Introduction
Retinoblastoma (RB) is the most common intraocular
malignancy that affects young children (1–3). It is
a rare disease with a 1:15,000/1:20,000 incidence worldwide
(4). The mortality rate ranges from
30 to 60% in developing countries, in opposition to 10% in other
countries such as the USA (4–9). The
low survival is due mainly to its late diagnosis (3,10).
Before the 1990’s, RB treatment consisted of the
removal of the eye and radiation, but the loss of eyes and facial
deformity as a consequence of the treatment resulted in severe
morbidity for the young patients (11). After the 1990’s, chemotherapy became
an effective treatment for cancer (12,13).
Extensive research has been carried out since then in RB management
to save the life and vision of the child. The most common
chemotherapy protocol currently in use consists of carboplatin,
vincristine and etoposide (14–16).
In general, carboplatin is given in 1 day doses (17–19)
and is then stopped for several weeks. During this interval, the
other anti-neoplastic drugs can be administered. The application of
these anti-neoplastic drugs aims to reduce the tumor size
(chemoreduction) but a second focal treatment such as cryotherapy
or brachytherapy is normally used giving this combined therapy a
positive prognosis. Adequate tumor reduction requires 2–6 cycles of
chemotherapy (20).
However, the systemic treatment of RB is effective
at the beginning of therapy but the long term use of chemotherapy
may be limited as these drugs cause serious adverse side-effects,
including myelosuppression, ototoxicity, nephrotoxicity and anemia
(21–24). In addition, tumor size is reduced by
only 3% after the third chemotherapeutic cycle, compared with a 30%
reduction after the first drug application (25). This suggests that chemotherapy
effectiveness in RB patients drops over time, increasing the risk
of developing multidrug-resistant RB cells, which increases the
chances of tumor re-growth and secondary metastases, thereby
limiting the application of chemotherapy in the treatment for RB
(26–28). As a result, there is a need for
alternative drugs that are effective, selective and with fewer
adverse side-effects for RB treatment to overcome the limitations
of the current chemotherapeutic drugs.
Artemisinin is a promising drug to test
anti-neoplastic activity against RB. Artemisinin is an herbal drug
that has been used in traditional Chinese medicine for thousands of
years (29) and it is clinically
used as an anti-malarial drug (30). Artemisinin has low solubility in
water or oil, poor bioavailability, and a short half-life in
vivo(30,31). However, some semi-synthetic
derivatives have been developed, such as artesunate,
dihydroartemisinin, artemether and arteether, that overcome the
problems associated with the natural product (30,31).
In recent years, artemisinin and its derivatives (Arts) have been
shown to inhibit cell growth in various types of cancer and cancer
cells, such as leukemia, fibrosarcoma, ovarian, breast cancer and
cervical cancer cells (32–35). In RB, the cytotoxicity and
specificity of these compounds has not been studied. It is a
promising drug to test anti-neoplastic activity against RB as Arts
induce practically no side-effects and are therefore suitable for a
long-term use (36).
Although the underlying mechanism is not clearly
known, it is likely that Arts work by a multiple mechanisms
dependent on iron (37). Several
studies suggested that the antitumor and anti-malarial activities
of Arts appear to be exerted through oxidative damage (38), by blocking the cell cycle
progression (35,39), by induction of cell apoptosis
(32), and others (40). Iron is a key player in the
anticancer activity of Arts as it mediates the production of
oxidative radicals and, also, as iron metabolism promotes cell
growth (34,36). Transferrin receptor 1 (TfR-1, also
known as CD71), a type II transmembrane protein, plays important
roles in the cellular iron uptake and iron metabolism (41). The expression of TfR-1 in cancer
cells is elevated compared to normal tissues, which helps absorb
more iron and keep the proliferative profile of the cancer cells
(42,43). However, it is not clear whether
there is any functional relationship between CD71 and Arts
cytotoxicity.
Therefore, Arts may be a good candidate to treat RB.
Nevertheless, the ability of these drugs to kill cancer cells is
variable and dependent on the tumor cell lines (33,44).
It is unknown whether these drugs could have a cytotoxic action on
RB cell lines. Therefore, in this study, we analyzed the cytotoxic
activity and specificity of artesunate (ART) in an RB cell line, in
comparison with its normal counterpart, the epithelial retina cell
line. We explored the possible relationship between CD71 and its
connection with ART cytotoxicity. In addition, we explored the
effect of ART on cell cycle progression in RB cells. We found that
the cytotoxic action of ART is specific to RB cells in a
dose-dependent manner, with low toxicity in normal retina cells.
Markedly, ART exerted high cytotoxicity in carboplatin-resistant RB
(RB-R) cells. Also, RB had higher CD71 levels at the membrane than
normal retina cells. ART internalization and ART cytotoxic action
was dependent, in part, on the CD71 receptor. In addition, ART
blocked the cell cycle progression at the G1 phase, even at low
doses. In summary, we showed that ART is a promising drug to be
used for RB treatment, highly cytotoxic against RB cells and
multidrug-resistant cells with limited function in normal retina
cells.
Materials and methods
Antibodies and reagents
ART was purchased from Guilin Pharmaceutical Co.,
Ltd. (Guilin, Guangxi, China; H10930195). Carboplatin was purchased
from Qilu Pharmaceutical Co., Ltd. (Jinan, Shandong, China;
H10920028). ART and carboplatin were freshly prepared and diluted
in 5% NaHCO3 to the required concentrations. CD71-FITC
and IgG1-FITC antibodies were purchased from BD Biosciences, USA.
Propidium iodide (PI) was purchased from the Beyotime Institute of
Biotechnology (Shanghai, China). shRNAs were purchased from
Shanghai GenePharma Co., Ltd. (Shanghai, China). Aurum Total RNA
Mini kit, iScript cDNA Synthesis kit, iQ SYBR Green SuperMix kit
were all purchased from Bio-Rad Laboratories, Inc.
Cell lines
Human RB cell line RB-Y79 was purchased from the
American Type Culture Collection (ATCC, Rockville, MD, USA), human
retinal pigment epithelium cell line (hTERT-RPE1) was purchased
from JENNIO Biological Company (Guangzhou, China). The cells were
cultured and passaged advisably in Complete Media (RPMI-1640
medium; Gibco, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco, Australia), 1% Penicillin-Streptomycin Solution (Gibco, USA)
at 37°C in a humidified atmosphere of 5% CO2.
Carboplatin-resistant RB-Y79 cells
RB-Y79 cells in logarithmic phase were incubated
with 40 μg/ml at 37°C in a humidified 5% CO2 incubator
for 2 h. After centrifuging and washing, the medium containing the
drug was discarded. Cells were then cultured in complete culture
medium. Once the culture growth was in the logarithmic phase again
the carboplatin treatment was repeated. The same procedure was
reiterated for several months until a stable resistant cell line at
40 μg/ml carboplatin was generated. To test RB cell resistance to
carboplatin, RB-Y79 and RB-R cells were cultured in the presence of
10, 20, 40, 50, 60, 70, 80, 90 or 100 μg/ml carboplatin
respectively. After 24 h, cell numbers were counted in the culture
using Counter Star with the Automated Cell Counter software
(unpublished data).
Cytotoxicity assay
To assess the potential inhibitory capacity of ART,
3×104 RB-Y79 and hTERT-RPE1 cells were seeded in
triplicate in 96-well plates at a density of 1×106
cells/ml. Four hours later, cells were cultured with different
concentrations of ART (0, 12.5, 25, 50, 100 and 200 μg/ml) and
carboplatin (50 μg/ml) for 24 and 48 h, respectively. Following
collection, cells were washed twice with PBS and stained with PI
(final concentration at 1 μg/ml) for 10 min in the dark, at room
temperature. Cytotoxicity analysis was carried out using the
FACSCalibur flow cytometer. Data were analyzed with CellQuest-Pro
software, and the percentage of dead cells was calculated. In all
experiments the cytotoxic activity was defined as the percentage of
dead cells after treatment minus the natural death percentage of
the respective cell type. To test the carboplatin cytotoxicity on
carboplatin-resistant RB cells, 3×104 cells were seeded
in triplicate in 96-well plates at a density of 1×106
cells/ml and cytotoxicity assay was performed by flow cytometry, as
explained above.
Membrane CD71 expression assay
To determine the CD71 expression level on cell
membrane, RB-Y79 and hTERT-RPE1 cells were seeded in triplicate in
T-25 flasks at a cell density of 1.5×106. Four hours
later, cells were treated with different ART concentrations (final
concentration, 0, 50 and 100 μg/ml). After 10 h, cells were washed
twice with PBS and incubated with CD71-FITC or IgG1-FITC as control
for 30 min at 4°C, according to the manufacturer’s instructions.
The CD71 expression levels were tested by FACSCalibur flow
cytometry, and 1×104 cells were acquired and analyzed
for each sample, respectively, using the CellQuest-Pro software.
The percentage of CD71 positive cells was defined as the CD71
expression levels.
CD71-RNAi
RB and hTERT-RPE1 cells were seeded in triplicate at
3×105/well in a 6-well plate. Transfections were
performed at ~30–50% confluency. Cells were incubated in an
antibiotic-free culture medium for 30 min before transfection.
Cells were transfected with validated shRNAs at 100 nM; scrambled
shRNA was used as a negative control, GAPDH was positive control
shRNA, and 2 CD71 shRNAs (CD71-homo-1569 and CD71-homo-1865), using
Lipofectamine® 2000 (Invitrogen) according to the
manufacturer’s instructions. Cells were cultured for 72 h and used
in the following experiments. Silencing was confirmed using
quantitative real-time PCR analysis (qRT-PCR). The specific
sequences of these shRNAs were: scramble negative control: 5′-UUC
UCC GAA CGU GUC ACG UTT-3′ and 5′-ACG UGA CAC GUU CGG AGA ATT-3′;
GAPDH positive control, 5′-GUA UGA CAA CAG CCU CAA GTT-3′ and
5′-CUU GAG GCU GUU GUC AUA CTT-3′; CD71-homo-1569, 5′-GCC CAG AUG
UUC UCA GAU ATT-3′ and 5′-UAU CUG AGA ACA UCU GGG CTT-3′;
CD71-homo-1865, 5′-GGC CAG CAA AGU UGA GAA ATT-3′ and 5′-UUU CUC
AAC UUU GCU GGC CTT-3′.
Preclusion of off-target effect
The BLAST program is a rapid sequence comparison
tool that uses a heuristic approach to construct alignments by
optimizing a measure of local similarity. It is widely used for
nucleic acid and protein database searches (45). In our experiment, the CD71 shRNAs
were blasted in the PubMed database to preclude off-target
effect.
Total RNA isolation and real-time PCR
analysis
Aurum total RNA mini kit was used to extract total
RNA from cells treated with shRNAs according to the manufacturer’s
instructions; 10 μl of total RNA was used for cDNA synthesis by
using iScript cDNA synthesis kit according to the manufacturer’s
instructions. Real-time PCR was performed sequentially by iQ SYBR
Green SuperMix kit. The primer sequences were: CD71, forward,
5′-ATCTCGGTCATCAGGATTGC-3′ and reverse, 5′-TTAAATGCAGGGACGAAAGG-3′;
GAPDH, forward, 5′-CGCATCTTCTTGTGCAGT-3′ and reverse,
5′-AATGAAGGGGTCGTTGATGG-3′.
Intracellular concentrations of ART
assay
Intracellular ART concentration was performed as
described by Okwelogu et al(47). Briefly, 2×107 RB-Y79
cells were seeded in triplicate in T-75 bottle. After 4 h, cells
were treated with different concentrations of ART (0, 15, 20, 25,
30, 35 and 40 μg/ml). After 24 h, cells were collected and
resuspended in 500 μl PBS. Repetitive freeze thaw method using
liquid nitrogen was used to extract cells, followed by alkaline
hydrolysis treatment with 0.1 mol/l NaOH at 83°C for 1 h. The UV
absorbance value at 237 nm was then registered by an ultraviolet
spectrophotometer. An ART standard curve was drawn under the same
conditions. Intracellular ART concentrations were calculated by
standard curve (47).
Cytotoxicity assay after CD71-RNAi
To test the cytotoxicity on RB cells,
3×104 cells were seeded in triplicate in 96-well plates
at a density of 1×106 cells/ml. After 4 h, cytotoxicity
analysis was tested by flow cytometry as explained above.
Cell cycle analysis
RB and hTERT-RPE1 cells were seeded in T-25 culture
bottle with 5×105 cells. After 24 h, cells were treated
with different ART concentrations (final concentration, 0, 5, 10,
15 and 20 μg/ml) for 24 h. Following collection, cells were washed
twice with PBS and fixed with 80% ice-cold ethanol overnight at
−20°C. The fixed cells were collected and incubated for 30 min in
PBS containing 50 μg/ml RNase A at 37°C, stained with 50 μg/ml PI
and 0.2% Triton X-100 for 10 min in the dark at room temperature.
DNA content analysis were carried out using the FACSCalibur flow
cytometer. G0/G1, S, G2/M cell cycle phase were analyzed with
ModFit software (Verity Software House, Topsham, ME, USA).
Statistical analysis
All data are presented as means ± SD. The
significance of the difference between groups was evaluated by
Paired t-test and one-way repeated measures analysis of variance
(ANOVA) and multiple comparisons with SPSS 17.0 software. A value
of P<0.05 was considered to indicate a statistically significant
difference.
Results
Specific ART cytotoxicity in the RB cell
line
The effect of ART in RB, either in vitro or
in vivo, has not been tested. We compared the cytotoxicity
of ART in an RB cell line (RB-Y79) vs. a normal retina pigment
epithelium cell line (hTERT-RPE1). Several ART concentrations
ranging from 12.5 to 200 μg/ml were used and the proportion of dead
cell was measured using flow cytometric analysis (FCM) at 24 and 48
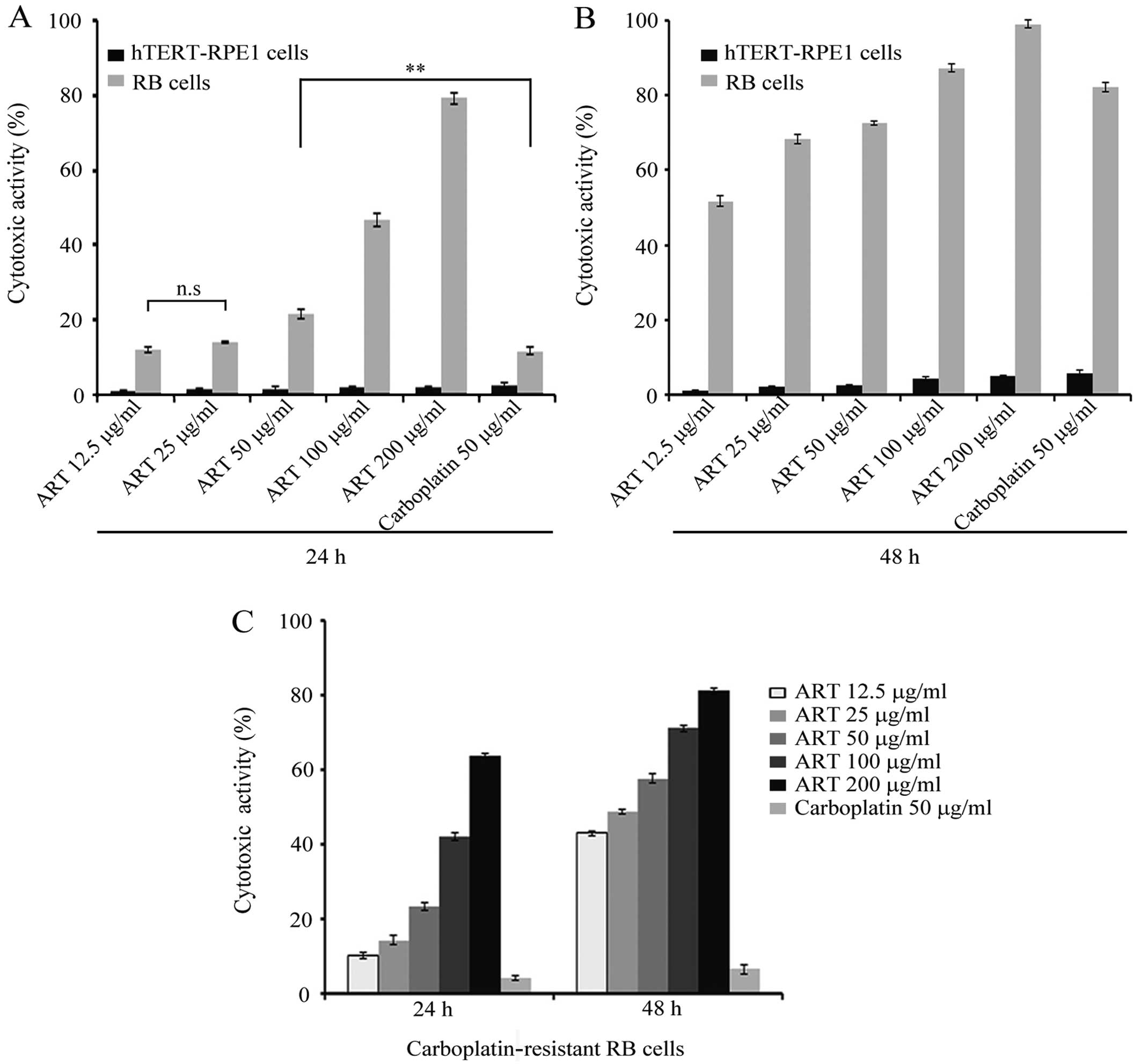
h as described in Materials and methods. At 12.5 μg/ml of ART,
17.5% of RB cells were dead after 24 h, as shown in Fig. 1A. A similar proportion was observed
when 25 μg/ml ART was used. However, the average for RB cell death
increased with higher ART doses. After 48 h, the number of RB dead
cells increased 2–3 times significantly compared with 24 h
treatment (Fig. 1B). ART killed
only a small proportion of hTERT-RPE1 cells, with values slightly
higher at 48 h than those observed with 24 h ART treatment.
This result shows that ART has a cytotoxic effect in
a dose-dependent manner against the RB cell line with negligible
effect on the normal retina cell line.
ART cytotoxicity in the
carboplatin-resistant RB cells
Carboplatin is one of the chemotherapeutic drugs
commonly used for RB treatment in the clinic (14). However, the effective drug
concentration used in clinical treatment causes unwanted secondary
effects (23). We compared the
cytotoxic activity of carboplatin and ART in the RB and hTERT-RPE1
cell lines. We tested the cytotoxicity of 50 μg/ml carboplatin on
both cell lines for 24 and 48 h (48,49).
The results showed that only 15% of RB cells died after 24 h
carboplatin treatment. This proportion was significantly lower than
that at the same ART concentration (P<0.001) (Fig. 1A). After 48 h, carboplatin
cytotoxicity increased up to 80%, similar to that observed at the
same ART concentration (Fig.
1B).
The generation of drug-resistant cells is considered
an important factor in the failure of chemotherapeutic cancer
treatment. A distinctive characteristic of RB is the fact that it
has high expression levels of the drug-resistant proteins that have
been suggested to confer resistance (at least in RB cell lines) to
drugs used commonly in the clinic for cancer treatment (21,23,50,51).
The relationship between the expression of those proteins and the
clinical outcome after chemotherapy treatment in RB remains unclear
(50,51). Nevertheless, we next explored
whether ART was capable of killing RB-R cells. We generated an RB
cell line unresponsive to 40 μg/ml of carboplatin in the laboratory
(as described in Materials and methods). This cell population was
shown to be unresponsive to 24 h treatment with 40 μg/ml of
carboplatin (data not shown). Therefore, we tested the cytotoxicity
in RB-R cells at different ART concentrations and 50 μg/ml
carboplatin for 24 and 48 h. As shown in Fig. 1C, ~15% of the RB-R cells were killed
by 12.5 μg/ml ART, in contrast to the 5% cell death observed in 50
μg/ml of carboplatin treatment. ART cytotoxicity on RB-R cells
increased with higher ART concentrations. After 48 h of treatment,
the cytotoxicity was significantly greater with all the ART
concentrations tested. However, carboplatin cytotoxicity on RB-R
remained low and similar at any incubation time tested (Fig. 1C).
Taken together, our results suggest that ART is
effective against RB cells in a dose-dependent manner and, more
importantly, it is capable of effectively killing RB-R cells.
Relative CD71 expression levels in RB and
hTERT-RPE1 cell lines
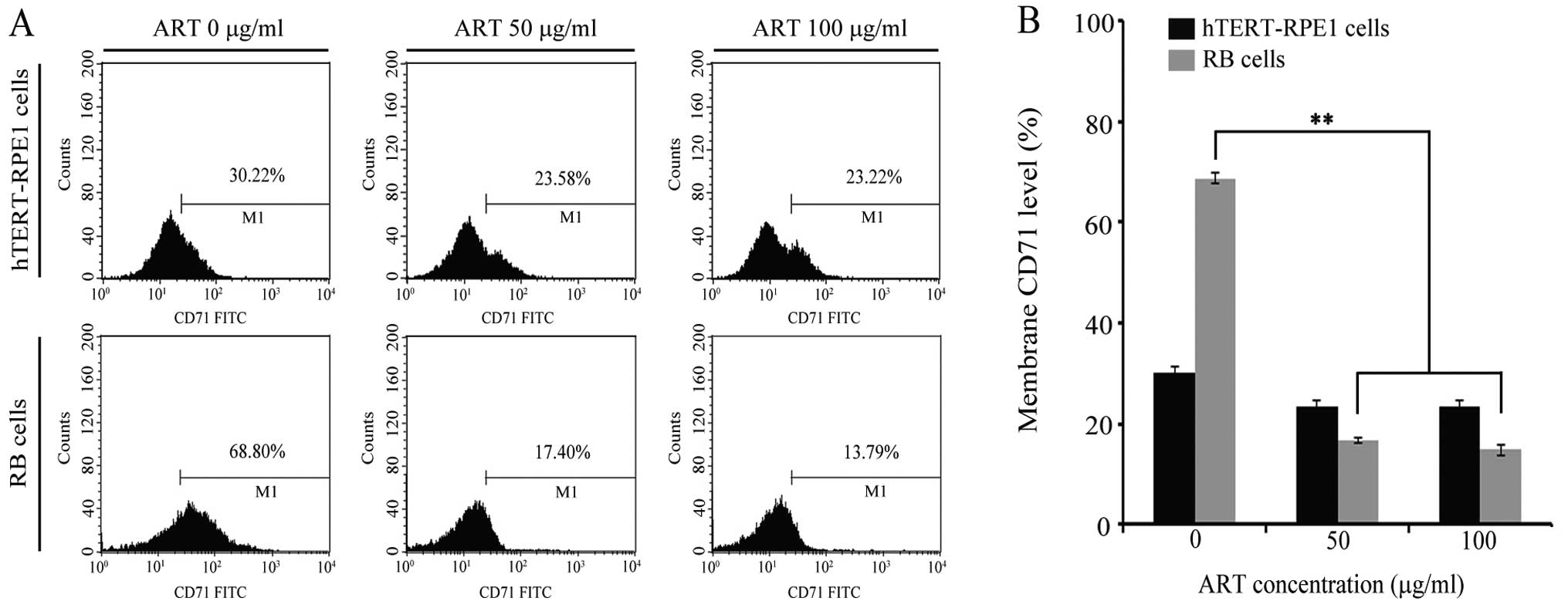
We next explored whether CD71 expression was related
to ART activity in RB cells. We quantitatively compared CD71
protein expression at the cell membrane in RB and hTERT-RPE1 cells
(Fig. 2). The relative level of
CD71 (in the absence of ART) in RB cells reached ~70%, displaying
values 2 times lower in hTERT-RPE1 cell lines (Fig. 2B). Next, RB and hTERT-RPE1 were
incubated with 50 μg/ml ART and 100 μg/ml ART for 10 h. We first
set up a curve dose response in RB cells and the cytotoxicity was
tested at different times ranging from 4 to 24 h (data not shown).
Based on the results, we selected the proper time for the drug to
cause <5% death. Therefore, we considered 10 h to be an
appropriate length of time for the drug to exert molecular action
without killing the cells. The CD71 level at the cell membrane was
measured in 1×104 cells in any experimental conditions
by FCM (Fig. 2). As shown in
Fig. 2, ART had no effect on CD71
at the cell membrane in hTERT-RPE1 cell lines. However, CD71
protein at the membrane decreased 4 times when RB cells were
incubated with ART, regardless of the dose, as demonstrated by FCM
(Fig. 2). These results show that
ART induced the CD71 downregulation at the cell membrane in RB
cells.
ART internalization depends partly on
CD71
In order to get an insight into the specific
cytotoxicity in the RB cell line and its relation with CD71, we
quantified the intracellular ART concentration. Therefore, we
proposed that if CD71 is involved in the internalization of ART,
then it would be expected that: i) cells with the highest surface
expression of CD71 will have higher intracellular levels of ART,
and ii) that suppressing CD71 expression in RB cells will render
these cells unresponsive to ART. To verify these, intracellular ART
concentration was measured by FCM in RB and hTERT-RPE1 cells after
the addition to the media culture of increasing doses of ART
ranging from 15 to 40 μg/ml. The results showed that intracellular
ART in RB cells increased with the extracellular ART dose. However,
low levels of ART were found inside the hTERT-RPE1 cell lines
(Fig. 3A) regardless of the dose
used.
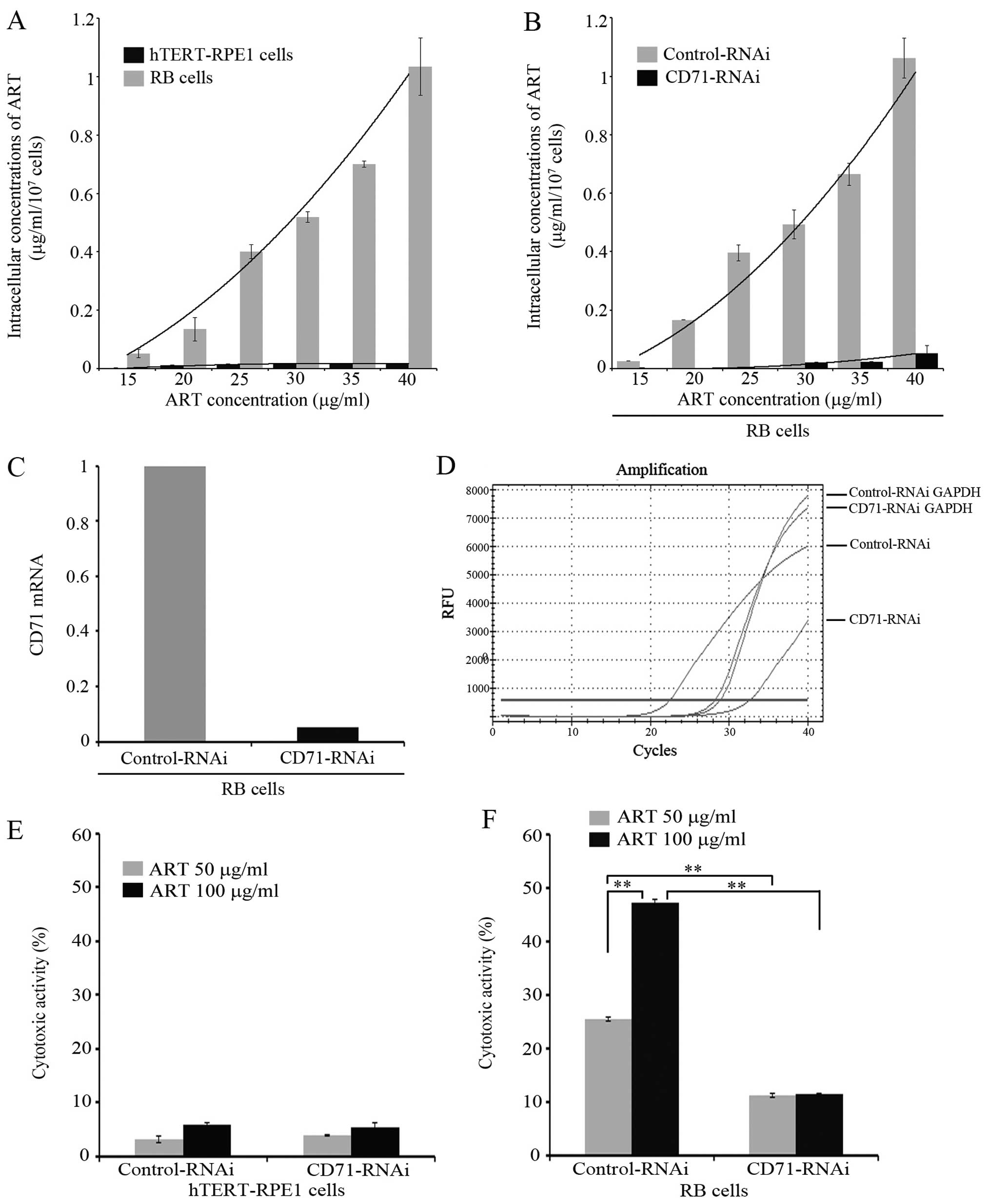 | Figure 3Intracellular concentration of
artesunate (ART) and cytotoxicity assay on retinoblastoma (RB) Y79
and hTERT-RPE1 cells after CD71-RNAi. (A) RB-Y79 and hTERT-RPE1
cells were treated with different concentrations of ART. After 24
h, intracellular concentration of ART was determined by ultraviolet
spectrophotometer at 237 nm. The intracellular concentration of ART
was calculated according to standard curve. Results are presented
as the mean ± SD (n=3). (B) RB-Y79 cells after CD-71 RNAi were
treated with different concentrations of ART. After 24 h,
intracellular concentration of ART was determined by ultraviolet
spectrophotometer at 237 nm. The intracellular concentration of ART
was calculated according to standard curve. Results are presented
as the mean ± SD (n=3). (C) RB-Y79 cells were transfected with
scrambled negative control shRNA, GAPDH positive control shRNA and
CD71-homo-1569/1865 shRNAs and detected by qRT-PCR. CD71 mRNA level
in CD71-RNAi RB-Y79 cells was calculated compared to the controls
treated with scrambled shRNA using GAPDH mRNA as control. (D) CD71
mRNA real-time curves of different groups treated with scrambled
negative control shRNA, GAPDH positive control shRNA and
CD71-homo-1569/1865 shRNAs in RB cells by qRT-PCR were performed.
(E) hTERT-RPE1 cells after CD71-RNAi were treated with different
concentrations of ART. After 24 h, cells were collected, stained
with propidium iodide (PI) (1 μg/ml) and 10,000 cells were measured
respectively by flow cytometry on a BD FACSCalibur. Cytotoxicity is
expressed as the percentage of dead cells, relative to the total
cell number in the culture corrected by subtracting the natural
cell death observed in the untreated control culture. Results are
presented as the mean ± SD (n=3). (F) RB-Y79 cells after CD71-RNAi
were treated with different concentrations of ART. After 24 h,
cells were collected, stained with PI (1 μg/ml) and 10,000 cells
were measured respectively by flow cytometry on a BD FACSCalibur.
Cytotoxicity is expressed as the percentage of dead cells, relative
to the total cell number in the culture corrected by subtracting
the natural cell death observed in the untreated control culture.
Results are presented as the mean ± SD (n=3,
**P<0.01). |
Since both the RB cell line and its normal
counterpart, the hTERT-RPE1 cell line, have different levels of
CD71 expression at the cell membrane, we further explored whether
the CD71 protein expression levels were correlated with ART
internalization in the RB cell line. To address this issue, we
knocked down CD71 in the RB cell line by using the RNA interference
technique (RNAi). We used siRNAs validated by the commercial
company (GenePharma Co., Ltd.) and they were blasted in the PubMed
database against CD71 to preclude off-target effect. Two
non-overlapping shRNAs were used for the CD71 and scramble shRNA
was used as a negative control (as explained in Materials and
methods). For siRNA validation, we used a quantitative RT-PCR. The
qRT-PCR was carried out using the housekeeping GAPDH mRNA in
parallel to facilitate comparison with the relative levels of CD71
transcripts purified from RB RNAi-treated cells. After 2 days of
siRNA treatment, undetectable CD71 mRNA amplification product was
obtained; only a negligible CD71 mRNA amount was detected at a very
high cycle number. This result showed that the mRNA knockdown
efficiency is acceptable (Fig. 3C and
D) under our experimental conditions. The intracellular ART
concentration was quantified by FCM in control RNAi and CD71
RNAi-treated cells. Intracellular ART increased with higher ART
concentration in the culture media in control RB cells but only a
low level of ART was found inside the cells in the CD71 knockdown
conditions (Fig. 3B). These results
suggest that CD71 may be involved in ART internalization.
Specific ART cytotoxicity is mediated by
CD71
We next explored whether CD71 was implicated in ART
cytotoxicity. Cytotoxicity was evaluated in RB and hTERT-RPE1 cells
in both CD71 RNAi and scrambled control conditions (Fig. 3E and F). A low proportion of cell
death was observed in control RNAi in hTERT-RPE1 cells, showing
values similar to those observed in Fig. 1A at 50 and 100 μg/ml ART. No
differences in cell death were observed in the CD71 knockdown cells
in the 2 ART concentrations tested. Moreover, significantly higher
values of cell death were measured in RB cells ranging from 20 to
50% at 50 and 100 μg/ml ART, respectively, consistent with the data
from Fig 1A. Conversely, a
significant 2.5-fold reduction in cytotoxicity was evident in the
CD71 RNAi-treated RB cells. These results suggested that ART
cytotoxicity could be explained in part by the relatively high CD71
protein expression in the RB cell line.
ART induces G1 phase cell cycle arrest in
human RB cells
An association between ART cytotoxicity and cell
cycle was previously reported (35,39).
We sought to determine whether ART has an effect on cell cycle in
RB cells. In order to examine this, we analyzed the cell cycle
phases in RB cells and in a normal retina cell line treated for 24
h with 5, 10, 15 or 20 μg/ml of ART. Since we intended to detect
any alteration in cell cycle without killing the cells, we used ART
concentrations that cause <10% cell death after 24 h of
incubation (Fig. 1A). The cell
cycle was unaffected by ART in the normal cell line at any drug
concentration tested (Fig. 4).
Markedly, a G0/G1 arrest was observed when RB cells were incubated
with ART at 5 μg/ml (Fig. 4A, D and
G) with additional effects at higher ART concentrations
(Fig. 4A and G). Moreover, S phase
was significantly affected by ART incubation (Fig. 4B and E) depicting values nearly 20%
lower than untreated RB cells but reaching values similar to the
hTERT-RPE1 untreated cells (P<0.05) (Fig. 4E). ART effect on S phase seems to be
independent of the drug concentration used in the experiment
(Fig. 4B). On the contrary, mitotic
phase seems to be unaffected regardless of the ART dose used in our
assay. These findings suggest that ART has an effect on G0/G1 and S
phase but no effect on the G2/M phase in the RB cell line.
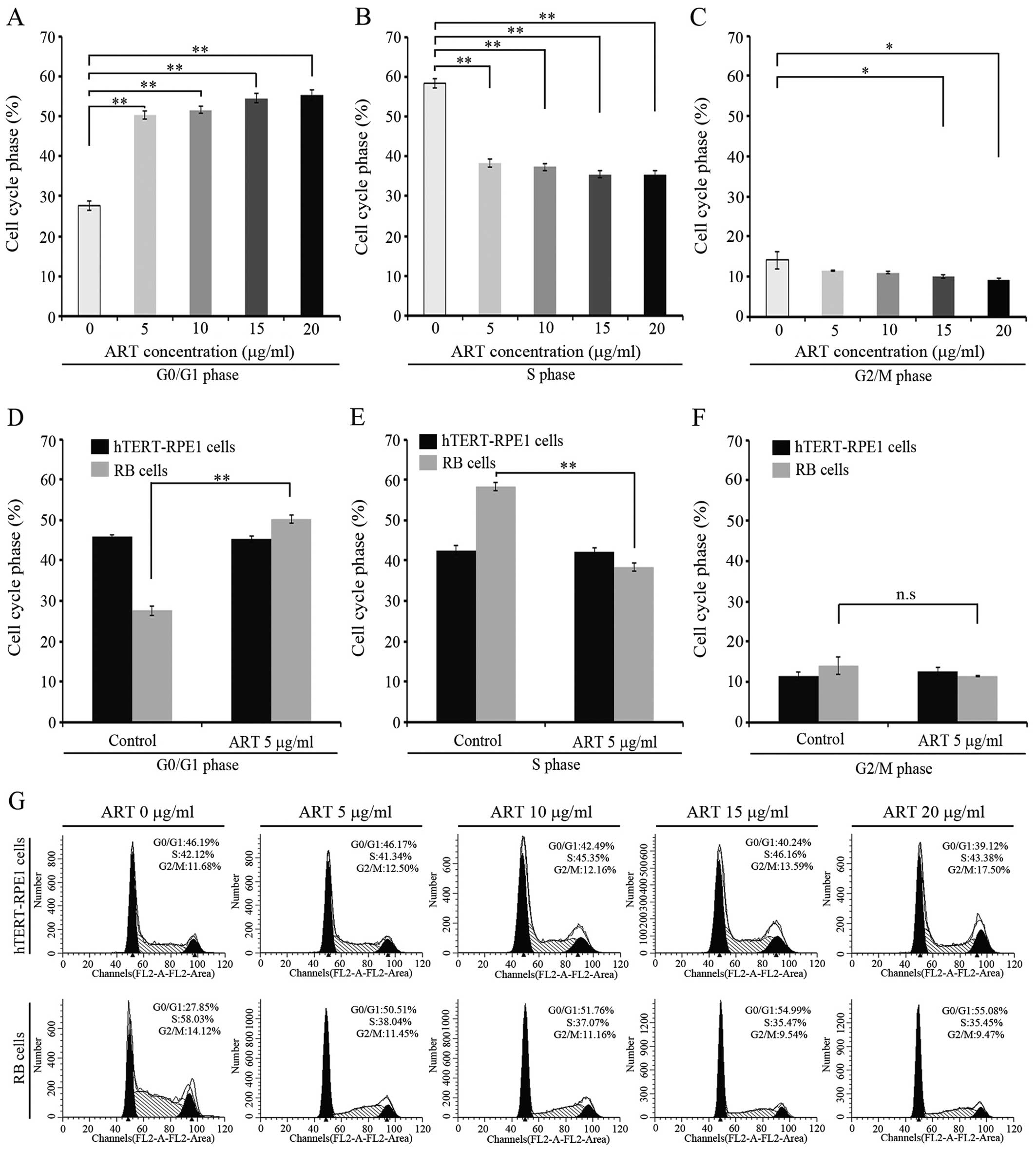 | Figure 4Cell cycle analysis on retinoblastoma
(RB) and hTERT-RPE1 cells. (A) RB-Y79 cells were treated with
different concentrations of artesunate (ART) for 24 h; 15,000 cells
were acquired and G0/G1 cell phase analysis were detected by flow
cytometry on a BD FACSCalibur. The percentage of G0/G1 cell phase
was analyzed using ModFit software compared to the controls treated
with the corresponding amounts of medium. Results are presented as
the mean ± SD (n=3, **P<0.01). (B) RB-Y79 cells were
treated with different concentrations of ART for 24 h; 15,000 cells
were acquired and S cell phase analysis were detected by flow
cytometry on a BD FACSCalibur. The percentage of S cell phase was
analyzed using ModFit software compared to the controls treated
with the corresponding amounts of medium. Results are presented as
the mean ± SD (n=3, **P<0.01). (C) RB-Y79 cells were
treated with different concentrations of ART for 24 h; 15,000 cells
were acquired and G2/M cell phase analysis was detected by flow
cytometry on a BD FACSCalibur. The percentage of G2/M cell phase
was analyzed using ModFit software compared to the controls treated
with the corresponding amounts of medium. Results are presented as
the mean ± SD (n=3, **P<0.01). (D) RB-Y79 and
hTERT-RPE1 cells were treated with 5 μg/ml ART; after 24 h, 15,000
cells were acquired and G0/G1 cell phase analysis was detected by
flow cytometry on a BD FACSCalibur. The percentage of G0/G1 cell
phase was analyzed using ModFit software compared to the controls
treated with the corresponding amounts of medium. Results are
presented as the mean ± SD (n=3, **P<0.01). (E)
RB-Y79 and hTERT-RPE1 cells were treated with 5 μg/ml ART; after 24
h, 15,000 cells were acquired and S cell phase analysis were
detected by flow cytometry on a BD FACSCalibur. The percentage of S
cell phase was analyzed using ModFit software compared to the
controls treated with the corresponding amounts of medium. Results
are presented as the mean ± SD (n=3, **P<0.01). (F)
RB-Y79 and hTERT-RPE1 cells were treated with 5 μg/ml ART; after 24
h, 15,000 cells were acquired and G2/M cell phase analysis was
detected by flow cytometry on a BD FACSCalibur. The percentage of
G2/M cell phase was analyzed using ModFit software comparing to the
controls treated with the corresponding amounts of medium. Results
are presented as the mean ± SD (n=3, **P<0.01). (G)
RB-Y79 and hTERT-RPE1 cells were treated with different
concentrations of ART; after 24 h, cell cycle was detected by flow
cytometry on a BD FACSCalibur. The percentage of different cell
phase was analyzed using ModFit software. Column diagrams are
shown. |
Discussion
This study presents evidence, for the first time,
that artesunate (ART) exerts a strong and selective cytotoxicity
against retinoblastoma (RB). First, we showed that ART cytotoxicity
in RB cells increases in a dose-dependent manner while the same
doses cause negligible cell death in normal retina cell lines.
Secondly, ART is more effective in RB than carboplatin with a
markedly strong cytotoxic effect on carboplatin-resistant RB (RB-R)
cells. Thirdly, ART internalization in RB cells is dependent upon
the expression of the transferrin receptor (CD71) and, finally, ART
influences cell cycle progression by arresting RB cells in G1 and
decreasing the proportion of RB cells in the S phase.
In 1992, Deng et al(52) first found that artemisinin and its
derivatives (Arts) have cytotoxicity in a murine leukemia cell line
(P388) (33) and, in the same year,
Sun et al(53) found that
Arts have cytotoxicity in a human hepatoma cell line (SMMC-7721)
and in human gastric carcinoma cells (SGC-7901) but only limited
cytotoxicity in the normal embryonic lung cell line (WI-38).
The first evidence of ART antitumor activity was
reported by Woerdenbag et al(54) and Yang et al(55). Since then, research has focused on
understanding the anti-neoplastic properties of ART. ART has
antitumor activity in a wide range of cell lines with variable
efficacy from one cancer cell line to another (56). For example, ART seems to be less
effective in breast cancer (MCF-7), gastric cancer (MKN) or some
prostate cancer cell lines (such as PC-3) (57,58)
compared with other cancer cells (59). This study provides the first
evidence that ART acts in an RB cell line, and it is specific for
RB with a negligible effect on normal retina cells. A comparison of
ART vs. carboplatin cytotoxicity showed that ART is active at
concentrations similar to those of established antitumor drugs
(59). Moreover, this study showed
that ART cytotoxicity is higher than carboplatin at the same dose.
It has been reported that the Arts are effective against a wide
range of resistant cancer cell lines including doxorubicin,
methotrexate and hydroxyurea-resistant lines with no
cross-resistance (44,60). In the present study we showed that
ART is effective in RB-R cells in a dose-dependent manner. These
characteristics make ART a suitable candidate as an anti-neoplastic
drug for RB treatment.
This study also showed that ART cytotoxicity
increases with longer incubation times. However, the serum
concentration of Arts declines quickly, with a half-life of the
order of an hour (30). Our
experimental evidence has been collected in vitro and it is
therefore only indicative of the pharmacological behavior of the
drug in vivo. The short half-life of ART in serum forces
clinicians to administrate the drug at least daily and typically
several times a day. For example, the WHO-approved adult dose of
ART of 2.4 mg/kg given at 0, 12 and 24 h for malaria management.
Nevertheless, in this study we found a very low cytotoxicity on
normal retina cell lines even after 48 h of ART incubation. Taking
into account the long treatment required for cancer management, we
cannot preclude a harmful effect on a daily administration during
the long period needed to manage an aggressive cancer. However,
clinical trials have shown promising results in cancer patients.
ART administration to a laryngeal squamous cell carcinoma patient
during nine months showed a 70% tumor reduction prolonging and
improving the quality of life of the patient (61).
Sustained proliferation and growth of malignant
cells require a high iron metabolism for cell survival and cancer
progression (62). Transferrin
receptor 1 (CD71) plays a key role in the uptake of iron and
regulation of its intracellular concentration (41,62).
Most cancer cells exhibit an increment in transferrin receptor
expression compared with their normal counterpart. However, CD71
expression levels in cancer cells depend on the cell line. For some
cancer cell lines (such as the astrocytoma U373 cell line) the
transferrin receptor expression is lower than for others cell lines
(such as the leukemia cells, CCRF-CEM), while it is still higher
than its normal (non-malignant) counterpart (37,63–65).
In the present study, we found that 70% of the RB cells expressed
CD71 protein at the plasma membrane and it is more than 2 times
higher than in normal retina cells, suggesting that the CD71
receptor could be a potential target for the ART cytotoxic activity
in RB cells.
Accordingly, experiments from other groups showed
that the use of a monoclonal antibody directed against the
transferrin receptor was able to block artemisinin action in
neoplastic cells (37). This raises
the question of the functional relationship between ART
cytotoxicity and the receptor. It is widely accepted that iron
content and metabolism are relevant in the selective antitumor
activity of artemisinins (42,43,66).
Consistent with this, we showed that ART decreased CD71 levels in
the cell membrane. A recent study by Ba et al(66) showed that CD71 internalization is
mediated by the artemisinin-derived compound, DHA, and this
internalization may disrupt cellular iron uptake, leading to cell
growth arrest and cell death. However, in our study we found that
the internalization of ART depends on the CD71 expression and,
consequently, it influences the ART cytotoxicity, indicating that
ART is internalized by an endocytic pathway together with CD71,
probably in a similar way that transferrin is used to internalize
iron into the cell (41). If this
mechanism is involved in the action of ART, then reducing the
expression of CD71 should render the cancer cells unresponsive to
ART (since ART will not be internalized and will be rendered
ineffective). Knocking down the CD71 receptor by RNAi lowered the
cytotoxicity associated to ART, in accordance with Efferth et
al(37) who used antibody to
block the transferrin receptor (44). However, reducing the CD71 expression
did not abolish ART-mediated cytotoxicity in RB. This residual ART
cytotoxicity may indicate that the cytotoxicity mediated by CD71
might not be the only mechanism by which ART is internalized and/or
exerts its cytotoxic action (44,67).
On the other hand, the residual CD71 at the membrane may be
sufficient for enough amounts of ART to enter the cell and exert
its action.
Finally, in agreement with other studies, low doses
of ART are sufficient to alter cell cycle progression (68). RB cells are arrested in the G1 phase
according to our data. In addition, the proportion of cells in the
S phase decreased significantly as it is expected if the iron
metabolism is affected (44).
Arts may act via multiple mechanisms. The toxicity
of artemisinin-related compounds is attributed to iron-mediated
oxidative damage by generating reactive oxygen species (ROS) and/or
carbon-centered radicals (69,70).
Both products may play an important role in inducing DNA damage,
mitochondrial depolarization and apoptosis (40,44).
Then, cancer cells may suffer more severe damage due to the
elevated iron levels that support their high cellular metabolism
(71–73). Transferrin receptor plays a key role
since it increases the iron level inside the cell. Cancer cells
have elevated levels of this receptor at the plasma membrane
(37,74). Ba et al(66) showed that in a hepatoma and breast
cancer cell line, DHA (an ART derivative) acts through regulating
cell-surface TfR-1. They proposed that in the presence of ART, the
CD71 receptor is internalized through a non-classical endocytic
pathway. In doing so, the iron uptake is altered. We showed here
that ART is internalized by the transferrin receptor CD71 (and
probably together with the receptor).
We propose that ART uses the CD71 endocytic pathway
to be internalized into the cell, reducing CD71 levels at the
plasma membrane, therefore blocking iron uptake, damaging the cells
by a mechanism independent of oxidative damage. However, our
results do not exclude that ART exerts additional cytotoxic actions
once it is inside the cell. For example, it has been shown that ART
may act on the activation of the mitochondrial intrinsic apoptotic
pathway leading to cell death (75), among others (60,76–78).
In summary, the present study showed that ART has a
strong cytotoxic effect on RB cells with low cytotoxicity on normal
retina cells. We propose that ART is a sound and potentially safe
candidate to treat RB. A randomized study in vivo may
provide further insight into the efficiency of the treatment.
References
|
1
|
Shields CL and Shields JA: Recent
developments in the management of retinoblastoma. J Pediatr
Ophthalmol Strabismus. 36:8–18; quiz 35–36. 1999.
|
|
2
|
Shields CL and Shields JA: Diagnosis and
management of retinoblastoma. Cancer Control. 11:317–327. 2004.
|
|
3
|
Dimaras H, Kahaki K, O’Dimba EA, et al:
Retinoblastoma. Lancet. 379:1436–1446. 2012. View Article : Google Scholar
|
|
4
|
Kivela T: The epidemiological challenge of
the most frequent eye cancer: retinoblastoma, an issue of birth and
death. Br J Ophthalmol. 93:1129–1131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bai S, Ren R, Shi J, et al: Retinoblastoma
in the Beijing Tongren Hospital from 1957 to 2006:
clinicopathological findings. Br J Ophthalmol. 95:1072–1076. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao J, Li S, Shi J and Wang N: Clinical
presentation and group classification of newly diagnosed
intraocular retinoblastoma in China. Br J Ophthalmol. 95:1372–1375.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Samaila MO: Malignant tumours of childhood
in Zaria. Afr J Paediatr Surg. 6:19–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
MacCarthy A, Draper GJ, Steliarova-Foucher
E and Kingston JE: Retinoblastoma incidence and survival in
European children (1978–1997). Report from the Automated Childhood
Cancer Information System project. Eur J Cancer. 42:2092–2102.
2006.
|
|
9
|
Broaddus E, Topham A and Singh AD:
Incidence of retinoblastoma in the USA: 1975–2004. Br J Ophthalmol.
93:21–23. 2009.
|
|
10
|
Chantada G, Fandiño A, Manzitti J, Urrutia
L and Schvartzman E: Late diagnosis of retinoblastoma in a
developing country. Arch Dis Child. 80:171–174. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schipper J, Tan KE and van Peperzeel HA:
Treatment of retinoblastoma by precision megavoltage radiation
therapy. Radiother Oncol. 3:117–132. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gallie BL, Budning A, DeBoer G, et al:
Chemotherapy with focal therapy can cure intraocular retinoblastoma
without radiotherapy. Arch Ophthalmol. 114:1321–1328. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kingston JE, Hungerford JL, Madreperla SA
and Plowman PN: Results of combined chemotherapy and radiotherapy
for advanced intraocular retinoblastoma. Arch Ophthalmol.
114:1339–1343. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Veal GJ and Boddy AV: Carboplatin dosing
in infants with retinoblastoma: a case for therapeutic drug
monitoring. J Clin Oncol. 30:34242012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rodriguez-Galindo C, Wilson MW, Haik BG,
et al: Treatment of intraocular retinoblastoma with vincristine and
carboplatin. J Clin Oncol. 21:2019–2025. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Varan A, Kiratli H, Aydin B, et al: The
treatment of retinoblastoma with four-drug regimen including
cisplatin, etoposide, vincristine, and cyclophosphamide. Pediatr
Hematol Oncol. 29:529–537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chantada G, Fandino A, Casak S, Manzitti
J, Raslawski E and Schvartzman E: Treatment of overt extraocular
retinoblastoma. Med Pediatr Oncol. 40:158–161. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao YJ, Qian J, Yue H, Yuan YF, Xue K and
Yao YQ: Clinical characteristics and treatment outcome of children
with intraocular retinoblastoma: a report from a Chinese
cooperative group. Pediatr Blood Cancer. 57:1113–1116. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leahey A: A cautionary tale: dosing
chemotherapy in infants with retinoblastoma. J Clin Oncol.
30:1023–1024. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shields CL, Kaliki S, Shah SU, et al:
Minimal exposure (one or two cycles) of intra-arterial chemotherapy
in the management of retinoblastoma. Ophthalmology. 119:188–192.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chan HS, Lu Y, Grogan TM, et al: Multidrug
resistance protein (MRP) expression in retinoblastoma correlates
with the rare failure of chemotherapy despite cyclosporine for
reversal of P-glycoprotein. Cancer Res. 57:2325–2330.
1997.PubMed/NCBI
|
|
22
|
Gobin YP, Dunkel IJ, Marr BP, Brodie SE
and Abramson DH: Intra-arterial chemotherapy for the management of
retinoblastoma: four-year experience. Arch Ophthalmol. 129:732–737.
2011.PubMed/NCBI
|
|
23
|
Qaddoumi I, Bass JK, Wu J, et al:
Carboplatin-associated ototoxicity in children with retinoblastoma.
J Clin Oncol. 30:1034–1041. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rizzuti AE, Dunkel IJ and Abramson DH: The
adverse events of chemotherapy for retinoblastoma: What are they?
Do we know? Arch Ophthalmol. 126:862–865. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abramson DH, Lawrence SD, Beaverson KL,
Lee TC, Rollins IS and Dunkel IJ: Systemic carboplatin for
retinoblastoma: change in tumour size over time. Br J Ophthalmol.
89:1616–1619. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marees T, van Leeuwen FE, de Boer MR,
Imhof SM, Ringens PJ and Moll AC: Cancer mortality in long-term
survivors of retinoblastoma. Eur J Cancer. 45:3245–3253. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Araki Y, Matsuyama Y, Kobayashi Y, et al:
Secondary neoplasms after retinoblastoma treatment: retrospective
cohort study of 754 patients in Japan. Jpn J Clin Oncol.
41:373–379. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Turaka K, Shields CL, Meadows AT and
Leahey A: Second malignant neoplasms following chemoreduction with
carboplatin, etoposide, and vincristine in 245 patients with
intraocular retinoblastoma. Pediatr Blood Cancer. 59:121–125. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tu Y: The discovery of artemisinin
(qinghaosu) and gifts from Chinese medicine. Nat Med. 17:1217–1220.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Balint G: Artemisinin and its derivatives:
an important new class of antimalarial agents. Pharmacol Ther.
90:261–265. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chaturvedi D, Goswami A, Saikia PP, Barua
NC and Rao PG: Artemisinin and its derivatives: a novel class of
anti-malarial and anti-cancer agents. Chem Soc Rev. 39:435–454.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Efferth T, Giaisi M, Merling A, Krammer PH
and Li-Weber M: artesunate induces ROS-mediated apoptosis in
doxorubicin-resistant T leukemia cells. PLoS One. 2:e6932007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lai HC, Singh NP and Sasaki T: Development
of artemisinin compounds for cancer treatment. Invest New Drugs.
31:230–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gong Y, Gallis BM, Goodlett DR, et al:
Effects of transferrin conjugates of artemisinin and artemisinin
dimer on breast cancer cell lines. Anticancer Res. 33:123–132.
2013.PubMed/NCBI
|
|
35
|
Gong XM, Zhang Q, Torossian A, Cao JP and
Fu S: Selective radiosensitization of human cervical cancer cells
and normal cells by artemisinin through the abrogation of
radiation-induced G2 block. Int J Gynecol Cancer. 22:718–724. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kerb R, Fux R, Mörike K, Kremsner PG, Gil
JP, Gleiter CH and Schwab M: Pharmacogenetics of antimalarial
drugs: effect on metabolism and transport. Lancet Infect Dis.
9:760–774. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Efferth T, Benakis A, Romero MR, et al:
Enhancement of cytotoxicity of artemisinins toward cancer cells by
ferrous iron. Free Radic Biol Med. 37:998–1009. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Berdelle N, Nikolova T, Quiros S, Efferth
T and Kaina B: artesunate induces oxidative DNA damage, sustained
DNA double-strand breaks, and the ATM/ATR damage response in cancer
cells. Mol Cancer Ther. 10:2224–2233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li YSF, Wu JM, Wu GS, Ding J, Xiao D, Yang
WY, Atassi G, Léonce S, Caignard DH and Renard P: Novel antitumor
artemisinin derivatives targeting G1 phase of the cell cycle.
Bioorg Med Chem Lett. 11:5–8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
O’Neill PM, Barton VE and Ward SA: The
molecular mechanism of action of artemisinin - the debate
continues. Molecules. 15:1705–1721. 2010.PubMed/NCBI
|
|
41
|
Hentze MW, Muckenthaler MU, Galy B and
Camaschella C: Two to tango: regulation of Mammalian iron
metabolism. Cell. 142:24–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shpyleva SI, Tryndyak VP, Kovalchuk O, et
al: Role of ferritin alterations in human breast cancer cells.
Breast Cancer Res Treat. 126:63–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Richardson DR, Kalinowski DS, Lau S,
Jansson PJ and Lovejoy DB: Cancer cell iron metabolism and the
development of potent iron chelators as anti-tumour agents. Biochim
Biophys Acta. 1790:702–717. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Crespo-Ortiz MP and Wei MQ: Antitumor
activity of artemisinin and its derivatives: from a well-known
antimalarial agent to a potential anticancer drug. J Biomed
Biotechnol. 2012:2475972012.PubMed/NCBI
|
|
45
|
Altschul SF, Madden TL, Schäffer AA, Zhang
J, Zhang Z, Miller W and Lipman DJ: Gapped BLAST and PSI-BLAST: a
new generation of protein database search programs. Nucleic Acids
Res. 25:3389–3402. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Green MD, Mount DL, Wirtz RA and White NJ:
A colorimetric field method to assess the authenticity of drugs
sold as the antimalarial artesunate. J Pharm Biomed Anal. 24:65–70.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Okwelogu C, Clark B, de Matas M, et al:
Design of a fixed-dose paediatric combination of artesunate and
amodiaquine hydrochloride. Int J Pharm. 387:19–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Calvert AH, Newell DR, Gumbrell LA, et al:
Carboplatin dosage: prospective evaluation of a simple formula
based on renal function. J Clin Oncol. 7:1748–1756. 1989.PubMed/NCBI
|
|
49
|
Shimokata T, Ando Y, Yasuda Y, et al:
Prospective evaluation of pharmacokinetically guided dosing of
carboplatin in Japanese patients with cancer. Cancer Sci.
101:2601–2605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Krishnakumar S, Mallikarjuna K, Desai N,
et al: Multidrug resistant proteins: P-glycoprotein and lung
resistance protein expression in retinoblastoma. Br J Ophthalmol.
88:1521–1526. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wilson MW, Fraga CH, Fuller CE, et al:
Immunohistochemical detection of multidrug-resistant protein
expression in retinoblastoma treated by primary enucleation. Invest
Ophthalmol Vis Sci. 47:1269–1273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Deng DA, Xu CH and Cai JC: Derivatives of
arteannuin B with antileukemia activity. Yao Xue Xue Bao.
27:317–320. 1992.(In Chinese).
|
|
53
|
Sun WC, Han JX, Yang WY, Deng DA and Yue
XF: Antitumor activities of 4 derivatives of artemisic acid and
artemisinin B in vitro. Zhongguo Yao Li Xue Bao. 13:541–543.
1992.(In Chinese).
|
|
54
|
Woerdenbag HJ, Moskal TA, Pras N, et al:
Cytotoxicity of artemisinin-related endoperoxides to Ehrlich
ascites tumor cells. J Nat Prod. 56:849–856. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang XP, Pan QC, Ling YJ, et al: Study on
antitumor effect of sodium artesunate. Cancer. 16:186–187. 1997.(In
Chinese).
|
|
56
|
Efferth T, Dunstan H, Sauerbrey A, Miyachi
H and Chitambar CR: The anti-malarial artesunate is also active
against cancer. Int J Oncol. 18:767–773. 2001.PubMed/NCBI
|
|
57
|
Buommino E, Baroni A, Canozo N, et al:
Artemisinin reduces human melanoma cell migration by
down-regulating alpha V beta 3 integrin and reducing
metalloproteinase 2 production. Invest New Drugs. 27:412–418. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Morrissey C, Gallis B, Solazzi JW, et al:
Effect of artemisinin derivatives on apoptosis and cell cycle in
prostate cancer cells. Anticancer Drugs. 21:423–432. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Du JH, Zhang HD, Ma ZJ and Ji KM:
Artesunate induces oncosis-like cell death in vitro and has
antitumor activity against pancreatic cancer xenografts in vivo.
Cancer Chemother Pharmacol. 65:895–902. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Efferth TSA, Olbrich A, Gebhart E, Rauch
P, Weber HO, Hengstler JG, Halatsch ME, Volm M, Tew KD, Ross DD and
Funk JO: Molecular modes of action of artesunate in tumor cell
lines. Mol Pharmacol. 64:382–394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Singh NP and Verma KB: Case report of a
laryngeal squamous cell carcinoma treated with artesunate. Arch
Oncol. 10:279–280. 2002. View Article : Google Scholar
|
|
62
|
Torti SV and Torti FM: Ironing out cancer.
Cancer Res. 71:1511–1514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Shterman N, Kupfer B and Moroz C:
Comparison of transferrin receptors, iron content and isoferritin
profile in normal and malignant human breast cell lines.
Pathobiology. 59:19–25. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Daniels TR, Delgado T, Rodriguez JA,
Helguera G and Penichet ML: The transferrin receptor part I:
biology and targeting with cytotoxic antibodies for the treatment
of cancer. Clin Immunol. 121:144–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Daniels TR, Delgado T, Helguera G and
Penichet ML: The transferrin receptor part II: targeted delivery of
therapeutic agents into cancer cells. Clin Immunol. 121:159–176.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ba Q, Zhou N, Duan J, et al:
Dihydroartemisinin exerts its anticancer activity through depleting
cellular iron via transferrin receptor-1. PLoS One. 7:e427032012.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Eichhorn T, Schloissnig S, Hahn B, et al:
Bioinformatic and experimental fishing for artemisinin-interacting
proteins from human nasopharyngeal cancer cells. Mol Biosyst.
8:1311–1318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhao Y, Jiang W, Li B, et al: Artesunate
enhances radiosensitivity of human non-small cell lung cancer A549
cells via increasing NO production to induce cell cycle arrest at
G2/M phase. Int Immunopharmacol. 11:2039–2046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mercer AE, Maggs JL, Sun XM, et al:
Evidence for the involvement of carbon-centered radicals in the
induction of apoptotic cell death by artemisinin compounds. J Biol
Chem. 282:9372–9382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mercer AE, Copple IM, Maggs JL, O’Neill PM
and Park BK: The role of heme and the mitochondrion in the chemical
and molecular mechanisms of mammalian cell death induced by the
artemisinin antimalarials. J Biol Chem. 286:987–996. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lai H and Singh NP: Selective cancer cell
cytotoxicity from exposure to dihydroartemisinin and
holotransferrin. Cancer Lett. 91:41–46. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lai H, Sasaki T and Singh NP: Targeted
treatment of cancer with artemisinin and artemisinin-tagged
iron-carrying compounds. Expert Opin Ther Targets. 9:995–1007.
2005. View Article : Google Scholar
|
|
73
|
Nakase I, Lai H, Singh NP and Sasaki T:
Anticancer properties of artemisinin derivatives and their targeted
delivery by transferrin conjugation. Int J Pharm. 354:28–33. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kwok JC and Richardson DR: The iron
metabolism of neoplastic cells: alterations that facilitate
proliferation? Crit Rev Oncol Hematol. 42:65–78. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hamacher-Brady A, Stein HA, Turschner S,
et al: Artesunate activates mitochondrial apoptosis in breast
cancer cells via iron-catalyzed lysosomal reactive oxygen species
production. J Biol Chem. 286:6587–6601. 2011. View Article : Google Scholar
|
|
76
|
Krishna S, Bustamante L, Haynes RK and
Staines HM: Artemisinins: their growing importance in medicine.
Trends Pharmacol Sci. 29:520–527. 2008. View Article : Google Scholar
|
|
77
|
Kelter G, Steinbach D, Konkimalla VB, et
al: Role of transferrin receptor and the ABC transporters ABCB6 and
ABCB7 for resistance and differentiation of tumor cells towards
artesunate. PLoS One. 2:e7982007. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hartwig CL, Rosenthal AS, D’Angelo J,
Griffin CE, Posner GH and Cooper RA: Accumulation of artemisinin
trioxane derivatives within neutral lipids of Plasmodium
falciparum malaria parasites is endoperoxide-dependent. Biochem
Pharmacol. 77:322–336. 2009. View Article : Google Scholar : PubMed/NCBI
|