
|
|
20
|
Carmeliet P, Dor Y, Herbert JM, Fukumura
D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R,
Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D and
Keshert E: Role of HIF-1α in hypoxia-mediated apoptosis, cell
proliferation and tumour angiogenesis. Nature. 394:485–490.
1998.
|
|
21
|
Kothari S, Cizeau J, McMillan WE, Israels
SJ, Bailes M, Ens K, Kirshenbaum LA and Gibson SB: BNIP3 plays a
role in hypoxic cell death in human epithelial cells that is
inhibited by growth factors EGF and IGF. Oncogene. 22:4734–4744.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Graham RM, Frazier DP, Thompson JW, Haliko
S, Li H, Wasserlauf BJ, Spiga MG, Bishopric NH and Webster KA: A
unique pathway of cardiac myocyte death caused by hypoxia-acidosis.
J Exp Biol. 207:3189–3200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen G, Ray R, Dubik D, Shi L, Cizeau J,
Bleackley RC, Saxena S, Gietz RD and Greenberg AH: The E1B
19K/Bcl-2-binding protein Nip3 is a dimeric mitochondrial protein
that activates apoptosis. J Exp Med. 186:1975–1983. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen G, Cizeau J, Velde CV, Park JH, Bozek
G, Bolton J, Shi L, Dubik D and Greenberg A: Nix and Nip3 form a
subfamily of pro-apoptotic mitochondrial proteins. J Biol Chem.
274:7–10. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Velde CV, Cizeau J, Dubik D, Alimonti J,
Brown T, Israels S, Hakem R and Greenberg AH: BNIP3 and genetic
control of necrosis-like cell death through the mitochondrial
permeability transition pore. Mol Cell Biol. 20:5454–5468. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Regula KM, Ens K and Kirshenbaum LA:
Inducible expression of BNIP3 provokes mitochondrial defects and
hypoxia-mediated cell death of ventricular myocytes. Circ Res.
91:226–231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang LE, Gu J, Schau M and Bunn HF:
Regulation of hypoxia-inducible factor 1alpha is mediated by an
O2 dependent degradation domain via the ubiquitin
proteasome pathway. Proc Natl Acad Sci USA. 95:7987–7992. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bruick RK and McKnight SL: A conserved
family of prolyl-4-hydroxylases that modify HIF. Science.
294:1337–1340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Epstein AC, Gleadle JM, McNeill LA,
Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI,
Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R,
Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ and Ratcliffe PJ:
C. elegans EGL9 and mammalian homologs define a family of
dioxygenases that regulate HIF by prolyl hydroxylation. Cell.
107:43–54. 2001. View Article : Google Scholar
|
|
30
|
Ivan M, Kondo K, Yang H, Kim W, Valiando
J, Ohh M, Salic A, Asara JM, Lane WS and Kaelin WG Jr: HIFα
targeted for VHL-mediated destruction by proline hydroxylation:
implications for O2 sensing. Science. 292:464–468.
2001.
|
|
31
|
Jaakkola P, Mole DR, Tian YM, Wilson MI,
Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji
M, Schofield CJ, Maxwell PH, Pugh CW and Ratcliffe PJ: Targeting of
HIF-α to the von Hippel-Lindau ubiquitylation complex by
O2-regulated prolyl hydroxylation. Science. 292:468–472.
2001.
|
|
32
|
Berra E, Benizri E, Ginouves A, Volmat V,
Roux D and Pouyssegur J: HIF prolyl-hydroxylase 2 is the key oxygen
sensor setting low steady-state levels of HIF-1α in normoxia. EMBO
J. 22:4082–4090. 2003.PubMed/NCBI
|
|
33
|
Feron O: Pyruvate into lactate and back:
from the Warburg effect to symbiotic energy fuel exchange in cancer
cells. Radiother Oncol. 92:329–333. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
DeBerardinis RJ, Mancuso A, Daikhin E,
Nissim I, Yudkoff M, Wehrli S and Thompson CB: Beyond aerobic
glycolysis: transformed cells can engage in glutamine metabolism
that exceeds the requirement for protein and nucleotide synthesis.
Proc Natl Acad Sci USA. 104:19345–19350. 2007. View Article : Google Scholar
|